Abstract
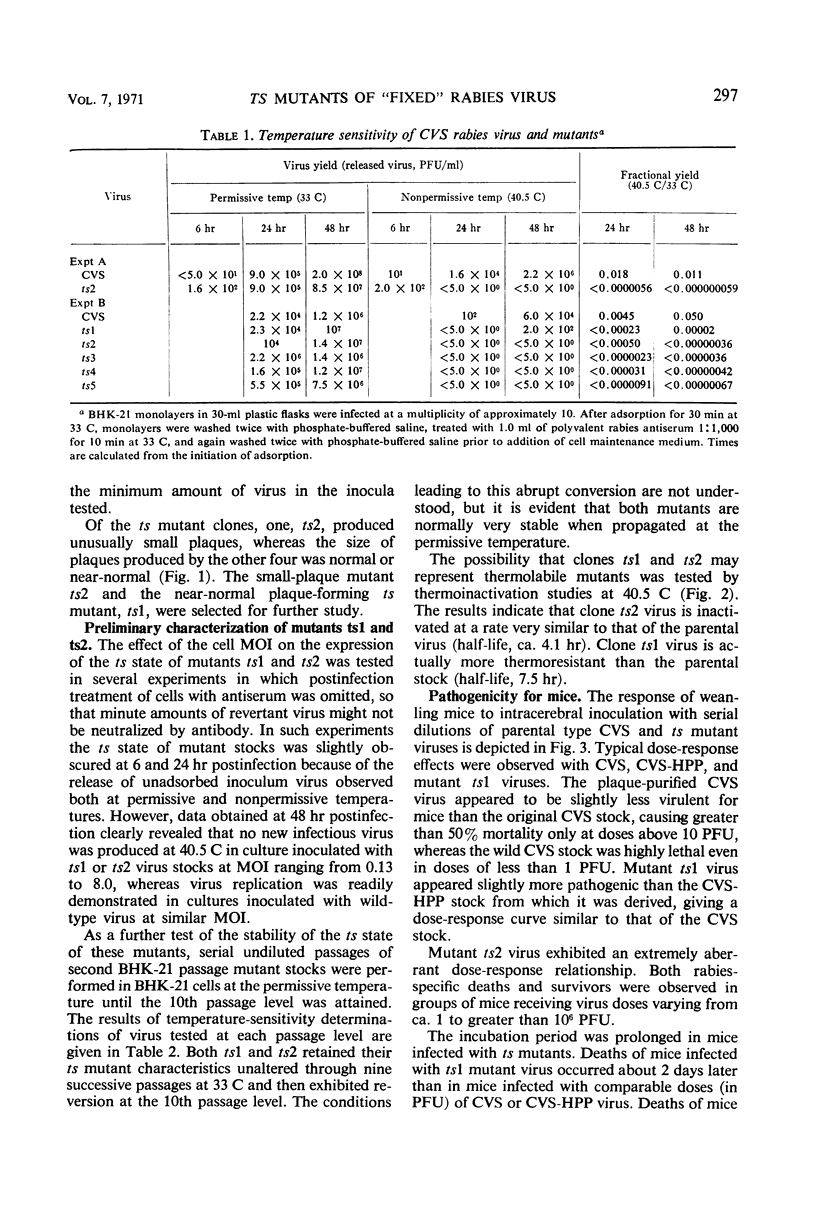
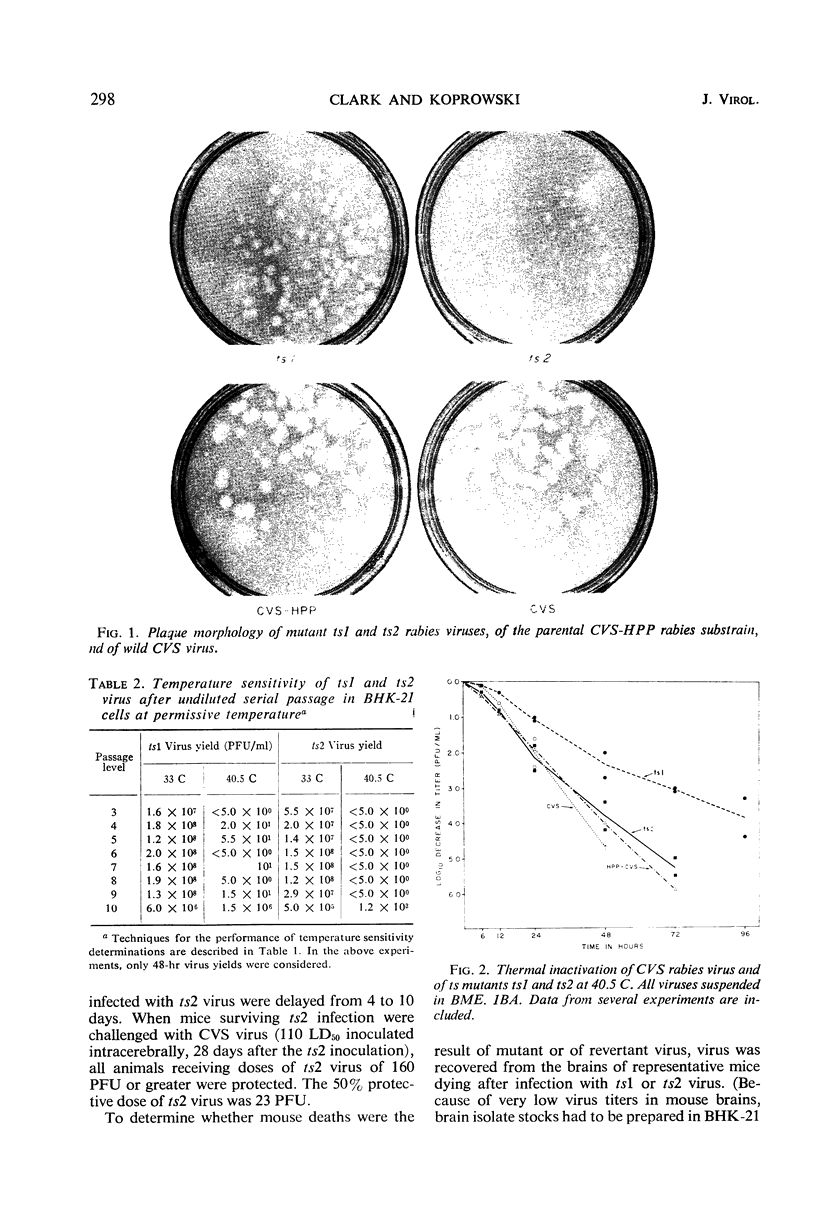
In an attempt to induce temperature-sensitive (ts) conditional lethal mutants of rabies virus, stocks of a plaque-purified substrain of strain CVS fixed rabies virus were subjected to mutagenesis by HNO2, 5-fluorouracil, or 5-azacytidine. It was necessary to prepare virus stocks from clones of mutagenized virus selected at random and to test subsequently each stock for possible ts characteristics by measuring its relative capacity for growth at permissive (33 C) and nonpermissive (40.5 C) temperatures. Five ts mutants were detected in tests of 161 clones of mutagenized virus. Each of the mutants exhibited a remarkably low incidence of reversion and little demonstrable “leakiness.” One of the five ts mutants (ts2), which formed formed very small plaques, and another (ts1), which formed plaques of only slightly reduced size, were further characterized. Virus ts1 was more thermostable at 40.5 C than the parental virus, but the ts2 mutant was unchanged in this respect. The ts1 virus exhibited normal pathogenicity for mice, but ts2 virus caused a very irregular death pattern. Both deaths and survivors immune to rabies virus challenge were noted in all groups of mice inoculated with ts2 virus, regardless of the virus dose.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fenner F. Conditional lethal mutants of animal viruses. Curr Top Microbiol Immunol. 1969;48:1–28. doi: 10.1007/978-3-642-46163-7_1. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire. Mise au point d'un test de complémentation. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 5;268(18):2305–2308. [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Induction of Newcastle disease virus mutants with nitrous acid. Virology. 1961 Apr;13:402–408. doi: 10.1016/0042-6822(61)90270-7. [DOI] [PubMed] [Google Scholar]
- Halle S. 5-Azacytidine as a mutagen for arboviruses. J Virol. 1968 Oct;2(10):1228–1229. doi: 10.1128/jvi.2.10.1228-1229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSLING R. E. Growth of rabies virus in non-nervous tissue culture. Proc Soc Exp Biol Med. 1958 Jun;98(2):223–225. doi: 10.3181/00379727-98-23997. [DOI] [PubMed] [Google Scholar]
- KISSLING R. E., REESE D. R. ANTI-RABIES VACCINE OF TISSUE CULTURE ORIGIN. J Immunol. 1963 Sep;91:362–368. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. S. Virulence of temperature-sensitive mutants of influenza virus. Br Med J. 1969 Sep 27;3(5673):757–758. doi: 10.1136/bmj.3.5673.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes R. Attenuation of foot-and-mouth disease virus by chemical means. Arch Gesamte Virusforsch. 1970;29(1):63–76. doi: 10.1007/BF01253881. [DOI] [PubMed] [Google Scholar]
- Mills J., Van Kirk J., Hill D. A., Chanock R. M. Evaluation of influenza virus mutants for possible use in a live virus vaccine. Bull World Health Organ. 1969;41(3):599–606. [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimov M. A., Nikitina L. F. Ob rct40-markere fibsirovannogo virusa beshenstva. Vopr Virusol. 1970 Mar-Apr;15(2):161–165. [PubMed] [Google Scholar]
- Shechmeister I. L., Streckfuss J., St John R. Comparative pathogenicity of vesicular stomatitis virus and its plaque type mutants. Arch Gesamte Virusforsch. 1967;21(2):127–132. doi: 10.1007/BF01241437. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Kaplan C. Some properties of fixed rabies virus. J Gen Virol. 1967 Oct;1(4):537–551. doi: 10.1099/0022-1317-1-4-537. [DOI] [PubMed] [Google Scholar]



