Abstract
Polyethylenimine (PEI) has been used widely in transient gene expression studies of mammalian cells. We performed transient gene expression in suspension Chinese hamster ovary cells using a one-step transfection procedure in which DNA and PEI were simultaneously added to a cell culture in suspension without prior PEI/DNA complex incubation. To further understand the effect of PEI/DNA formation on the transfection and expression of exogenous gene in shaking state, we investigated the diameter and overcharge of the PEI/DNA complex. The results showed that the diameter of the complex was smaller with more positive charge when the PEI/DNA ratio was higher. Moreover, DNA more easily penetrated cells and nuclei at higher PEI concentrations. The highest transcription level, transfection efficiency, and GFP expression were obtained when the PEI/DNA ratio was 5:1.
Keywords: Transient gene expression, Polyethylenimine (PEI), CHO cells, One-step transfection
Introduction
Chinese hamster ovary (CHO) cells are widely used in the biotechnology industry to produce recombinant proteins (Derouazi et al. 2004; Wurm and Bernard 1999). The most rapid and least expensive method for recombinant protein expression in mammalian cells is transient gene expression (TGE). This approach has made it possible to produce milligram-to-gram quantities of a recombinant protein within days or weeks (Wurm and Bernard 1999; Derouazi et al. 2004). Its ability to generate proteins within a relatively short timeframe enables TGE to evaluate functionalities and toxicities much earlier and therefore provide valuable information about drug candidates (Girard et al. 2002; Jordan et al. 1996). Transiently expressed materials are ideal for preclinical and early clinical trials because their use represents a faster and less expensive approach than stable expression (Derouazi et al. 2004).
Gene transfer is a critical step in TGE. Many chemical agents have been used for the transfer of DNA into cultivated mammalian cells, including inorganic compounds, cationic polymers, and cationic lipids (Jordan et al. 1996). The most efficient cationic polymer is polyethylenimine (PEI), which exhibits high positive charge density, because of its high efficiency in a broad range of cell lines, low cell toxicity, and low cost (Boussif et al. 1995; Schlaeger and Christensen 1999; Baldi et al. 2007; Hanzlíková et al. 2011). PEI spontaneously forms stable complexes with DNA that can be used for gene delivery both in vivo and in vitro (Boussif et al. 1995; Coll et al. 1999; Bertschinger et al. 2006). As a result of its tertiary amino groups and tertiary structure, PEI demonstrates a buffering capacity over a wide pH range (Tang and Szoka 1997; Kichler et al. 2001). Thus, PEI prevents hydrolysis in endosomes and helps the complex escape from the endosomal compartment through a proton sponge mechanism (Behr 1997; Remy et al. 1998; Akinc et al. 2005). Commercially available PEIs ranging in molecular weight from 25,000 to 800,000 Da, most notably linear or branched PEI (25 kDa), have been successfully used for in vivo and in vitro gene delivery of DNA (Boussif et al. 1995; Baker and Cotten 1997; Baker et al. 1997; Meunier-Durmort et al. 1997; Fischer et al. 1999; Schlaeger and Christensen 1999; Derouazi et al. 2004; Han et al. 2007, 2009).
According to the classic protocol for transfection, PEI and DNA are mixed and incubated in 100 mM NaCl for a few minutes to form complex before transfection. A new and simpler method of TGE without priori formation of the PEI/DNA complex has been reported recently, and it has been confirmed to be better than a priori complex formation under certain conditions (Backliwal et al. 2008). In the present study, we found that a one-step transfection process could produce higher transfection efficiency compared with the standard two-step process; however, there is no significant difference in GFP expression between these methods. In the one-step process, the PEI/DNA complex would form and be absorbed by cells in a turbulent environment, which significantly differs from the resting state. We thus investigated the formation of the PEI/DNA complex using different PEI/DNA ratios and the effects of PEI/DNA in situ formation on transfection under the above-described conditions.
Materials and methods
Preparation of plasmid and PEI solution
Plasmid DNA encoding enhanced green fluorescent protein (eGFP) under the human cytomegalovirus promoter was amplified in Escherichia coli and isolated using an MN NucleoBondXtra Maxi Plus kit (Macherey-Nagel, Dueren, Germany). The pIRESneo3-eGFP plasmid was confirmed by agarose gel electrophoresis. The concentrations and purity of the plasmids were determined by measuring absorbance at 260 and 280 nm. Linear PEI (25 kDa; Polysciences, Warrington, PA, USA) was dissolved to 1 mg/mL in ultrapure water and filtered by a 0.2 μm filter (Millipore, Billerica, MA, USA).
PEI/DNA complex diameter distribution
DNA plasmid (12.5 μg) was added to ProCHO5 medium (Lonza, Verviers, Belgium) in TubeSpin Bioreactor 50 tubes (TPP, Trasadingen, Switzerland), to which different volumes of PEI solution were then added to maintain the PEI/DNA weight ratio (w/w) at 1:1, 3:1, 5:1, and 7:1. The mixtures were kept at 37 °C with 5 % CO2 and 85 % humidity, with agitation at 180 rpm. The samples were detected using Malvern Zetasizer Nano ZS (Malvern Instruments, Westborough, MA, USA) at different times.
Gel retardation assay
PEI/DNA complexes were formed by shaking, and samples were taken 0, 10, and 60 min after mixing. A series of PEI/DNA weight ratios (w/w) with loading dye were loaded on the agarose gel (10 mL of the sample containing 0.2 mg of DNA). Electrophoresis was carried out at a constant voltage of 100 V for 20 min in TBE buffer (4.45 mM Tris–base, 1 mM sodium EDTA, and 4.45 mM boric acid, pH 8.3) containing 0.5 g/mL of ethidium bromide. The plasmid DNA bands were then visualized under a UV transilluminator at a wavelength of 365 nm (Maeda et al. 2005).
Covalent labeling of PEI and DNA
pIRESneo3-eGFP was covalently labeled and purified with a Mirus Label IT Cy3 Labeling Kit (Mirus Bio LLC, Madison, WI, USA) following the manufacturer’s protocol. PEI was dissolved to 1 mg/mL in 0.1 M NaHCO3 (pH 9.3). Cy5 mono-reactive dye (0.01 mg) was added to 1 mL of PEI solution, and the reaction was incubated for 1 h at room temperature with intermittent mixing. A 10 kDa ultrafiltration tube (Millipore) centrifuged at 13,400 rpm for 10 min was used to eliminate free Cy5. The concentration of PEI was quantified by spectrophotometric analysis (Han et al. 2009).
Cell culture and transfection
Suspension-adapted CHO DG44 cells were routinely cultivated in ProCHO5 medium supplemented with 54.4 mg/L of hypoxanthine (Sigma, St. Louis, MO, USA), 15.5 mg/L of thymine (Sigma), and 4 mM of l-glutamine (Sigma). Cells were maintained in suspension in TubeSpin Bioreactor 50 tubes at 37 °C and 5 % CO2. They were subcultured twice a week, with the densities kept within the range of 0.5–2 × 106 cells/mL.
On the day of transfection, the plasmid DNA and 25 kDa linear PEI were sequentially added to the cells at a density of 2 × 106 cells/mL in 5 mL of ProCHO5 medium. The transfected culture was incubated at 37 °C with 5 % CO2 and 85 % humidity, with agitation at 180 rpm. The cell density at the time of transfection and the amounts of PEI and plasmid DNA were varied to optimize the transfection method. All transfections were performed in triplicate at a final volume of 5 mL in TubeSpin tubes.
Confocal laser scanning microscopy
Cells were transfected with Cy5-PEI/Cy3-DNA using the same transfection procedures as those described above, and samples were collected at 1 and 8 h. Cells were washed with PBS twice and then treated with 4 % paraformaldehyde at 4 °C for 1 h. Subsequently, the cells were washed with PBS twice, used to stain the nuclei with DAPI staining solution (Beyotime Inst. Biotechnol., Haimen, China) for 5 min, and washed again with PBS twice. The samples were examined using Carl Zeiss Laser Scanning System LSM 510. Laser with excitation lines at 405, 543, and 633 nm was used to induce fluorescence. DAPI, Cy3-DNA, and Cy5-PEI fluorescence were detected using BP420-480, LP560, and LP615 lenses, respectively.
Quantitative real-time PCR analysis
Cells were first lysed by TRIZOL (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted by chloroform and purified using 70 % alcohol. First-strand cDNA was prepared with an LK-RT-0050 reverse transcription kit (Land, China). Quantitative real-time PCR analysis was performed with 40 cycles of melting at 94 °C for 15 s and extension at 60 and 75 °C for 15 s each. Thunderbird SYBR qPCR Mix (TOYOBO, Shanghai, China) was used as replication enzyme.
GFP fluorescence intensity quantification
Cells were harvested 48 h after transfection, followed by washing twice with PBS, and then resuspended in PBS at a cell density of 2 × 106 cells/mL. One hundred microliters of the suspension was added to each well of a 96-well plate and measured by TECAN Saphire II plate-reading fluorometer (TECAN, Shanghai, China). GFP was excited at 485 nm with a bandwidth of 10 nm, and the emission fluorescence was measured at 515 nm with a bandwidth of 10 nm. All samples were analyzed in triplicate.
Results
Comparison between the one-step process and the two-step process
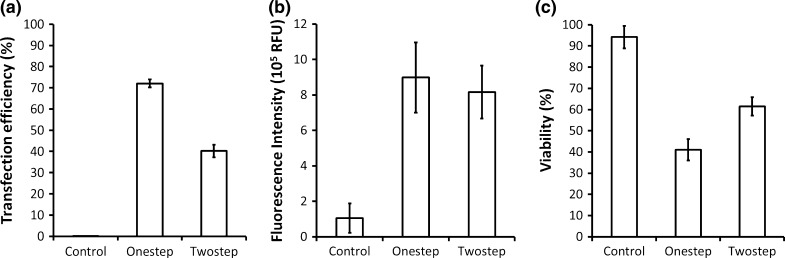
The CHO DG44 cells were maintained in suspension in TubeSpin bioreactor 50 tubes at 37 °C and 5 % CO2. In the one-step process, 12.5 μg of pIRESneo3-eGFP plasmid was directly added to 107 cells in each tube, followed by the addition of 62.5 μg of PEI into the tubes to keep the PEI/DNA ratio at 5:1. In the two-step process, plasmid and PEI were first mixed and incubated for 5 min. The mixture was added to the cells to initiate the transfection. Treatment with the one-step process resulted in transfection efficiency rates as high as 72 %, whereas that with the two-step process yielded rates of up to 40 % (Fig. 1a). Significant differences in eGFP fluorescence intensity between the two groups were not detected (Fig. 1b); however, cell viability in the one-step group was lower than that in the two-step group (Fig. 1c).
Fig. 1.
Comparison between one-step process and two-step process. CHO DG44 cells were transfected with PEI and DNA of the ratio 5:1. Transfection efficiency, fluorescence intensity and cell viability were analysed at the 48th h after transfection. a Transfection efficiency of two different processes; b Fluorescence measurement system detected total fluorescence intensity (relative fluorescence unit (RFU)); c Percentages of living cells of total populations
Dynamics of PEI/DNA complex formation
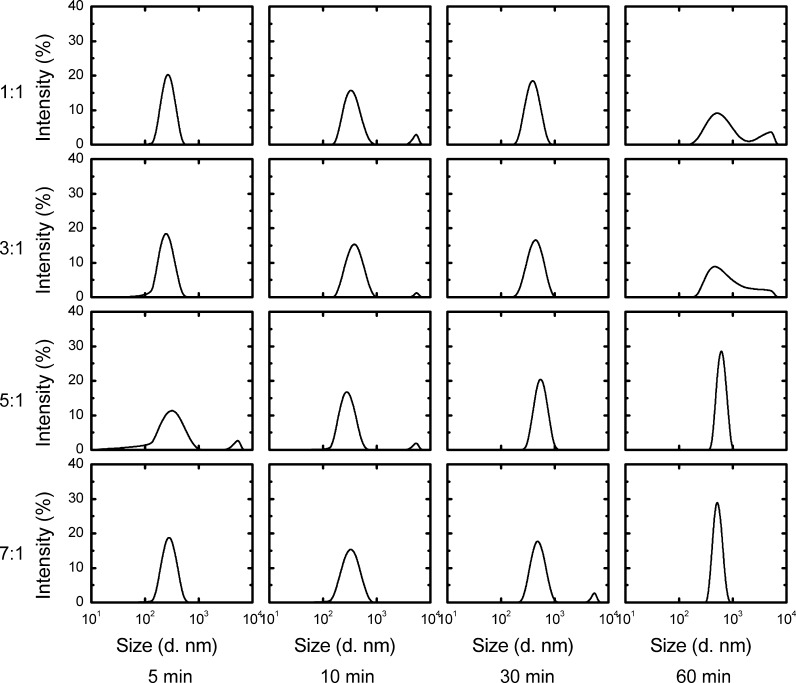
Due to the opposite electric charges of PEI and DNA, they naturally attach to each other and form a complex in physical environments (Dunlap et al. 1997). The extent of condensation depends on the PEI/DNA ratio (Minagawa et al. 1991; Godbey et al. 1999). Different PEI/DNA ratios affect the diameter and stability of the complex, which further influence gene transfection and expression.In this study, different PEI/DNA ratios were prepared and analyzed using Malvern Zetasizer. During the first 10 min, the different PEI/DNA ratios did not exhibit any variance. The diameters of all PEI/DNA complexes were approximately 277 nm after 5 min and 339 nm after 10 min. However, the diameters of complexes with different PEI/DNA ratios varied after 30 min. The mean diameters of complexes with low PEI ratios (1:1 and 3:1) were larger than those of complexes with high PEI ratios (5:1 and 7:1). Moreover, diameter distribution significantly differed between complexes with low PEI ratios and those with high PEI ratios. After 60 min, diameters varied from approximately 150 to 5500 nm and from 250 to 5000 nm when the PEI/DNA ratios were 1:1 and 3:1, respectively. When the amount of PEI increased, the diameter distribution became narrower. The diameter distribution varied from 450 to 950 nm and from 400 to 700 nm when the PEI/DNA ratios were 5:1 and 7:1, respectively (Fig. 2).
Fig. 2.
Dynamics of different PEI/DNA ratio complexes formation. DNA concentration was 2.5 μg mL−1, PEI concentration was 2.5 μg mL−1, 7.5 μg mL−1, 12.5 μg mL−1, 17.5 μg mL−1 representing the 1:1, 3:1, 5:1, 7:1 groups respectively. Reactions took place in 5 mL PBS in TubeSpin 50. Complex sizing was performed on Malvern Zetasizer at 5, 10, 30 and 60 min after mixing
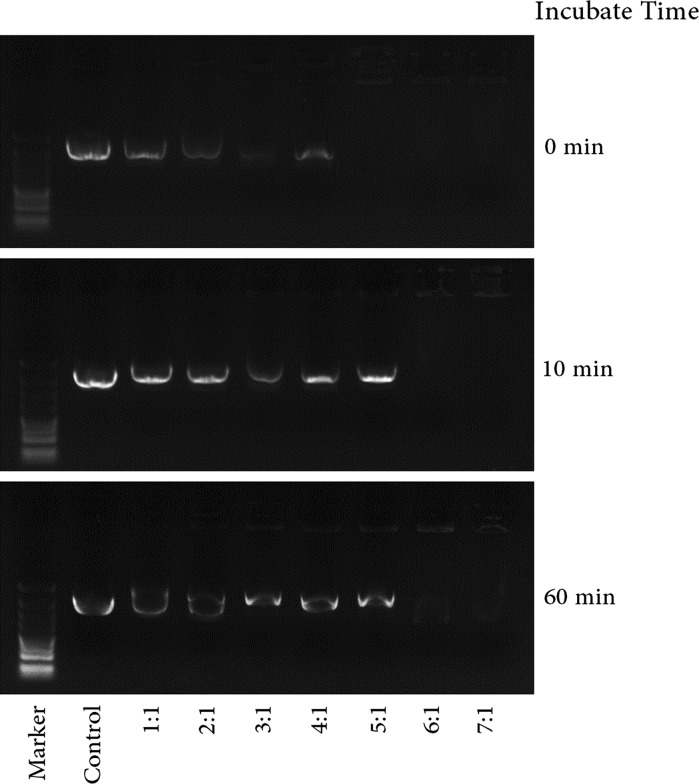
Apart from the size of the complex, the overall charge of PEI/DNA particles is another parameter that affects transfection efficiency. Moreover, different PEI/DNA ratios could change the complex charge (Davis et al. 1993; Kircheis et al. 2001; Backliwal et al. 2008). The interaction and stability of all PEI/DNA complexes were confirmed by gel retardation assay. Figure 2 shows that DNA interacted with PEI at higher concentrations (5:1 and 7:1)at the beginning of incubation and that some remained uncomplexed when the PEI/DNA ratios were 1:1 and 3:1. However, more uncomplexed DNA was found in the mixture with longer incubation time. The tertiary structure of DNA also changed, and some DNA existed as supercoiled plasmids. DNA could be neutralized by positive PEI completely only when the weight/weight ratio (PEI/DNA) was very high (more than 6:1) (Fig. 3).
Fig. 3.
Gel retardation of plasmid DNA using acetylated branched polyethylenimine with different weight/weight ratios (PEI/DNA) at different incubation times. Numbers below each lane are PEI/DNA weight/weight ratios. Plasmid DNA alone was used as a control
Delivery process of DNA by PEI
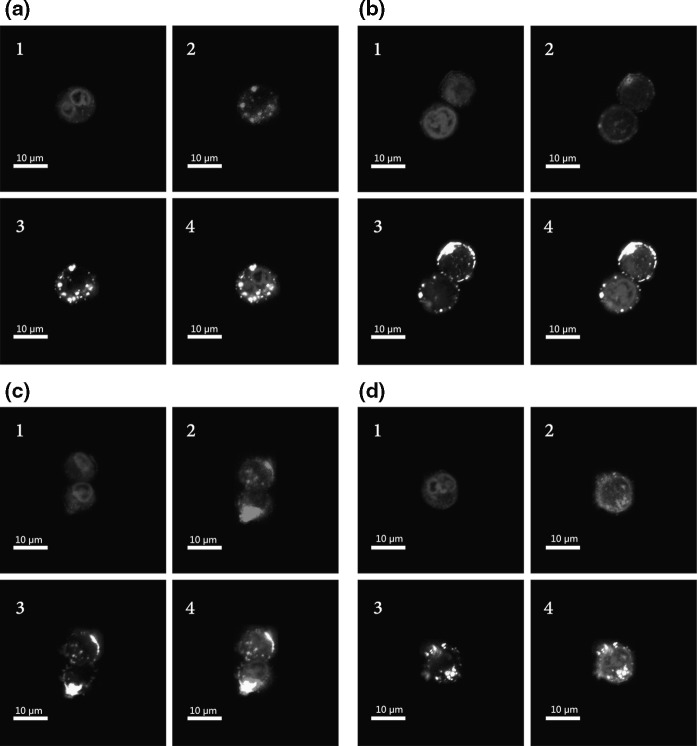
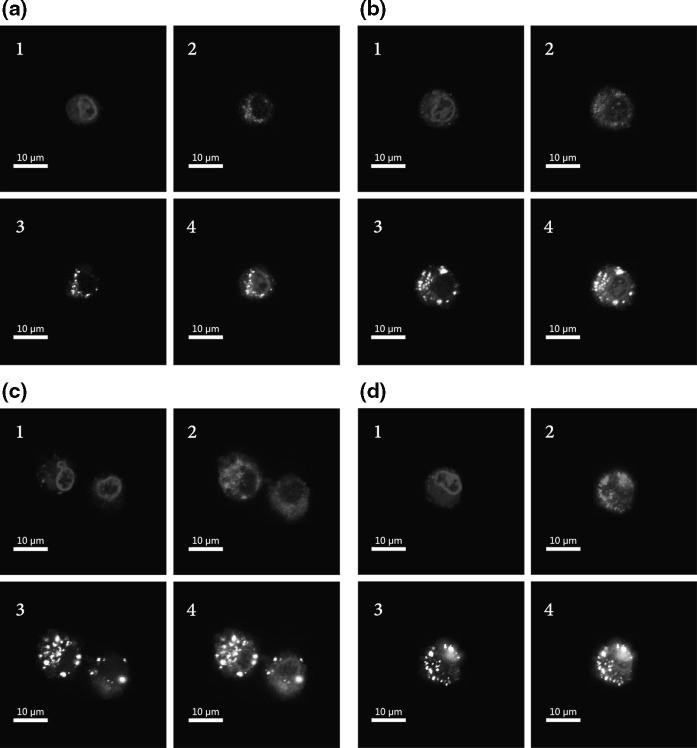
The DNA delivery process in eukaryotic cells can be divided into two steps. The first step is the transfer of DNA into the cytoplasm, and the second step is the transfer of DNA into the nucleus. To explore the DNA delivery process, we monitored the behavior of these molecules by confocal laser scanning microscopy. As shown in Fig. 4, PEI/DNA particles were absorbed into the cell cytoplasm 1 h after transfection. They gathered closely around the nuclei, but most of them still remained outside the nuclei. Many unbound PEI molecules permeated into the nuclei without DNA when the PEI/DNA ratios were 5:1 and 7:1 (Fig. 4). At 8 h post-transfection, DNA gradually entered the nucleoplasm, in which the exogenous gene could be recognized by the RNA polymerase to accomplish the function. More DNA entered the nuclei when the PEI ratio was higher. In addition, we found that the distribution pattern of DNA was not in agreement with that of PEI (Fig. 5), suggesting that some DNA dissociated from the PEI/DNA complex after its entry into the nucleus.
Fig. 4.
CLSM images of CHO DG44 cells transfected with Cy5-PEI/Cy3-pEGFP-N1 with PEI/DNA ratio of 1:1 (a), 3:1 (b), 5:1 (c), 7:1 (d) for 1 h. 1 DAPI stained nuclei (blue); 2 Cy5-PEI (red); 3 Cy3-pEGFP-N1 (yellow); 4 overlay of Cy5-PEI (red), Cy3-pEGFP-N1 (yellow), DAPI stained nuclei (blue). (Color figure online)
Fig. 5.
CLSM images of CHO DG44 cells transfected with Cy5-PEI/Cy3-pEGFP-N1 with PEI/DNA ratio of 1:1 (a), 3:1 (b), 5:1 (c), 7:1 (d) for 8 h. 1 DAPI stained nuclei (blue); 2 Cy5-PEI (red); 3 Cy3-pEGFP-N1 (yellow); 4 overlay of Cy5-PEI (red), Cy3-pEGFP-N1 (yellow), DAPI stained nuclei (blue). (Color figure online)
Transcription and expression of GFP
The CHO DG44 cells were maintained in suspension in TubeSpin bioreactor 50 tubes at 37 °C and 5 % CO2. In total, 12.5 μg of the pIRESneo3-eGFP plasmid was added to 107 cells in each tube; 12.5, 37.5, 62.5, and 87.5 μg of PEI were added to the tubes to keep the PEI/DNA ratio at 1:1, 3:1, 5:1, and 7:1, respectively. Cells were collected 48 h after transfection, and RNA was extracted. The transcription levels of GFP with different PEI/DNA ratios were investigated using real-time PCR. The transcription level was highest when PEI/DNA was 5:1, followed by 3:1 and 7:1. When the PEI/DNA ratio was 1:1, the transcription level was very low (Fig. 6a). At the same time, transfection efficiency was determined by FCM. The results showed that high PEI concentrations could significantly improve the transfection efficiency and that the transfection efficiency was nearly zero when PEI/DNA was 1:1 (Fig. 6b). The effect of PEI/DNA on fluorescence intensity was almost the same as that on transfection efficiency except when the PEI/DNA ratio was 7:1. The total fluorescence intensity improved with an increase in PEI levels, and the highest fluorescence intensity was obtained when PEI/DNA was 5:1, which was nine times that at the PEI/DNA ratio of 1:1. However, fluorescence intensity decreased when the PEI/DNA ratio was 7:1 (Fig. 6c).
Fig. 6.
CHO DG44 cells transfected with different ratios of PEI/pEGFP-N1 complexes 48 h after transfection. a Quantitative real-time PCR detected eGFP transcription level; b FACS analyses of percentages of eGFP positive cells in total populations; c Fluorescence measurement system detected total fluorescence intensity (relative fluorescence unit (RFU)); d Cell densities with a unit of 106 cells per mL; e Percentages of living cells of total populations; f Single cell fluorescence intensity (RFU/cell)
The relationship between increasing PEI and transfection efficiency is different from that between PEI concentration and fluorescence intensity. When the PEI/DNA ratio was 7:1 the fluorescence intensity was much lower than when it was 5:1, although the transfection efficiencies at these levels were the same. This finding could be explained by the cytotoxicity of PEI at high concentration. Cell density and viability decreased with an increase in PEI concentration. The cell density and viability were 0.64 × 106 cells/mL and 25 %, respectively, when the PEI/DNA ratio was 7:1, whereas the corresponding values were 1.5 × 106 cells/mL and 44 %, respectively, when the PEI/DNA ratio was 5:1 (Fig. 6d, e). The highest specific fluorescence per cell was obtained when the PEI/DNA ratio was 5:1 (Fig. 6f), possibly because this level exhibited the highest transfection and gene expression and moderate cell density.
Discussion
In our proposed method, PEI and DNA were added to cells directly in one step without prior complex formation. Compared with the standard two-step process, which requires an additional sterile vessel to premix PEI and DNA and then transfers the content into a bioreactor without sterile filtration (PEI/DNA particles cannot be sterile filtered), in situ complex formation is more amenable to upscaling and GMP compliance because of the lower contamination risk and easier handling involved. Research has suggested that in situ PEI/DNA complex formation can achieve higher reproducibility as small changes in maturation time can have a significant impact on titers (Backliwal et al. 2008). In the present study, a higher transfection efficiency was obtained with the one-step transfection procedure. However, the environment of PEI/DNA formation is very different from the resting state, indicating that the parameters affecting transfection in the one-step procedure may be different from those of the standard protocol. In fact, research has shown that in situ complex formation outperforms a priori complex formation only at higher cell densities and higher concentrations of PEI and DNA (Backliwal et al. 2008). In our study, cell viability in the one-step group was lower than that in the two-step group; however, these groups exhibited the same total GFP expression, although the former group produced a higher transfection efficiency (Fig. 1). The PEI/DNA particles in shaking state were not stable (Fig. 3), demonstrating that the cytotoxicity of free PEI was higher in the one-step method. On the other hand, some DNA could dissociate from PEI, especially under lower PEI/DNA ratios. These highlight the importance of higher cell densities and higher concentrations of PEI and DNA in one-step transfection.
We also found that the PEI/DNA ratio is essential to TGE using the one-step protocol. When the ratio was lower (1:1 and 1:3), a larger complex (150 to 5500 nm) was obtained. In contrast, when the PEI/DNA ratios were 5:1 and 7:1, the diameters varied from 450 to 950 nm and from 400 to 700 nm, respectively (Fig. 2). Previous research has suggested that compact PEI/DNA complexes are usually obtained only at higher polycation/DNA ratios with a strong positive net charge (Kircheis et al. 2001). Large particles do not easily enter the cell, and their loose structure is not stable enough to resist the low pH environment and lysosomal enzymes in the cell. High PEI concentrations could not only produce compact particles with small sizes but also increase the quantity of PEI bound to DNA, thus increasing the particle’s positive charge, which helps facilitate entry into cells and nuclei (Bertschinger et al. 2006). Furthermore, the excess of bound PEI may enhance the efficiency of endosomal escape by PEI/DNA particles (Backliwal et al. 2008). The results of the present study revealed that more DNA entered the cells and nuclei when the PEI ratio was higher and that many uncomplexed PEI molecules also permeated the nuclear membrane (Figs. 4, 5). DNA entry into the nucleus was low when the PEI/DNA ratio was 1:1, thereby resulting in low transfection efficiency (Fig. 6b).
However, small and compact particles also have a negative effect on the expression of exogenous genes. If the PEI/DNA interaction is too strong, DNA could not be completely released from the complex in the nucleus and may reduce the transcription efficiency and TGE (Kircheis et al. 2001; Backliwal et al. 2008). This can explain why the expression of GFP (total fluorescence intensity) was lower at the PEI/DNA ratio of 7:1 than that at the ratio of 5:1 even if their transfection efficiencies were the same. Another possible reason is that higher PEI retios show higher cell toxicity inducing cell death (Fig. 6). Therefore, increasing PEI within a certain range could improve the transfection efficiency and gene expression, but too much PEI will induce higher cytotoxicity and thus decrease total protein production. Only if the DNA and PEI are well balanced will the highest expression level be achieved.
Acknowledgments
This study was supported by grants from the Guangdong Provincial project for cooperation of industry and academy (2010B090400500) and Major national science and technology project for New medicine research (2012ZX09202-301-001).
Footnotes
Xie Qiuling and Guo Xinyong contributed equally to this work.
References
- Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- Backliwal G, Hildinger M, Hasija V, Wurm FM. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioeng. 2008;99:721–727. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- Baker A, Cotten M. Delivery of bacterial artificial chromosomes into mammalian cells with psoralen-inactivated adenovirus carrier. Nucleic Acids Res. 1997;25:1950–1956. doi: 10.1093/nar/25.10.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Saltik M, Lehrmann H, Killisch I, Mautner V, Lamm G, Christofori G, Cotten M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4:773. doi: 10.1038/sj.gt.3300471. [DOI] [PubMed] [Google Scholar]
- Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett. 2007;29:677–684. doi: 10.1007/s10529-006-9297-y. [DOI] [PubMed] [Google Scholar]
- Behr JP. The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA Int J Chem. 1997;51:34–36. [Google Scholar]
- Bertschinger M, Backliwal G, Schertenleib A, Jordan M, Hacker DL, Wurm FM. Disassembly of polyethylenimine-DNA particles in vitro: Implications for polyethylenimine-mediated DNA delivery. J Controlled Release. 2006;116:96–104. doi: 10.1016/j.jconrel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Nat Acad Sci. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JL, Chollet P, Brambilla E, Desplanques D, Behr JP, Favrot M. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum Gene Ther. 1999;10:1659–1666. doi: 10.1089/10430349950017662. [DOI] [PubMed] [Google Scholar]
- Davis T, Wickham T, McKenna K, Granados R, Shuler M, Wood H. Comparative recombinant protein production of eight insect cell lines. In Vitro Cell Dev Biol Anim. 1993;29:388–390. doi: 10.1007/BF02633986. [DOI] [PubMed] [Google Scholar]
- Derouazi M, Girard P, Van Tilborgh F, Iglesias K, Muller N, Bertschinger M, Wurm FM. Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng. 2004;87:537–545. doi: 10.1002/bit.20161. [DOI] [PubMed] [Google Scholar]
- Dunlap DD, Maggi A, Soria MR, Monaco L. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997;25:3095. doi: 10.1093/nar/25.15.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/A:1014861900478. [DOI] [PubMed] [Google Scholar]
- Girard P, Derouazi M, Baumgartner G, Bourgeois M, Jordan M, Jacko B, Wurm FM. 100-liter transient transfection. Cytotechnology. 2002;38:15–21. doi: 10.1023/A:1021173124640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbey W, Wu KK, Mikos AG. Poly (ethylenimine) and its role in gene delivery. J Controlled Release. 1999;60:149–160. doi: 10.1016/S0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- Han X, Sun L, Fang Q, Li D, Gong X, Wu Y, Yang S, Shen BQ. Transient expression of osteopontin in HEK 293 cells in serum-free culture. Enzyme Microb Technol. 2007;41:133–140. doi: 10.1016/j.enzmictec.2006.12.013. [DOI] [Google Scholar]
- Han X, Fang Q, Yao F, Wang X, Wang J, Yang S, Shen BQ. The heterogeneous nature of polyethylenimine-DNA complex formation affects transient gene expression. Cytotechnology. 2009;60:63–75. doi: 10.1007/s10616-009-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlíková M, Ruponen M, Galli E, Raasmaja A, Aseyev V, Tenhu H, Urtti A, Yliperttula M. Mechanisms of polyethylenimine-mediated DNA delivery: free carrier helps to overcome the barrier of cell-surface glycosaminoglycans. J Gene Med. 2011;13:402–409. doi: 10.1002/jgm.1587. [DOI] [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichler A, Leborgne C, Coeytaux E, Danos O. Polyethylenimine-mediated gene delivery: a mechanistic study. J Gene Med. 2001;3:135–144. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]
- Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53:341–358. doi: 10.1016/S0169-409X(01)00202-2. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kusakabe T, Man Lee J, Miyagawa Y, Koga K, Kawaguchi Y. Efficient nonviral gene transfer mediated by polyethylenimine in an insect cell line. J Insect Biotechnol Sericol. 2005;74:21–26. [Google Scholar]
- Meunier-Durmort C, Grimal H, Sachs L, Demeneix B, Forest C. Adenovirus enhancement of polyethylenimine-mediated transfer of regulated genes in differentiated cells. Gene Ther. 1997;4:808. doi: 10.1038/sj.gt.3300450. [DOI] [PubMed] [Google Scholar]
- Minagawa K, Matsuzawa Y, Yoshikawa K, Matsumoto M, Doi M. Direct observation of the biphasic conformational change of DNA induced by cationic polymers. FEBS Lett. 1991;295:67–69. doi: 10.1016/0014-5793(91)81386-M. [DOI] [PubMed] [Google Scholar]
- Remy JS, Abdallah B, Zanta MA, Boussif O, Behr JP, Demeneix B. Gene transfer with lipospermines and polyethylenimines. Adv Drug Deliv Rev. 1998;30:85–95. doi: 10.1016/S0169-409X(97)00109-9. [DOI] [PubMed] [Google Scholar]
- Schlaeger EJ, Christensen K. Transient gene expression in mammalian cells grown in serum-free suspension culture. Cytotechnology. 1999;30:71–83. doi: 10.1023/A:1008000327766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Szoka F. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4(8):823. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol. 1999;10:156–159. doi: 10.1016/S0958-1669(99)80027-5. [DOI] [PubMed] [Google Scholar]