Abstract
The Snail/Gfi-1 (SNAG) family of zinc finger proteins is a group of transcriptional repressors that have been intensively studied in mammals. SNAG family members are similarly structured with an N-terminal SNAG repression domain and a C-terminal zinc finger DNA binding domain, however, the spectrum of target genes they regulate and the ranges of biological functions they govern vary widely between them. They play active roles in transcriptional regulation, formation of repressive chromatin structure, cellular signaling and developmental processes. They can also result in disease states due to deregulation. We have performed a thorough investigation of the relevant literature and present a comprehensive mini-review. Based on the available information, we also propose a mechanism by which SNAG family members may function.
Keywords: SNAG repression domain, SNAG-ZFP target genes, Transcription Regulation, Cellular signaling and Development, EMT and Cancer
1. INTRODUCTION
1.1 Transcription factors and transcriptional regulation
Transcription, the process of synthesis of an RNA transcript from a DNA template, is an important event in all aspects of biology since it leads to gene expression. Eukaryotic genomes are highly complex and contain several thousand genes. These genes are transcribed with the help of regulatory proteins known as transcription factors, which can either activate or silence target gene expression in a spatiotemporal fashion. The human genome is comprised of approximately 20,500 polypeptide-coding genes (1), of which, approximately 1,400 encode transcription factors (2) that function in regulating the expression of the remaining genes.
There are roughly 700 C2H2 zinc finger-type transcription factors, named for the specific presence of zinc chelating modules in their structure. Each finger consists of a pair of cysteine residues in the beta helix and a pair of histidine residues in the alpha helix (3). These zinc fingers function as DNA binding domains that allow for the interaction of the DNA’s major groove with the divalent cation (4). The C2H2 family of transcriptional repressors is classified into sub-families such as SNAG, BTB/POZ (Broad Complex, Tramtrack, Bric-a-brac/Pox virus Zinc finger), and KRAB (Krüppel Associated Box) based on the repressor domains they contain (5).
Transcriptional regulation mainly occurs at the initiation of transcription of the target gene, where transcription factors function either as activators or repressors turning genes on or off respectively. Transcriptional repressors may function by directly interacting with components of basal transcription machinery, remodeling the chromatin in an ATP-dependent manner, or site-specific modifications of the histone tails (6, 7). A transcriptional repressor can inhibit transcription in many ways upon binding to its target gene promoter in a sequence-specific manner. It can block the function of an activator in a process termed active repression (8) or involve chromatin compaction leading to the loss of binding of the polymerase II holoenzyme complex at the promoter, which is needed to transcribe the DNA into RNA (9). The repressors can inhibit transcription either directly or indirectly by recruiting a corepressor (8, 10, 11).
1.2 SNAG domain family members and their classification
The SNAG domain family of transcription factors is a group of transcriptional repressors named after the presence of this domain in two founding members of the family – Snail (first identified in Drosophila melanogaster) (12) and Gfi-1 (Growth factor independence-1) (13) in human. Various members of this family have been implicated in developmental and disease states. A BLAST analysis using the minimal SNAG domain (amino acids MPRSFLV) identified a collection of over 70 genes (including homologs) from several organisms (Figure 1). The presence of a strong conservation among these members that perform diverse functions in a variety of organisms is indicated in the highlighted portion. Except for the Gfi-1 isoform 2 of M.musculus, in all others the SNAG domain is located at the very N-terminus and begins with initiator methionine. Also, the first seven amino acids are highly conserved in all of them. Only the fourth amino acid position varies and is comprised of Ser, Ala, Arg or Gly. This residue is dispensable since alanine substitution in this position will not eliminate the SNAG repressor activity (data not shown).
Figure 1.
SNAG domain homology. BLAST program was used to analyze the SNAG domain genes from a variety of organisms. Strong conservation is indicated in the highlighted region. At the bottom, “*” indicates identical residues and “:” indicates good conservation.
A diagrammatic representation of the architecture of SNAG family members in Homo sapiens is shown in Figure 2. All SNAG family members share highly conserved domains at their N- and C-termini. Structurally, they are comprised of an N-terminal SNAG domain and a variable number (four to six) of zinc fingers at the C-terminus. Snail2 has a domain of unknown function within its linker region as do Scratch1 and Scratch2. Distinct regions in SNAG family transcription factors allow for numerous interactions with DNA and/or protein, which is essential for influencing a variety of biological functions. Snail and other members use a combination of zinc finger mediated DNA binding and SNAG domain mediated transcription repression when they localize to SNAG-regulated target promoters (14, 15).
Figure 2.
Architecture of SNAG family members in human. Canonical structure illustrates the presence of an N-terminal SNAG repression domain and a C-terminal C2H2 zinc finger DNA-binding domain. Snail2, Scratch1 and Scratch2 also contain a domain of unknown function.
The various members of the SNAG family can be classified into distinct sub-families. The largest sub-family is comprised of Snail1 (Snail), Snail2 (Slug), Snail3 (Smuc), Scratch 1 and Scratch 2 (16). In a classical review article, the evolutionary significance of this Snail/Scratch sub-family is thoroughly discussed (17). The Gfi-1 sub-family consists of Gfi-1, Gfi-1B and their isoforms and homologs. Gfi-1 was discovered through a retroviral insertional mutagenesis screen approach (13). The insulinoma-associated-1 sub-family includes Insm1 (previously known as IA-1), Insm2, and their homologs. Insm1 is an intronless gene and was discovered by subtraction screening of a human insulinoma library (18). Other members include Ol-insm1a, Ol-insm1b (19), Dr-insm 1a, Dr-insm 1b (20), and Mlt-1 (Methylated in liver tumor or Insm2 in Mus musculus) (21).
New members have been discovered that are not previously grouped into the aforementioned families and that possess the SNAG domain and DNA binding zinc fingers. This includes Ovol1 (19, 22), and genes found in other organisms including Worniu and Escargot in D. melanogaster and CES-1 gene in C. elegans, see (14) for a review.
1.3 Characteristics of SNAG domain family
In all SNAG family members, the amino terminal end contains the SNAG domain, which functions as a transcriptional repressor domain. Gfi-1 isoform 2 in Mus musculus is an exception to this paradigm in which the SNAG domain is not located at the very N-terminal end. In this variant, an alternate 5’ exon is present, creating a distinct and longer N-terminus relative to isoform 1) (see Figure 1). The SNAG domain is a highly conserved sequence composed of eight amino acids MPRSFLVK. A single point mutation of proline in position 2 to alanine (P2A) can inhibit transcriptional repression by Gfi-1 (13). Similarly, if FLV is changed to AAA, again the SNAG domain will lose function (11). Although the biological functions influenced by these SNAG domain transcriptional repressors have been extensively studied, the exact molecular mechanisms by which they act in transcription repression remained largely unknown, but recent research has added significant information in this area.
The second structural identifier of SNAG domain family members is the presence of carboxy terminal C2H2 zinc fingers. As shown in Figure 2 the number of zinc fingers varies between different family members, which may reflect the variations in the length of DNA being recognized. In the human genome, majority of transcriptional repressors are zinc finger proteins which contain sequence-specific DNA binding zinc finger motifs. In general, binding of the zinc fingers to the upstream regulatory region in a DNA sequence-specific manner can selectively inhibit the transcription of the target gene.
Additionally, the Snail2 and Scratch zinc finger proteins have conserved domains (SLUG and SCRT) within their linker regions (between the SNAG domain and C2H2 zinc fingers as diagrammatically shown in Figure 2). Though not much is known about these conserved domains, we speculated owing to their well-conserved nature in a number of organisms and retention during the evolutionary time that they carry out significant functions. Interestingly, a recent study has shown that the SLUG domain is necessary for efficient Snail2-mediated repression. Deletion of it can impair repression of E-cadherin promoter (23). Additionally, yeast two-hybrid screen studies in our laboratory have identified a RNA polII-CTD interacting protein when this domain was used as bait (data not shown). Our screen also identified a lysine-specific histone demethylase (KDM3A) as an interactor of Snail3 (data not shown). Perhaps these conserved domains may aid in SNAG domain function by recruiting additional corepressors to facilitate transcriptional repression.
1.4 Transcriptional repression via SNAG domain, co-repressors, and histone deacetylases
Although the SNAG domain is well characterized as a repression domain and is highly conserved among a number of transcription factors, the mechanism by which it acts is not completely understood. There are a number of co-repressor proteins and additional molecules that allow epigenetic modifications and that can act independently or co-dependently to form a multi-molecular structure in order for the repression to occur.
We identified a multiple LIM domain protein called Ajuba to function as a corepressor for the SNAG domain, allowing for enhanced transcriptional repression as part of a multi-protein complex that also includes histone tail modifiers. Ajuba co-localizes with the domain, shuttling between the cytoplasm and nucleus, while assembling repression complexes at target promoters within the nucleus (11). We also proposed that SNAG domain mediated transcriptional repression may involve histone deacetylases (HDAC) since trichostatin A (TSA), a HDAC inhibitor was able to reverse this repression (11). We also showed that in the molecular assembly, protein arginine methyltransferase 5 (PRMT5) acts as an effector, gets recruited by Snail, interacts with Ajuba, and functions to repress E-cadherin gene transcription (24). It was further shown that the Ajuba LIM proteins are essential for Snail family repressor complex assembly during the repression of E-cadherin by Snail. Ajuba is important in neural crest development as well as in Snail1 and Snail2 functions (25). Other studies have shown that Ajuba is SNAG-independent as it can bind directly to Gfi-1 as well in order to repress transcription. In this study, a multi-protein complex containing Ajuba and histone deacetylases is suggested (26).
In another study, lysine-specific demethylase 1 (LSD1) is reported to function as a SNAG corepressor. It interacts with the SNAG domain of Snail and also helps in the recruitment of LSD1 to target gene promoters by forming a ternary complex that provides stability and function along with the corepressor, CoREST. This function of SNAG as a binding domain allows for stability of Snail1 (27). The SNAG repression domain in Gfi-1 and Gfi-1B have also been found to interact with CoREST, LSD1, and histone deacetylases (HDACs) 1 and 2, which are recruited to the target genes of these transcription factors. The same study also confirmed that a point mutation (proline to alanine) in the SNAG domain could inactivate Gfi-1 function (28). Gfi-1B recruits LSD1-CoREST complex via dimethylation of SNAG domain in order to control erythroid differentiation (29). Amino-terminal SNAG domain region of Snail1 also works with CtBP corepressor, which possesses interaction motifs to help in the Snail1 mediated repression (30, 31). A recent study has demonstrated nuclear corepressor, NCoR’s ability to interact with Snail2 through the SNAG domain in the repression of E-cadherin (23).
Similar to Ajuba functioning as a corepressor for the SNAG domain family, KRAB-Associated Protein-1 (KAP-1) functions as a corepressor for the KRAB domain family of transcriptional repressors that has more than 400 members in human. KAP-1 directly interacts with M31 and hHP1α (found in heterochromatin) as well as with M32 and hHP1γ (found in euchromatin). These HP1-like gene products are possibly recruited by a KRAB-zinc finger protein-KAP-1 complex to regulate gene repression (10, 32). We envision that such a mechanism of recruitment of a multi-protein complex is occurring at the SNAG-ZFP regulated target genes.
As mentioned earlier, the SNAG domain can also work with HDACs in order to repress transcriptional activity. A number of SNAG family members recruit HDACs in conjunction with other corepressor proteins that allow for chromatin modification and epigenetic regulation. Snail works as a mediator of other E-cadherin repressors such as EZH2 via HDAC1 and HDAC2 multi-molecular complex (33). Ovol1 represses basal and activated transcription of the Ovol1 promoter by necessarily recruiting HDACs 1, 2, and 3 via its SNAG domain (22). Gfi-1 recruits histone lysine methyltransferase G9a and HDAC1 (34), Gfi-1 and Gfi-1B associate with ETO (MTG8), another potential corepressor, via its zinc fingers and not the SNAG domain. This study confirmed the involvement of HDACs 1, 2, and 3 since transcription repression was occurring in a TSA-sensitive manner as suggested earlier in our study (11, 35). Similarly, Insm-1 has been shown to mediate transcriptional repression via recruitment of cyclin D1 and HDAC3 (36).
2. RECOGNITION SEQUENCES IN TARGET GENES OF SNAG FAMILY MEMBERS
The zinc finger motifs of C2H2 transcription factors can direct sequence-specific binding to DNA, which gives insight into their DNA recognition capabilities. The binding sites of several members of the SNAG family transcription factors have been identified, allowing for the discovery of genes targeted by each of them. Most of the SNAG domain family members have similar binding site consensus sequences. Generally, sequences binding to zinc finger domains in random oligonucleotide selection assays are aligned to construct a consensus sequence.
One of the most commonly derived sequences for many members of the SNAG family transcription factors is E-box, a hexanucleotide sequence characterized by the sequence CANNTG. In Drosophila melanogaster, Snail1 binds to a highly conserved core of ACAGGTG (37) as does Snail2 (38), while Scratch binds to E-box motifs, CAGGTG specifically (39). Snail1 and Scratch have been shown bind to E-box elements in the proximal promoter region of E-cadherin (CDH1) (40, 41). This E-box can itself be regulated by other transcription factors, as noticed in the case of Insm-1 (42). Also, when Snail1 binds to an E-box within its own promoter, it serves as a feed-back mechanism for its self-regulation (43). Three putative E-boxes in the Insm-1 promoter region are regulated by neuroD1 and E47 heterodimers; both are transcription factors critical to transcribe the Insm-1 gene (44).
In the Gfi-1 binding site TAAATCAC(A/T)GCA, the sequence AATC/AAGC is invariant (45) and has been shown to be recognized by the fourth and fifth zinc fingers (46). Gfi-1 target genes include those involved in stem cell differentiation (47). Tsichlis group has also demonstrated that Gfi-1B can recognize a similar sequence, TAAATCACTGC(A/T), also containing an invariant AATC (48). This study reveals that Gfi-1B may also regulate Gfi-1 and itself by binding to the AATC sites in their promoters (49). The sequence, T(G/T)(C/T)(C/T)(T/A)GGGG(G/T)C(G/A) was identified for Insm-1 (50) while a similar sequence GTCCAGGGGGCA was used to identify a binding site in the insulin promoter, which is shown to be regulated with via recruitment of cyclin D1 and HDAC3 (51). Binding sites have been identified for other SNAG domain family transcription factors such as Ovol1, which recognizes the CCGTTA sequence (22).
As discussed earlier, a number of positions may vary within a derived consensus sequence. It may be possible that different combination of zinc fingers may be participating in DNA recognition, thus providing an explanation for multiple consensus sequences for a single transcription factor. This feature allows for slight variations in the binding sites and thus result in different target genes being recognized. In our study, we have observed consensus-binding sites that are different from the ones reported in the literature (data not shown). This includes an extension of the consensus sequence on either side of the E-box. This feature points to the fact that only certain zinc fingers may participate in the recognition of the invariant core CANNTG while others may facilitate binding to additional nucleotides and strengthen overall interaction. Different internal dinucleotide (NN) combinations within E-box can also affect the binding affinity of different zinc finger transcription factors with their target DNA (52). Corollary to this hypothesis, a ClustalW alignment of the zinc finger regions of the E-box binding SNAG family members such as Snail1, 2, 3 and Scratch revealed that a high degree of similarity existed in only two zinc fingers (Figure 3). Based on our observation, we propose that these fingers may mediate binding to the invariant core CANNTG. Future site-directed mutagenesis and DNA-protein interaction studies would confirm this.
Figure 3.

ClustalW alignment of zinc finger regions of E-box binding SNAG family members. This analysis reveals the presence of a strong conservation between two specific zinc fingers (exact matches are highlighted in black boxes and denoted by "*").
3. SNAG FAMILY MEMBERS PLAY DIVERSE FUNCTIONAL ROLES
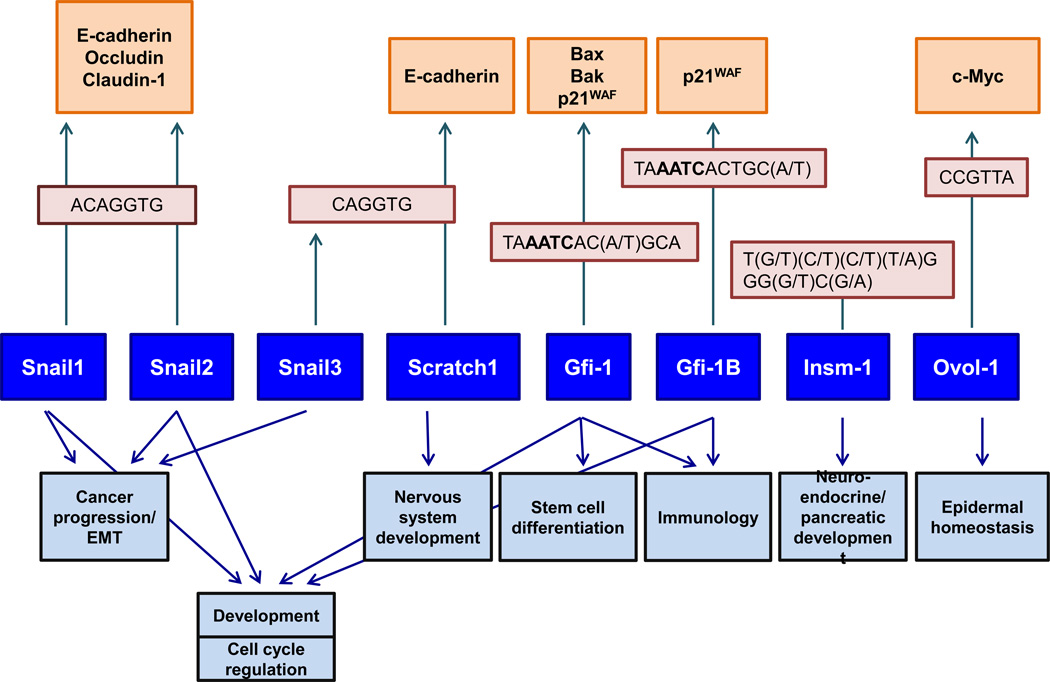
The nature of consensus DNA binding sites for each member varies extensively between the three main SNAG subfamilies. As expected, the functional roles that each member plays also vary to a great extent. Figure 4 shows the commonalities and differences existing among these members functionally. SNAG zinc finger proteins have been implicated in numerous development processes as well as in diseases. These transcription factors have been found in a wide array of tissues and are involved in the development of many organs. Understanding the roles these transcription factors play and the molecular pathways they are involved in, is important for the characterization of these members functionally. Our analysis also reveals that despite a similar structure and conservation in some areas, each SNAG member is unique, and possibly arose as a result of evolutionary divergence from an ancestral point.
Figure 4.
Transcriptional control by SNAG domain transcription factors. Binding site consensus sequences are indicated in light pink boxes; For selected SNAG members, target genes and biological processes of significance are shown in dark pink and light blue boxes respectively.
3.1 Cell adhesion, epithelial to mesenchymal transition, and cancer
Multiple members of the SNAG family participate in the progression or manifestation of a variety of cancers. This can be seen in the high level expression of the SNAG zinc finger proteins in cancerous tissues. During developmental processes they repress cell adhesion molecules that ultimately results in epithelial to mesenchymal transition (EMT). Many of these findings have been reviewed by Cano’s group (53), but in more recent years, additional roles have been discovered for the SNAG family members. Though most SNAG members play a role in cancer, Scratch is a notable exception. It is neither detectable in tumor tissues, nor has been shown to be involved in tumorigenesis (54).
The most notable SNAG family member interaction that results in cancer is that of Snail1’s ability to target E-cadherin promoters. This results in the loss of an important gene in cell adhesion, E-cadherin that will ultimately lead to highly malignant and invasive human tumor progression (55). E-cadherin plays a role in EMT, and its repression by Snail1 has been established in both murine and human cell lines and tumors (40). Snail2, which is expressed in chick and Xenopus embryo EMT regions, has also been found to be an E-cadherin repressor (56, 57), more specifically in breast cancer (58).
The induction of EMT is a multi-step process involving a number of molecules. It is triggered by phosphorylation of Snail2 within the SNAG domain at serine 4 (23). GSK-3β binds to and phosphorylates its substrate Snail1 at two consensus motifs to control EMT (59). Snail1, via its C-terminal, interacts with Akt which binds to E-cadherin’s promoter (60). The recruited polycomb repressive complex 2 (PRC2) is also required to efficiently repress E-cadherin gene (61). Additionally, when Snail is overexpressed, histones H3 and H4 are deacetylated at the E-cadherin promoter. Chromatin-modifying activities allow the SNAG domain to recruit HDAC1 and 2 and the corepressor mSin3A, thus forming a multi-molecular complex that can repress E-cadherin gene (62). Another recent study has shown that interaction of the SNAG domain of Snail1 with the catalytic SET domain of Suv39H1, and subsequent DNA methylation of the E-cadherin promoter changes the chromatin conformation to a closed form (63).
The role that Snail1 plays in EMT makes it an ideal therapeutic target to block tumor progression. Silencing of Snail expression can lead to re-expression of E-cadherin, revert from mesenchymal to epithelial transition, and ultimately decrease tumor growth and invasiveness in MDCK and epithelial cell lines (64). Apart from E-cadherin, other tight junction proteins such as claudin-1, occludin, and ZO-1, which participate in EMT are also down regulated by Snail (65). Many studies have shown that multiple members of the SNAG family are up regulated in breast cancer, including Snail1, Snail2, and Twist (66). Despite their presence in mammary cancers, both Snail1 and Snail2 are also shown to be highly expressed in invasive human breast tumors (67), play distinct roles in the disease’s progression, and act as repressors of the genes encoding tight junction components and tumor suppressor gene BRCA2 (68).
Several other instances where SNAG members have been shown to be involved in cancer manifestation are as follows: Snail is expressed in hepatocellular carcinoma cells and shown to up-regulate MMP expression, which accelerates cancer invasion (69). Snail1 is also involved in renal EMT (70). Snail2 expression is up regulated in human bladder cancer tissue while Snail1 is not detectable (71). It is also highly expressed in prostate tumors (72) and in human gliomas (73). Insm-1 is specifically expressed in neuroendocrine tumors (74) and in small-cell lung cancers (SCLC), making it a target for transcription-based targeted therapy for these cancers (75). Insm-1 mRNA is highly expressed and also detected in 97% of SCLC lines; thus Insm-1 expression can serve as a biomarker (76). Contrary to these observations, Mlt 1, a newly discovered Insm-2 homolog in Mus musculus, is down regulated during tumor progression, suggesting that this ZFP may function as a growth or tumor suppressor (21).
3.2 Immunology and apoptosis
SNAG domain family members have been shown to play critical roles in a number of immunological processes. Gfi-1B represses p21WAF to block IL-6 induced G1 arrest in IL-6-dependent B-cell lines (48). Gfi-1 is involved in the control of CLP1-dependent IL7-receptor mediated B cell differentiation (77). Its involvement in the immunological processes has been documented by the observation that Gfi-1 knockout mice had a severe neutropenia phenotype and associated inflammatory reactions (78, 79). Corollary to this, severe congenital neutropenia or non-immune chronic idiopathic neutropenia can be seen in patients with mutations in Gfi-1 gene (80).
Programmed cell death (apoptosis) is intricately linked to immunological processes. A number of SNAG family members are involved in regulating apoptosis, especially those in the Gfi-1 sub-family. Overexpression of Gfi-1 inhibits T-cell death by down regulating mRNA and protein levels of Bax, a proapoptotic regulator, via direct repression of binding sites in the gene’s promoter (81). Gfi-1 also represses Bak (another proapoptotic regulator), a member of the Bcl-2 gene family (81). It can also inhibit G1 arrest and contribute to T-cell activation, even upon interleukin-2 withdrawal, by repressing genes that inhibit cell proliferation (13). Ol-insm1b, a homolog of Insm1b in Orysias latipes (a teleost), is a regulator of cell proliferation. It slows down the cell cycle without inducing apoptosis and causing cell cycle arrest in early embryos (19). Snail1 and Snail2 regulate p53 and other pro-apoptotic genes to promote cell survival after genotoxic stress (82), while Snail2, activated by E2A-HLF promotes survival of IL-3-dependent murine pro-B cells (38).
3.3 Developmental regulation
Ubiquitous presence of SNAG domain transcription factors in a wide array of cells and tissue types suggest that they might play significant roles in several developmental processes. Indeed studies have shown that they participate in the regulation of processes from early embryonic development to adult neurogenesis. Snail1 participates in mesoderm invagination and is necessary for gastrulation in Drosophila (30). Snail1 was also found necessary for the early development of mouse (83). Snail1 and Snail2 genes are both involved in palate development in mice (84). Snail2 was found to be expressed in neural crest and mesodermal cells (85). Expression of the Scratch family of transcription factors is restricted to the nervous system. Abundant expression is observed in the brain and spinal cord and neurons (39) and in the developing and mature adult brain (86). Gfi-1 and Gfi-1B play a role in haematopoiesis as well as in inner ear development (87). Insm-1 necessary for the development of pancreatic β cells and intestinal endocrine cells (88). Insm-1 mRNA expression is detected in a range of neural areas from embryonic to adult neurogenesis (89), and in the developing mouse neocortex (90).
3.4 Cell signaling
Many signaling pathways employ SNAG domain members in order to regulate cell cycle, regulation of small molecules, and other miscellaneous mechanisms. Although some commonalities exist in function, each member plays a distinct role in these pathways. Snail1 collaborates with Twist during the regulation of E2A-activated p21 gene transcription (91). Snail blocks cell cycle progression by repressing cyclin D2, restricting progress from early to late G1. It also confers resistance to cell death, protecting cells from TNF-α induced cell death (92). Snail1 overexpression promotes migration of bone mesenchymal stem cells through a PI3K/Akt-dependent pathway, induces MMP-2 secretion, and confers resistance to apoptosis (93). Forced Snail2 overexpression can lead to decreased cyclin D1 expression and therefore elimination of cell cycle arrest in prostate cancer cell lines (72).
Those in the Gfi-1 subfamily are indirectly linked to various cell signaling processes. Gfi-1B expression is restricted to hematopoietic stem cells, erythroblasts, and megakaryocytes (94). Gfi-1 was also discovered in hematopoietic stem cells and shown to regulate their self-renewal (47). Their expression patterns are complementary during hematopoiesis and lymphopoiesis (95). Differentiation of normal and erythroid progenitor cells requires Gfi-1B (96). Gfi-1B directly represses p21WAF1 by binding to a site in the gene’s promoter. IL-6 down regulates Gfi-1B, which in turn induces p21WAF1 that ultimately contribute to G1 arrest and differentiation (48). Socs1 and Socs3 genes contain Gfi-1B binding sites within their promoters and function as direct repression targets. This action is mediated through cytokines such as erythropoietin that activates STAT5 (97).
Insm1 participates in the induction of cell cycle arrest necessary to facilitate cellular differentiation (74). Other potential downstream targets of mouse Insm1 include several genes involved in cell cycle regulation such as Elavl4, Nhlh1, Ebf3, Net1, St18, and Rassf2 (90). They promote pancreatic duct cell trans-differentiation and induce Panc-1 cell cycle arrest either with or without islet cell transcription factors (98). Ovol1 represses c-Myc and can regulate the growth arrest of embryonic cells (99).
4. PROPOSED MODEL FOR THE MECHANISM OF TRANSCRIPTIONAL REPRESSION BY SNAG FAMILY TRANSCRIPTION FACTORS
Based on the current literature including our recent studies in this field, we propose the following model for the SNAG-domain mediated transcriptional repression of its target genes (Figure 5). Similar to most transcription factors, a variety of methods may be employed to repress the target gene expression. In this model, target gene transcription may be initiated at the transcription start site (indicated by the bent arrow) if a transcriptional activator binds to a specific DNA sequence in the proximal promoter region. This process can be counteracted by a transcriptional repressor, which can also bind to specific regions via their zinc fingers. The SNAG repressor domain can directly prevent the RNA polymerase holoenzyme II and associated proteins from binding to the TATA box, thereby preventing initiation of transcription. Alternatively, the SNAG domain can interact with co-repressors such as Ajuba, which can recruit other proteins such as PRMT5, MEP50 and HDAC that can modify local chromatin structure to conceal the TATA box from RNA polymerase II holoenzyme binding.
Figure 5.
Proposed mechanism for SNAG domain-mediated transcriptional repression. Zinc fingers bind to promoter and SNAG domain interacts with Ajuba, which can recruit PRMT5, MEP50 and HDAC to condense local chromatin structure and prevent transcription initiation (see text for details).
In our model, the LIM domains in Ajuba corepressor can mediate efficient protein-protein interactions and lead to the recruitment of a multi-protein repression complex at the promoters of the SNAG zinc finger transcription factor regulated target genes. We witness evidence for our hypothetical model in the current literature as multiple SNAG family members have been shown to recruit not only other corepressors such as Co-REST and LSD, but also HDACs to create a multi-molecular complex in order for the transcriptional repression to occur (Figure 5). Our proposed model integrates available evidences for explaining SNAG-domain function. In this model, Ajuba serves as a central molecule, which has the potential to recruit other members such as HDACs or other corepressors into the repression complex. Future studies will provide more evidence for this model and may also refine it further.
5. FINAL REMARKS
The SNAG family represents the smallest family of zinc finger transcription factors. Yet they serve in the regulation of a number of significant, biologically relevant genes that are involved in many developmental and disease states. This feature makes them a unique set of transcription factors worth investigating continuously. Indeed, many of their regulated genes may be potential targets for developing therapeutic treatments for diseases, while others may reveal critical cellular, developmental or signaling events. Although the first member was discovered over three decades ago, we are yet to understand the complexity of this group of transcription factors. As evidenced in this review of literature for the SNAG domain transcription factor family, we find that despite the classification within the same family, each member is unique. Not only are the targeted genes different for the different members, even the repression activity may be mediated by a host of different molecules. Even though all members of the family are similarly structured, they have been found to function mechanistically in different ways. Based on the proteins’ primary structure alone one cannot derive any information about which co-repressor/ HDACs proteins will be recruited. Therefore, each SNAG zinc finger member must be studied with regard to what is currently known about it. This approach will further our understanding of this family and successfully explain the mechanisms employed by the different members in this transcription factor family.
ACKNOWLEDGMENTS
Funding assistance from the National Institutes of Health to K. A. (grant 5K01CA95620) and from the Department of Biological Sciences, FAU (grant-in-aid to K.A.) to carry out this work is gratefully acknowledged.
Biographies

Cindy Chiang
Cindy Chiang received both her M.S. and Ph.D. degrees from Florida Atlantic University in Boca Raton, Florida in the lab of Dr. Kasirajan Ayyanathan. During this time her research focused on the gene regulation of the SNAG family of transcription factors. She studied both the DNA and protein interactions to identify possible target genes and co-repressors of these regulatory molecules. Her current research interests include understanding the molecular mechanisms of cancer and possible therapeutic treatments.

Kasirajan Ayyanathan
Kasirajan Ayyanathan received his Ph.D. degree from the Department of Biochemistry, Indian Institute of Science, one of the premier research institutions in India. Subsequently, at Temple University School of Medicine, USA, he conducted post-doctoral research on the signal transduction by purinergic receptors in cancer cells. Next, he was trained at the Wistar Institute, USA studying transcription regulation, chromatin and epigenetic mechanisms in cancer before becoming an Associate Professor at Florida Atlantic University. Currently he is at the Center for Molecular Biology and Biotechnology as a Research Associate Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clamp M, Fry B, Kamal M, et al. Distinguishing protein-coding and noncoding genes in the human genome. Proc Natl Acad Sci U S A. 2007;104:19428–19433. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM. A census of human transcription factors: function, expression and evolution. Nat Rev Genet. 2009;10:252–263. doi: 10.1038/nrg2538. [DOI] [PubMed] [Google Scholar]
- 3.Tadepally HD, Burger G, Aubry M. Evolution of C2H2-zinc finger genes and subfamilies in mammals: species-specific duplication and loss of clusters, genes and effector domains. BMC Evol Biol. 2008;8:176. doi: 10.1186/1471-2148-8-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. Embo J. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins T, Stone JR, Williams AJ. All in the family: the BTB/POZ, KRAB, and SCAN domains. Mol Cell Biol. 2001;21:3609–3615. doi: 10.1128/MCB.21.11.3609-3615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 7.Tsukiyama T, Wu C. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell. 1995;83:1011–1020. doi: 10.1016/0092-8674(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 8.Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 9.Fondell JD, Brunel F, Hisatake K, Roeder RG. Unliganded thyroid hormone receptor alpha can target TATA-binding protein for transcriptional repression. Mol Cell Biol. 1996;16:281–287. doi: 10.1128/mcb.16.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayyanathan K, Lechner MS, Bell P, et al. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 2003;17:1855–1869. doi: 10.1101/gad.1102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyanathan K, Peng H, Hou Z, et al. The Ajuba LIM domain protein is a corepressor for SNAG domain mediated repression and participates in nucleocytoplasmic Shuttling. Cancer Res. 2007;67:9097–9106. doi: 10.1158/0008-5472.CAN-07-2987. [DOI] [PubMed] [Google Scholar]
- 12.Grau Y, Carteret C, Simpson P. Mutations and Chromosomal Rearrangements Affecting the Expression of Snail, a Gene Involved in Embryonic Patterning in DROSOPHILA MELANOGASTER. Genetics. 1984;108:347–360. doi: 10.1093/genetics/108.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol Cell Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 15.Hemavathy K, Guru SC, Harris J, Chen JD, Ip YT. Human Slug is a repressor that localizes to sites of active transcription. Mol Cell Biol. 2000;20:5087–5095. doi: 10.1128/mcb.20.14.5087-5095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manzanares M, Locascio A, Nieto MA. The increasing complexity of the Snail gene superfamily in metazoan evolution. Trends Genet. 2001;17:178–181. doi: 10.1016/s0168-9525(01)02232-6. [DOI] [PubMed] [Google Scholar]
- 17.Barrallo-Gimeno A, Nieto MA. Evolutionary history of the Snail/Scratch superfamily. Trends Genet. 2009;25:248–252. doi: 10.1016/j.tig.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Lan MS, Li Q, Lu J, Modi WS, Notkins AL. Genomic organization, 5'-upstream sequence, and chromosomal localization of an insulinoma-associated intronless gene, IA-1. J Biol Chem. 1994;269:14170–14174. [PubMed] [Google Scholar]
- 19.Candal E, Alunni A, Thermes V, Jamen F, Joly JS, Bourrat F. Ol-insm1b, a SNAG family transcription factor involved in cell cycle arrest during medaka development. Dev Biol. 2007;309:1–17. doi: 10.1016/j.ydbio.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Lukowski CM, Ritzel RG, Waskiewicz AJ. Expression of two insm1-like genes in the developing zebrafish nervous system. Gene Expr Patterns. 2006;6:711–718. doi: 10.1016/j.modgep.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Tateno M, Fukunishi Y, Komatsu S, et al. Identification of a novel member of the snail/Gfi-1 repressor family, mlt 1, which is methylated and silenced in liver tumors of SV40 T antigen transgenic mice. Cancer Res. 2001;61:1144–1153. [PubMed] [Google Scholar]
- 22.Nair M, Bilanchone V, Ortt K, Sinha S, Dai X. Ovol1 represses its own transcription by competing with transcription activator c-Myb and by recruiting histone deacetylase activity. Nucleic Acids Res. 2007;35:1687–1697. doi: 10.1093/nar/gkl1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina-Ortiz P, Villarejo A, MacPherson M, et al. Characterization of the SNAG and SLUG domains of Snail2 in the repression of E-cadherin and EMT induction: modulation by serine 4 phosphorylation. PLoS One. 2012;7:e36132. doi: 10.1371/journal.pone.0036132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Z, Peng H, Ayyanathan K, et al. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol. 2008;28:3198–3207. doi: 10.1128/MCB.01435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ, 3rd, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montoya-Durango DE, Velu CS, Kazanjian A, et al. Ajuba functions as a histone deacetylase-dependent co-repressor for autoregulation of the growth factor-independent-1 transcription factor. J Biol Chem. 2008;283:32056–32065. doi: 10.1074/jbc.M802320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Wu Y, Li J, et al. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. Embo J. 2010;29:1803–1816. doi: 10.1038/emboj.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27:562–572. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 29.Laurent B, Randrianarison-Huetz V, Frisan E, et al. A short Gfi-1B isoform controls erythroid differentiation by recruiting the LSD1-CoREST complex through the dimethylation of its SNAG domain. J Cell Sci. 2012;125:993–1002. doi: 10.1242/jcs.095877. [DOI] [PubMed] [Google Scholar]
- 30.Hemavathy K, Hu X, Ashraf SI, Small SJ, Ip YT. The repressor function of snail is required for Drosophila gastrulation and is not replaceable by Escargot or Worniu. Dev Biol. 2004;269:411–420. doi: 10.1016/j.ydbio.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Kruppel and Snail in the Drosophila embryo. Embo J. 1998;17:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan RF, Schultz DC, Ayyanathan K, et al. KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong ZT, Cai MY, Wang XG, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–594. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 34.Duan Z, Zarebski A, Montoya-Durango D, Grimes HL, Horwitz M. Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1. Mol Cell Biol. 2005;25:10338–10351. doi: 10.1128/MCB.25.23.10338-10351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGhee L, Bryan J, Elliott L, et al. Gfi-1 attaches to the nuclear matrix, associates with ETO (MTG8) and histone deacetylase proteins, and represses transcription using a TSA-sensitive mechanism. J Cell Biochem. 2003;89:1005–1018. doi: 10.1002/jcb.10548. [DOI] [PubMed] [Google Scholar]
- 36.Liu WD, Wang HW, Muguira M, Breslin MB, Lan MS. INSM1 functions as a transcriptional repressor of the neuroD/beta2 gene through the recruitment of cyclin D1 and histone deacetylases. Biochem J. 2006;397:169–177. doi: 10.1042/BJ20051669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inukai T, Inoue A, Kurosawa H, et al. SLUG, a ces-1-related zinc finger transcription factor gene with antiapoptotic activity, is a downstream target of the E2A-HLF oncoprotein. Mol Cell. 1999;4:343–352. doi: 10.1016/s1097-2765(00)80336-6. [DOI] [PubMed] [Google Scholar]
- 39.Nakakura EK, Watkins DN, Schuebel KE, et al. Mammalian Scratch: a neural-specific Snail family transcriptional repressor. Proc Natl Acad Sci U S A. 2001;98:4010–4015. doi: 10.1073/pnas.051014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 41.Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA. Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun. 1997;241:453–458. doi: 10.1006/bbrc.1997.7831. [DOI] [PubMed] [Google Scholar]
- 42.Kataoka H, Murayama T, Yokode M, et al. A novel snail-related transcription factor Smuc regulates basic helix-loop-helix transcription factor activities via specific E-box motifs. Nucleic Acids Res. 2000;28:626–633. doi: 10.1093/nar/28.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peiro S, Escriva M, Puig I, et al. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–2084. doi: 10.1093/nar/gkl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breslin MB, Zhu M, Lan MS. NeuroD1/E47 regulates the E-box element of a novel zinc finger transcription factor, IA-1, in developing nervous system. J Biol Chem. 2003;278:38991–38997. doi: 10.1074/jbc.M306795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16:4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Doddapaneni K, Hogue A, McGhee L, Meyers S, Wu Z. Solution structure of Gfi-1 zinc domain bound to consensus DNA. J Mol Biol. 2010;397:1055–1066. doi: 10.1016/j.jmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Duan Z, Horwitz M. Gfi-1 takes center stage in hematopoietic stem cells. Trends Mol Med. 2005;11:49–52. doi: 10.1016/j.molmed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Tong B, Grimes HL, Yang TY, et al. The Gfi-1B proto-oncoprotein represses p21WAF1 and inhibits myeloid cell differentiation. Mol Cell Biol. 1998;18:2462–2473. doi: 10.1128/mcb.18.5.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassen L, Fiolka K, Mahlmann S, Moroy T. Direct transcriptional repression of the genes encoding the zinc-finger proteins Gfi1b and Gfi1 by Gfi1b. Nucleic Acids Res. 2005;33:987–998. doi: 10.1093/nar/gki243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breslin MB, Zhu M, Notkins AL, Lan MS. Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: identification of consensus IA-1 binding sequence. Nucleic Acids Res. 2002;30:1038–1045. doi: 10.1093/nar/30.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HW, Muguira M, Liu WD, et al. Identification of an INSM1-binding site in the insulin promoter: negative regulation of the insulin gene transcription. J Endocrinol. 2008;198:29–39. doi: 10.1677/JOE-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang C, Ayyanathan K. Characterization of the E-box binding affinity to SNAG-zinc finger proteins. Molekulyarnaya Biologiya. 2012;46 In Press. [PubMed] [Google Scholar]
- 53.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 54.Bastid J, Bouchet BP, Ciancia C, et al. The SNAIL family member SCRATCH1 is not expressed in human tumors. Oncol Rep. 2010;23:523–529. [PubMed] [Google Scholar]
- 55.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 56.Savagner P, Kusewitt DF, Carver EA, et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 57.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 58.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 59.Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 60.Villagrasa P, Diaz VM, Vinas-Castells R, et al. Akt2 interacts with Snail1 in the E-cadherin promoter. Oncogene. 2011 doi: 10.1038/onc.2011.562. [DOI] [PubMed] [Google Scholar]
- 61.Herranz N, Pasini D, Diaz VM, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong C, Wu Y, Wang Y, et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene. 2012 doi: 10.1038/onc.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olmeda D, Jorda M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–1874. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Estrada OM, Culleres A, Soriano FX, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 68.Tripathi MK, Misra S, Khedkar SV, et al. Regulation of BRCA2 gene expression by the SLUG repressor protein in human breast cells. J Biol Chem. 2005;280:17163–17171. doi: 10.1074/jbc.M501375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyoshi A, Kitajima Y, Kido S, et al. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92:252–258. doi: 10.1038/sj.bjc.6602266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshino J, Monkawa T, Tsuji M, Inukai M, Itoh H, Hayashi M. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2007;362:63–68. doi: 10.1016/j.bbrc.2007.07.146. [DOI] [PubMed] [Google Scholar]
- 71.Yu Q, Zhang K, Wang X, Liu X, Zhang Z. Expression of transcription factors snail, slug, and twist in human bladder carcinoma. J Exp Clin Cancer Res. 2010;29:119. doi: 10.1186/1756-9966-29-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Uygur B, Zhang Z, et al. Slug inhibits proliferation of human prostate cancer cells via downregulation of cyclin D1 expression. Prostate. 2010;70:1768–1777. doi: 10.1002/pros.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang HW, Menon LG, Black PM, Carroll RS, Johnson MD. SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer. 2010;10:301. doi: 10.1186/1471-2407-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lan MS, Breslin MB. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. Faseb J. 2009;23:2024–2033. doi: 10.1096/fj.08-125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pedersen N, Pedersen MW, Lan MS, Breslin MB, Poulsen HS. The insulinoma-associated 1: a novel promoter for targeted cancer gene therapy for small-cell lung cancer. Cancer Gene Ther. 2006;13:375–384. doi: 10.1038/sj.cgt.7700887. [DOI] [PubMed] [Google Scholar]
- 76.Lan MS, Russell EK, Lu J, Johnson BE, Notkins AL. IA-1, a new marker for neuroendocrine differentiation in human lung cancer cell lines. Cancer Res. 1993;53:4169–4171. [PubMed] [Google Scholar]
- 77.Rathinam C, Geffers R, Yucel R, et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 2005;22:717–728. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Karsunky H, Zeng H, Schmidt T, et al. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat Genet. 2002;28:28. doi: 10.1038/ng831. [DOI] [PubMed] [Google Scholar]
- 79.Moroy T, Zeng H, Jin J, Schmid KW, Carpinteiro A, Gulbins E. The zinc finger protein and transcriptional repressor Gfi1 as a regulator of the innate immune response. Immunobiology. 2008;213:341–352. doi: 10.1016/j.imbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Kazanjian A, Gross EA, Grimes HL. The growth factor independence-1 transcription factor: new functions and new insights. Crit Rev Oncol Hematol. 2006;59:85–97. doi: 10.1016/j.critrevonc.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimes HL, Gilks CB, Chan TO, Porter S, Tsichlis PN. The Gfi-1 protooncoprotein represses Bax expression and inhibits T-cell death. Proc Natl Acad Sci U S A. 1996;93:14569–14573. doi: 10.1073/pnas.93.25.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 84.Murray SA, Oram KF, Gridley T. Multiple functions of Snail family genes during palate development in mice. Development. 2007;134:1789–1797. doi: 10.1242/dev.02837. [DOI] [PubMed] [Google Scholar]
- 85.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 86.Marin F, Nieto MA. The expression of Scratch genes in the developing and adult brain. Dev Dyn. 2006;235:2586–2591. doi: 10.1002/dvdy.20869. [DOI] [PubMed] [Google Scholar]
- 87.Fiolka K, Hertzano R, Vassen L, et al. Gfi1 and Gfi1b act equivalently in haematopoiesis, but have distinct, non-overlapping functions in inner ear development. EMBO Rep. 2006;7:326–333. doi: 10.1038/sj.embor.7400618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gierl MS, Karoulias N, Wende H, Strehle M, Birchmeier C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 2006;20:2465–2478. doi: 10.1101/gad.381806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duggan A, Madathany T, de Castro SC, Gerrelli D, Guddati K, Garcia-Anoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J Comp Neurol. 2008;507:1497–1520. doi: 10.1002/cne.21629. [DOI] [PubMed] [Google Scholar]
- 90.Farkas LM, Haffner C, Giger T, et al. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi E, Funato N, Higashihori N, Hata Y, Gridley T, Nakamura M. Snail regulates p21(WAF/CIP1) expression in cooperation with E2A and Twist. Biochem Biophys Res Commun. 2004;325:1136–1144. doi: 10.1016/j.bbrc.2004.10.148. [DOI] [PubMed] [Google Scholar]
- 92.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zha YH, He JF, Mei YW, Yin T, Mao L. Zinc-finger transcription factor snail accelerates survival, migration and expression of matrix metalloproteinase-2 in human bone mesenchymal stem cells. Cell Biol Int. 2007;31:1089–1096. doi: 10.1016/j.cellbi.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 94.Osawa M, Yamaguchi T, Nakamura Y, et al. Erythroid expansion mediated by the Gfi-1B zinc finger protein: role in normal hematopoiesis. Blood. 2002;100:2769–2777. doi: 10.1182/blood-2002-01-0182. [DOI] [PubMed] [Google Scholar]
- 95.Vassen L, Okayama T, Moroy T. Gfi1b:green fluorescent protein knock-in mice reveal a dynamic expression pattern of Gfi1b during hematopoiesis that is largely complementary to Gfi1. Blood. 2007;109:2356–2364. doi: 10.1182/blood-2006-06-030031. [DOI] [PubMed] [Google Scholar]
- 96.Garcon L, Lacout C, Svinartchouk F, et al. Gfi-1B plays a critical role in terminal differentiation of normal and transformed erythroid progenitor cells. Blood. 2005;105:1448–1455. doi: 10.1182/blood-2003-11-4068. [DOI] [PubMed] [Google Scholar]
- 97.Jegalian AG, Wu H. Regulation of Socs gene expression by the proto-oncoprotein GFI-1B: two routes for STAT5 target gene induction by erythropoietin. J Biol Chem. 2002;277:2345–2352. doi: 10.1074/jbc.M105575200. [DOI] [PubMed] [Google Scholar]
- 98.Zhang T, Wang H, Saunee NA, Breslin MB, Lan MS. Insulinoma-associated antigen-1 zinc-finger transcription factor promotes pancreatic duct cell trans-differentiation. Endocrinology. 2010;151:2030–2039. doi: 10.1210/en.2009-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nair M, Teng A, Bilanchone V, Agrawal A, Li B, Dai X. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J Cell Biol. 2006;173:253–264. doi: 10.1083/jcb.200508196. [DOI] [PMC free article] [PubMed] [Google Scholar]