Background: αvβ3 binds to IGF1, and the αvβ3-IGF1-IGF1R complex is formed in non-transformed cells.
Results: IGF1 induces signals with the complex formation in anchorage independence. IGF1R or Src inhibitors did not suppress the complex formation.
Conclusion: αvβ3-ECM interaction is not required for IGF signaling. The complex formation occurs before IGF1R activation.
Significance: This study identifies new therapeutic targets in IGF signaling.
Keywords: Cell Surface Receptor, Growth Factors, Insulin-like Growth Factor (IGF), Integrin, Signal Transduction
Abstract
Integrin αvβ3 plays a role in insulin-like growth factor 1 (IGF1) signaling (integrin-IGF1 receptor (IGF1R) cross-talk) in non-transformed cells in anchorage-dependent conditions. We reported previously that IGF1 directly binds to αvβ3 and induces αvβ3-IGF1-IGF1R ternary complex formation in these conditions. The integrin-binding defective IGF1 mutant (R36E/R37E) is defective in inducing ternary complex formation and IGF signaling, whereas it still binds to IGF1R. We studied if IGF1 can induce signaling in anchorage-independent conditions in transformed Chinese hamster ovary cells that express αvβ3 (β3-CHO) cells. Here we describe that IGF1 signals were more clearly detectable in anchorage-independent conditions (polyHEMA-coated plates) than in anchorage-dependent conditions. This suggests that IGF signaling is masked by signals from cell-matrix interaction in anchorage-dependent conditions. IGF signaling required αvβ3 expression, and R36E/R37E was defective in inducing signals in polyHEMA-coated plates. These results suggest that αvβ3-IGF1 interaction, not αvβ3-extracellular matrix interaction, is essential for IGF signaling. Inhibitors of IGF1R, Src, AKT, and ERK1/2 did not suppress αvβ3-IGF-IGF1R ternary complex formation, suggesting that activation of these kinases are not required for ternary complex formation. Also, mutations of the β3 cytoplasmic tail (Y747F and Y759F) that block β3 tyrosine phosphorylation did not affect IGF1R phosphorylation or AKT activation. We propose a model in which IGF1 binding to IGF1R induces recruitment of integrin αvβ3 to the IGF-IGF1R complex and then β3 and IGF1R are phosphorylated. It is likely that αvβ3 should be together with the IGF1-IGF1R complex for triggering IGF signaling.
Introduction
Insulin-like growth factor 1 (IGF1)2 is a polypeptide hormone that has a high degree of structural similarity to proinsulin. IGF1 acts through binding to the type 1 IGF receptor (IGF1R), a receptor tyrosine kinase. Ligand binding induces phosphorylation of specific tyrosine residues of IGF1R. These phosphotyrosines then bind to adaptor molecules such as Shc and insulin receptor substrate (IRS) 1. Phosphorylation of these proteins leads to activation of the PI3K and MAPK signaling pathways (1).
IGF1 has been implicated in cancer progression (2). IGF1 is involved in cell growth and consequently IGF1 inhibition is being pursued as a potential measure for treating and preventing cancer. Many cancer cells secrete abnormally high levels of IGF1. Once released by cancer cells, IGF1 binds and activates IGF1R on their surface. This autocrine receptor activation causes the release of intracellular signals that are strongly anti-apoptotic, notably through their ability to activate the PI3K/AKT pathway. Thereby IGF1 confers resistance to chemotherapy and radiation therapy. Several strategies to target IGF1 signaling have been developed, including siRNA and monoclonal antibodies for IGF1R and kinase inhibitors to inhibit the enzymatic activity of the receptor (2).
It has been well established that integrin αvβ3 plays a critical role in regulating IGF1 signaling (2). In a current model, “ligand occupancy” of αvβ3 (i.e. the binding of extracellular matrix (ECM) proteins such as vitronectin to αvβ3) enhances signaling induced by IGF1 binding to IGF1R (2). Indeed, antagonists to αvβ3 block IGF1 signaling. Anti-αvβ3 mAb and echistatin, a snake venom disintegrin that specifically inhibits αvβ3, blocks IGF1-induced cell migration (3). Also, echistatin blocks IGF1-stimulated DNA synthesis and IRS-1 phosphorylation and attenuates IGF1R-linked downstream signaling events, such as activation of PI3K and ERK1/2 (4).
We discovered recently that IGF1 directly and specifically binds to αvβ3 and generated an integrin-binding defective mutant (R36E/R37E) of IGF1 (5). R36E/R37E is defective in inducing cell survival and in inducing IGF signaling, although the mutant still binds to IGF1R (5). Also, WT IGF1 induces a ternary complex formation (αvβ3, IGF1, and IGF1R) but R36E/R37E does not. This suggests that the direct binding of integrins to IGF1 is critical for IGF signaling and a potential mechanism of IGF1R-integrin cross-talk. These findings are not consistent with the current model as described above, in which αvβ3-ECM interaction plays a major role in IGF signaling (2). It is unclear whether αvβ3-ECM ligand interaction or αvβ3-IGF interaction is related to cancer progression. Previous studies used non-transformed cells (NIH3T3, C2C12, and smooth muscle cells) (6–9), and it has not been tested whether IGF can induce signals in the absence of cell-matrix interaction because non-transformed cells do not survive in anchorage-independent conditions. Also, we do not know the role of the ternary complex formation (αvβ3, IGF1, and IGF1R) induced by WT IGF1 in IGF1R activation.
In this study, we studied whether IGF1 can induce signaling in anchorage-independent conditions in transformed Chinese hamster ovary (CHO) cells that express human β3 (β3-CHO) cells. We describe that IGF1 signals were more clearly detectable in anchorage-independent conditions (in polyHEMA-coated plates) than in anchorage-dependent conditions (in regular tissue culture plates). This suggests that IGF signaling is masked by signals from cell-matrix interaction in anchorage-dependent conditions. IGF signaling required αvβ3 expression, and R36E/R37E was defective in inducing signals in anchorage-independent conditions. These results suggest that αvβ3-IGF1 interaction, not cell-matrix interaction, is essential for IGF signaling. We also asked whether IGF1-induced phosphorylation of IGF1R and downstream signaling pathways and/or β3 is required for the ternary complex formation. We used anchorage-independent conditions for studying the role of αvβ3 in IGF signaling because αvβ3-ECM interaction itself may induce β3 phosphorylation. Notably, inhibitors of IGF1R (PPP), Src (PP2), PI3K (LY294002), or ERK1/2 (PD098059) did not suppress αvβ3-IGF-IGF1R ternary complex formation, suggesting that activation of these kinases is not required for ternary complex formation. Also, mutations of the β3 cytoplasmic tail (Y747F and Y759F) that block β3 tyrosine phosphorylation did not affect IGF1R phosphorylation or AKT activation. Thus it appears that β3 phosphorylation is not required for ternary complex formation in anchorage-independent conditions. We propose a model, in which IGF1 binding to IGF1R induces recruitment of integrin αvβ3 to the IGF-IGF1R complex, and then β3 and IGF1R are phosphorylated. It is likely that αvβ3 should be together with the IGF1-IGF1R complex for triggering IGF signaling.
EXPERIMENTAL PROCEDURES
Materials
Recombinant wt and R36E/R37E IGF1 were synthesized as described (5). CHO cells were obtained from ATCC. CHO cells expressing human integrin β1 (β1-CHO) or β3 (β3-CHO) were described (10). Anti-phospho-ERK1/2 (Thr-202 and Tyr-204), anti-phospho-AKT (Ser-473), anti-phospho-IGF1Rβ (Tyr1135/1136), anti-ERK1/2, anti-AKT, and anti-IGF1Rβ were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti-integrin β3 was purchased from Cell Signaling Technology or BD biosciences. Anti-phospho-integrin β3 (Tyr747) was purchased from Invitrogen. HRP-conjugated anti-His tag antibody was purchased from Qiagen (Valencia, CA). 7E3 (anti-human integrin β3) hybridomas was obtained from ATCC. PP2 was purchased from Calbiochem. PPP was purchased from Santa Cruz Biotechnology. LY294002 and PD098059 were purchased from Promega.
DNA Synthesis
DNA synthesis was measured by BrdU assays (cell proliferation ELISA kit (Roche)). β1-CHO cells or β3-CHO cells were plated in regular tissue culture plates (5000 cells/well) and serum-starved in DMEM at 37 °C for 24 h in the presence of WT IGF1. Cells were then incubated with BrdU for 2 h. After removing the labeling medium, the cells were fixed, and the DNA was denatured in one step by adding FixDenat (fixative). Incorporated BrdU was measured by using peroxidase-conjugated anti-BrDU antibody and tetramethyl-benzidine at 370 nm. In polyHEMA-coated plates, β1-CHO cells or β3-CHO cells were plated in polyHEMA-coated plates (20,000 cells/well) and serum-starved in DMEM at 37 °C for 24 h in the presence of WT IGF1 or R36E/R37E. Cells were then incubated with BrdU for 2 h. The cells were transferred to regular tissue culture plates, centrifuged at 300 × g for 15 min, and the labeling medium was removed. The cells were dried using a hair dryer for 15 min and fixed with FixDenat. Incorporated BrdU was measured as described above.
Signaling Assays
We cultured β1-CHO or β3-CHO cells in regular tissue culture to near confluence in DMEM with 10% FCS and then serum-starved them in DMEM without FCS overnight. We stimulated the starved cells with WT and/or R36E/R37E IGF1 for 10–20 min. We solubilized cells in lysis buffer (20 mm HEPES (pH 7.4), 100 mm NaCl, 10% glycerol, 1% Nonidet P-40, 1 mm MgCl2, 1 mm PMSF, 20 mm NaF, 1 mm Na3VO4, and protease inhibitor mixture (Sigma-Aldrich)). We analyzed cell lysates by Western blotting using specific antibodies. Bound IgG was detected using HRP-conjugated second antibody and Super Signal WestPico (Thermo Scientific). We analyzed the images using a Fuji LAS 4000 mini luminescent image analyzer and Multi Gauge v.3.0 software (Fujifilm, Tokyo, Japan). PolyHEMA-coated plates were prepared as described (11), except that the final polyHEMA concentration was 1.2 mg/cm2. In polyHEMA-coated plates, we performed signaling assays as described above, except that we serum-starved them for 3 h in DMEM without FCS. We stimulated the starved cells with WT or R36E/R37E IGF1 for 5 to 30 min. In some experiments, cells were pretreated with PPP (10 μm) or PP2 (10 μm) for 60 min.
Transfection of CHO or β3-CHO Cells
We subcloned the cDNA fragment encoding WT and mutant IGF1 (5) into the BamHI/EcoRI site of the pSec TagB secretion vector, which has been modified to have the 6-His and S tags at the amino terminus of the protein (12). We generated CHO or β3-CHO cells that stably secrete WT or mutant IGF1 by transfecting WT or mutant IGF1 cDNA in the modified pSec TagB vector together with the neomycin-resistant plasmid using FuGENE (Promega) as described (5). After selection with G418 we used them without further enrichment. To detect the expression of IGF1 (6-His-tagged) from the cells that stably express WT IGF1 or R36E/R37E, cells were cultured with DMEM with 10% FCS. The culture medium was concentrated five times and analyzed by Western blotting using anti-6-His antibodies (IGF1 has an N-terminal 6-His tag).
The Tyr-747- or Tyr-759-to-Phe mutants (the Y747F or Y759F mutants) of β3 were generated by quick-change mutagenesis. These β3 mutant expression constructs were transfected into CHO cells together with a neomycin-resistant plasmid using FuGENE (Promega). After selection with G418 cells stably expressing mutant β3 integrin were further enriched by limited dilution to obtain about 80% positive populations.
Soft Agar Colony Formation Assays
Soft agar colony formation assays were performed as described previously (13). We cultured cells to near confluence in DMEM with 10% FCS and then serum-starved them in DMEM with 0.4% FCS overnight. We suspended starved cells (5 × 104 cells) in DMEM with 0.4% FCS containing 0.3% agar and layered them on a bottom layer of DMEM with 0.4% FCS containing 1.0% agar. We overlaid DMEM with 10% FCS and cultured cells for 3 weeks at 37 °C. We replaced the medium twice a week. We determined the number of the colonies from the digital images of colonies using National Institutes of Health ImageJ software.
Coprecipitation of integrin β3, IGF1R, and IGF
β3-CHO cells were cultured to be nearly confluent in DMEM/10% FCS. Cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h or plated in regular tissue culture plates and serum-starved in DMEM for 12 h. The cells were incubated with 100 ng/ml WT IGF1 or R36E/R37E for 30 min. In some experiments, cells were pretreated with PPP (10 μm), PP2 (10 μm), PD098059 (50 μm), or LY294002 (50 μm) for 60 min. The cells were lysed in lysis buffer (20 mm HEPES (pH 7.4), 100 mm NaCl, 10% glycerol, 1% Nonidet P-40, 1 mm MgCl2, 1 mm PMSF, and protease inhibitors mixture (Sigma-Aldrich)). Cell lysates were incubated with anti-integrin β3 antibody or anti-IGF1R antibody overnight at 4 °C, and the immune complex was recovered by incubating with protein A-Sepharose (GE Healthcare) for 1 h at 4 °C and washed three times with wash buffer (20 mm HEPES (pH 7.4), 100 mm NaCl, 10% glycerol, 0.5% Nonidet P-40, 1 mm MgCl2, 1 mm PMSF, and protease inhibitors). The immunoprecipitated materials were analyzed by Western blotting with anti-IGF1R antibody or anti-integrin β3 antibody.
Other Methods
MTS assays (14) were performed as described. Statistical significance was calculated using Prism 5 (GraphPad software).
RESULTS
IGF1 Induces Signals in Anchorage-independent Conditions in an αvβ3-dependent Manner
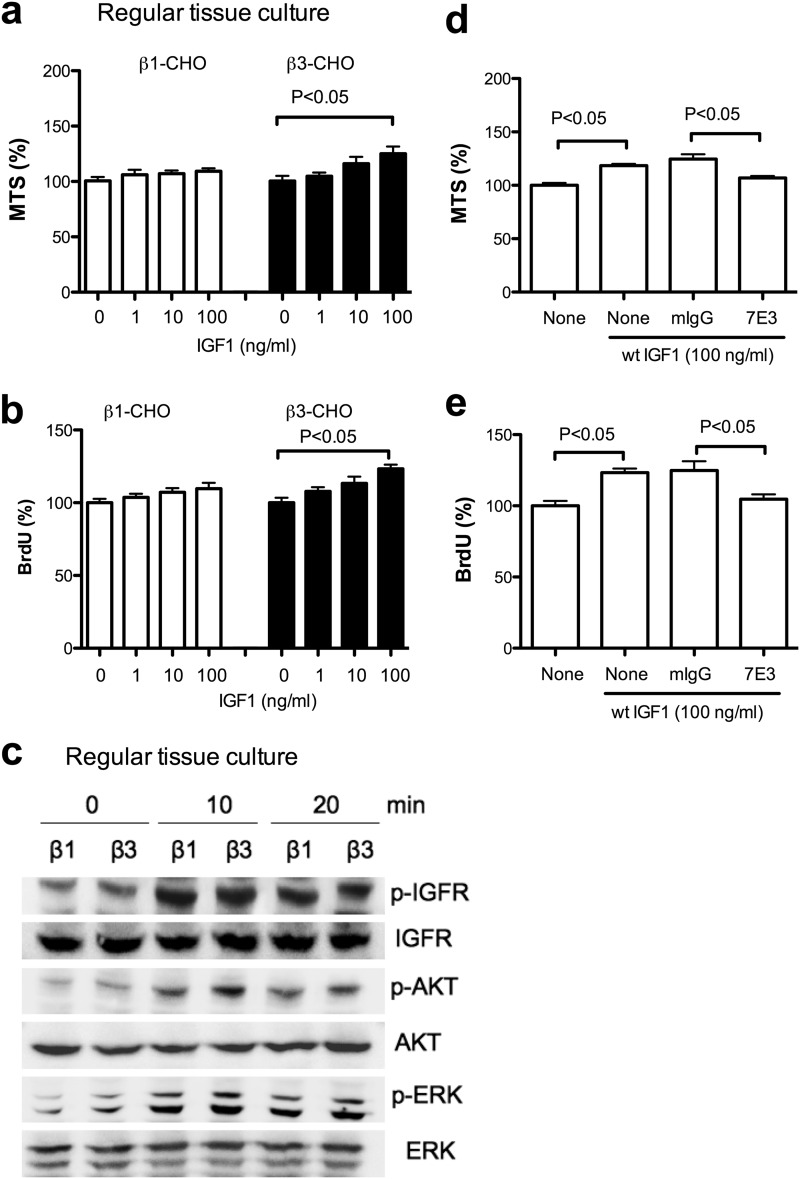
IGF1 directly binds to integrin αvβ3 and induces integrin-IGF1-IGF1R ternary complex formation in non-transformed cells in anchorage-dependent conditions (5). The integrin-binding defective R36E/R37E mutant is defective in these functions. These findings suggest that αvβ3-IGF1 interaction is critical for IGF signaling (5). These findings are not consistent with a current paradigm in which IGF1 binds to IGF1R, αvβ3 binds to ECM ligands, and two separate signals merge inside the cells (1, 15–19). αvβ3 is a receptor for many ECM and non-ECM ligands. Previous studies used non-transformed cells (e.g. smooth muscle cells) as a model of IGF signaling in anchorage-dependent conditions in which cells attach to ECM. IGF signaling in anchorage-independent conditions has not been studied. We thus studied CHO cells (ovarian cancer cells) that overexpress αvβ3 (β3-CHO cells) to identify the role of αvβ3-ECM in IGF signaling. In anchorage-dependent conditions (regular tissue culture plates without an ECM coating), we found that IGF1 induced a slightly higher cell survival (Fig. 1a) and cell proliferation (b) in β3-CHO cells than that of control CHO cells that express human β1 (β1-CHO cells), and there was only small enhancement in ERK1/2 and AKT activation between β3- and β1-CHO cells (c). Although the enhancement in IGF1-induced cell survival and proliferation in β3-CHO cells is small, we showed that anti-β3 mAb (7E3) suppressed it (Fig. 1, d and e), suggesting that IGF1-induced cell survival and proliferation in anchorage-dependent condition is αvβ3-dependent.
FIGURE 1.
IGF signaling in CHO cells in anchorage-dependent conditions. a, cell survival. β1-CHO and β3-CHO cells were serum-starved in DMEM and cultured in regular tissue culture plates with WT IGF (1, 10, and 100 ng/ml) for 48 h. Cell survival was measured by MTS assays. The data is shown as means ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. b, cell proliferation. β1-CHO and β3-CHO cells were serum-starved in DMEM and cultured in regular tissue culture plates with WT IGF(1, 10, and 100 ng/ml) for 24 h. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. c, intracellular signals induced by WT IGF1. p, phospho. β1-CHO and β3-CHO cells were serum-starved in DMEM for 12 h in regular tissue culture plates. Cells were stimulated with WT IGF1(100 ng/ml) for 10–20 min. Cell lysates were analyzed with Western blotting. d, anti-β3 mAb 7E3 inhibits cell survival induced by WT IGF1. β3-CHO cells were plated in regular tissue culture plates and serum-starved in DMEM and cultured with WT IGF (100 ng/ml) in the presence or absence of 7E3 (20 μg/ml) or mouse IgG (20 μg/ml) for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. e, anti-β3 mAb 7E3 inhibits cell proliferation induced by WT IGF1. β3-CHO cells were plated in regular tissue culture plates, serum-starved in DMEM, and cultured with WT IGF (100 ng/ml) in the presence or absence of 7E3 (20 μg/ml) or mouse IgG (20 μg/ml) for 24 h. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test.
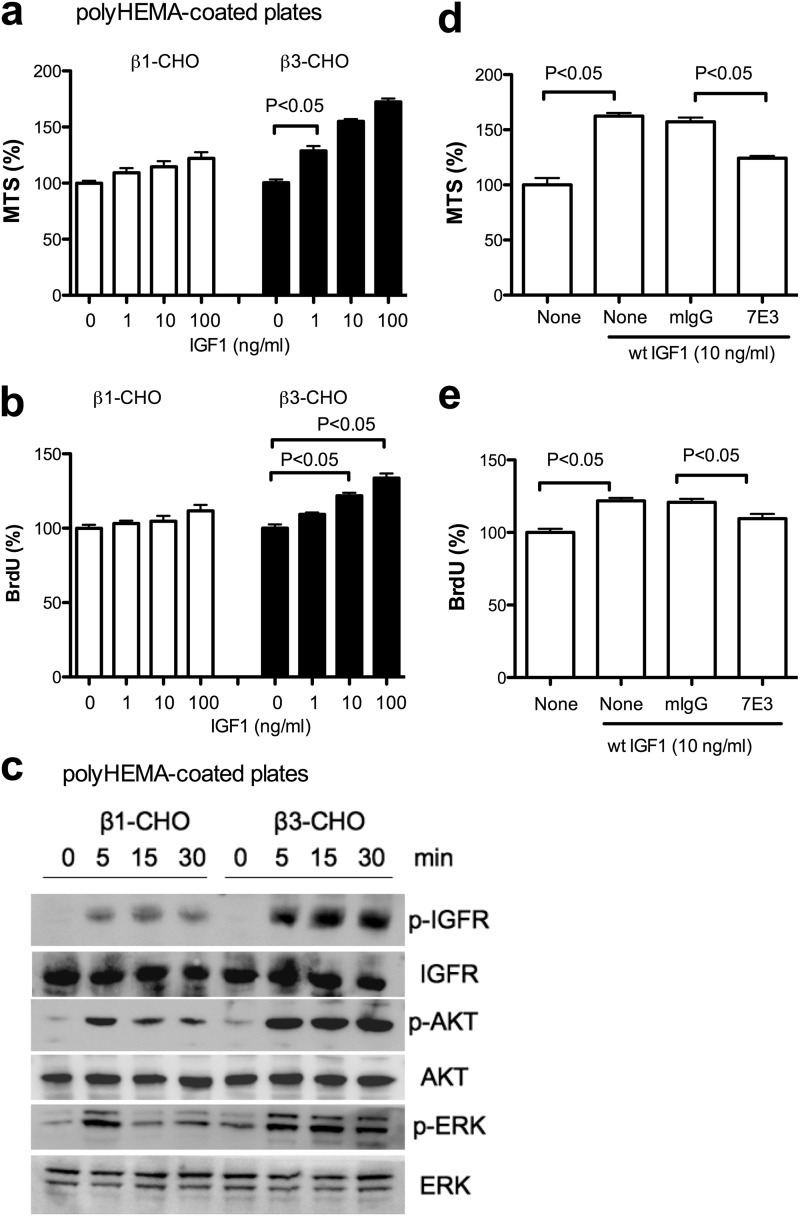
We used polyHEMA-coated plates to suppress cell matrix adhesion (anchorage independence) (11, 20). In anchorage-independent conditions, WT IGF1 enhanced cell survival (Fig. 2a) in β3-CHO cells to much higher levels than in β1-CHO cells that responded to IGF1 only weakly. Also, IGF1-induced cell proliferation of β3-CHO cells was slightly higher than in anchorage-dependent conditions (Fig. 2b). In these conditions, WT IGF1 induced ERK1/2 and AKT activation in β3-CHO cells, whereas WT IGF1 induced only weak ERK1/2 and AKT activation and the signals quickly reduced in β1-CHO cells (Fig. 2c). Anti-β3 mAb 7E3 suppressed the enhanced cell survival and proliferation of β3-CHO cells induced by WT IGF1 in anchorage-dependent conditions (Fig. 2, d and e). These results suggest that enhanced IGF signaling in β3-CHO in anchorage-independent conditions is dependent on αvβ3.
FIGURE 2.
IGF signaling in CHO cells in anchorage-independent conditions. a, cell survival. β1-CHO and β3-CHO cells were serum-starved in DMEM and cultured with WT IGF (1, 10, and 100 ng/ml) for 48 h in polyHEMA-coated plates. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. b, cell proliferation. β1-CHO and β3-CHO cells were serum-starved in DMEM and cultured with WT IGF (1, 10, and 100 ng/ml) for 24 h in polyHEMA-coated plates. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. c, intracellular signals induced by WT IGF1. p, phospho. β1-CHO and β3-CHO cells were serum-starved in DMEM for 3 h in polyHEMA-coated plates. Cells were stimulated with WT IGF1 (100 ng/ml) for 5–15 min. Cell lysates were analyzed by Western blotting. d, anti-β3 mAb 7E3 inhibits cell survival induced by WT IGF1. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM and cultured with WT IGF (10 ng/ml) in the presence or absence of 7E3 (20 μg/ml) or mouse IgG (20 μg/ml) for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. e, anti-β3 mAb 7E3 inhibits cell proliferation induced by WT IGF1. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF (10 ng/ml) in the presence or absence of 7E3 (20 μg/ml) or mouse IgG (20 μg/ml) for 24 h. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test.
Our results suggest that IGF signaling in anchorage-dependent conditions is masked by cell matrix interaction in CHO cells. This is consistent with a previous report that cell matrix adhesion masks heparin-binding EGF signaling in cancer cells (21).
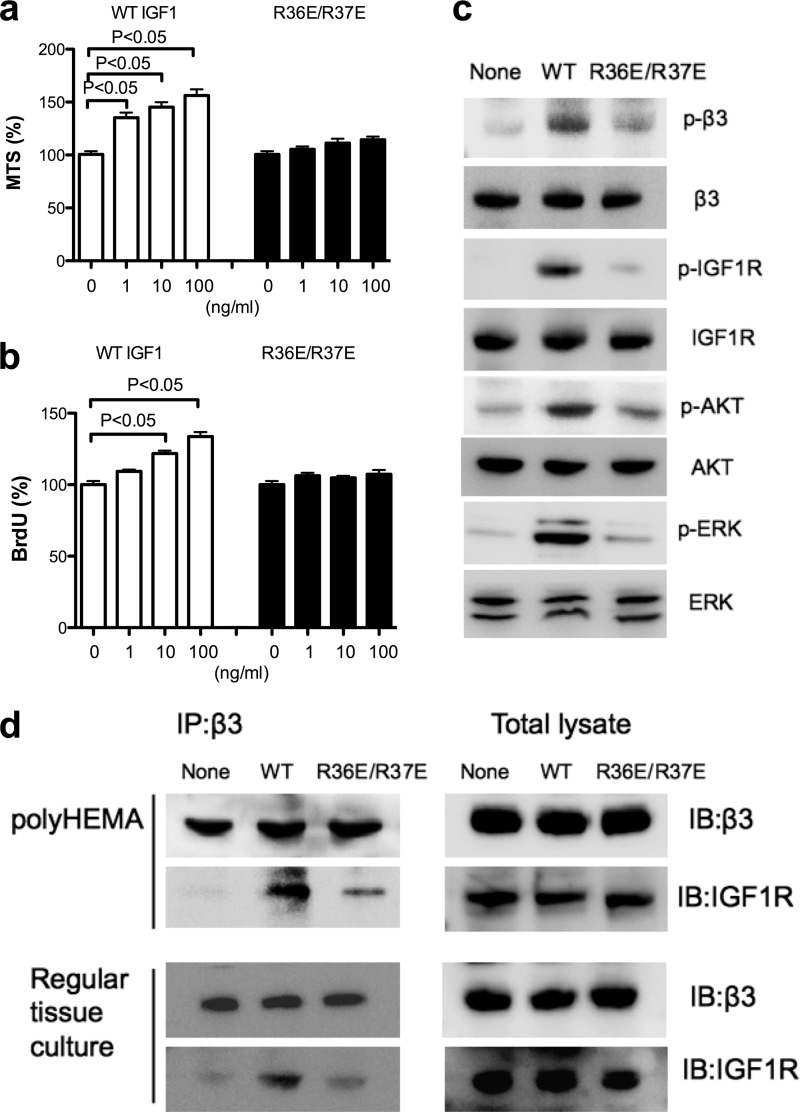
We reported previously that R36E/R37E is defective in enhancing cell survival and inducing phosphorylation of IGF1R, β3, AKT, and ERK1/2 and in ternary complex formation in non-transformed cells in anchorage-dependent conditions (5). We studied whether R36E/R37E is defective in these functions in anchorage-independent conditions. In polyHEMA-coated plates, R36E/R37E was defective in enhancing cell survival (Fig. 3a); cell proliferation (b); and inducing phosphorylation of IGF1R, β3, AKT, and ERK1/2 (c) in β3-CHO cells. Also, WT IGF1 induced coprecipitation of IGF1R and integrin β3 in β3-CHO cells, whereas R36E/R37E was much less effective in this function (Fig. 3d). These findings suggest that integrin αvβ3-IGF1 interaction is involved in IGF signaling in anchorage-independent conditions. This suggests that the ternary complex formation through direct αvβ3-binding to IGF1 is critical for IGF signaling in β3-CHO cells. Therefore it is expected that αvβ3-IGF1 interaction, but not αvβ3-ECM interaction, is critical for IGF signaling. Interestingly, we obtained essentially the same results in IGF1-induced ternary complex formation in anchorage-dependent conditions (Fig. 3d). This suggests that the ternary complex formation through direct αvβ3-binding to IGF1 is involved in IGF signaling in β3-CHO cells in anchorage-dependent conditions as well.
FIGURE 3.
The effect of R36E/R37E on IGF1 signaling in β3-CHO cells in anchorage-independent conditions. a, cell survival. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF or R36E/R37E (1, 10, and 100 ng/ml) for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. b, cell proliferation. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF or R36E/R37E (1, 10, and 100 ng/ml) for 24 h. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. c, intracellular signals induced by IGF1. p, phospho. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were stimulated with WT IGF1or R36E/37E (100 ng/ml) for 30 min. Cell lysates were analyzed by Western blotting. d, coprecipitation of IGF1R with integrin β3. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h or in regular tissue culture plates and serum-starved in DMEM for 12 h. Cells were stimulated with WT IGF1or R36E/37E (100 ng/ml) for 30 min. β3 was immunopurified (IP) with anti-β3 antibodies from cell lysates. The immunoprecipitated materials or total cell lysates were analyzed with anti-IGF1R or β3 antibodies by Western blotting (IB).
Direct Binding of αvβ3 to IGF1 Is Required for IGF1 Signaling in Anchorage-independent Conditions
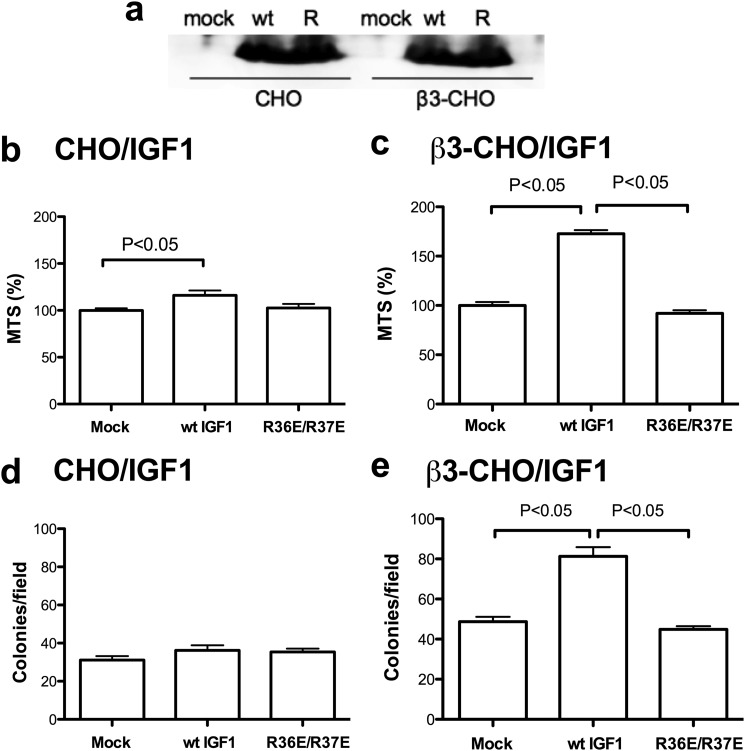
We stably expressed WT IGF1 or R36E/R37E in β3-CHO or CHO cells in the pSec-TagB secretion vector. Transfected cells secrete WT IGF1 or R36E/R37E at comparable levels (Fig. 4a). We first determined the survival of cells stably secreting WT IGF1 or R36E/R37E in polyHEMA-coated plates. WT IGF1 or R36E/R37E expression did not affect the survival of CHO cells (Fig. 4b). In contrast, WT IGF1 markedly enhanced cell survival of β3-CHO cells, whereas R36E/R37E did not (Fig. 4c), suggesting that IGF1 enhanced cell survival of β3-CHO cells requires αvβ3 expression and αvβ3-IGF1 interaction in anchorage-independent conditions. These results are consistent with the results with exogenous IGF1 (Figs. 1 and 2).
FIGURE 4.
The role of β3-integrin in IGF signaling in anchorage-independent conditions. a, secretion of WT IGF1 or R36E/R37E in CHO and β3-CHO cells. Cells were cultured with DMEM with 10% FCS. The culture medium was concentrated five times and analyzed by Western blotting using anti-6-His antibodies (IGF1 has an N-terminal 6-His tag). R, R36E/R37E. b and c, cell survival of the transfectants in polyHEMA-coated plates. The transfected cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. d and e, colony formation of the transfectants in three-dimensional culture (in soft agar). The transfected cells were cultured in soft agar for 3 weeks, and the number of colonies was counted from digital images using ImageJ software. Statistical differences were tested using ANOVA and Tukey's multiple comparison test (n = 10).
We studied whether IGF1 affects colony formation in three-dimensional culture, which mimics in vivo tumorigenesis, using CHO or β3-CHO cells that express WT and mutant IGF1. The cells were cultured in soft agar for 3 weeks, and the number of colonies was counted. We found that WT IGF1 had no effect in CHO cells (Fig. 4d) but markedly enhanced colony formation in β3-CHO cells (e). R36E/R37E did not enhance colony formation in β3-CHO or CHO cells. These findings suggest that the enhancing effect of WT IGF1 requires αvβ3 expression and direct αvβ3-IGF1 interaction in three-dimensional culture. Taken together, our results are consistent with the model in which IGF1-αvβ3 interaction, rather than αvβ3-ECM interaction, is required for IGF signaling.
IGF1R Is Not Related to β3 Phosphorylation, and Src Is Related to β3 Phosphorylation but Not IGF1R Phosphorylation
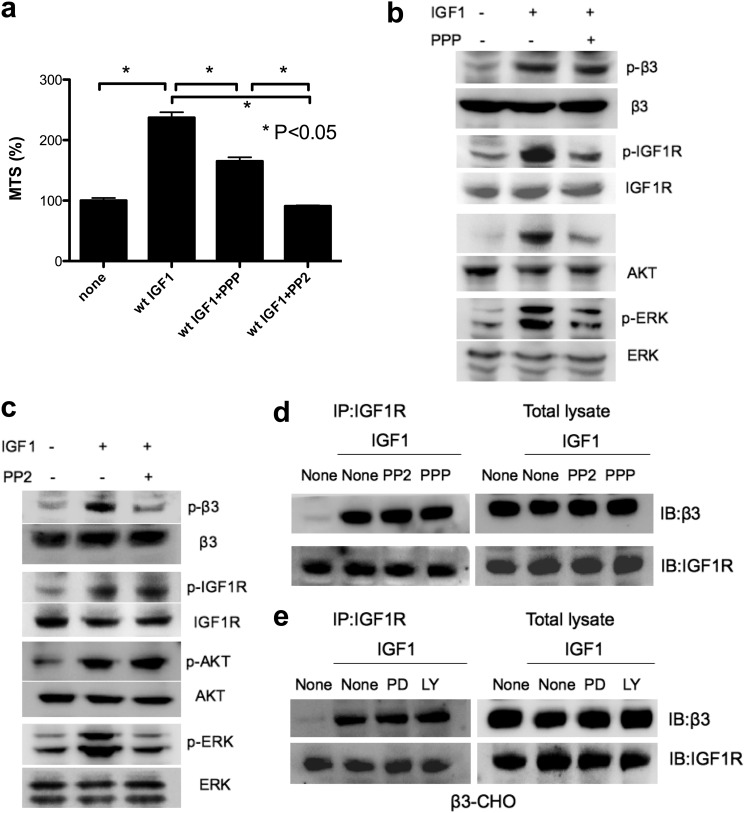
Our results so far suggest that IGF signaling depends exclusively on αvβ3 expression and its direct binding to IGF1 in β3-CHO cells in anchorage-independent conditions. It has been proposed that the β3 cytoplasmic domain binds constitutively and selectively to the SH3 domain of primed c-Src, generating a oncogenic signaling complex (22). The αvβ3-c-Src complex was shown to enhance cell survival in a cell adhesion-independent manner and promote tumor progression (23). Thus it is suggested that the c-Src is present in the αvβ3-IGF1-IGF1R complex. What is the role of Src in the complex? To address this question, we stimulated β3-CHO cells with IGF1 in the presence of PPP, a kinase inhibitor of IGF1R, or PP2, a Src inhibitor. We found that PPP and PP2 suppressed the enhanced cell survival of β3-CHO cells induced by WT IGF1 in anchorage-independent conditions (Fig. 5a). We found that PPP suppressed phosphorylation of IGF1R, AKT, and ERK1/2 but did not affect phosphorylation of β3 (Fig. 5b). PP2 suppressed β3 phosphorylation and ERK1/2 activation but did not affect IGF1R phosphorylation and AKT activation (Fig. 5c). These results suggest that IGF1R phosphorylation is required for AKT and ERK1/2 activation but not in β3 phosphorylation and that Src is required for β3 phosphorylation and ERK1/2 activation but not in IGF1R phosphorylation or AKT activation. However, we cannot rule out the possibility that phosphorylation of other residues of β3 (e, g Tyr-759) is involved. Future studies will address this point.
FIGURE 5.
The effect of PPP (IGF1R kinase inhibitor) and PP2 (Src inhibitor) on IGF1 signaling in β3-CHO cells in anchorage-independent conditions. a, the effect of PPP and PP2 on cell survival. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM for 48 h, and cultured with IGF1 (100 ng/ml) in the presence or absence of PPP (10 μm) or PP2 (10 μm). Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. b and c, the effect of PPP and PP2 on phosphorylation of β3 integrin, IGF1R, AKT, and ERK. p, phospho. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were pretreated with PPP (b) (10 μm) or PP2 (10 μm) (c) for 60 min and then with WT IGF1 (100 ng/ml) for 30 min. Cell lysates were analyzed by Western blotting. d, the effect of PPP and PP2 on ternary complex formation. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were pretreated with PPP (10 μm) or PP2 (10 μm) for 60 min and stimulated with WT IGF1 (100 ng/ml) for 30 min. IGF1R was immunopurified (IP) with anti-IGF1R antibodies from cell lysates, and the immunopurified materials were analyzed with anti-IGF1R or β3 antibodies by Western blotting (IB). e, the effect of PD098059 and LY294002 on ternary complex formation. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were pretreated with PD098059 (50 μm) (PD) or LY294002 (50 μm) (LY) for 60 min and stimulated with WT IGF1 (100 ng/ml) for 30 min. IGF1R was immunopurified with anti-IGF1R antibodies from cell lysates, and the immunopurified materials were analyzed with anti-IGF1R or β3 antibodies by Western blotting.
Suppression of IGF1R, Src, ERK1/2, or PI3K Does Not Affect Ternary Complex Formation
We next asked whether the suppression of IGF1R or Src activity affects ternary complex formation. Interestingly, PPP or PP2 did not affect coprecipitation of β3 and IGF1R (Fig. 5d), suggesting that ternary complex formation does not require IGF1R or β3 phosphorylation. We also found that inhibitors of ERK1/2 (PD098059) or PI3K (LY294002) did not affect coprecipitation of β3 and IGF1R (Fig. 5e), suggesting that ternary complex formation is not regulated by signaling downstream of IGF1R. Because R36E/R37E is defective in ternary complex formation and IGF1R and β3 phosphorylation, one possibility is that ternary complex formation is required for IGF1R or β3 phosphorylation and that IGF1R or β3 phosphorylation occurs after ternary complex formation.
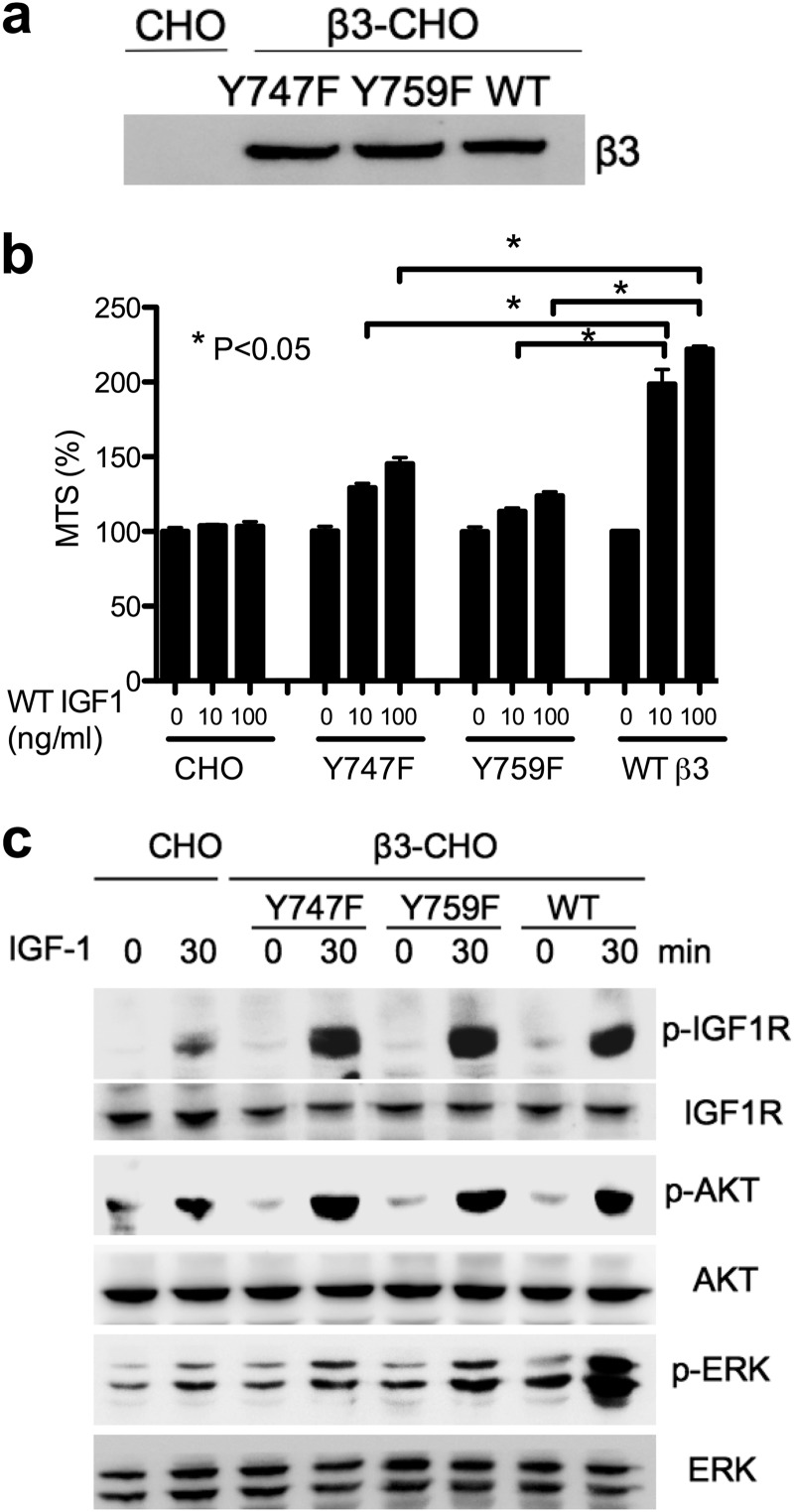
Phosphorylation of Integrin β3 Is Not Required for IGF1R Phosphorylation
Our results suggest that Src (as the β3-Src complex) plays a role in IGF signaling. Src is known to induce β3 phosphorylation (22). To study the role of β3 phosphorylation on IGF signaling, we generated CHO cells that express β3 with mutations in the tyrosine residues at position 747 and 759 of the β3 cytoplasmic domain, which are phosphorylated upon ligand binding (24). We selected CHO cells stably expressing the WT and mutant (Y747F and Y759F) and further enriched the populations that express β3 at comparable levels (Fig. 6a). We found that the Y747F and Y759F mutations suppressed the enhanced cell survival of β3-CHO cells induced by WT IGF1 in anchorage-independent conditions (Fig. 6b) consistent with previous studies (6). Notably, the Y747F and Y759F mutations suppressed ERK1/2 phosphorylation but did not affect IGF1R phosphorylation upon IGF1 stimulation (Fig. 6c). These results suggest that Src-mediated β3 phosphorylation is related to ERK1/2 phosphorylation but is not related to IGF1R phosphorylation or AKT activation. It is thus likely that Src plays a prominent role in regulating β3-mediated IGF1 signaling independent of IGF1R activation.
FIGURE 6.
The effect of Y747F and Y759F mutation of the β3 cytoplasmic domain on IGF1 signaling. a, the expression of integrin β3. Cells were cultured in DMEM with 10% FCS. Cell lysates were analyzed by Western blotting. Human integrin β3 (WT, Y747F, or Y759F) was stably expressed in CHO cells. b, cell survival in anchorage-independent conditions. CHO and β3-CHO (WT, Y747F, or Y579F) cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF1 (10 or 100 ng/ml) for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. c, intracellular signals induced by WT IGF1 in anchorage-independent conditions. p, phospho. Cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were stimulated with WT IGF1 (100 ng/ml) for 30 min. Cell lysates were analyzed by Western blotting.
Taken together, we propose a model in which IGF1 induces ternary complex formation and thereby induces phosphorylation of IGF1R and β3 cytoplasmic tail, which triggers two separate downstream signaling cascades.
DISCUSSION
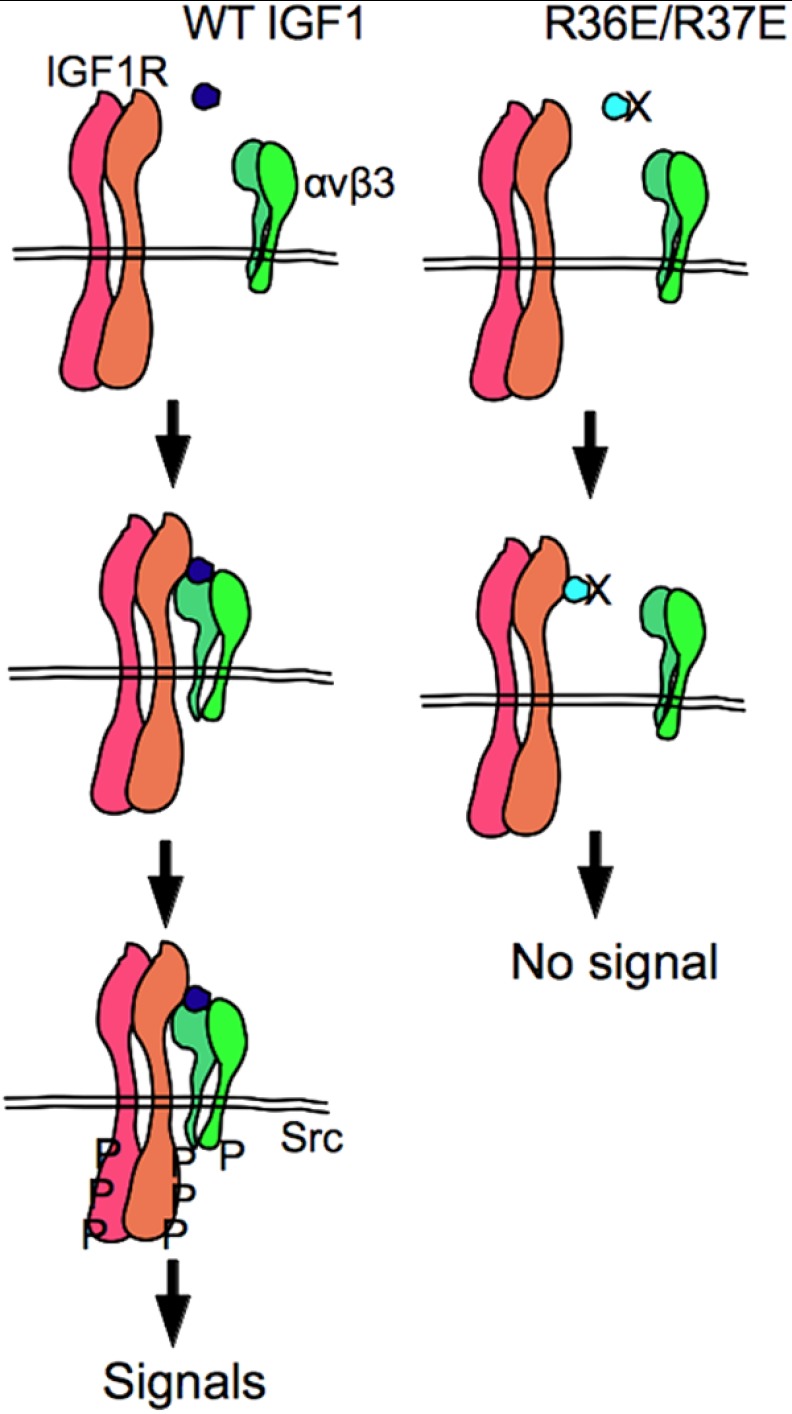
In this study we establish that IGF1 induces signaling in anchorage-independent conditions in an αvβ3-dependent manner using β3-CHO cells. These results are not consistent with the current model in which the binding of αvβ3 to ECM proteins enhances signaling induced by IGF1 binding to IGF1R (2). Our findings suggest that integrin αvβ3-IGF interaction, rather than αvβ3-ECM interaction, is critical for IGF signaling. We propose a model in which IGF1 binds to IGF1R and integrin αvβ3 is recruited to the complex through direct binding to IGF1 (IGF1R-IGF1-integrin complex formation) (Fig. 7).
FIGURE 7.
A model of IGF signaling. IGF1 binds to IGF1R, and then integrin αvβ3 (and α6β4 and perhaps other integrins as well) is recruited to the IGF1-IGF1R complex. Recruitment of integrin to the IGF1-IGF1R complex plays a critical role in triggering IGF1R phosphorylation. Consistent with this model, the integrin-binding defective IGF1 mutant (R36E/R37E) binds to IGF1R but is defective in inducing ternary complex formation, IGF1R phosphorylation, and activation of AKT and ERK1/2. P, phosphorylation.
In regular culture conditions, IGF signaling is not clearly detectable in β3-CHO cells, probably because IGF signaling is masked by the massive signals from cell-matrix adhesion. These findings are consistent with a previous report that cell matrix adhesion masks the heparin-binding EGF signaling but that it is possible to detect the proliferative effect of heparin-binding EGF on cancer cells in vitro in three-dimensional culture or two-dimensional culture in which cell-matrix interaction is reduced (21). Cell proliferation in anchorage-dependent conditions is much faster than that in anchorage-independent conditions, probably reflecting the amount of proliferative signals from cell matrix adhesion.
We studied IGF signaling in anchorage-independent conditions using β3-CHO cells. We found that R36E/R37E was defective in inducing signals, suggesting that direct integrin binding to IGF1 is required for signaling as in non-transformed cells. It is likely that αvβ3 should be physically in the IGF1/IGF1R complex through direct binding to IGF1 to properly induce intracellular signals.
The contribution of αvβ3-IGF interaction and αvβ3-ECM interaction in IGF signaling is unclear in non-transformed cells used in previous studies (smooth muscle cells (6–9), NIH3T3 cells, and C2C12 cells (5)) because non-transformed cells can only be maintained in anchorage-dependent conditions. However, we assume that αvβ3-IGF interaction is critical in non-transformed cells because R36E/R37E is defective in inducing IGF signing in these cells as well.
This study demonstrates that the ternary complex formation is induced by WT IGF1 (not R36E/R37E) in anchorage-independent conditions. We used anchorage-independent conditions (polyHEMA-coated plates) for studying the role of αvβ3 in IGF signaling because αvβ3-ECM interaction itself may induce β3 phosphorylation. Our results suggest that IGF1 itself induces β3 phosphorylation without contribution of ECM. Because R36E/R37E did not induce β3 phosphorylation, the direct binding of IGF1 to αvβ3 and/or ternary complex formation are critical for the β3 phosphorylation. Interestingly, inhibitors of IGF1R, Src kinases, PI3K, or ERK1/2 did not affect IGF1-induced ternary complex formation. Src is involved in β3 phosphorylation but not in IGF1R phosphorylation (6). β3 phosphorylation is not required for IGF1R phosphorylation (6, 25). This suggests that IGF1R or β3 phosphorylation is not required for ternary complex formation and that ternary complex formation is not regulated by downstream signaling pathways. We propose that ternary complex formation precedes IGF1R or β3 phosphorylation (Fig. 7). If this is the case, the defect of R36E/R37E in inducing ternary complex formation is tightly related to its defect in inducing IGF1R phosphorylation and β3 phosphorylation. PPP, an inhibitor of IGF1R, did not affect β3 phosphorylation, whereas it suppressed IGF1R, AKT, and ERK1/2 phosphorylation. This is the first indication that β3 phosphorylation and IGF1R phosphorylation induce independent downstream signaling while they are in the ternary complex. Then why do they have to be in the ternary complex for inducing signaling? One possibility is that ternary complex formation triggers dimerization of the αvβ3-Src oncogenic complex, leading to Src activation and β3 phosphorylation. Another possibility is that recruitment of integrin αvβ3 into the complex may trigger conformational changes in IGF1R, leading to IGF1R activation. It would be interesting to address these possibilities in future studies.
Integrin αvβ3 is expressed on invasive melanoma but not benign nevi or normal melanocytes (26). Levels of expression of αvβ3 closely correlate with levels of cell invasion and metastasis (27, 28). Antagonists of αvβ3 block melanoma growth by inducing apoptosis (29, 30). Also, αvβ3 has a key role in endothelial cell survival and migration during angiogenesis (31, 32). It has been reported that αvβ3 on endothelial cells is expressed in response to angiogenic growth factors and tumors in angiogenesis and tumor growth (31–34). Antagonists of αvβ3 inhibit angiogenesis in cancer and in ocular disease (31–35). αvβ3 antagonists induce endothelial cell apoptosis, increase the activity of the tumor suppressor p53, increase levels of the cell cycle inhibitor p21, and decrease levels of the anti-apoptotic protein BAX (36). αvβ3 antagonists activate a caspase 8-dependent cell death program (37). Thus, previous studies suggest that αvβ3 promotes angiogenesis and endothelial cell survival and that antagonists to αvβ3 suppress angiogenesis by inducing endothelial cell apoptosis in vitro and in vivo. These studies are, however, on the basis of the assumption that αvβ3 interacts almost exclusively with ECM ligands (e.g. vitronectin) and antagonists to αvβ3 block αvβ3-ECM interaction. We discovered that αvβ3 plays a role in IGF signaling through direct binding to IGF1 and subsequent ternary complex formation (αvβ3-IGF1-IGF1R) (this study and Ref. 5). It is likely that antagonists to αvβ3 suppress αvβ3-IGF interactions and subsequent IGF signaling in addition to αvβ3-ECM interactions. This may be particularly true in anchorage-independent conditions, in which IGF plays a role in enhancing cell survival and proliferation. It is also important to note that integrin-growth factor interaction is not limited to IGF1. We have reported that FGF1 and neuregulin-1 directly bind of αvβ3 and that these interactions play a role in FGF signaling (38, 39) and neuregulin-1 signaling (14), respectively. It would be important to evaluate the role of αvβ3-growth factor interaction in the pathogenesis of the diseases in future studies.
This work was supported, in whole or in part, by National Institutes of Health Grants CA13015 and DOD W81XWH-10-1-0312 (to Y. T.).
- IGF
- insulin-like growth factor
- IGF1R
- insulin-like growth factor 1 receptor
- ECM
- extracellular matrix
- CHO
- Chinese hamster ovaries
- PPP
- picropodophyllin
- PP2
- (4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine
- polyHEMA
- poly(2-hydroxyethyl methacrylate)
- ANOVA
- analysis of variance
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium).
REFERENCES
- 1. Clemmons D. R., Maile L. A. (2005) Interaction between insulin-like growth factor-I receptor and αVβ3 integrin linked signaling pathways. Cellular responses to changes in multiple signaling inputs. Mol. Endocrinol. 19, 1–11 [DOI] [PubMed] [Google Scholar]
- 2. Clemmons D. R. (2007) Modifying IGF1 activity. An approach to treat endocrine disorders, atherosclerosis and cancer. Nat. Rev. Drug Discov. 6, 821–833 [DOI] [PubMed] [Google Scholar]
- 3. Jones J. I., Doerr M. E., Clemmons D. R. (1995) Cell migration. Interactions among integrins, IGFs and IGFBPs. Prog. Growth Factor Res. 6, 319–327 [DOI] [PubMed] [Google Scholar]
- 4. Zheng B., Duan C., Clemmons D. R. (1998) The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J. Biol. Chem. 273, 8994–9000 [DOI] [PubMed] [Google Scholar]
- 5. Saegusa J., Yamaji S., Ieguchi K., Wu C. Y., Lam K. S., Liu F. T., Takada Y. K., Takada Y. (2009) The direct binding of insulin-like growth factor-1 (IGF-1) to integrin αVβ3 is involved in IGF-1 signaling. J. Biol. Chem. 284, 24106–24114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ling Y., Maile L. A., Clemmons D. R. (2003) Tyrosine phosphorylation of the β3-subunit of the αVβ3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol. Endocrinol. 17, 1824–1833 [DOI] [PubMed] [Google Scholar]
- 7. Ling Y., Maile L. A., Badley-Clarke J., Clemmons D. R. (2005) DOK1 mediates SHP-2 binding to the αVβ3 integrin and thereby regulates insulin-like growth factor I signaling in cultured vascular smooth muscle cells. J. Biol. Chem. 280, 3151–3158 [DOI] [PubMed] [Google Scholar]
- 8. Ling Y., Maile L. A., Lieskovska J., Badley-Clarke J., Clemmons D. R. (2005) Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol. Biol. Cell 16, 3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xi G., Maile L. A., Yoo S. E., Clemmons D. R. (2008) Expression of the human β3 integrin subunit in mouse smooth muscle cells enhances IGF-I-stimulated signaling and proliferation. J. Cell Physiol. 214, 306–315 [DOI] [PubMed] [Google Scholar]
- 10. Takagi J., Kamata T., Meredith J., Puzon-McLaughlin W., Takada Y. (1997) Changing ligand specificities of αVβ1 and αVβ3 integrins by swapping a short diverse sequence of the β subunit. J. Biol. Chem. 272, 19794–19800 [DOI] [PubMed] [Google Scholar]
- 11. Fukazawa H., Mizuno S., Uehara Y. (1995) A microplate assay for quantitation of anchorage-independent growth of transformed cells. Anal. Biochem. 228, 83–90 [DOI] [PubMed] [Google Scholar]
- 12. Akakura N., Hoogland C., Takada Y. K., Saegusa J., Ye X., Liu F. T., Cheung A. T., Takada Y. (2006) The COOH-terminal globular domain of fibrinogen γ chain suppresses angiogenesis and tumor growth. Cancer Res. 66, 9691–9697 [DOI] [PubMed] [Google Scholar]
- 13. Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. (1971) Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc. Natl. Acad. Sci. U.S.A. 68, 1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ieguchi K., Fujita M., Ma Z., Davari P., Taniguchi Y., Sekiguchi K., Wang B., Takada Y. K., Takada Y. (2010) Direct binding of the EGF-like domain of neuregulin-1 to integrins ({α}V{β}3 and {α}6{β}4) is involved in neuregulin-1/ErbB signaling. J. Biol. Chem. 285, 31388–31398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz M. A., Ginsberg M. H. (2002) Networks and crosstalk. Integrin signalling spreads. Nat. Cell Biol. 4, E65–68 [DOI] [PubMed] [Google Scholar]
- 16. Clemmons D. R., Maile L. A. (2003) Minireview. Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology 144, 1664–1670 [DOI] [PubMed] [Google Scholar]
- 17. Clemmons D. R., Maile L. A., Ling Y., Yarber J., Busby W. H. (2007) Role of the integrin αVβ3 in mediating increased smooth muscle cell responsiveness to IGF-I in response to hyperglycemic stress. Growth Horm. IGF Res. 17, 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Legate K. R., Wickström S. A., Fässler R. (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418 [DOI] [PubMed] [Google Scholar]
- 19. Soung Y. H., Clifford J. L., Chung J. (2010) Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep. 43, 311–318 [DOI] [PubMed] [Google Scholar]
- 20. Folkman J., Moscona A. (1978) Role of cell shape in growth control. Nature 273, 345–349 [DOI] [PubMed] [Google Scholar]
- 21. Mizushima H., Wang X., Miyamoto S., Mekada E. (2009) Integrin signal masks growth-promotion activity of HB-EGF in monolayer cell cultures. J. Cell Sci. 122, 4277–4286 [DOI] [PubMed] [Google Scholar]
- 22. Huveneers S., Danen E. H. (2010) The interaction of SRC kinase with β3 integrin tails. A potential therapeutic target in thrombosis and cancer. ScientificWorldJournal 10, 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desgrosellier J. S., Barnes L. A., Shields D. J., Huang M., Lau S. K., Prévost N., Tarin D., Shattil S. J., Cheresh D. A. (2009) An integrin α(v)β(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 15, 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blystone S. D. (2002) Kinetic regulation of β 3 integrin tyrosine phosphorylation. J. Biol. Chem. 277, 46886–46890 [DOI] [PubMed] [Google Scholar]
- 25. Maile L. A., Badley-Clarke J., Clemmons D. R. (2001) Structural analysis of the role of the β 3 subunit of the α V β 3 integrin in IGF-I signaling. J. Cell Sci. 114, 1417–1425 [DOI] [PubMed] [Google Scholar]
- 26. Gehlsen K. R., Davis G. E., Sriramarao P. (1992) Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin. Exp. Metastasis 10, 111–120 [DOI] [PubMed] [Google Scholar]
- 27. Felding-Habermann B., Fransvea E., O'Toole T. E., Manzuk L., Faha B., Hensler M. (2002) Involvement of tumor cell integrin α v β 3 in hematogenous metastasis of human melanoma cells. Clin. Exp. Metastasis 19, 427–436 [DOI] [PubMed] [Google Scholar]
- 28. Nip J., Shibata H., Loskutoff D. J., Cheresh D. A., Brodt P. (1992) Human melanoma cells derived from lymphatic metastases use integrin α v β 3 to adhere to lymph node vitronectin. J. Clin. Invest. 90, 1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petitclerc E., Strömblad S., von Schalscha T. L., Mitjans F., Piulats J., Montgomery A. M., Cheresh D. A., Brooks P. C. (1999) Integrin α(v)β3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 59, 2724–2730 [PubMed] [Google Scholar]
- 30. Mitjans F., Sander D., Adán J., Sutter A., Martinez J. M., Jäggle C. S., Moyano J. M., Kreysch H. G., Piulats J., Goodman S. L. (1995) An anti-α V-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J. Cell Sci. 108, 2825–2838 [DOI] [PubMed] [Google Scholar]
- 31. Brooks P. C., Clark R. A., Cheresh D. A. (1994) Requirement of vascular integrin α v β 3 for angiogenesis. Science 264, 569–571 [DOI] [PubMed] [Google Scholar]
- 32. Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. (1994) Integrin α V β 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 33. Brooks P. C., Strömblad S., Klemke R., Visscher D., Sarkar F. H., Cheresh D. A. (1995) Antiintegrin α V β 3 blocks human breast cancer growth and angiogenesis in human skin. J. Clin. Invest. 96, 1815–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., Cheresh D. A. (1995) Definition of two angiogenic pathways by distinct α V integrins. Science 270, 1500–1502 [DOI] [PubMed] [Google Scholar]
- 35. Friedlander M., Theesfeld C. L., Sugita M., Fruttiger M., Thomas M. A., Chang S., Cheresh D. A. (1996) Involvement of integrins α V β 3 and α V β 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. U.S.A. 93, 9764–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strömblad S., Becker J. C., Yebra M., Brooks P. C., Cheresh D. A. (1996) Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin αVβ3 during angiogenesis. J. Clin. Invest. 98, 426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stupack D. G., Puente X. S., Boutsaboualoy S., Storgard C. M., Cheresh D. A. (2001) Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155, 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mori S., Wu C. Y., Yamaji S., Saegusa J., Shi B., Ma Z., Kuwabara Y., Lam K. S., Isseroff R. R., Takada Y. K., Takada Y. (2008) Direct binding of integrin αVβ3 to FGF1 plays a role in FGF1 signaling. J. Biol. Chem. 283, 18066–18075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaji S., Saegusa J., Ieguchi K., Fujita M., Mori S., Takada Y. K., Takada Y. (2010) A novel fibroblast growth factor-1 (FGF1) mutant that acts as an FGF antagonist. PLoS ONE 5, e10273. [DOI] [PMC free article] [PubMed] [Google Scholar]