Abstract
OBJECTIVES
Antibodies to infliximab (ATIs) have been associated with loss of clinical response and lower serum infliximab (IFX) levels in some studies of patients with inflammatory bowel disease (IBD). This has important implications for patient management and development of novel biologic therapies. The objective of this study was to perform a systematic review and meta-analysis of studies that reported clinical outcomes and IFX levels according to patients’ ATI status.
METHODS
MEDLINE, Web of Science, CINAHL, Scopus, and EMBASE were searched for eligible studies. Quality assessment was undertaken using GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria. Raw data from studies meeting inclusion criteria was pooled for meta-analysis of effect estimates. Sensitivity analysis was performed for all outcomes. Funnel plot was performed to assess for publication bias.
RESULTS
Thirteen studies met the inclusion criteria, and reported results in 1,378 patients with IBD. All included studies had a high risk of bias in at least one quality domain. The pooled risk ratio (RR) of loss of clinical response to IFX in patients with IBD who had ATIs was 3.2 (95 % confidence interval (CI): 2.0–4.9, P < 0.0001), when compared with patients without ATIs. This effect estimate was predominantly based on data from patients (N = 494) with Crohn’s disease (RR: 3.2, 95 % CI: 1.9–5.5, P < 0.0001). Data only from patients with ulcerative colitis (n = 86) exhibited a non-significant RR of loss of response of 2.2 (95 % CI: 0.5–9.0, P = 0.3) in those with ATIs. Heterogeneity existed between studies, in both methods of ATI detection, and clinical outcomes reported. Three studies (n = 243) reported trough serum IFX levels according to ATI status; the standardized mean difference in trough serum IFX levels between groups was −0.8 (95 % CI −1.2, −0.4, P < 0.0001). A funnel plot suggested the presence of publication bias.
CONCLUSIONS
The presence of ATIs is associated with a significantly higher risk of loss of clinical response to IFX and lower serum IFX levels in patients with IBD. Published studies on this topic lack uniform reporting of outcomes. High risk of bias was present in all the included studies.
INTRODUCTION
Infliximab (IFX), a chimeric monoclonal antibody directed against tumor necrosis factor (TNF), is approved for the induction and maintenance of remission in both Crohn’s disease (CD) and ulcerative colitis (UC) (1,2). Clinical trials and case series have reported induction of remission in 40–60 % of patients treated with this agent, with the majority continuing with maintenance therapy every 8 weeks (2,3). Despite its proven efficacy in maintenance of remission, a significant proportion of patients lose their clinical response over time despite maintenance treatment (4). This loss of response (LOR) occurs in up to 70 % of patients treated with IFX, and usually requires escalation of dosing or change in anti-TNF agent to re-capture clinical remission (5–7).
There are several mechanisms of LOR to IFX; however, immunogenicity to the antibody itself appears to be a commonly identified factor (8,9). Since IFX is a chimeric mouse–human IgG1 molecule, antibodies to IFX (antibodies to infliximab (ATIs)) are primarily directed against the murine F(ab)2 fragment of the agent (10,11). ATIs are reported to develop in 8–60 % of patients with inflammatory bowel disease (IBD), depending on IFX dosing schedule, administration of concomitant steroids, or immunomodulators and the method of measuring ATI in the blood (10,12–16). These antibodies can appear as soon as after the first IFX infusion, and can persist in the blood stream for up to 1–4.5 years even after discontinuation of IFX therapy (17,18).
The problem of immunogenicity of anti-TNF agents was not described in the early pivotal trials in IBD. It has since been observed that clearance of IFX is greatly increased in the presence of ATIs, and results in low IFX trough levels (10,19,20). Low serum IFX concentrations have been associated with a lack of clinical response in both CD and UC (16,21,22). Multiple studies in IBD patients have linked the development of ATI with loss of treatment response, shorter duration of response, and infusion reactions (10,12,15,16,23). Conversely, others have shown no difference in clinical outcomes between ATI-positive or ATI-negative patients (14,21).
The association of ATIs with trough IFX levels and response to therapy with IFX has been inconsistent due to a lack of standardization of methods of measurement of serum IFX or antidrug antibodies. The presence of detectable drug in the serum typically impairs the performance of a solid-phase enzyme-linked immunosorbent assay (ELISA) and western blot (10). With classic ELISA, antibodies remain undetectable as long as the drug is present in the blood. The type of detection assays also affects the reported incidence of ATIs (24). Drug trough levels are less liable to interassay variations and may prove to be a more relevant surrogate marker for loss of clinical response than ATIs (25). Although ATIs are well-described, other humanized therapeutic monoclonal antibodies that lack the murine F(ab) fragment are also associated with anti-drug antibodies (26–28).
For clinicians, patients, and developers of biologic agents, loss of clinical remission due to immunogenicity is a potential major limitation of this class of drug, leading to clinical relapse, impaired quality of life, and increased cost of care. In addition, the focus by regulatory authorities on the immunogenicity of pioneer biologics has implications for the future development and approval of generic biologics (so-called “biosimilars”) (29,30). Although the potential negative impact of ATIs on trough IFX levels and clinical outcomes is widely acknowledged, no study has quantified the extent of this effect via meta-analysis of raw data from published studies. In order to provide a pooled estimate of the impact of immunogenicity, we sought to perform a systematic review and meta-analysis of the impact of ATIs on clinical outcomes and serum IFX levels in patients with IBD.
METHODS
Literature search
A literature search was performed to identify all published and unpublished studies in any language for consideration for inclusion studies that had measured serum antibodies to IFX (ATI) and/or trough serum IFX levels and reported on clinical outcomes of IFX therapy in patients with IBD. A systematic search of the following databases was performed: MEDLINE (Pubmed)–1966 to February 2012, Web of Science–2000 to February 2012), Allied Health Literature (CINAHL)–1990 to February 2012, Scopus–2000 to February 2012 and EMBASE 2000–August 2012. The following search strategy was constructed by using a combination of MeSH subject headings and text-words relating to antibodies to IFX: “infliximab,” “immunogenicity,” “anti-drug antibodies,” “infliximab levels,” “antibodies to infliximab (ATIs),” “human anti-chimeric antibodies (HACAs),” “ulcerative colitis,” “Crohn’s disease,” “loss of response,” “remission.” Abstracts from American Digestive Diseases Week and the United European Gastroenterology Week (2002–2011) were searched manually, and reference lists of all articles read and several previously published reviews were scrutinized to disclose additional literature on the topic.
Inclusion and exclusion criteria
We included all studies (controlled trials, observational studies, cohort studies) that reported prevalence of ATIs and clinical outcomes and/or serum IFX levels in patients who were treated with IFX for UC or CD. The primary outcome measure was “LOR,” defined as relapse of clinical symptoms in patients who were in clinical remission from, or had responded to, IFX. No pre-specified Crohn’s Disease Activity Index (CDAI) score was used to determine this outcome, as we expected there would be no universal definition of LOR across the studies. We did not require that included studies report the objective confirmation of active inflammation as the cause of symptoms. The included studies all came from experienced tertiary-referral IBD groups with extensive clinical trial history, and most reported exclusion of infection and/or biochemical confirmation of inflammation as part of their follow-up assessment of patients in their methods. Studies that measured ATIs by any method were included if they also reported clinical outcomes and/or serum IFX levels according to ATI status.
We excluded studies if they (i) were review articles (ii) examined IFX use in non-IBD patients, (iii) did not measure either ATI or IFX trough levels, (iv) did not report on numbers of patients who were ATI positive or negative, (v) did not report clinical outcomes of IFX therapy or serum IFX levels, and (vi) were studies using other anti-TNFs only (adalimumab or certolizumab).
Study selection
Two authors (K.S.N. and A.S.C.) independently scanned the abstract of every trial identified by the search to determine eligibility. Blinding to source was not performed. Full articles were selected for further assessment if the abstract suggested the study included patients with CD or UC, ATIs or serum IFX were measured, and clinical outcomes were reported. If these criteria were unclear from the abstract, the full article was retrieved for clarification. Papers not meeting the inclusion criteria were excluded. Any disagreements were resolved by discussion, and if required, by consultation with the senior author (A.C.M.).
Data extraction and quality assessment
The following data were retrieved (where possible) from published reports using standardized forms with disagreements resolved by discussion between the reviewers: number of patients in the study, method of selection of cohorts, schedule of anti-TNF administration, assay type used to measure ATI, numbers of ATI-positive and -negative patients, mean (s.d.) and median (interquartile range) IFX trough levels, methods of measurement of clinical outcomes, and reporting outcomes used. Assessment of quality of randomized controlled trials and observational studies was performed using The GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria (31). For observational studies, methodological quality was assessed by determining the eligibility criteria, degree of measurement of both exposure and outcome, control for confounders, and completeness of follow-up (32).
Statistical analysis
Data were analyzed and reported consistent with the consensus guidelines by the Meta-analysis of Observational Studies in Epidemiology group (33). Data were pooled for meta-analysis if the outcomes were sufficiently similar (determined by consensus of authors) and data were homogenous (determined by the degree of clinical and statistical heterogeneity). Data were pooled for meta-analysis if the criteria for “LOR” in each study were determined by consensus to represent clinically comparable events in the context of disease activity. Raw data from included studies (absolute numbers) were used to construct 2×2 contingency tables, and unadjusted risk ratios (RRs) were calculated using Review Manager (RevMan 5.1, Nordic Cochrane Centre, Copenhagen, Denmark) for dichotomous outcomes. Standardized mean difference was used to report the summary statistic for comparison of outcomes presented as continuous scales, to account for difference in methods of measurement of IFX levels and ATIs. The random effects model was used to account for variations between studies and give a more conservative pooled estimate (34). The Q test was used to assess for heterogeneity and I2 statistic to quantify the percentage of heterogeneity due to between-study variation; a value of P < 0.10 was considered statistically significant (35). Funnel plots and the Egger’s test were used to evaluate for publication bias (36). The NNH (number needed to harm) was calculated where informative (37). Sensitivity analyses were performed for all outcomes where three or more studies were included. Sensitivity analysis included sensitivity based on individual studies, sample size, method or ATI determination, episodic or scheduled IFX, length of study. Significance levels were set at a P < 0.05.
RESULTS
Study characteristics
Supplementary Figure 1 illustrates the study selection process; of the 99 references identified from database searches, 29 articles were selected for full text review. From these, only 13 articles were deemed suitable and met the pre-specified inclusion criteria. Sixteen articles/abstracts were excluded for various reasons detailed in Supplementary Table 1 (12,15,17,18,38–48,49). Table 1 summarizes the main characteristics of the included studies. Ten studies had suitable data to be included in the immunogenicity meta-analysis (six CD only, one UC only, two pooled results from patients with IBD, and one that presented separate results for patients with UC and CD). Two studies reported combined data from both CD and UC patients and were thus classified as “mixed IBD” for the purpose of meta-analysis. Three articles (21,50,51) met the criteria for inclusion, but did not report raw data in a manner suitable for inclusion in the meta-analysis.
Table 1.
Description of the main variables of the included studies (eight with Crohn’s disease patients only, two with ulcerative colitis patients, three with both UC and CD patients)
| Study | Year | N | Definition of “loss of response” | ATI assay type | ATI measured |
|---|---|---|---|---|---|
| Crohn’s disease | |||||
| Farrell et al. (13) | 2003 | 53 | Clinicians assessment | DA ELISA | Yes |
| Hanauer et al. (14) | 2004 | 514 | CDAI | DA ELISA | Yes |
| Maser et al. (21) | 2006 | 105 | HBI | DA ELISA | Yes |
| Candon et al. (58) | 2006 | 28 | HBI/surgery/non-healing fistula | DA ELISA | Yes |
| Ainsworth et al. (16) | 2008 | 33 | Clinicians assessment | Fluid-phase RIA | Yes |
| Yamada et al. (50) | 2010 | 31 | CDAI/CRP | N/A | No |
| Pariente et al. (20) | 2011 | 56 | HBI/clinicians assessment | DA ELISA | Yes |
| Steenholdt et al. (22) | 2011 | 85 | Clinicians assessment | Fluid-phase RIA | Yes |
| Ben Horin et al. (10) | 2011 | 51 | Clinicians assessment | AHLC ELISA | Yes |
| Kopylov et al. (52) | 2011 | 63 | Clinicians assessment | DA ELISA and AHLC ELISA | Yes |
| Imaeda et al. (19) | 2012 | 58 | CDAI | DA ELISA and IC based ELISA | Yes |
| Ulcerative colitis | |||||
| Seow et al. (23) | 2010 | 115 | Mayo score | DA ELISA | Yes |
| Arias et al. (59) | 2011 | 136 | Clinicians assessment/CRP/albumin | DA ELISA | Yes |
| Pariente et al. (20) | 2011 | 18 | SCCAI/clinicians assessment | DA ELISA | Yes |
| Steenholdt et al. (22) | 2011 | 21 | Clinicians assessment | Fluid-phase RIA | Yes |
| Ben Horin et al. (10) | 2011 | 11 | Clinicians assessment | DA ELISA | Yes |
AHLC, antihuman λ chain; ATI, antibodies to infliximab; CD, Crohn’s disease; CDI, Crohn’s disease index; DA ELISA, double antigen enzyme-linked immunoassay; HBI, Harvey Bradshaw index; IC, immunoaffinity chromatography; IFX, infliximab; LOR, loss of response; RIA, radioimmunoassay; SCCAI, Simple Clinical Colitis Activity Index; UC, ulcerative colitis.
Patients with CD were included in 11 studies (n = 1,077). Length of follow-up in these studies ranged from 16 weeks to 3 years. In studies that reported the “number of IFX infusions,” the range was from 7 to 28 infusions. In the quality assessment of included studies, 7/11 (63%) of studies were graded 2/4 for quality and only 1 study (9 %) graded 3/4 (Supplementary Table 2).
Patients with UC were included in five studies (n = 301). Length of follow-up in these studies ranged from 14 months to 38 months, or a median of 8–13 IFX infusions. In the quality assessment of included studies, 80 % of studies graded 2/4 for quality and one graded 3/4.
Serum ATIs in the included studies were measured using several different assays, including double antigen (DA) ELISA, antihuman λ chain ELISA, fluid-phase RIA (radioimmunoassay), immunoaffinity chromatography-based ELISA, and western blot (Table 1). Serum IFX levels in the included studies were measured using DA ELISA. The primary outcome for inclusion in this meta-analysis, “LOR,” was reported based on clinicians’ assessment, CDAI, or HBI (Harvey Bradshaw Index) in patients with CD, and clinicians’ assessment Partial Mayo score, or Simple Colitis Clinical Activity Index in patients with UC (Table 1).
Effects of ATIs on LOR
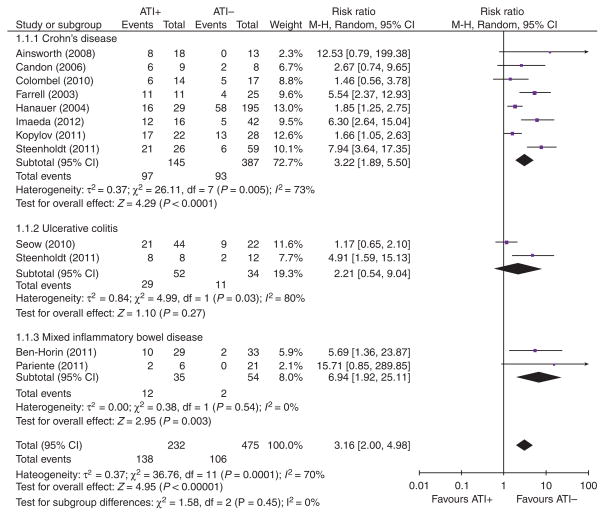
Ten studies of patients with IBD reported data on loss of clinical response according to ATI status, with data on a total of 668 patients (Figure 1). The pooled RR of “loss of clinical response” in all patients with IBD treated with IFX who developed ATIs was 3.2 (95 % confidence interval (CI): 2.0–4.9, P < 0.0001). The number of patients needed to develop ATIs for one of them to lose response (NNH) was 3.
Figure 1.
Forrest plot of meta-analysis of “loss or response” according to patients’ antibodies to infliximab (ATI) status (ATI+ or ATI−).
Seven studies of patients with CD were suitable for inclusion into a subgroup meta-analysis, with pooled data on 494 patients. The RR of LOR in patients with ATIs was 3.2 (95 % CI: 1.9–5.5, P < 0.0001) when compared with patients without ATIs. The number of patients needed to develop ATIs for one of them to lose response (NNH) was 2. There was statistical heterogeneity between the studies (I2 = 73 %; P < 0.001). Two included studies were not suitable for the ATI meta-analysis; Maser et al. (21) was excluded from the meta-analysis due to the ambiguous clinical end point (“percentage of patients that achieved the median duration of clinical remission”), and Yamada et al. (50) due to the lack of reported ATI levels.
Only two studies reported raw data on the outcome of patients with UC alone, stratified according to ATI status or IFX levels (22,23). The pooled RR of “loss of clinical response” in patients with UC treated with IFX who developed ATIs was 2.2 (95 % CI: 0.5–9). However, the test for overall effect was non-significant (P = 0.3), and there was significant statistical heterogeneity between the two studies (I2 = 80%; P < 0.03).
Two studies reported results in a mixed population of patients with IBD, both CD and UC (10,20). The prevalence of ATI ranged from 22.4 to 46 %. RR of LOR in the presence of ATIs was 6.9 (95 % CI: 1.9–25; P < 0.003). NNH calculated was 3. There was no statistical heterogeneity between the two studies (I2 = 0 %; P < 0.5).
Finally, the prior analyses grouped patients according to dichotomous ATI status (present/absent). Four studies reported mean/median ATI absolute levels according to patients’ clinical outcomes and all concluded that patients who lost response had significantly higher ATI levels than patients who maintained remission (Supplementary Table 3). These data were not suitable for meta-analysis due to the lack of reporting of means and standard deviations in all studies.
Effects of ATIs on serum IFX levels
The effect of ATI status on serum IFX levels was reported in one study of patients with CD (n = 58), and two studies that pooled patients with UC and CD together (n = 185). All three studies reported significantly lower trough serum IFX levels in patients with detectable ATIs. Raw data on mean or median trough IFX levels according to ATI status are summarized in Table 2a. A meta-analysis of the pooled standardized mean difference confirmed a significant difference in trough serum IFX levels between ATI+ and ATI− patients (standardized mean difference −0.8, 95% CI: −1.2, −0.4, P < 0.0001, random effects model) (Figure 2).
Table 2a.
Serum infliximab trough levels (μg/ml) in anti-infliximab antibody (ATI)-positive and ATI-negative patients (mean±s.d. unless otherwise stated)
| Study | n | ATI+ | ATI− | P value |
|---|---|---|---|---|
| Pariente et al. (20) | 76 | 1.61 (±6.13) | 4.45 (±3.92) | < 0.0001 |
| Ben-Horin et al. (10) | 109 | 3.3 (±4.6) | 11.8 (±12) | < 0.001 |
| Imaeda et al.a (19) | 58 | 0.18 | 3.41 | < 0.01 |
ATI, antibodies to infliximab; IQR, interquartile range.
Data are represented as median values (IQR not given).
Figure 2.
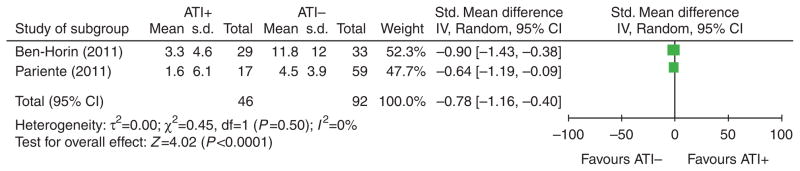
Forrest plot of meta-analysis of “mean trough serum infliximab (IFX) level” according to patients’ status (ATI+ or ATI−). ATI, antibodies to infliximab.
Since low serum IFX levels have also been associated with LOR to IFX, we also examined the serum IFX levels according to LOR in patients with IBD. Raw data on trough IFX levels according to maintenance of response to therapy were reported in six studies in patients with IBD; four reported significant differences in serum IFX level according to clinical outcomes, but two did not. Median or mean IFX levels in each group are provided in Table 2b. Only one study reported mean and standard deviation for serum IFX levels, so these data were not suitable for performing a meta-analysis of the results.
Table 2b.
Infliximab trough levels (μg/ml) in IBD patients who had loss of response vs. no loss of response (median and IQR range, unless otherwise stated)
| Study | N | Lost response | Maintained remission | P value |
|---|---|---|---|---|
| Ainsworth et al. (16) | 27 | 0 (0–0.1) | 2.9 (0.9–4.3) | 0.002 |
| Yamada et al.a (50) | 31 | 6.3 | 4.7 | NS |
| Steenholdt et al. (22) | 69 | 0 (0–0) | 2.8 (0.8–5.3) | < 0.0001 |
| Pariente et al.b (20) | 76 | 3.3 (±4.1) | 2.3 (±2.2) | NS |
| Steenholdt et al.c (22) | 13 | 0 (0–0) | 3.8 (1.1–8.5) | < 0.0001 |
| Arias et al.c (59) | 136 | 0.3 (0.3–3.6) | 4.9 (1.7–8.2) | 0.01 |
IBD, inflammatory bowel disease; IQR, interquartile range.
No IQR given.
Data are represented as mean values (±s.d.).
Indicates patients with ulcerative colitis.
Sensitivity analysis
Sensitivity analysis was performed for the overall effect estimate for outcomes where there were >3 studies in a meta-analysis (LOR in all IBD patients, and in those with CD only). Exclusion of studies that showed no significant difference in LOR according to ATI status (14,23,52) increased the pooled effect estimate (Supplementary Table 4A and B). Analyses based on effect estimates (fixed or random), IFX schedule (episodic or scheduled), type of ATI assay (RIA or DA ELISA), criteria for LOR (physicians’ assessment or CDAI/HBI, reported exclusion of other causes of symptoms) did not significantly change the effect estimate. No adjusted data were reported in the included studies for further subgroup analyses.
In order to identify the determinants of the statistical heterogeneity seen in the main subgroup analysis (CD), each individual study was excluded, and the I2 value measured for heterogeneity. Exclusion of the Imaeda and Steenholdt studies lowered the I2 result to 40% (heterogeneity “might not be important”) (35). The RR for the effect of ATIs decreased to 2.2 (95 % CI: 1.5–3.4, P = 0.0002) when these studies were excluded.
Test for publication bias
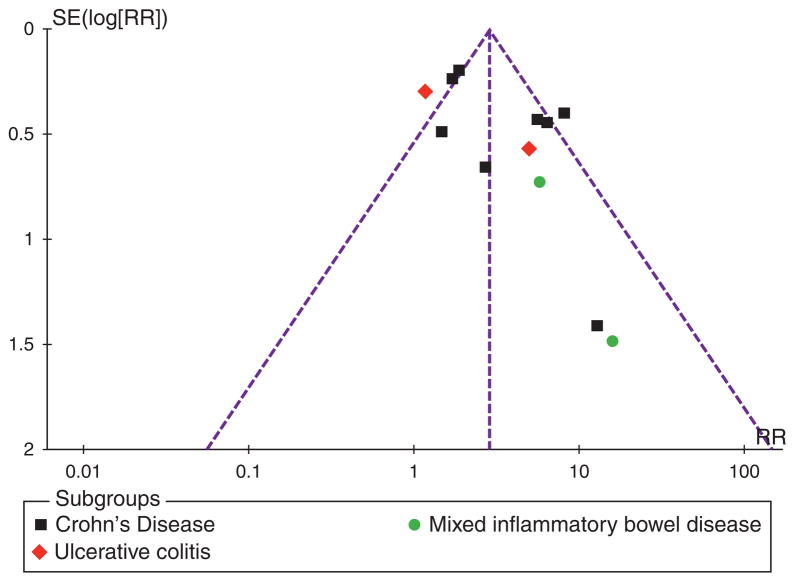
The funnel plot (standard error by RR) for publication bias is illustrated in Figure 3. Formal tests for funnel plot asymmetry were not performed as the power of these tests is too low to distinguish chance from real asymmetry if < 10 studies are included (53). The overall appearance of the funnel plot suggests an inverse linear association between precision and effect size; the most precise studies (the ones with larger size and more events) produced RRs closer to 1 (no effect).
Figure 3.
Funnel plot of risk ratio (x axis) by standard error (y axis). Dashed line represents expected distribution of studies on graph in the absence of publication bias.
DISCUSSION
The association between clinical efficacy with anti-TNF-α drug levels and the presence of ATI has been examined by a number of groups, with inconsistent results (54). The development of ATI and low serum IFX concentrations have been implicated as important cause of therapeutic failure in patients receiving IFX (12,16,22,23). There is ongoing debate as to the relevance of ATI development in clinical care; two recent systematic reviews came to different conclusions on the significance of ATIs on clinical outcomes (9,55). Our study is the first meta-analysis to investigate the pooled impact of immunogenicity on clinical outcomes with IFX therapy in patients with IBD.
We found an association between ATI and LOR to therapy in our meta-analysis involving 10 studies and 668 patients. Overall, patients with IBD on IFX who develop ATI have a threefold risk of LOR to therapy. There was significant heterogeneity among the studies, which implies that the summary RR should be interpreted with caution. On subgroup and sensitivity analyses, the positive association between ATI and LOR was retained. The presence of ATI is also associated with lower serum IFX levels in the pooled results of two studies. Lower IFX levels were associated with LOR in three of five studies that examined this topic, but these data were not suitable for meta-analysis.
The link between immunogenicity and LOR was first reported by Baert et al. (12). They demonstrated that the presence of ATI was associated with a shorter duration of clinical response. Since then numerous other observational and randomized controlled trial have investigated this association with conflicting results. Ben-Horin et al. (17) showed that in their cohort of 62 patients on scheduled IFX dosing, LOR was associated with higher frequency of ATI (78 vs. 17 % no LOR, P < 0.01). Maser et al. (21) performed a study on CD patients, most of who were on scheduled maintenance treatment with IFX and found no difference in the duration of clinical response in patients with detectable IFX serum levels with or without ATIs (66 vs. 67 %). A subanalysis of ACCENT 1 data showed that remission rates at 54 weeks were similar (41 and 39 %) in patients who were ATI-positive and ATI-negative (14).
Higher IFX trough levels have been associated with sustained clinical remission in several studies. Seow et al. (23) showed that in their UC population of 106 patients treated with scheduled IFX dosing, detectable IFX trough was associated with significant positive predictive value for clinical remission, endoscopic improvement, and avoidance of colectomy (all P < 0.001). Similarly, Maser et al. (21) showed that in a subgroup of 90 patients on maintenance therapy, higher IFX levels were associated with improved clinical remission, CRP, and endoscopic healing (P < 0.001). Steenholdt et al. (22) investigated 106 mixed IBD patients and found that higher median IFX trough levels were associated with maintenance of response (P < 0.0001). They were also able to determine clinically relevant threshold values for trough IFX and ATI concentrations. However, Pariente et al. (20), in a retrospective study of 76 IBD patients, 39 of whom had dose intensification of IFX following initial non-response to therapy, showed no difference in mean IFX trough between patients who responded (69 %) to dose intensification vs. those who did not.
The strength of our study lies in the uniform selection criteria used for including studies in the meta-analysis. We chose to focus on studies that reported prevalence of ATIs according to clinical outcomes and/or serum IFX levels in patients who were treated with maintenance IFX. We chose a primary end point that could clinically be comparable across similar patient population studies, therefore excluding some studies (12,21,49). We also performed an assessment of study quality using the GRADE criteria (32).
As with all meta-analyses, caution needs to be used when making conclusions based on pooled studies with heterogeneous patient populations. In this context, we have included studies that did not share universal methods for enrolling or determining the primary end point, and used different methods for detection of ATIs. To incorporate for clinical heterogeneity, we only extracted data on patients receiving maintenance therapy, and assumed that “LOR” looks similar in practice, whether measured with CDAI, HBI, Mayo score, or a physician’s assessment. It has been noted by others that a physician’s global assessment of clinical status correlates well with more complicated disease activity scores such as the CDAI (56). To incorporate for statistical heterogeneity in the meta-analysis, we used a random effects model to analyze all outcomes, and used standardized mean difference for reporting continuous outcomes. We also performed subgroup and sensitivity analyses to examine differences in the overall effect estimate (57). It should also be noted that all included studies were judged to be at “high risk” of bias in at least one quality domain (Supplementary Table 2). In addition, the funnel plot (Figure 3) is asymmetric, suggesting a publication bias in this literature.
It should be noted that we did not extract data for analysis on patients who were ATI inconclusive (ATI negative but IFX detectable). There is evidence that ATI inconclusive patients tend not to have early LOR; however, it may be an indicator for future potential LOR (52). Maser et al. (21) showed that ATI inconclusive group had longer median duration of clinical remission (100 %) compared with both ATI-positive (66 %) and ATI-negative groups (67 %) (P < 0.001). Similar observations have been made by Colombel et al. (38), whereby this ATI result was associated with higher steroid free remission rates and Seow et al. (23) showing improved remission rates, endoscopic improvement and reduced colectomy rates. Others have shown ATI inconclusive patients do not differ to ATI-positive or -negative patients in terms of clinical outcomes (14). The higher remission rate for ATI inconclusive patients is likely related to higher serum IFX concentrations relative to ATI-positive or -negative patients. Kopylov et al. (52) showed that in their study, LOR in ATI inconclusive patients was only associated with low IFX levels in 50 % of the cases, which indicates the possibility of an alternative, non-immune mediated pathway for development of LOR. As previously described, the presence of ATI can impede the clinical response by affecting the drug’s bioavailability, pharmacokinetics, and pharmacodynamics (51). The true incidence of ATI in IBD patients treated with IFX remains unknown due to the different administration schedules, timing of ATI measurements, methods used in ATI detection, and the presence of serum IFX.
In conclusion, the present meta-analysis quantifies the impact of ATIs on maintenance of clinical remission in patients with IBD; patients on IFX therapy who develop ATI have a threefold higher increased risk of LOR to therapy compared with those who do not develop ATIs. ATI development is also associated with lower serum IFX levels in these patients. This has implications for the management of patients on biologic therapy, and the development of biosimilars.
Supplementary Material
Acknowledgments
We thank Brian Healy, PhD, Department of Biostatistics, Harvard School of Public Health for statistical advice.
Financial support: A.C.M. has served on Advisory Boards for Janssen, Abbott, and UCB, and acted as a consultant on an unrelated study for Prometheus Laboratories and SQI Laboratories. He was supported by NIH Grant K23DK084338. KSN is an IBD fellow at BIDMC, supported by an unrestricted educational grant to the Mater Misericordiae University Hospital by MSD (Ireland).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Alan C. Moss, MD, FACG.
Specific author contributions: Acquisition of data, analysis and interpretation of data, and wrote the manuscript: Kavinderjit S. Nanda; critical revision of the manuscript for important intellectual content: Adam S. Cheifetz; study concept and design, critical revision of the manuscript for important intellectual content, statistical analysis, and study supervision: Alan C. Moss. All authors discussed the results and implications and commented on the manuscript at all stages.
Potential competing interests: None.
References
- 1.Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106:S2–S25. doi: 10.1038/ajg.2011.58. [DOI] [PubMed] [Google Scholar]
- 2.Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133:312–39. doi: 10.1053/j.gastro.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–98. doi: 10.1038/ajg.2011.103. [DOI] [PubMed] [Google Scholar]
- 4.Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1–10. doi: 10.1111/j.1365-2036.2011.04679.x. [DOI] [PubMed] [Google Scholar]
- 5.Rostholder E, Ahmed A, Cheifetz A, et al. Outcomes after escalation of infliximab therapy in ambulatory patients with moderately active ulcerative colitis. Aliment Pharmacol Ther. 2012;35:562–7. doi: 10.1111/j.1365-2036.2011.04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–7. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 7.Regueiro M, Siemanowski B, Kip KE, et al. Infliximab dose intensification in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1093–9. doi: 10.1002/ibd.20177. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–95. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 9.Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn’s disease: a critical systematic review. Inflamm Bowel Dis. 2009;15:1264–75. doi: 10.1002/ibd.20899. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F (ab) 2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. 2011;60:41–8. doi: 10.1136/gut.2009.201533. [DOI] [PubMed] [Google Scholar]
- 11.Svenson M, Geborek P, Saxne T, et al. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology. 2007;46:1828–34. doi: 10.1093/rheumatology/kem261. [DOI] [PubMed] [Google Scholar]
- 12.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 13.Farrell R, Alsahli M, Jeen Y, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology. 2003;124:917–24. doi: 10.1053/gast.2003.50145. [DOI] [PubMed] [Google Scholar]
- 14.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 15.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–31. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ainsworth M, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol. 2008;103:944–8. doi: 10.1111/j.1572-0241.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Horin S, Mazor Y, Yanai H, et al. The decline of anti-drug antibody titres after discontinuation of anti-TNFs: implications for predicting re-induction outcome in IBD. Aliment Pharmacol Ther. 2012;35:714–22. doi: 10.1111/j.1365-2036.2012.04997.x. [DOI] [PubMed] [Google Scholar]
- 18.Steenholdt C, Al-khalaf M, Brynskov J, et al. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22910. Epub 29 August. [DOI] [PubMed] [Google Scholar]
- 19.Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012;47:136–43. doi: 10.1007/s00535-011-0474-y. [DOI] [PubMed] [Google Scholar]
- 20.Pariente B, de Chambrun GP, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;18:1199–206. doi: 10.1002/ibd.21839. [DOI] [PubMed] [Google Scholar]
- 21.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn’s disease. Scand J Gastroenterol. 2011;46:310–8. doi: 10.3109/00365521.2010.536254. [DOI] [PubMed] [Google Scholar]
- 23.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 24.Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies--toward improved methods of anti-antibody measurement. Curr Opin Immunol. 2008;20:431–5. doi: 10.1016/j.coi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Rutgeerts P, Vermeire S, Van Assche G. Predicting the response to infliximab from trough serum levels. Gut. 2010;59:7–8. doi: 10.1136/gut.2009.191411. [DOI] [PubMed] [Google Scholar]
- 26.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–40. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 27.West R, Zelinkova Z, Wolbink G, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28:1122–6. doi: 10.1111/j.1365-2036.2008.03828.x. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber S, Khaliq-Kareemi M, Lawrance I, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–49. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed I, Kaspar B, Sharma U. Biosimilars: impact of biologic product life cycle and European experience on the regulatory trajectory in the United States. Clin Ther. 2012;34:400–19. doi: 10.1016/j.clinthera.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Lahiff C, Kane S, Moss A. Drug development in inflammatory bowel disease: the role of the FDA. Inflamm Bowel Dis. 2011;17:2585–93. doi: 10.1002/ibd.21712. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G, Oxman A, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Stroup D, Berlin J, Morton S, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cates CJ. Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Med Res Methodol. 2002;2:1–4. doi: 10.1186/1471-2288-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 39.Drastich P, Kozeluhova J, Jaresova M, et al. Infliximab serum trough levels and deep remission in patients with IBD. Gastroenterology. 2011;140 (Suppl 1):S292. [Google Scholar]
- 40.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate for the prevention of antibodies to infliximab in patients with Crohn’s disease. Gastroenterology. 2010;138 (Suppl 1):S167–8. [Google Scholar]
- 41.Vermeire S, Gabriels F, Ballet V, et al. The effect of dose escalation on trough levels in patients who lost response to infliximab. Gut. 2010;59:A81. [Google Scholar]
- 42.Afif W, Loftus EV, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Moerkercke W, Ackaert C, Compernolle G, et al. High infliximab trough levels are associated with mucosal healing in Crohn’s disease. Gastroenterology. 2010;138 (Suppl 1):S60. [Google Scholar]
- 44.Lichtenstein G, Diamond R, Wagner C, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD: subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–26. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Assche G, Magdelaine–Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–8. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Miele E, Markowitz JE, Mamula P, et al. Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr. 2004;38:502–8. doi: 10.1097/00005176-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Fasanmade A, Masters P, Munsanje E, et al. Infliximab pharmacokinetics and improvement in fistulizing Crohn’s disease. Gastroenterology. 2003;124 (Suppl 1):A61. [Google Scholar]
- 48.Settesoldi A, Giannotta M, Genise S, et al. Loss of efficacy and adverse drug reactions during infliximab therapy in IBD patients are related to the appearance of anti-infliximab antibodies. Digestive Liver Dis. 2012;44 (Suppl 2):S194. [Google Scholar]
- 49.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 50.Yamada A, Sono K, Hosoe N, et al. Monitoring functional serum anti-tumor necrosis factor antibody level in Crohn’s disease patients who maintained and those who lost response to anti-TNF. Inflamm Bowel Dis. 2010;16:1898–04. doi: 10.1002/ibd.21259. [DOI] [PubMed] [Google Scholar]
- 51.Jürgens M, Laubender RP, Hartl F, et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811–9. doi: 10.1038/ajg.2010.95. [DOI] [PubMed] [Google Scholar]
- 52.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18:1628–33. doi: 10.1002/ibd.21919. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library; 2008. [Google Scholar]
- 54.Colombel J, Feagan B, Sandborn W, et al. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349–58. doi: 10.1002/ibd.21831. [DOI] [PubMed] [Google Scholar]
- 55.Chaparro M, Guerra I, Muñoz-Linares P, et al. Systematic review: antibodies and anti-TNF-α levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012 doi: 10.1111/j.1365-2036.2012.05057.x. E-pub 22 March. [DOI] [PubMed] [Google Scholar]
- 56.Sandler RS, Jordan MC, Kupper LL. Development of a Crohn’s index for survey research. J Clin Epidemiol. 1988;41:451–8. doi: 10.1016/0895-4356(88)90046-7. [DOI] [PubMed] [Google Scholar]
- 57.Higgins J, Thompson S, Deeks J, et al. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7:51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 58.Candon S, Mosca A, Ruemmele F, et al. Clinical and biological consequences of immunization to infliximab in pediatric Crohn’s disease. Clin Immunol. 2006;118:11–9. doi: 10.1016/j.clim.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Arias MT, Drobne D, Vande Castelle N, et al. Influence of trough serum levels and immunogenicity on long term outcome of infliximab therapy in ulcerative colitis. Gut. 2011:60:A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.