Abstract
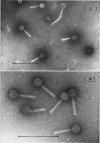
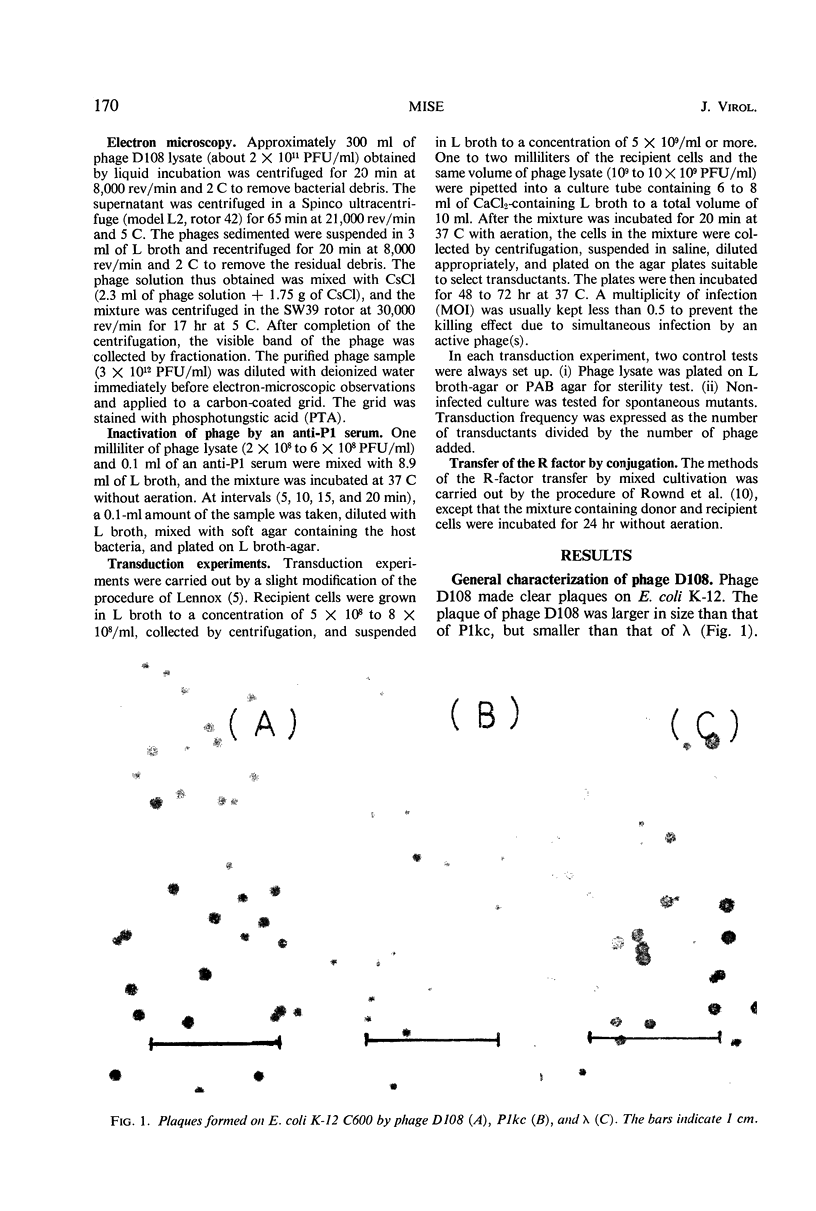
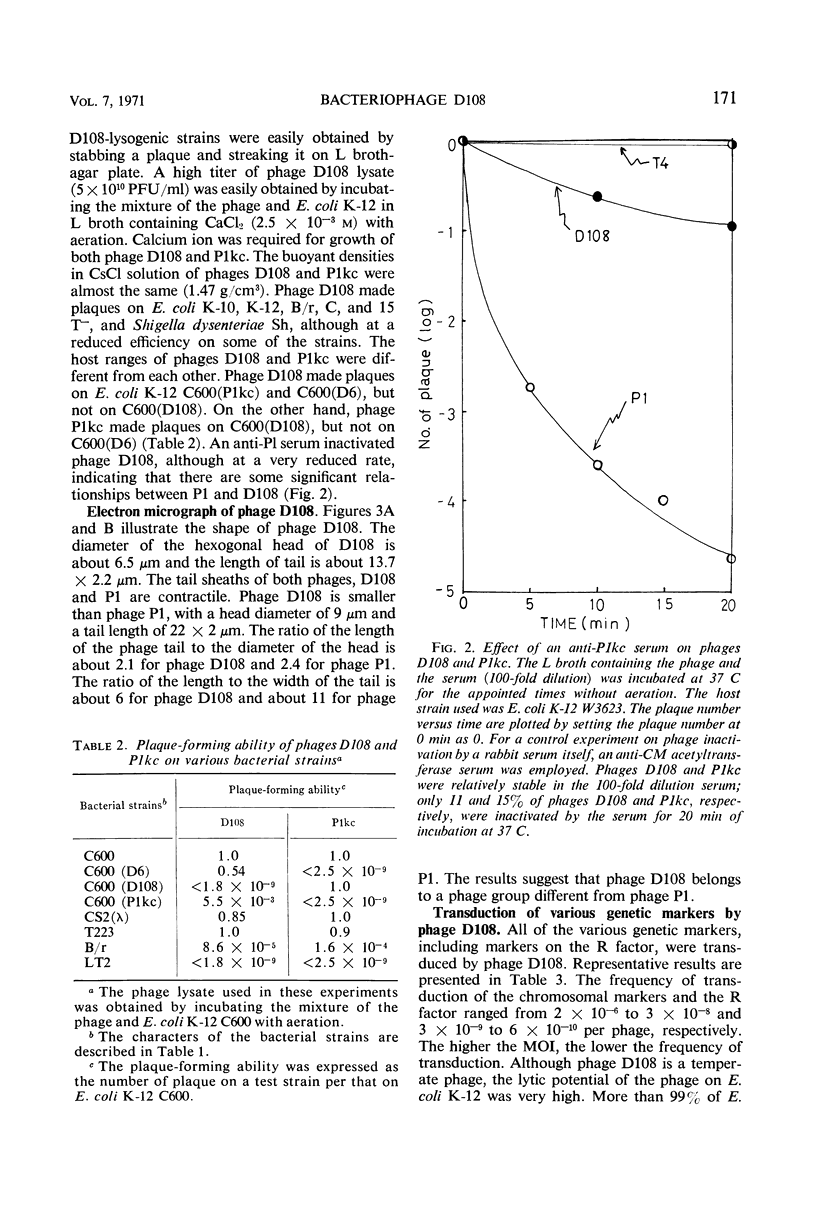
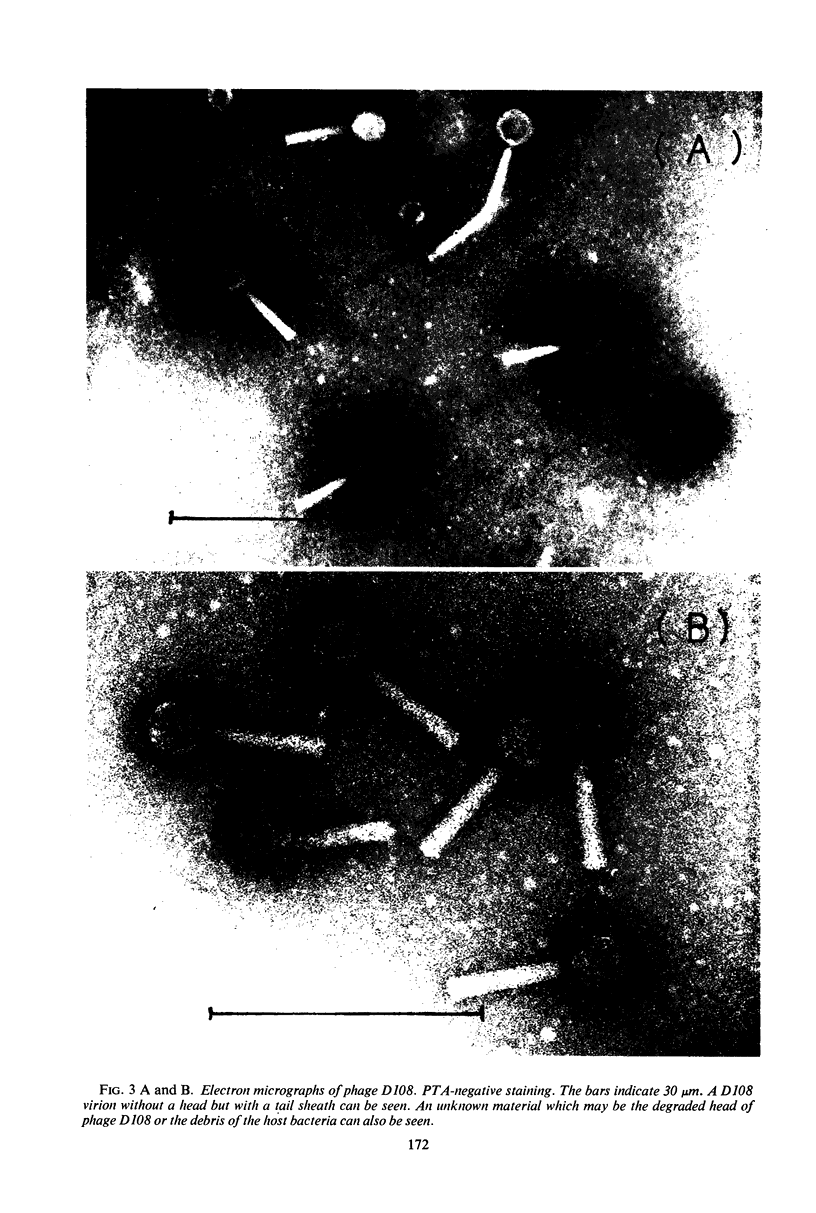
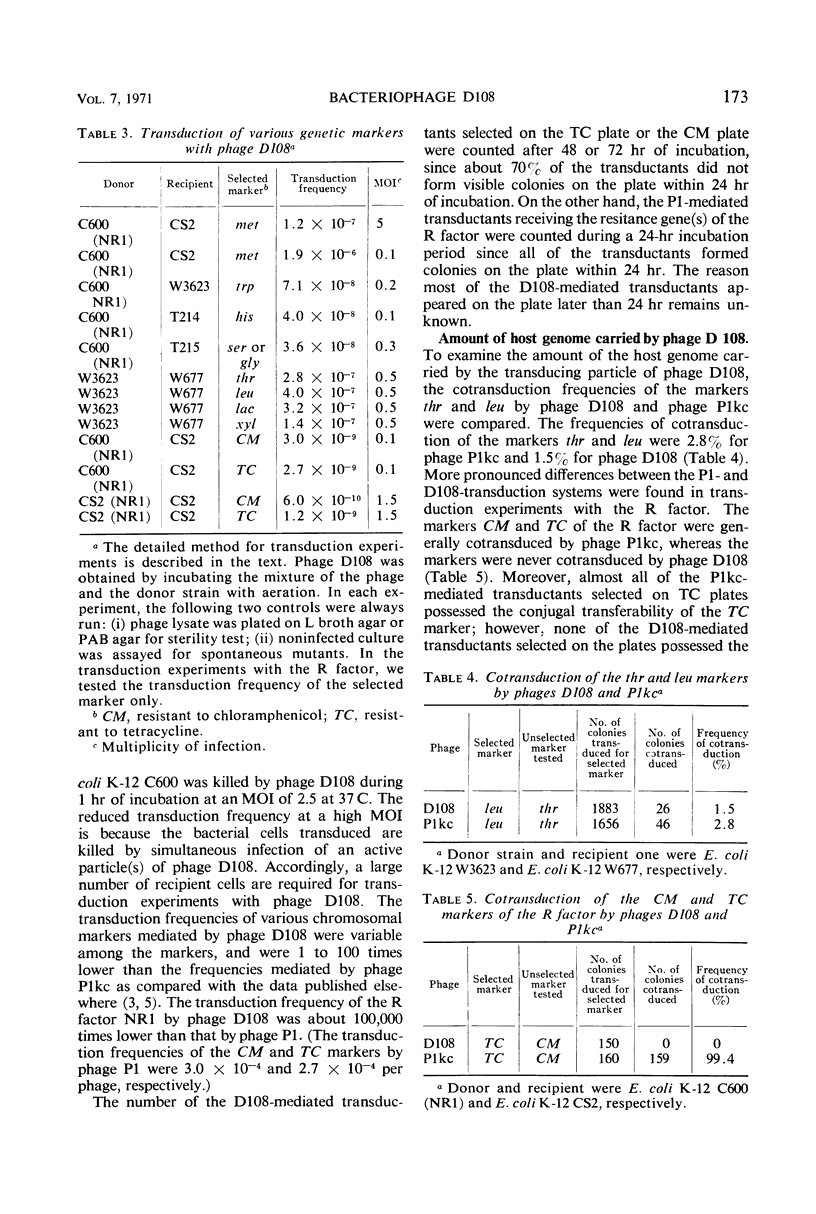
A new generalized transducing bacteriophage in the Escherichia coli system was isolated and characterized. This phage, designated D108, makes clear plaques on E. coli K-10, K-12, K-12(P1kc), K-12(D6), B/r, C, and 15 T−, and Shigella dysenteriae. The plaque of phage D108 is larger in size than that of phage P1kc. Electron-microscopic observation revealed that phages D108 and P1kc are morphologically different from each other, suggesting that phage D108 belongs to a phage group different from phage P1. The fact that all of the 10 markers tested were transduced by phage D108 indicates that this phage is a generalized transducing phage in the E. coli system. The transduction frequency by phage D108 of chromosomal markers and of a drug resistance factor (R factor) ranged from 2 × 10−6 to 3 × 10−8 and 3 × 10−9 to 6 × 10−10 per phage, respectively. The cotransduction frequency of the thr and leu markers was 2.8% for phage P1kc and 1.5% for phage D108. The CM and TC markers (chloramphenicol-resistant and tetracycline-resistant markers, respectively) of the R factor were not cotransduced by phage D108, but the markers were generally cotransduced by phage P1kc. The results suggest that the transducing particle of phage D108 contains a smaller amount of host deoxyribonucleic acid than does phage P1kc.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOWES R. C., ROWLEY D. Some observations on linkage effects in genetic recombination in Escherichia coli K-12. J Gen Microbiol. 1954 Oct;11(2):250–260. doi: 10.1099/00221287-11-2-250. [DOI] [PubMed] [Google Scholar]
- Drexler H. Transduction by bacteriophage T1. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1083–1088. doi: 10.1073/pnas.66.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- JACOB F. Transduction of lysogeny in Escherichia coli. Virology. 1955 Jul;1(2):207–220. doi: 10.1016/0042-6822(55)90017-9. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Mise K., Suzuki K. New generalized transducing bacteriopahge in Echerichia coli. J Virol. 1970 Aug;6(2):253–255. doi: 10.1128/jvi.6.2.253-255.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., HIROTA Y. Conjugal fertility associated with resistance factor R in Escherichia coli. J Bacteriol. 1962 Nov;84:902–910. doi: 10.1128/jb.84.5.902-910.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar P. D., Garen A. THE ORIENTATION AND EXTENT OF GENE TRANSFER IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1956 Sep;42(9):619–624. doi: 10.1073/pnas.42.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]