Abstract
Background
The long-lasting high affinity opioid buprenorphine has complex pharmacology including ceiling effects with respect to analgesia and respiratory depression. Plasma concentrations of the major buprenorphine metabolites norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide approximate or exceed those of the parent drug. Buprenorphine glucuronide metabolites pharmacology is undefined. This investigation determined binding and pharmacological activity of the two glucuronide metabolites, and in comparison with buprenorphine and norbuprenorphine.
Methods
Competitive inhibition of radioligand binding to human mu, kappa, delta opioid and nociceptin receptors was used to determine glucuronide binding affinities for these receptors. Common opiate effects were assessed in vivo in Swiss Webster mice. Antinociception was assessed using a tail-flick assay, respiratory effects were measured using unrestrained whole-body plethysmography, and sedation was assessed by inhibition of locomotion measured by open-field testing.
Results
Buprenorphine-3-glucuronide had high affinity for human mu (Ki = 4.9±2.7 pM), delta (Ki = 270±0.4 nM), and nociceptin (Ki = 36±0.3 μM) but not kappa receptors. Norbuprenorphine-3-glucuronide had affinity for human kappa (Ki = 300±0.5 nM) and nociceptin (Ki= 18±0.2 μM) but not mu or delta receptors. At the dose tested, buprenorphine-3-glucuronide had a small antinociceptive effect. Neither glucuronide had significant effects on respiratory rate, but norbuprenorphine-3-glucuronide decreased tidal volume. Norbuprenorphine-3-glucuronide also caused sedation.
Conclusions
Both glucuronide metabolites of buprenorphine are biologically active at doses relevant to metabolite exposures which occur after buprenorphine. Activity of the glucuronides may contribute to the overall pharmacology of buprenorphine.
Introduction
Buprenorphine is a long-lasting, high affinity opioid, available for three decades for treating pain and opiate addition.1 Buprenorphine is marketed for addiction therapy in sublingual tablets or films, both alone and coformulated with naloxone (to discourage diversion and parenteral administration). Initially approved for treatment of pain, buprenorphine has more recently been used for opiate withdrawal therapy and is now being considered for other drug addictions such as cocaine and ethanol.2 A transdermal formulation was recently approved for the treatment of moderate-severe chronic pain.3 Buprenorphine displays unusual pharmacology.4 It is a partial mu agonist, delta and kappa antagonist, and nociceptin receptor (formerly termed the opioid receptor-like ORL1 receptor) agonist. It has ceiling effects with respect to both analgesia and respiratory depression.5–9 Despite years of clinical use, the mechanisms by which buprenorphine exerts its pharmacological effects remain undefined.
Buprenorphine is extensively metabolized in humans, with minimal parent drug excreted in urine.10,11 The primary route is N-dealkylation to norbuprenorphine, catalyzed mainly (80–90%) by the cytochrome P450 enzymes CYP3A4/5, with contribution from CYP2C8 and CYP2C9.12–14 Both buprenorphine and norbuprenorphine undergo glucuronidation by UDP-glucuronosyl transferases (UGT) to buprenorphine-3-glucuronide (B3G) and norbuprenorphine-3-glucuronide (N3G).15 B3G formation is catalyzed mainly by UGT2B7 and UGT 1A1, with some contribution from UGT1A3 and 2B17, and N3G formation is catalyzed predominantly by UGT1A3 and UGT1A1.16,17 Based on molar area under the plasma concentration versus time curves, glucuronides constitute 70% of a buprenorphine dose. In humans, peak plasma norbuprenorphine concentrations equal or exceed that of buprenorphine, and relative exposures of norbuprenorphine, B3G, and N3G based on molar area under the concentration in plasma versus time curve are 200%, 100%, and 600% those of buprenorphine.13,18–20 If buprenorphine metabolites are pharmacologically active, then buprenorphine metabolism could constitute a bioactivation pathway.
Metabolism of buprenorphine to norbuprenorphine was initially considered to be an inactivation pathway, because norbuprenorphine in rats had 1/50th the analgesic potency of buprenorphine (based on intravenous dose) and 1/4th the potency based on intracerebroventricular dose.21 Evidence now suggests that dealkylation of buprenorphine to norbuprenorphine is actually a bioactivation pathway. Norbuprenorphine is a potent opioid agonist, with high affinities for mu, delta, and kappa opioid receptors.22 In rats, norbuprenorphine caused dose-dependent respiratory depression and was 10 times more potent than buprenorphine.8,23 Norbuprenorphine respiratory depression was opioid-receptor mediated, and also antagonized by buprenorphine.8 In sheep, norbuprenorphine also had respiratory depressant effects.24 Unlike buprenorphine, which is a partial mu receptor agonist with slow receptor dissociation rates, norbuprenorphine in rats has rapid mu receptor binding and is a full agonist, causing full respiratory depression.8,25 Since clinical plasma norbuprenorphine concentrations equal or exceed those of buprenorphine, norbuprenorphine formation may be a bioactivation rather than inactivation pathway in humans.
No information is available about the pharmacology of the buprenorphine and norbuprenorphine glucuronides. While drug glucuronidation is generally considered a detoxification and inactivation pathway, there is precedence for active 6-glucuronide metabolites of drugs.26,27 Opioids are a particularly noteworthy and clinically important example, best exemplified by morphine-6-glucuronide.28,29 Morphine-6-glucuronide has mu and delta receptor affinity similar to morphine, and is 300 times more potent than morphine when administered intracerebroventricularly. Clinically, approximately 10% of morphine is metabolized to morphine-6-glucuronide. Although initial studies of morphine-6-glucuronide at doses (0.04–0.1 mg/kg) approximating concentrations resulting from in vivo morphine glucuronidation showed little effect, higher doses produced effective and long-lasting analgesia, and morphine-6-glucuronide has been used clinically.29 Glucuronides of dihydromorphine and codeine have also been implicated in the biological effects of their parent drugs.28,30 Therefore, glucuronidation may theoretically be a buprenorphine bioactivation pathway, and the pharmacological activity of buprenorphine or norbuprenorphine glucuronides could have significant clinical effects. This would also be the first example of active 3-glucuronides. Nonetheless, pharmacological effects of buprenorphine and norbuprenorphine glucuronides are unknown. This investigation tested the hypothesis that these glucuronide metabolites are pharmacologically active. Also, this work pertains to the Food and Drug Administration guidance on drug metabolites. The US Food and Drug Administration defines a major metabolite as comprising >10% of parent drug systemic exposure (area under the curve) at steady state, and suggests it be considered for safety assessment.*
Materials and Methods
Reagents
Unless otherwise noted, reagents were from Sigma Aldrich (St. Louis, MO). 3H-diprenorphine and 3H-nociceptin were from Perkin Elmer (Waltham, MA). Buprenorphine, norbuprenorphine and B3G were from the National Institute on Drug Abuse. N3G was synthesized according to Fan et al.31 Naloxone was from Cerillant (Round Rock, TX). Membrane preparations from Chinese hamster ovary cells expressing human mu or delta receptors and from human embryonic kidney cells expressing the human nociceptin receptor were purchased from Perkin Elmer (Waltham, MA). Chinese hamster ovary cells stably expressing the human kappa opioid receptor were obtained from the laboratory of R. Rothman (National Institutes of Health).32
Preparation of cell membranes
Membranes from Chinese hamster ovary cells stably expressing the human kappa opioid receptor were prepared for ligand binding assays as described by Zhu et al,33 with modifications. Adherent cells were washed 3 times in ice-cold PBS, harvested in hypotonic lysis buffer (20 mM Tris HCl pH 7.5 with 5 mM MgCl2), and incubated in lysis buffer on ice for one hour. After incubation, the cells were further disrupted by sonication using a probe sonicator three times for 18 sec each. The sonicated samples were centrifuged at 40,000g for 30 min. The pellet was resuspended in binding assay buffer plus 5% protease inhibitors (Complete protease inhibitor cocktail tablets, EDTA free, Santa Cruz Biotechnology, Santa Cruz, CA) and passed through a 26 gauge syringe for homogenization. Protein content was determined by protein assay (Bio-Rad Protein Assay, Bio-Rad Laboratories, Hercules, CA), using bovine serum albumin as the standard. Presence of the kappa receptor was confirmed by western blot. Aliquots were flash frozen in liquid nitrogen and stored at −80°C.
Opioid Receptor Affinity Assays
Mu and delta opioid receptor and nociceptin receptor membrane preparations (Perkin Elmer) were diluted according to manufacturer’s recommendations. Competitive displacement of radioligand binding was performed using a method modified from Huang et al.22 Competitive displacement of 3H-diprenorphine (0.4 nM) binding to mu, delta, and kappa receptors by buprenorphine, norbuprenorphine, B3G and N3G was performed in the absence or presence of at least 7 concentrations of each test compound. Non-specific binding was determined by the addition of the specific inhibitor naloxone (10 μM). Binding was carried out in binding assay buffer (50 mM Tris HCl with 1 mM EGTA). Bound 3H was separated from unbound by vacuum filtration over GF/C filters (Whatman) presoaked in buffer (50 mM Tris HCl, pH 4.0, with 1 mM EGTA, 0.4% BSA, 0.01% polylysine). Filters were rinsed 4 times with 4 ml ice-cold 50 mM Tris HCl pH 4.0 and dried overnight at room temperature.
Competitive displacement of 3H-nociceptin (0.1 nM) binding to nociceptin receptors was determined in the absence or presence of at least 7 various concentrations of each test compound. Non-specific binding was determined by the addition of the specific inhibitor dynorphin A (20 μM). Binding was carried out in assay buffer (50 mM HEPES pH 7.4, 10 mM MgCl2, 1 mM EDTA). Bound 3H was separated from unbound by vacuum filtration over GF/C filters presoaked in assay buffer with 0.5% polylysine. Filters were rinsed 4 times with 4 mL ice-cold assay buffer and dried overnight at room temperature.
Remaining bound radioactivity was determined by liquid scintillation counting. Each experiment was performed in triplicate and repeated at least two times. Specific and nonspecific binding of 3H-diprenorphine to the mu, kappa, and delta receptors was ~2500 cpm and 300 cpm, respectively. Specific and nonspecific binding of 3H-nociceptin to the nociceptin receptor was ~3000 cpm and 700 cpm, respectively. Ki values of each compound were determined by non-linear regression analysis and the Cheng-Prusoff equation34 (Sigma Plot 11.2, Systat Corp, San Jose, CA) after subtracting non-specific binding. Kd values of the nonspecific inhibitors used in the calculations were from the manufacturer of the cell membrane preparations.
Animals
Experiments were performed in accordance with the Guidelines of the National Institutes of Health and were approved by the Animal Care and Use Committee of Washington University (St. Louis, MO). Male Swiss-Webster mice (Taconic Farms, Germantown, NY), age 7 – 9 weeks (35–45g), were used in all experiments. All mice were group housed on a 12:12-h light/dark schedule with ad libitum access to food and water. Doses of norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide were chosen to reflect the relative exposure of each metabolite in humans following a buprenorphine dose.13,17–19 An initial tail-flick nociception experiment was performed to determine the dose of buprenorphine (0.1, 0.3, 1, 10, or 100 mg/kg) that produced the maximum possible effect (MPE). Results showed that 0.3 mg/kg (0.6 μmol/kg) had the greatest analgesic response, with an MPE of 100% at 30 minutes. Therefore, norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide were dosed at 2, 1, and 6 times 0.6 μmol/kg.
Tail Flick Assay
A tail flick assay was used to test the antinociceptive effect of the glucuronides.5,35 Tail flick latency, defined by the time in seconds for tail withdrawal from a warm water bath (52°C) was measured using an IITC 500 warm water tail immersion test analgesia meter (IITC Life Science, Woodland Hills, CA). Mice (10/group) were dosed subcutaneously with either saline vehicle (control) or drug (0.1 – 100 mg/kg buprenorphine, 1 mg/kg B3G, 1 mg/kg norbuprenorphine, and 2.22 and 22.2 mg/kg N3G). Each animal was injected only once. Tail flick latency was measured every 15 min for 90 min after drug administration. A separate experiment (no drug) was performed to determine the baseline tail flick latency for each mouse. A cut-off of 10 sec was used to prevent tissue damage. Animals not responding within 3 sec were excluded from the assay. Maximum Possible Effect (MPE) was calculated as: [(T1−T0)/(T2−T0)] × 100, where T0 and T1 represent latencies before and after drug administration, and T2 is the cut-off time.
Unrestrained Whole Body Plethysmography
Measurements of ventilation parameters were obtained using unrestrained whole body plethysmography (Buxco Research Systems, Wilmington, NC). The plethysmograph consisted of eight animal chambers with orifices for entry and exit of breathing air, and a 1-mL syringe permitting calibrations, connected to a differential pressure transducer. The air entry orifice was connected to a source of compressed breathing air. Each chamber was calibrated with 1 ml of room air immediately prior to each experiment. Each awake mouse was placed in a chamber. Ventilation parameters were recorded for 20 minutes pre-dosing. Each animal was removed from the chamber, administered drug subcutaneously, and replaced in the chamber. Post-dosing ventilation parameters were recorded for one hour. Four animals were studied in each group. Respiratory values were calculated by Biosystems XA software (Buxco Research Systems).
Open-Field Locomotor Testing
Locomotor activity was measured in an open-field using a VersaMax Animal Activity Monitor (Accuscan Instruments, Inc. Columbus OH), as previously described.36 After habituation to the test room, a single mouse was administered test compound subcutaneously and then immediately placed in the test chamber. Locomotor activity was assessed by recording photobeam breaks for 60 min. Total distance traveled, time spent moving, and the numbers of beam breaks (horizontal activity) were calculated for the entire chamber. Data were combined and reported as total activity/time. Four mice were tested in each group.
Disposition of norbuprenorphine glucuronides
To test the hypothesis that pharmacologic activity of B3G and N3G could be due to hydrolysis of the glucuronides back to the aglycones, plasma and brain concentrations of buprenorphine, norbuprenophine, B3G and N3G were determined after subcutaneous injection of either B3G or N3G. Drug naïve Swiss-Webster mice (4 per group) were administered B3G (1 mg/kg) or N3G (2.22 mg/kg). After 60 min, mice were anesthetized with sevoflurane and blood was collected by cardiac puncture into heparinized microtainers (BD Biosciences, Franklin Lakes, NJ) and then centrifuged at 14,000 rpm for 1 minute to separate plasma. After exsanguination, whole brains were collected and flash frozen. Plasma and brain were stored at −80°C until analysis.
Analytical methods
Analysis of buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide in brain and plasma was performed on an API 4000 QTRAP mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA)-Agilent 1100 series HPLC system (Agilent, Wilmington, DE). The mass spectrometer was equipped with a Turbo Ion Spray ionization source operating in positive ionization mode. Chromatographic separation was performed on a Waters XBridge C8 column (150 × 2.1mm, 3.5 μm) (Waters Corp, Milford, MA). The injection volume was 30 μl and the oven temperature was 25°C. The HPLC mobile phase (0.25 ml/min) was (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The gradient program was 5 % B for 0 min, linear gradient to 40% B between 0 and 0.5 min, held at 40%B until 2.5 min, linear gradient to 90% between 2.5 and 5 min, held at 90% B until 8 min, then re-equilibrated to initial conditions (5% B) between 8.01 and 15.0 min. Under these conditions, retention times were 7.62, 6.73, 6.52 and 6.00 min, respectively, for buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide. Both Q1 and Q3 quadrupoles were optimized to unit mass resolution, and the mass spectrometer conditions were optimized for each analyte. The instrument was operated in positive-ion mode with an ion spray voltage of 5200 V. The curtain gas was set at 15, ion source gas 1 at 40, ion source gas 2 at 50 and collision gas set at the high setting. Multiple reaction monitoring transitions for each analyte and internal standard were m/z 468.5>55.2 for buprenorphine, m/z 414.3>82.9 for norbuprenorphine, m/z 644.3>468.5 for buprenorphine glucuronide, m/z 590.4>414.3 for norbuprenorphine glucuronide, m/z 472.5>59.2 for buprenorphine d4 and m/z 417.3>82.9 for norbuprenorphine d3. Analytes were quantified using area ratios and standard curves prepared using calibration standards in blank media.
Brains samples were prepared immediately prior to analysis, by dounce homogenization with 4 mL Hanks buffered salt solution buffer to 1 g of mouse brain. Mouse brain calibration standards and quality control samples were prepared by similarly homogenizing mouse brain, and 500 uL of buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide solution (mixture at 50 mg/mL each in methanol) added to 9.5 mL of mouse brain homogenate to prepare 2.5 mg/mL working stock solution. Calibration standards for buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide in brain homogenate were prepared at 0.12, 0.62, 1.25, 6.25, 12.5, and 25 ng/mL. Quality control samples were prepared at 0.1, 1.0 and 10 ng/mL. Mouse plasma calibration standards and quality control samples were prepared by adding 500 uL of buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide solution (mixture at 50 mg/mL each in methanol) to 9.5 mL of mouse plasma to prepare 2.5 mg/mL working stock solution. Calibration standards in mouse plasma were prepared at 0.62, 1.25, 2.5, 5, 10, 25, 50, 100, 250, and 500 ng/mL while quality control samples were prepared at 1, 10 and 100 ng/mL. Sample preparation steps for both mouse brain and plasma were as follows: Experimental, quality control, and calibration samples (100 μL) were mixed with 400 uL of acetonitrile containing buprenorphine d4 and norbuprenorphine d3 (10 ng/mL each) and vortexed for 2 min. The samples were centrifuged at 3000 rpm for 5 min. The supernatant (250 μL) were removed and evaporated to dryness and reconstituted in 100 μL of 0.1% acetic acid for brain samples and 500 μL of 0.1% acetic acid for plasma samples.
Statistical Analysis
The results are expressed as the mean ± SD. Two-way analysis of variance (time, drug group) was used, followed by the Student-Newman-Keuls test, to test for significant differences between groups (Sigma Plot 11.2). Statistical significance was assigned at p< 0.05. Nonnormal data were log-transformed for analysis of variance.
Results
Binding Affinity for Opioid Receptors
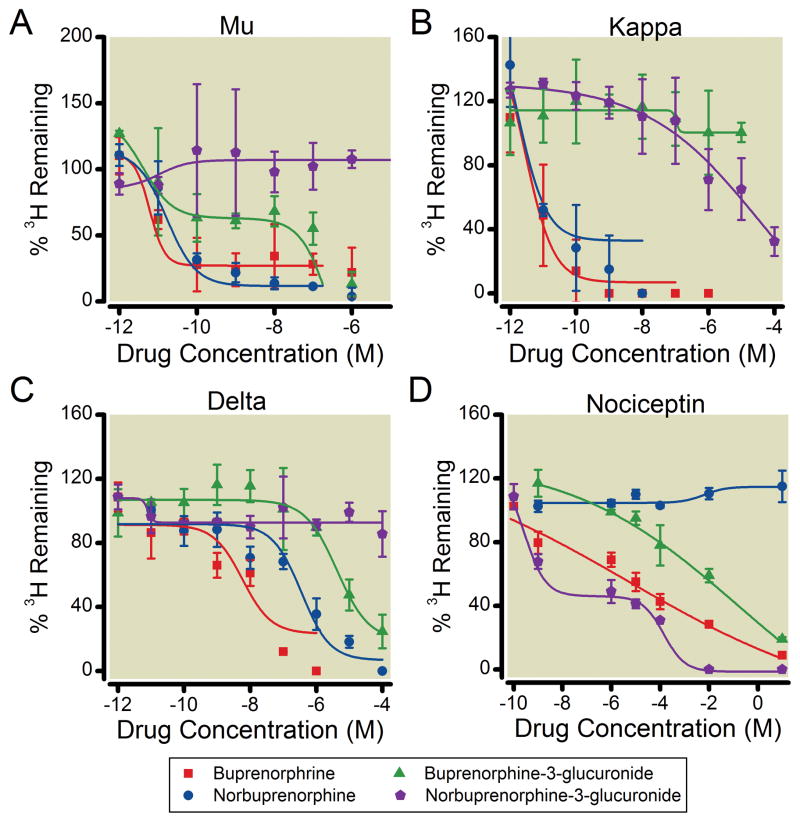
Competitive inhibition of 3H-diprenorphine to opiate receptors was used to determine the binding affinities of buprenorphine, norbuprenorphine, B3G and N3G for the mu, kappa, and delta opioid receptors (Table 1, figure 1). B3G inhibited 3H-diprenorphine mu receptor binding with high affinity, with a Ki in the picomolar range. N3G did not inhibit 3H-diprenorphine mu receptor binding even at concentrations as high as 2.5 mM. B3G also inhibited 3H diprenorphine binding to the delta opioid receptor with a Ki in the nanomolar range. N3G did not inhibit 3H-diprenorphine binding to the delta opioid receptor even at concentrations as high as 2.5 mM. N3G but not B3G inhibited radioligand binding to the kappa opioid receptor. Buprenorphine and norbuprenorphine had affinities for the receptors in the subnanomolar and nanomolar ranges, which is consistent with reports from other laboratories.22
Table 1.
Receptor affinity of buprenorphine and buprenorphine metabolites
| Mu | Delta | Kappa | Nociceptin | |
|---|---|---|---|---|
| Buprenorphine | 2.7 ± 0.4 pM | 33 ± 1.6 nM | 2.1 ± 0.2 pM | 25 ± 0.3 μM |
| Norbuprenorphine | 1.8 ± 0.4 pM | 1.3 ± 0.2 μM | 1.3 ± 0.3 pM | N.B. |
| Buprenorphine-3-Glucuronide | 4.9 ± 2.7 pM | 270 ± 0.4 nM | N.B. | 36 ± 0.3 μM |
| Norbuprenorphine-3-Glucuronide | N.B. | N.B. | 300 ± 0.5 nM | 18 ± 0.2 μM |
Results are shown as apparent Ki values of buprenorphine, norbuprenorphine, B3G, and N3G for human mu, delta and kappa opioid receptors and the human nociceptin receptor. Apparent Ki values were calculated from the equation Ki = IC50/(1+ ([L]/Kd)). IC50 values were derived from the competition curves shown in Figure 1.
N.B. = No Binding.
Figure 1. Competitive inhibition by buprenorphine, norbuprenorphine, B3G, and N3G of 3H-diprenorphine to human mu, kappa, and delta opioid receptors, and 3H nociceptin binding to the nociceptin receptor.
Binding to the opioid receptors and the nociceptin receptor was carried out with 0.4 nM 3H diprenorphine and 0.1 nM 3H-nociceptin, respectively, in the presence or absence of varying concentrations of buprenorphine (●), norbuprenorphine (■), B3G (○), and N3G (□). Data were normalized to percentage of specific binding. Each data point represents the mean ± SD (n=9). Lines are predicted results based on the Ki values obtained by nonlineaer regression analysis of the observed data, using the equation shown in Table 1. Apparent Ki values are shown in Table 1.
Competitive inhibition of 3H-nociceptin was used to determine the binding affinities of the metabolites for the nociceptin receptor. Both glucuronide metabolites displayed Ki values for the nociceptin receptor in the micromolar range. Consistent with previous reports,22 buprenorphine had low affinity for this receptor, however norbuprenorphine did not displace the radioligand.
Antinociceptive Activity of B3G and N3G
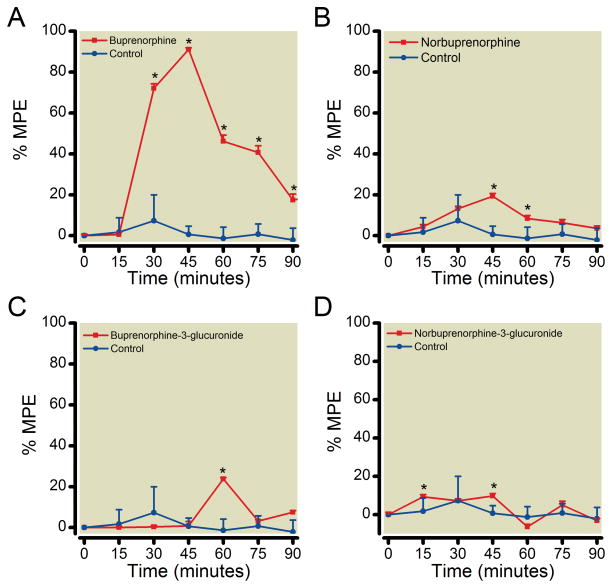
The antinociceptive effect of each glucuronide was tested using a hot water tail flick latency assay. At the doses tested, both glucuronides had antinociceptive effects (Figure 2). The response to B3G was brief, with onset, time to peak, and return to baseline within 60 min. N3G had a small but statistically significant analgesic effect lasting approximately 45 min with a peak at 45 min. A slight decrease in tail flick latency compared to control was seen with the starting dose of N3G tested. To test whether this was a submaximal, dose-dependent response, a 10-fold greater dose was also tested. A similar slight decrease in the tail flick latency was noticed, but was not statistically different from the lower dose, and neither was different from control (data not shown). The antinociceptive effect of 1 mg/kg norbuprenorphine was approximately one-fifth that of 0.3 mg/kg buprenorphine. As shown in previous reports,21 buprenorphine and norbuprenorphine effects followed the same time course, with the same time to onset and time to peak. The time to onset for both compounds was 15 minutes, with peak effects at 45 minutes.
Figure 2. Antinociceptive effects of buprenorphine, norbuprenorphine, B3G, and N3G in an acute pain model.
Time to withdrawal of the tail from a hot water (52°C) bath, or tail flick latency (TFL) was measured after subcutaneous injection of vehicle or buprenorphine (0.3 mg/kg; 0.6 μmol/kg), norbuprenorphine (1 mg/kg; 1.2 μmol/kg), B3G (1 mg/kg; 0.6 μmol/kg), or N3G (2.2 mg/kg, 3.8 μmol/kg). The % maximum possible effect (MPE) was calculated every 15 min for 90 min. Each data point is the mean ± SD (n=10 per group). *Significantly different than control (p < 0.05) by two-way analysis of variance.
Respiratory effects of B3G and N3G
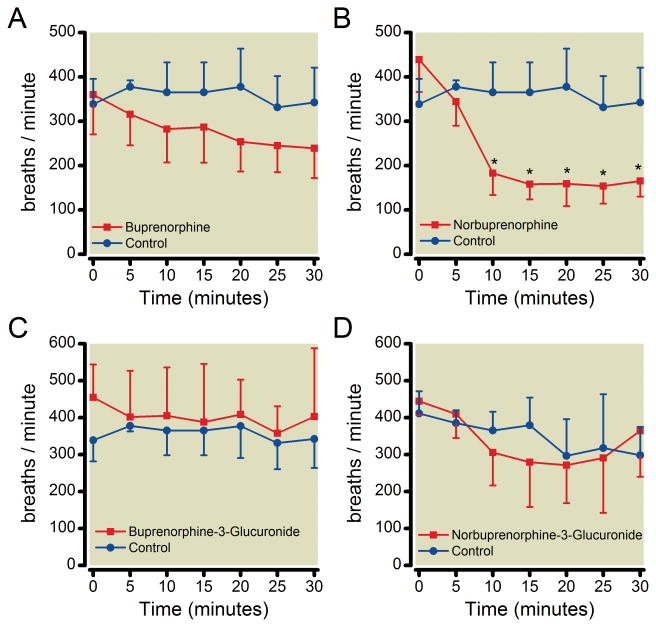
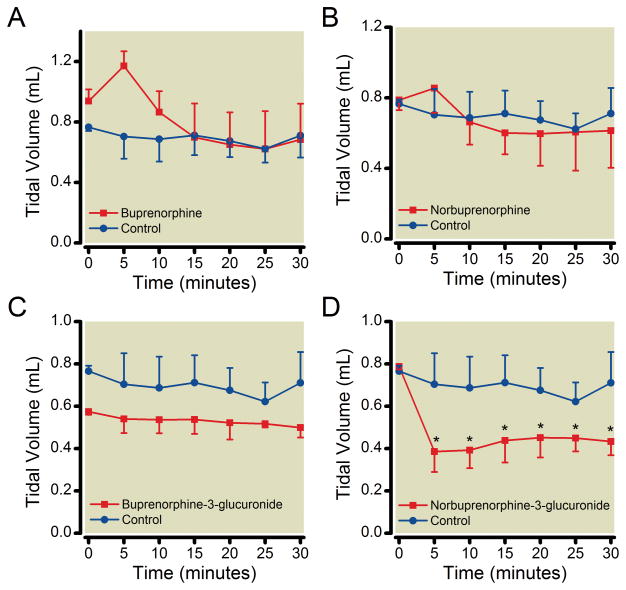
The effect of B3G, N3G, buprenorphine and norbuprenorphine on respiratory rate was measured using unrestrained whole body plethysmography. Neither B3G nor N3G had a significant effect on respiratory rate at the dose tested (Figure 3), however, N3G did significantly decrease tidal volume (Figure 4). Buprenorphine (0.3 mg/kg) did not have a significant effect on respiratory rate while 1 mg/kg norbuprenorphine elicited a pronounced reduction in respiratory rate, with an onset within 10 min of drug administration. Buprenorphine and norbuprenorphine effects on respiratory rate were similar to those previously reported,23,25 and neither compound had an effect on tidal volume. Neither buprenorphine nor B3G affected minute ventilation, while both norbuprenorphine and N3G decreased minute ventilation, in an equivalent manner.
Figure 3. Effect of buprenorphine, norbuprenorphine, B3G, and N3G on respiratory rate.
Unrestrained whole-body plethysmography was used to study the effect on respiratory rate. Data were recorded for 30 min following subcutaneous injection of vehicle or buprenorphine (0.3 mg/kg; 0.6 μmol/kg), norbuprenorphine (1 mg/kg; 1.2 μmol/kg), B3G (1 mg/kg; 0.6 μmol/kg), or N3G (2.2 mg/kg, 3.8 μmol/kg). Norbuprenorphine caused a marked decrease in respiratory rate 10 min after drug dose. Results are the mean ± SD (n=4 per group). *Significantly different than control (p <0.05) by two-way analysis of variance.
Figure 4. Effect of buprenorphine, norbuprenorphine, B3G, and N3G on tidal volume.
Unrestrained whole-body plethysmography was used to study the effect on tidal volume. Data were recorded for 30 min following subcutaneous injection of vehicle or buprenorphine (0.3 mg/kg; 0.6 μmol/kg), norbuprenorphine (1 mg/kg; 1.2 μmol/kg), B3G (1 mg/kg; 0.6 μmol/kg), or N3G (2.2 mg/kg, 3.8 μmol/kg). N3G caused a marked decrease in tidal volume 5 min after drug dose. Results are the mean ± SD (n=4 per group). * Significantly different than control (p < 0.05) by two-way analysis of variance.
Effects of buprenorphine, norbuprenorphine, B3G, and N3G on locomotor activity
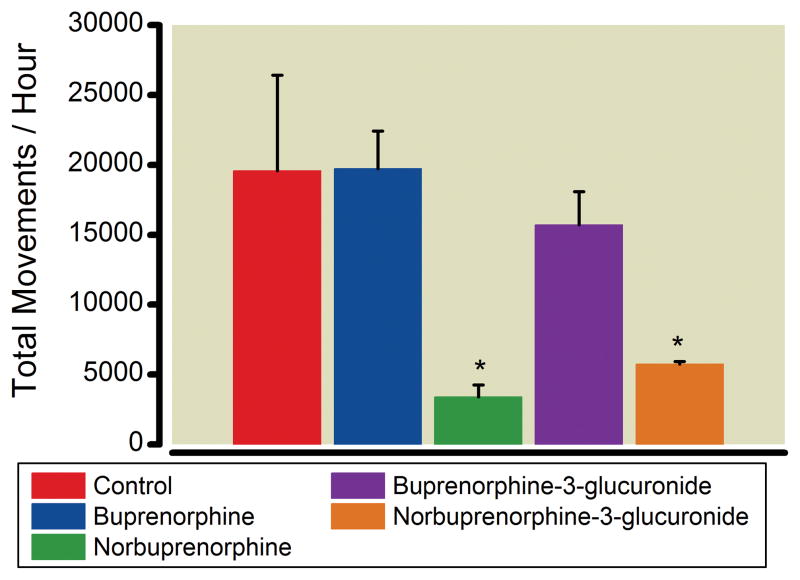
Open-field testing was performed to identify and quantify effects of buprenorphine, norbuprenorphine B3G and N3G on locomotor activity (Figure 5). This test measures the exploratory activity of animals in a novel environment. Drug-induced sedation will override the desire to explore a novel environment resulting in a reduced number of movements per unit time. Each animal was monitored for an hour immediately after subcutaneous drug administration. Both norbuprenorphine and N3G caused a significant decrease in total activity compared to control. Buprenorphine and B3G did not cause a decrease in activity compared to control.
Figure 5. Sedative effects of buprenorphine, norbuprenorphine, B3G, and N3G.
The effect of buprenorphine, norbuprenorphine, B3G and N3G on locomotion was quantified in an open-field test. A single mouse was habituated to the test room, administered vehicle or buprenorphine (0.3 mg/kg; 0.6 μmol/kg), norbuprenorphine (1 mg/kg; 1.2 μmol/kg), B3G (1 mg/kg; 0.6 μmol/kg), or N3G (2.2 mg/kg, 3.8 μmol/kg) and placed in a test chamber. Total activity was recorded by photobeam breaks for one hour. Each result is the mean ± SD (n= 4 per group). *Significantly different than control (p < 0.05) by one-way analysis of variance.
Brain and plasma concentrations of buprenorphine, norbuprenorphine, B3G and N3G
The extent of hydrolysis of each glucuronide to either of the aglycones was investigated by analysis of plasma and brain homogenate 60 minutes after subcutaneous injection of either B3G or N3G (Table 2). There was minimal aglycone detected in plasma or brain after administration of either glucuronide. In brains of mice administered B3G, there was no buprenorphine detected, and norbuprenorphine was 2% of the glucuronide concentration. In plasma, no buprenorphine was detected, and norbuprenorphine was 1% of the glucuronide concentration. In mice administered N3G, brain norbuprenorphine concentration was 9% of the glucuronide, and in plasma was <1%. There was no buprenorphine detected in brain or plasma of these mice.
Table 2.
Plasma and brain concentrations of buprenorphine and buprenorphine metabolites after administration of buprenorphine glucuronides
| Concentration (ng/ml) | Compound dosed
|
||
|---|---|---|---|
| Buprenorphine-3-glucuronide (1 mg/kg) | Norbuprenorphine-3-glucuronide (2.2 mg/kg) | ||
| Plasma | buprenorphine-3-glucuronide | 133 ± 44 | ND |
| norbuprenorphine-3-glucuronide | 1.5±0.8 | 360±111 | |
| buprenorphine | ND | ND | |
| norbuprenorphine | ND | 0.65±0.20 | |
| Brain Homogenate | buprenorphine-3-glucuronide | 1.4 ± 0.7 | ND |
| norbuprenorphine-3-glucuronide | ND | 2.1±0.7 | |
| buprenorphine | ND | ND | |
| norbuprenorphine | 0.03 ± 0.05 | 0.19±0.14 | |
ND, not detected
Results are the mean ± SD (n=4)
Discussion
Buprenorphine has unusual and complex pharmacology. Like other mu agonists, it causes analgesia, respiratory depression, miosis, and mood changes, but there is a ceiling effect at higher doses - both intravenous and sublingual.6,37,38 Although buprenorphine ceiling effects have been attributed to partial mu antagonism, and to differential opioid receptor-mediated actions at different concentrations, buprenorphine pharmacology remains a mechanistic conundrum. Particularly, the contribution(s) of buprenorphine metabolite(s) toward the clinical effects of buprenorphine remain undefined. Only the N-demethylated metabolite, norbuprenorphine, has been evaluated in animals. This investigation provides, for the first time, evidence that two glucuronide metabolites of buprenorphine are pharmacologically active. Also, it is the first example of active opioid-3-glucuronides.
The activity profile of B3G included mu, delta, and nociceptin receptor binding, and an antinociceptive effect in an acute pain model. At the clinically relevant dose tested, the magnitude of B3G antinociception was approximately one-fourth that of buprenorphine. Also, the onset and peak of antinociception occurred at 60 min, compared with 30 and 45 min with buprenorphine. There are several potential explanations for both the lesser antinociception and the slower onset of B3G compared to buprenorphine. The low response could reflect lower potency and/or efficacy. In support of this hypothesis, the affinity of B3G for the mu receptor was half that of buprenorphine. Similarly, the mu receptor affinity of morphine-6-glucuronide was less than that of the parent drug morphine.39 Nevertheless, morphine-6-glucuronide elicited an analgesic response similar to morphine when the dose of morphine-6-glucuronide reflected plasma concentrations which occur following a morphine dose.29 Further studies are warranted to determine the full dose-response effect of B3G, and its potency and efficacy relative to buprenorphine. Whether B3G exhibits an inverse-U shaped dose-response curve, analogous to that of buprenorphine, is still unknown. While this investigation shows that B3G has high affinity for the mu and delta receptors, and moderate affinity for the nociceptin receptor, it does not address whether B3G binding activates or antagonizes these receptor pathways. However, all known active opioid glucuronides are receptor agonists. The later onset of analgesic effect of B3G compared to buprenorphine could be due to differences in brain access between the more hydrophilic glucuronide and the highly lipophilic aglycone, and/or to differences in receptor kinetics between the two compounds. Given that there was no buprenorphine detected in the brains of mice administered B3G, and that the minimal norbuprenophine detected in brain was much less than has been shown to elicit any physiological response,21 it can be concluded that the observed pharmacologic effect of B3G was due to the glucuronide itself, rather than hydrolysis to and activity of the aglycone (buprenorphine).
The activity profile of N3G included kappa and nociceptin receptor binding, reduction of tidal volume and marked reduction of locomotor activity. The significant decrease in tidal volume but not respiratory rate suggests that N3G may have activity at receptors other than the opioid receptors. The lack of effect on respiratory rate may also be attributable to the lack of N3G binding to the mu opioid receptor. The sedative effect of N3G was comparable to that of norbuprenorphine and could be mediated through either the kappa or the nociceptin receptor, since activation of either receptor acts on dopamine neurotransmission and can result in decreased locomotor activity.40–42 Despite not having binding affinity for the mu receptor, N3G did have a small antinoceptive effect, much less than that of all three other compounds tested. The antinoceptive effect of N3G may be the result of kappa receptor activation. Kappa receptor mediated analgesia has been shown, generally in animal models.42 Activation of the nociceptin receptor has not been associated with antinociception; conversely, it has been shown to elicit a hyperalgesic or anti-analgesic response in rodent models of acute pain.40,43 While synergy and/or opposition of nociceptin and other opioid receptor-associated pathways is not yet fully understood, evidence suggests that nociceptin activation may at least partially antagonize mu receptor-mediated analgesia.41,44 If the same is true for kappa-mediated analgesia, then the limited analgesic response to N3G may be due to intrinsically low potency or to opposition of the nociceptin and kappa receptors with respect to analgesia. Whether N3G is a kappa-receptor agonist or antagonist remains to be fully defined. Norbuprenorphine was detected in the brains of mice administered N3G, however, the amount was <10% of the total glucuronide present, and less than the concentration shown to elicit a pharmacologic effect.21 Moreover, whereas norbuprenorphine decreased respiratory rate, N3G did not. Therefore it can be concluded that the effect of N3G was due to the glucuronide itself, rather than hydrolysis to and activity of the aglycone (norbuprenorphine).
The activity profile of norbuprenorphine included affinity for the mu, delta and kappa receptors (but not the nociceptin receptor), respiratory depression, inhibition of locomotion, and an analgesic effect approximately one-fourth that of a lower dose (0.3 mg/kg) of buprenorphine (compared with 1 mg/kg norbuprenorphine). The onset and peak of analgesia following norbuprenorphine was at 45 and 60 min, respectively, mirroring the analgesic response to buprenorphine. The respiratory and sedative effects, however, occurred at 15 minutes. The activity profile of buprenorphine included affinity for all four receptors, and an analgesic effect of approximately 100% MPE. The antinoceptive effect of buprenorphine was the greatest of the four compounds tested in this experiment, both in magnitude and duration. Unlike norbuprenorphine, buprenorphine did not cause respiratory depression, nor did it have an effect on locomotion/sedation.
Comparison of buprenorphine and the three metabolites is shown in Table 3. Each compound has distinct pharmacological effects, with B3G effects most similar to those of the parent drug. The effects of norbuprenorphine and N3G are pronounced and strikingly different from those of the parent drug. However, buprenorphine respiratory depression and sedation are not typically reported in animals, raising the question of whether buprenorphine antagonizes the effects of its metabolites, possibly through ORL1 agonism or through differences in affinities for receptor subtypes. Indeed, buprenorphine could both protect against and reverse norbuprenorphine-induced respiratory depression in rats.8 In rats, induction of CYP3A by dexamethasone increased plasma norbuprenorphine concentrations but did not result in respiratory depression following high-dose buprenorphine.45 Since the central nervous system activity of drug metabolites relies both on their formation (metabolism) and their accessibility to the brain (diffusion or transport across the blood-brain barrier), genetic variants or drug interactions with metabolizing enzymes and/or transport proteins could potentially affect the clinical response to buprenorphine via its metabolites. For example, norbuprenorphine but not buprenorphine is a substrate for the brain efflux transporter P-glycoprotein.46,47
Table 3.
Major pharmacological effects of buprenorphine and buprenorphine metabolites
| Receptor affinity
|
Effect
|
||||||
|---|---|---|---|---|---|---|---|
| Mu | Delta | Kappa | Nociceptin | Analgesia | Respiratory Depression | Sedation | |
| Buprenorphine | + | + | + | + | + | − | − |
| Norbuprenorphine | + | + | + | − | + | + | + |
| Buprenorphine-3-glucuronide | + | + | − | + | + | − | − |
| Norbuprenorphine-3-glucuronide | − | − | + | + | − | + | + |
Notably, of the four compounds tested, norbuprenorphine is the only one that causes respiratory depression and also does not have affinity for the nociceptin receptor. This may suggest a role for nociceptin activation in attenuation of mu receptor mediated respiratory depression. This hypothesis is supported by recent work with experimental compounds having activity at both mu and nociceptin receptors.2 That inhibition of locomotion was observed only with norbuprenorphine and N3G, and yet these two compounds had very different receptor affinity profiles, is intriguing. As before mentioned, inhibition of locomotion could be mediated by activation of the nociceptin receptor, yet N3G and not norbuprenorphine has affinity for that receptor. This suggests that either the same effect is mediated through different pathways activated by the different receptors, or that the effect is mediated through a receptor for which the two compounds both have affinity, such as the kappa receptor. Conversely, it is also intriguing that B3G and buprenorphine, both with moderate affinity for the nociceptin receptor, do not elicit sedative effects. Experimental compounds with mixed nociceptin/mu receptor activation are sedative, suggesting that a mechanism other than activation of these receptors is preventing or antagonizing a sedative effect following a dose of buprenorphine or B3G.48
In conclusion, both B3G and N3G have receptor binding and pharmacological activity. This is the first example of active opioid-3-glucuronides. Buprenorphine and its 3 major metabolites, norbuprenorphine, B3G, and N3G, have distinct pharmacological profiles. Potential contribution of these metabolites to the biological effects of buprenorphine adds to the complexity of buprenorphine pharmacology. Further investigation of B3G and N3G is warranted. B3G might ultimately merit exploration as a potential clinical analgesic, particularly if further studies confirm that it does not have adverse respiratory side effects.
Final Box Summary.
What we already know about this topic
Buprenorphine is an opioid that has a complex pharmacology, including ceiling analgesic and respiratory depressant effects.
Relative exposure to buprenorphine metabolites exceedsexposure to buprenorphine in humans.
One metabolite, norbuprenorphine, causes dose-dependent full respiratory depression in rats.
What this article tells us that is new
Buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide are the first active opioid-3-glucuronide metabolites to be identified.
Buprenorphine-3-glucuronide had mild antinociceptive activity in an acute mouse pain model.
Norbuprenorphine-3-glucuronide had a sedative effect and decreased tidal volume in mice.
Acknowledgments
Supported by National Institutes of Health grants R01 DA02931 and K24 DA00417 to EDK. Additional support was provided by the National Institutes of Health Neuroscience Blueprint Interdisciplinary Center Core Grant P30 NS057105 (to Washington University).
We thank Dr. R. Rothman for the generous gift of CHO cells expressing human kappa opioid receptors, Brad Manion and Dr. Zi-wei Chen for their assistance with the radioligand binding experiments, and Rolly (Ed) Johnson of Reckitt Benckiser Pharmaceuticals and Dr. Moo Park of the National Institutes on Drug Abuse for their assistance in obtaining norbuprenorphine.
Individuals or organizations to be acknowledged (Complete name, degrees, academic rank, department, institutional affiliation, city, state, and country):
Rolley (Ed) Johnson, Pharm.D., Vice President for Scientific and Regulatory Affairs, Reckitt Benckiser Pharmaceuticals, 10710 Midlothian Turnpike, Suite 430, Richmond, VA 23235, USA (804) 423-8913, ed.johnson@reckittbenckiser.com
Richard B. Rothman, MD, Ph.D., Clinical Psychopharmacology Section, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD 21224, USA. 443 740 2652
Moo Kwang Park, Ph.D., Program Director for Pharmaceutics and Clinical Supplies, Chemistry & Pharmaceutics Branch (CPB), Division of Pharmacotherapies and Medical Consequences of Drug Abuse, National Institute on Drug Abuse, 6001 Executive Boulevard Room 4123, MSC 9551, Bethesda, MD 20892-9551, USA 301-443-9813, mp264a@nih.gov
Brad Manion, B.A., Research Assistant, Department of Anesthesiology, Washington University School of Medicine, 660 S. Euclid Ave., St. Louis MO, 63110. USA 314-362-8562, manionb@morpheus.wustl.edu
Zi-wei Chen, Ph.D., Research Instructor in Anesthesiology, Department of Anesthesiology, Washington University School of Medicine, 660 S. Euclid Ave St. Louis MO, 63110. USA 314-362-8562, chenz@anest.wustl.edu
Footnotes
Guidance for Industry, Safety Testing of Drug Metabolites; Food and Drug Administration; Center for Drug Evaluation and Research, February 2008; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079266.pdf, last accessed August 12, 2011
References
- 1.Heel RC, Brogden RN, Speight TM, Avery GS. Buprenorphine. A review of its pharmacological properties and therapeutic efficacy. Drugs. 1979;17:81–110. doi: 10.2165/00003495-197917020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L. The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): Characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther. 2011;336:952–61. doi: 10.1124/jpet.110.175620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans G, Robert D. Transdermal buprenorphine - A critical appraisal of its role in pain management. J Pain Res. 2009;2:117–34. doi: 10.2147/jpr.s6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutfy K, Cowan A. Buprenorphine. A unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–45. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 7.Gueye PN, Borron SW, Risede P, Monier C, Buneaux F, Debray M, Baud FJ. Lack of effect of single high doses of buprenorphine on arterial blood gases in the rat. Toxicol Sci. 2001;62:148–54. doi: 10.1093/toxsci/62.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Megarbane B, Marie N, Pirnay S, Borron SW, Gueye PN, Risede P, Monier C, Noble F, Baud FJ. Buprenorphine is protective against the depressive effects of norbuprenorphine on ventilation. Toxicol Appl Pharmacol. 2006;212:256–67. doi: 10.1016/j.taap.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627–32. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 10.Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–97. [PubMed] [Google Scholar]
- 11.Moody DE, Chang Y, Huang W, McCance-Katz EF. The in vivo response of novel buprenorphine metabolites, M1 and M3, to antiretroviral inducers and inhibitors of buprenorphine metabolism. Basic Clin Pharmacol Toxicol. 2009;105:211–5. doi: 10.1111/j.1742-7843.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K, Yamamoto T, Chiba K, Tani M, Shimada N, Ishizaki T, Kuroiwa Y. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos. 1998;26:818–21. [PubMed] [Google Scholar]
- 13.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem. 2002;306:31–9. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 14.Picard N, Cresteil T, Djebli N, Marquet P. In vitro metabolism study of buprenorphine: evidence for new metabolic pathways. Drug Metab Dispos. 2005;33:689–95. doi: 10.1124/dmd.105.003681. [DOI] [PubMed] [Google Scholar]
- 15.Bruce RD, McCance-Katz E, Kharasch ED, Moody DE, Morse GD. Pharmacokinetic interactions between buprenorphine and antiretroviral medications. Clin Infect Dis. 2006;43 (Suppl 4):S216–23. doi: 10.1086/508186. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Moody DE. Glucuronidation of buprenorphine and norbuprenorphine by human liver microsomes and UDP-glucuronosyltransferases. Drug Metab Lett. 2009;3:101–7. doi: 10.2174/187231209788654117. [DOI] [PubMed] [Google Scholar]
- 17.Rouguieg K, Picard N, Sauvage FL, Gaulier JM, Marquet P. Contribution of the different UDP-glucuronosyltransferase (UGT) isoforms to buprenorphine and norbuprenorphine metabolism and relationship with the main UGT polymorphisms in a bank of human liver microsomes. Drug Metab Dispos. 2010;38:40–5. doi: 10.1124/dmd.109.029546. [DOI] [PubMed] [Google Scholar]
- 18.McCance-Katz EF, Moody DE, Morse GD, Friedland G, Pade P, Baker J, Alvanzo A, Smith P, Ogundele A, Jatlow P, Rainey PM. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43 (Suppl 4):S224–34. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 19.McCance-Katz EF, Moody DE, Smith PF, Morse GD, Friedland G, Pade P, Baker J, Alvanzo A, Jatlow P, Rainey PM. Interactions between buprenorphine and antiretrovirals. II. The protease inhibitors nelfinavir, lopinavir/ritonavir, and ritonavir. Clin Infect Dis. 2006;43 (Suppl 4):S235–46. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 20.McCance-Katz EF, Moody DE, Morse GD, Ma Q, DiFrancesco R, Friedland G, Pade P, Rainey PM. Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug Alcohol Depend. 2007;91:269–78. doi: 10.1016/j.drugalcdep.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtani M, Kotaki H, Sawada Y, Iga T. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharm Exp Ther. 1995;272:505–10. [PubMed] [Google Scholar]
- 22.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–95. [PubMed] [Google Scholar]
- 23.Ohtani M, Kotaki H, Nishitateno K, Yasufumi S, Iga T. Kinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphine. J Pharmacol Exp Ther. 1997;281:428–33. [PubMed] [Google Scholar]
- 24.Jensen ML, Foster D, Upton R, Grant C, Martinez A, Somogyi A. Comparison of cerebral pharmacokinetics of buprenorphine and norbuprenorphine in an in vivo sheep model. Xenobiotica. 2007;37:441–57. doi: 10.1080/00498250701251126. [DOI] [PubMed] [Google Scholar]
- 25.Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats. J Pharmacol Exp Ther. 2007;321:598–607. doi: 10.1124/jpet.106.115972. [DOI] [PubMed] [Google Scholar]
- 26.Ritter JK. Roles of glucuronidation and UDP-glucuronosyltransferases in xenobiotic bioactivation reactions. Chem Biol Interact. 2000;129:171–93. doi: 10.1016/s0009-2797(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 27.Coller JK, Christrup LL, Somogyi AA. Role of active metabolites in the use of opioids. Eur J Clin Pharmacol. 2009;65:121–39. doi: 10.1007/s00228-008-0570-y. [DOI] [PubMed] [Google Scholar]
- 28.Lötsch J. Opioid metabolites. J Pain Symptom Manage. 2005;29:S10–24. doi: 10.1016/j.jpainsymman.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.van Dorp EL, Morariu A, Dahan A. Morphine-6-glucuronide: Potency and safety compared with morphine. Expert Opin Pharmacother. 2008;9:1955–61. doi: 10.1517/14656566.9.11.1955. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt H, Vormfelde SV, Walchner-Bonjean M, Klinder K, Freudenthaler S, Gleiter CH, Gundert-Remy U, Skopp G, Aderjan R, Fuhr U. The role of active metabolites in dihydrocodeine effects. Int J Clin Pharmacol Ther. 2003;41:95–106. doi: 10.5414/cpp41095. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Brown SM, Tu Z, Kharasch ED. Chemical and enzyme-assisted syntheses of norbuprenorphine-3-β-D-glucuronide. Bioconjug Chem. 2011;22:752–8. doi: 10.1021/bc100550u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Wang X, Partilla JS, Bishop-Mathis K, Benaderet TS, Dersch CM, Simpson DS, Prisinzano TE, Rothman RB. Differential effects of opioid agonists on G protein expression in CHO cells expressing cloned human opioid receptors. Brain Res Bull. 2008;77:49–54. doi: 10.1016/j.brainresbull.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Yin J, Law PY, Claude PA, Rice KC, Evans CJ, Chen C, Yu L, Liu-Chen LY. Irreversible binding of cis-(+)-3-methylfentanyl isothiocyanate to the d opioid receptor and determination of its binding domain. J Biol Chem. 1996;271:1430–4. doi: 10.1074/jbc.271.3.1430. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 35.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507:87–98. doi: 10.1016/j.ejphar.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 36.Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RWt. The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther. 2009;330:834–43. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–34. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 38.Yassen A, Olofsen E, Romberg R, Sarton E, Danhof M, Dahan A. Mechanism-based pharmacokinetic-pharmacodynamic modeling of the antinociceptive effect of buprenorphine in healthy volunteers. Anesthesiology. 2006;104:1232–42. doi: 10.1097/00000542-200606000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Geisslinger G, Brune K, Kobal G, Lötsch J. Intravenous morphine-6-glucuronide (M6G) is devoid of analgesic activity in man. Pain. 1997;70:289–90. [PubMed] [Google Scholar]
- 40.Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- 41.Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L. Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/μ-opioid receptor agonists. J Pharmacol Exp Ther. 2009;331:946–53. doi: 10.1124/jpet.109.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26:S10–5. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 43.Butour JL, Moisand C, Mazarguil H, Mollereau C, Meunier JC. Recognition and activation of the opioid receptor-like ORL 1 receptor by nociceptin, nociceptin analogs and opioids. Eur J Pharmacol. 1997;321:97–103. doi: 10.1016/s0014-2999(96)00919-3. [DOI] [PubMed] [Google Scholar]
- 44.Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Buprenorphine-induced antinociception is mediated by μ-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–7. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hreiche R, Megarbane B, Pirnay S, Borron SW, Monier C, Risede P, Milan N, Descatoire V, Pessayre D, Baud FJ. Dexamethasone hepatic induction in rats subsequently treated with high dose buprenorphine does not lead to respiratory depression. Toxicol Appl Pharmacol. 2006;217:352–62. doi: 10.1016/j.taap.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Hassan HE, Myers AL, Coop A, Eddington ND. Differential involvement of P-glycoprotein (ABCB1) in permeability, tissue distribution, and antinociceptive activity of methadone, buprenorphine, and diprenorphine: In vitro and in vivo evaluation. J Pharm Sci. 2009;98:4928–40. doi: 10.1002/jps.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Decleves X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) Int J Neuropsychopharmacol. 2010;13:905–15. doi: 10.1017/S1461145709990848. [DOI] [PubMed] [Google Scholar]
- 48.Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, Zaveri NT. Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol. 2008;153:609–19. doi: 10.1038/sj.bjp.0707598. [DOI] [PMC free article] [PubMed] [Google Scholar]