Erythropoiesis stimulating agent (ESA) treatment failure is associated with poor prognosis for patients with IPSS1 low and intermediate-1 (‘lower risk’) myelodysplastic syndromes (MDS)2 and, to date, there is no standard treatment option. In this subgroup of patients, disease modifying approaches may be needed to change disease evolution.3 Azacitidine (AZA) improves survival in patients with higher risk MDS,4 although the majority of responses are limited to hematologic improvement (HI). This pattern of response fits with the objectives of treatment needed for lower risk MDS patients who carry other poor prognostic factors, or with resistance to ESAs. Use of AZA in these patients5,6 resulted in hematologic responses (especially erythroid responses) in 30-40% of cases, but data on survival were limited. We previously reported that the outcome of patients with higher-risk MDS is very poor after AZA failure.7 The increasing use of AZA for lower risk patients raises the issue of patient outcome following AZA treatment failure. This information will be important to define treatment strategies and to evaluate new agents in clinical trials. In the present report, we examined the outcome of lower risk MDS after failure of azacitidine.
Inclusion criteria in the present study were: i) a diagnosis of MDS or non-proliferative CMML (WBC<13×109/L) according to WHO classification;8 ii) IPSS low or intermediate-1 before onset of AZA treatment; iii) patient having received at least one cycle of AZA; iv) primary or secondary AZA failure; v) no previous exposure to chemotherapy before AZA. A total of 59 patients from the French AZA compassionate program (n=50) and 2 clinical studies from Johns Hopkins University (J9950 and J0443, n=9) treated between 2000 and 2011 fulfilled these criteria. Details of methodology and azacitidine regimens have been previously described.7 Response to initial AZA treatment had been assessed with IWG 2000 criteria.9 Date of AZA failure was defined by the date of evaluation of response after the last cycle of AZA. Disease status at the end of AZA was categorized as primary failure in the absence of any response to AZA, secondary failure if disease progressed after a first response (loss of HI or bone marrow progression) or AZA intolerance (if AZA was stopped because of adverse events regardless of clinical response). Response to salvage therapy was assessed according to IWG 2000 criteria. Survival was calculated from the date of AZA failure to the date of death or last follow up. Statistical analysis was performed using the R.2.3.0. software (R Development Core Team, Vienna, Austria).
Details of patients' characteristics are shown in Table 1. Median age was 71 years and median duration of MDS before onset of AZA was 13 months. Most patients at onset of AZA belonged to the ‘high-risk’ subpopulation of lower risk IPSS MDS, with 81% of the patients having RBC transfusion requirements and 73% of patients having intermediate or high WPSS score. Thirty-eight patients (64%) had received treatment before AZA, including 11 patients with 2 or 3 prior regimens. The median number of AZA cycles was 6 (range 1-41). Twenty-five patients (42%) achieved a response (15 major HI, 3 PR, and 7 CR). Treatment failure was classified as primary failure for 33 patients (56%), secondary failure for 24 patients (41%), and toxic failure for 2 patients (3%). Median survival after azacitidine failure was 16.7 months (Figure 1A). As expected, survival of this series of low-risk MDS patients was better than the survival of higher risk MDS with AZA fail ure that we had previously published (median overall survival (OS) 7.5 months, P=0.001).7 Patients 70 years and older had a shorter survival as compared to younger patients (12.5 months vs. 27.8 months, respectively, P=0.03). Finally, patients with high WPSS before AZA (n=28) had a poorer outcome than patients with very low to intermediate WPSS (n=31) (8.2 months vs. 31 months, respectively, P=0.001, Figure 1B). No other patient-related or treatment-related variable seems to impact survival. Data on treatments administered after AZA failure were available for 53 patients. Twenty-six patients (49%) were treated with best supportive care (BSC) and their median survival was 8.6 months. Twenty-seven patients received other treatments with a median survival of 12.8 months: 7 patients received chemotherapy for disease progression (including AML-like regimen in 5 patients), 1 patient decitabine, and 1 patient vorinostat. Nine patients received thalidomide (n=4) or lenalidomide (n=5). Allogeneic transplantation was performed for 8 others. All but one received transplant immediately after AZA failure. Six of the 8 patients were alive in CR at last follow up (1-year survival estimate 83%). Overall, there was no survival benefit for being actively treated compared to best supportive care (13 months vs. 9 months, P=0.56), but median survival was not reached in patients treated with allogene-ic transplantation (P=0.14 vs. BSC). Although allografted patients represent a highly selected subset and the number of patients was small, these results suggest that allogeneic transplantation should be considered in this situation when feasible and this needs to be evaluated prospectively.
Table 1.
Baseline characteristics of the patients with lower risk MDS who failed azacitidine treatment.
Figure 1.
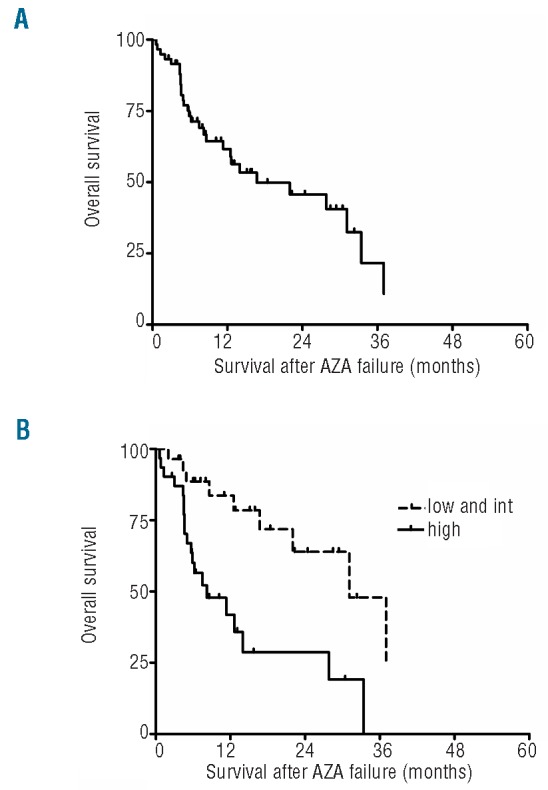
(A) Kaplan-Meier estimates of the overall survival after azacitidine failure in low and int-1 IPSS MDS patients. (B) Survival estimates according to WPSS. Curves represent survival estimates of patients. Each tick mark represents a censored patient. Low and Int: includes patients with very low, low and intermediate WPSS scores.
Despite a relatively small number of patients, our results showed that the outcome of lower risk IPSS patients who have failed AZA is poor with a median OS of 17 months, and that WPSS score may be able to predict outcome. A majority of the patients (56%) of our cohort had bone marrow progression after AZA treatment even if median MDS duration before AZA was relatively short (median 13 months) reflecting the severity of the underlying disease in this group of patients. We were not able to demonstrate that active treatment strategy after AZA failure was associated with any survival benefit apart from the potential benefit of allogeneic transplantation. Treatment options following failure of ESAs are limited, in particular in elderly patients unfit for intensive treatments. Besides the potential interest of using immunomodulatory grugs (IMID),10,11 investigational agents will have to be tested in those settings.3,12 Our data, by determining the outcome of lower risk MDS after AZA treatment failure, provide a background for such future trials.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodys-plastic syndromes. Blood. 1997;89(6):2079-88 [PubMed] [Google Scholar]
- 2.Park S, Grabar S, Kelaidi C, Beyne-Rauzy O, Picard F, Bardet V, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574-82 [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol. 2011;29(5):516-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musto P, Maurillo L, Spagnoli A, Gozzini A, Rivellini F, Lunghi M, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer. 2010;116(6):1485-94 [DOI] [PubMed] [Google Scholar]
- 6.Lyons RM, Cosgriff TM, Modi SS, Gersh RH, Hainsworth JD, Cohn AL, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850-6 [DOI] [PubMed] [Google Scholar]
- 7.Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29(24):3322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-51 [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671-4 [PubMed] [Google Scholar]
- 10.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-65 [DOI] [PubMed] [Google Scholar]
- 11.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86-93 [DOI] [PubMed] [Google Scholar]
- 12.Stone RM. How I treat patients with myelodysplastic syndroms. Blood. 2009;113:6296-303 [DOI] [PubMed] [Google Scholar]


