Abstract
For clinical trials of therapeutic monoclonal antibodies (mAbs) to be successful, their efficacy needs to be adequately evaluated in preclinical experiments. However, in many cases it is difficult to evaluate the candidate mAbs using animal disease models because of lower cross-reactivity to the orthologous target molecules. In this study we have established a novel humanized Castleman's disease mouse model, in which the endogenous interleukin-6 receptor gene is successfully replaced by human IL6R, and human IL6 is overexpressed. We have also demonstrated the therapeutic effects of an antibody that neutralizes human IL6R, tocilizumab, on the symptoms in this mouse model. Plasma levels of human soluble IL6R and human IL6 were elevated after 4-week treatment of tocilizumab in this mouse model similarly to the result previously reported in patients treated with tocilizumab. Our mouse model provides us with a novel means of evaluating the in vivo efficacy of human IL6R-specific therapeutic agents.
Worldwide trends in the development of therapeutic agents are towards the use of molecularly targeted drugs such as monoclonal antibodies (mAbs), which have revolutionized therapy for many intractable diseases. However, novel issues have emerged when evaluating their preclinical efficacy and safety1,2,3. It is usually difficult to evaluate the efficacy of therapeutic mAbs in animal experiments because, in most cases, they have no or low cross-reactivity to orthologous molecules of animals other than primates (phylogenetically the closest species to human). This means that systems using smaller experimental animals, such as mice and rats, are not applicable despite their obvious advantages. These advantages are that they are well-characterized after a long history of contributing to thousands of studies in various research fields of medical science, and their smaller body sizes require relatively small amounts of candidate agents. This latter advantage is especially useful at the early stage of drug development when a wider variety of drug candidates needs to be screened to select the best agent. As for the use of primates, this has been limited by disease outbreak risks, legislative changes and logistical problems with supply2. Moreover, it has also been pointed out that even an examination using primates would not be sufficient to perfectly predict clinical outcomes1.
Some reviews propose the use of genetically engineered rodents and/or surrogate antibodies in order to predict the efficacy and safety of drug candidate antibodies in preclinical studies1,2,3, but various attributes of the two tools need to be taken into account. For example, it will be costly and time-consuming to develop surrogate antibodies only for preclinical animal experiments and, even then, the surrogate antibodies would not necessarily work in the same manner as fully developed therapeutic antibodies. Genetic engineering in mice is a powerful technique to make loss-of-function or gain-of-function mutants for analyzing in vivo gene function and to develop animal models for human diseases, but the type of transgenic mouse established needs to correspond to its end purpose. To evaluate the pharmacokinetics, pharmacodynamics, in vivo efficacy, etc. of a drug, we must produce a genetically humanized mouse by the gene knock-in technique, in which a human target gene would be substituted and controlled to express in a similar spatial and temporal pattern to that of the endogenous orthologous gene. By using such genetically humanized mice, we can expect to evaluate the in vivo efficacy of the drug candidate antibodies themselves, instead of using surrogate antibodies.
We have previously reported that transgenic mice with human interleukin-6 (hIL6) driven by the major histocompatibility complex class I H-2Ld gene promoter develop symptoms similar to Castleman's disease in human4,5,6 such as lymphadenopathy, massive immunoglobulin G1 plasmacytosis, splenomegaly, mesangial proliferative glomerulonephritis, thrombocytopenia, leukocytosis, anemia and muscle atrophy7,8. We also demonstrated that a mAb to mouse IL-6 receptor, the surrogate antibody MR16-1, completely blocked their symptoms8. These findings indicate that neutralization of IL-6 signaling by a mAb to IL-6 receptor would be an effective therapeutic strategy for IL-6-related diseases. However, it is not possible to use these transgenic mice to evaluate the in vivo efficacy of drug candidate antibodies directly because they express murine IL-6 receptor (Il6ra) instead of human IL-6 receptor (hIL6R). A possible solution is to use a double transgenic mouse established by crossing an H-2Ld-hIL6 transgenic mouse with an hIL6R transgenic mouse. As far as we know, two lines of hIL6R transgenic mice were previously reported9,10. However, these hIL6R transgenic mice cannot be used to evaluate therapeutic mAbs because they express not only hIL6R but also endogenous mouse Il6ra, which is well known as responding to human IL6. Therefore, it is necessary to neutralize or disrupt the endogenous mouse Il6ra before evaluating drug efficacy. Moreover, these hIL6R transgenic mice express extremely higher levels of hIL6R, driven by relatively stronger promoters. Therefore we predict that using these hIL6R transgenic mice to evaluate the therapeutic efficacy of neutralizing antibody to hIL6R would be difficult because the antibody, mediated by antigen, would disappear extremely rapidly from blood.
In this study we have generated a novel Castleman's disease mouse model, in which, in addition to the H-2Ld-hIL6 transgene described above, mouse endogenous Il6ra gene is successfully replaced by hIL6R with the gene knock-in technique to establish a humanized ligand-receptor system for IL6 in mice. We have also demonstrated that symptoms of this model were almost completely blocked by administering tocilizumab, a humanized antibody against hIL6R11. These results demonstrate that genetically humanized mice will be powerful tools for directly evaluating in vivo efficacy of not only mAbs but also a wide variety of future therapeutic agents that are highly specific to human target molecules.
Results
Establishing a human IL6R knock-in mouse
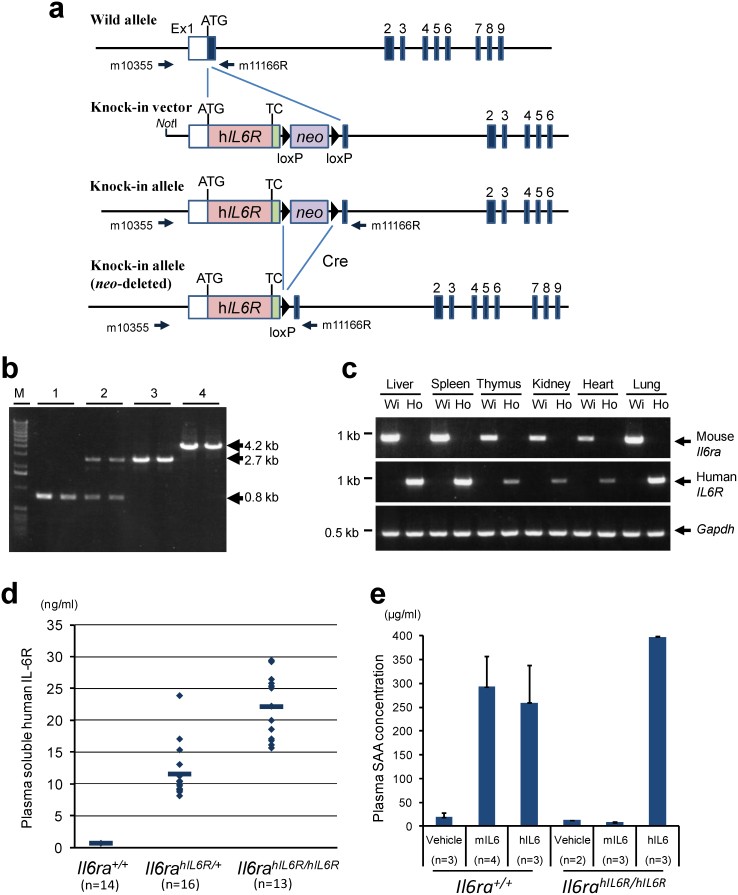
The scheme for generating an hIL6R gene knock-in mouse is presented in Fig. 1a. Correctly targeted ES cell clones with the targeting vector were microinjected into the blastocysts of C57BL/6J (B6) mouse to make chimera mice. Male chimera mice were crossed with B6 females to obtain offspring with the hIL6R knock-in locus. Genomic PCR analysis of the offspring revealed that the full length of hIL6R cDNA with a floxed neomycin resistant gene (neo) cassette was correctly inserted in the target region by homologous recombination, and the knock-in allele was transmitted through the germline. To establish the hIL6R knock-in allele without the neo cassette, the Cre expression plasmid vector was microinjected into the pronuclei of fertilized eggs12 that were obtained by crossing male heterozygous knock-in mice with C57BL/6J females. PCR product, amplified with the primer set depicted in Fig. 1a, reduced the size from 4.2 kb to 2.7 kb; this difference of 1.5 kb indicates the length of the neo cassette excised from the knock-in allele (Fig. 1b). Heterozygous mice without the neo cassette were intercrossed to obtain homozygous knock-in mice. This strain of the hIL6R knock-in mouse has been named B6;129S6-Il6ratm1(hIL6R)Csk. No apparent abnormalities were observed in hIL6R knock-in mice.
Figure 1. Generation of human IL6 receptor (IL6R) gene knock-in mouse.
(a) Schematic representation of the knock-in strategy for the hIL6R gene. A knock-in vector was constructed by inserting hIL6R cDNA with neo cassette flanked by two loxP sites into the mouse Il6ra genomic locus in the frame of a BAC genomic clone. A knock-in allele and a neo-deleted knock-in allele are also shown. Arrows indicate PCR primers (m10355 and m11166R) for genotyping. TC, terminal codon. (b) A representative result of genotyping to confirm the neo-depleted hIL6R knock-in allele and homozygosity of the hIL6R knock-in allele. Wild-type allele and knock-in allele were detected as signals of 0.8 kb and 4.2 kb, respectively, whereas knock-in allele after removing neo cassette was detected as a signal of 2.7 kb. M, DNA molecular marker. Numbers above the gel denote the mouse genotypes, (1) Il6ra+/+, (2) Il6rahIL6R/+, (3) Il6rahIL6R/hIL6R and (4) Il6rahIL6R(neo)/hILR6(neo). (c) Representative results of RT-PCR analysis for tissue distribution of Il6ra+/+ (Wi) and Il6rahIL6R/hIL6R (Ho) mice. (d) Plasma levels of soluble hIL6R in Il6ra+/+ (n = 14), Il6rahIL6R/+ (n = 16) and Il6rahIL6R/hIL6R mice (n = 13). (e) Species-specific ligand response was confirmed after intraperitoneal injection of mouse Il6 (mIL6) or human IL6 (hIL6) in Il6ra+/+ and in Il6rahIL6R/hIL6R mice. Ligand responses were evaluated by the elevation of plasma SAA levels after injection of vehicle (n = 3), mIL6 (n = 4) and hIL6 (n = 3) in Il6ra+/+ and those of vehicle (n = 2), mIL6 (n = 3) and hIL6 (n = 3) in Il6rahIL6R/hIL6R.
The results of RT-PCR for hIL6R or mouse Il6ra cDNA show that each reaction amplified the specific target correctly; that is, in the cDNA samples of homozygous Il6rahIL6R/hIL6R mice, the human-specific IL6R target sequence was exclusively amplified and the mouse Il6ra sequence was not and, in the cDNA samples of wild-type (Il6ra+/+) littermates, the mouse-specific Il6ra sequence was amplified and the hIL6R sequence was not. Signal intensities detected in the same organs were almost similar between hIL6R in Il6rahIL6R/hIL6R mice and mouse Il6ra in Il6ra+/+ mice (Fig. 1c).
Plasma soluble hIL6R in homozygous Il6rahIL6R/hIL6R mice was detected at a range of 15 ng/mL–30 ng/mL (Fig. 1d), which is substantially similar to that reported in human13,14,15. Soluble hIL6R levels in heterozygous Il6rahIL6R/+ mice were at a range of 8 ng/mL–24 ng/mL, about half of those in homozygous Il6rahIL6R/hIL6R mice, which indicates that soluble hIL6R levels in plasma would be dependent on the gene-dosage of knocked-in hIL6R. As determined by the plasma levels of serum amyloid A (SAA), which is produced by the hepatocytes in response to IL616, the hIL6R knock-in mice can respond to human IL6 but not mouse Il6, whereas wild type mice can respond to both human IL6 and mouse Il6 (Fig. 1e).
Establishing a humanized Castleman's disease model mouse
We have crossed the hIL6R knock-in mouse and the H-2Ld-hIL6 transgenic mouse to establish a humanized Castleman's disease mouse model, which is named B6(Cg);129-Il6ratm1(hIL6R)Csk-Tg(IL6)40Csk. Enlargement of systemic lymph nodes and splenomegaly, typical symptoms of Castleman's disease4,5,6, were observed in hIL6 transgenic mice whether their Il6ra gene alleles were wild-type (Il6ra+/+) (Fig. 2b) or humanized (Il6rahIL6R/hIL6R) (Fig. 2c). Histological observation revealed that the number of plasma cells and white pulps were increased in the spleen of both Il6rahIL6R/hIL6R-hIL6 transgenic mice (Table 1, Fig. 3b), as compared to hIL6 non-transgenic control mice (Il6rahIL6R/hIL6R mice), shown in Fig. 3a.
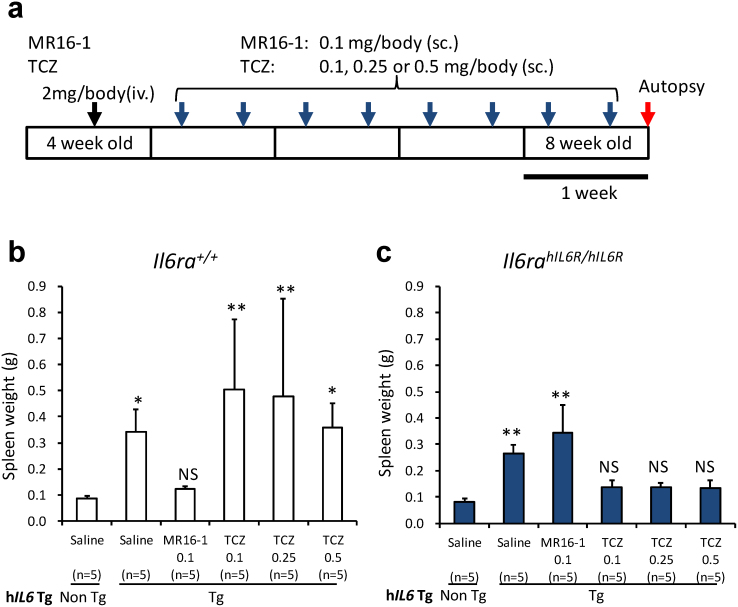
Figure 2. Treatment with an hIL6R-neutralizing antibody in humanized Castleman's disease model mice.
(a) Protocol for 4-week treatment with the anti-mouse Il6ra antibody, MR16-1, or the anti-human IL6R antibody, tocilizumab (TCZ). (iv.), intravenous injection; (sc.) subcutaneous injection. (b) Spleen weights of Il6ra+/+-hIL6 transgenic mice and (c) Il6rahIL6R/hIL6R-hIL6 transgenic mice after 4-week treatment (n = 5 per group). Statistical significances were determined by nonparametric comparisons with control using the Dunn method for joint ranking. The data of each treatment group were compared with those of the respective hIL6 non-transgenic mouse group in each genotype of interleukin-6 receptor, Il6ra+/+ (b) and Il6rahIL6R/hIL6R (c). *, p < 0.05, **, p < 0.01 and NS, not significant. Non Tg, hIL6 non-transgenic mice; Tg, hIL6 transgenic mice.
Table 1. Incidence of histopathological findings of splenic lymphocytes in humanized Castleman's disease model mice with or without tocilizumab treatment.
| Il6rahIL6R/hIL6R | ||||
|---|---|---|---|---|
| Non Tg | hIL6 Tg | hIL6 Tg | ||
| Findings | Severity | Saline | Saline | Treated† |
| *Increased plasma cells | − | 3/3 | 0/3 | 4/5 |
| ± | 0/3 | 2/3 | 1/5 | |
| + | 0/3 | 1/3 | 0/5 | |
| **Increased number of white pulp | − | 3/3 | 0/3 | 1/5 |
| + | 0/3 | 0/3 | 4/5 | |
| ++ | 0/3 | 3/3 | 0/5 | |
Severity of findings: *, increased amount of plasma cells in the marginal zone compared to non-transgenic mice; +, aggregates of plasma cells observed in the marginal zone. ** +, increased incidence of white pulp compared to non-transgenic mice; ++, increased incidence of white pulp with enlargement of the total spleen area.
Non Tg, hIL6 non-transgenic mice; hIL6 Tg, human IL6 transgenic mice.
Numerals indicate the number of animals examined.
†, treated with tocilizumab.
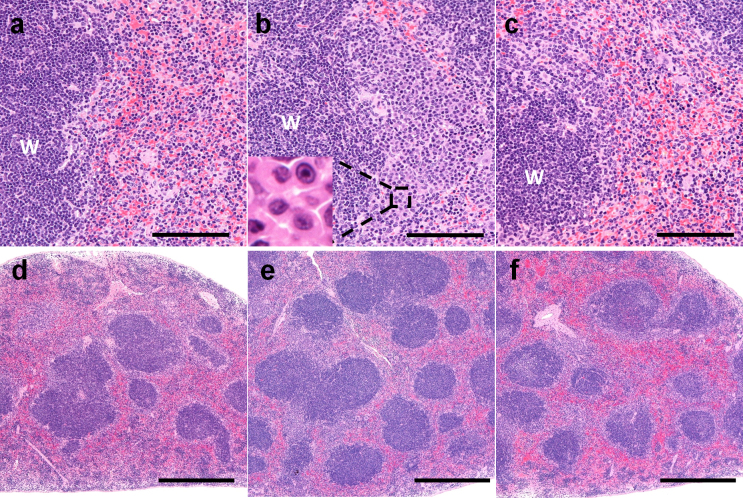
Figure 3. Spleen tissue of an Il6rahIL6R/hIL6R mouse (a, d), an Il6rahIL6R/hIL6R-hIL6 transgenic mouse (b, e), and a tocilizumab-treated Il6rahIL6R/hIL6R-hIL6 transgenic mouse (c, f).
Increase of plasma cells and increased numbers of white pulp in the Il6rahIL6R/hIL6R-hIL6 transgenic mouse (b, e) were ameliorated after 4-week treatment with tocilizumab (c, f). Plasma cells are shown in insert (b). Bars: (a–c), 100 μm; (d–f), 500 μm. W, white pulp.
Treatment with an hIL6R-neutralizing antibody in a humanized Castleman's disease mouse model
We then examined whether this novel humanized Castleman's disease mouse model can be used to evaluate the efficacy of hIL6R-specific therapeutic agents. We treated Il6rahIL6R/hIL6R-hIL6 transgenic mice and Il6ra+/+-hIL6 transgenic mice with tocilizumab and MR16-1 (Fig. 2a). As we previously reported, tocilizumab has a neutralizing activity specifically against hIL6R but not against mouse Il6ra, whereas MR16-1 has a specific neutralizing activity to mouse Il6ra but not to hIL6R17.
The spleen weights (mean ± SD) markedly increased to 0.26 ± 0.03 g in the vehicle-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice. These increased spleen weights were significantly different from those of the hIL6 non-transgenic Il6rahIL6R/hIL6R mice group (0.08 ± 0.01 g). Treatment with tocilizumab markedly prevented the development of splenomegaly in male Il6rahIL6R/hIL6R-hIL6 transgenic mice (Fig. 2c; Supplementary Fig. S1): the spleen weights at the end of 4-week treatment were decreased to 0.14 ± 0.03 g, 0.14 ± 0.02 g and 0.13 ± 0.03 g in groups treated with 0.1, 0.25 and 0.5 mg/body of tocilizumab, respectively. These values were not significantly different to those of the hIL6 non-transgenic Il6rahIL6R/hIL6R mice group. Spleen weights of the group treated with MR16-1, an antibody to mouse Il6ra (0.34 ± 0.11 g) increased to the same level as the vehicle-treated group.
In contrast, in Il6ra+/+-hIL6 transgenic mice tocilizumab treatment does not show preventive effects on the splenomegaly (0.45 ± 0.26 g) observed in the vehicle-treatment group (0.34 ± 0.09 g), whereas MR16-1 markedly prevented splenomegaly at a dose of 0.1 mg/body, with spleen weights decreasing to 0.12 ± 0.01 g. These values were not significantly different to those of hIL6 non-transgenic Il6ra+/+ mice (0.08 ± 0.01 g) (Fig. 2b; Supplementary Fig. S1). The spleen weights also displayed great interindividual variability in Il6ra+/+-hIL6 transgenic mice treated with tocilizumab (Fig. 2b).
An increase in the amount of plasma cells in the marginal zone was observed in all of the saline-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice (Table 1, Fig. 3b) compared to non-transgenic mice (Table 1, Fig. 3a), and aggregates of plasma cells were observed in one of three animals (Table 1, Fig. 3b). Additionally, increased numbers of white pulp was observed in saline-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice (Table 1, Fig. 3e) compared to non-transgenic mice (Table 1, Fig. 3d), which was evidenced by the incidence of white pulp, and this finding was accompanied by enlargement of the total spleen area (Table 1, Fig. 3e). Pathological symptoms of the spleen in tocilizumab-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice were substantially ameliorated upon histological observation at the end of the 4-week treatment (Table 1, Fig. 3, c and f) compared with saline-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice.
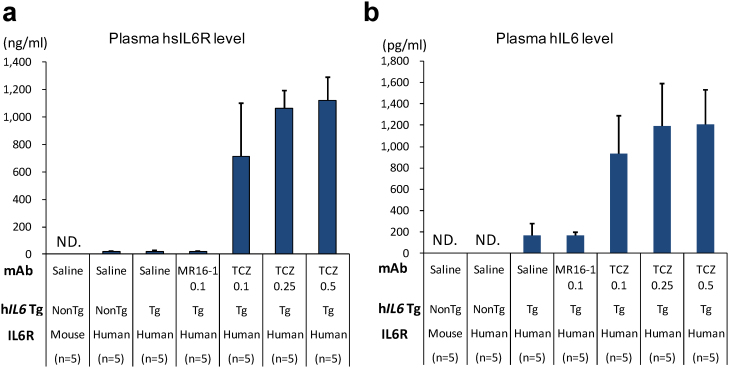
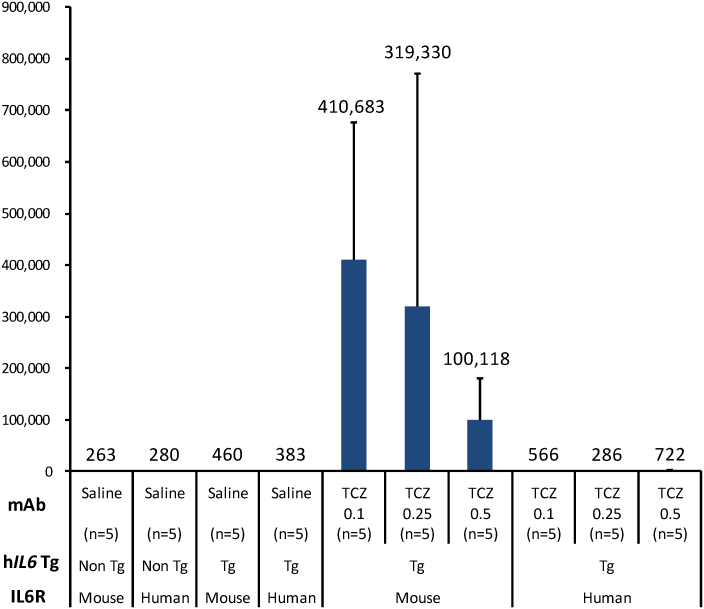
Plasma levels of human soluble IL6R and human IL6 were markedly increased at the end of a 4-week tocilizumab treatment in Il6rahIL6R/hIL6R-hIL6 transgenic mice (Fig. 4, a and b). Antibodies to the drug were minimally detected in Il6rahIL6R/hIL6R-hIL6 transgenic mice even after repeated subcutaneous administration of tocilizumab (Fig. 5).
Figure 4. Plasma levels of soluble hIL6R (a) and hIL6 (b) concentration after 4-week treatment with 0.1, 0.25 and 0.5 mg/body of TCZ in each genotype of mouse (n = 5 per group).
(a) Plasma soluble hIL6R concentrations were approximately 21 ng/ml in saline-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice, whereas marked elevation of plasma soluble hIL6R levels, approximately 40–50 times higher than those of vehicle control, was observed after 4 weeks in TCZ-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice. (b) Plasma hIL6 was detected at the level of 163 pg/ml in saline-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice, whereas the hIL6 levels were markedly elevated to the levels of 936–1204 pg/ml after 4-week treatment of TCZ. ND, not detected. Non Tg, hIL6 non-transgenic mice; Tg, hIL6 transgenic mice.
Figure 5. Titers of plasma anti-drug antibodies after 4-week treatment with 0.1, 0.25 and 0.5 mg/body of TCZ in each genotype of mouse (n = 5 per group).
Extremely high levels of plasma anti-TCZ-antibody titers were detected in Il6ra+/+-hIL6 transgenic mice, whereas those in TCZ-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice were minimally detected. Non Tg, hIL6 non-transgenic mice; Tg, hIL6 transgenic mice.
Discussion
We have established a line of hIL6R knock-in mice, in which endogenous mouse Il6ra gene is successfully replaced by hIL6R cDNA. Results of RT-PCR analysis indicate that tissue distribution of the knocked-in hIL6R expression is well-controlled by endogenous transcription mechanisms (Fig. 1c). Membrane-bound hIL6R expressed on the cell surface in these mice would be normally released to the blood as soluble hIL6R, which lacks the transmembrane and cytoplasmic region18,19, and the plasma levels of soluble hIL6R are revealed to be similar to those reported in Castleman's disease13,14, rheumatoid arthritis patients14 and healthy volunteers14,15. According to our survey, two lines of hIL6R transgenic mice were previously established9,10. Both of them were reported to have higher serum levels of soluble hIL6R than healthy humans. Peters et al. established a line of hIL6R transgenic mice, driven by phosphoenolpyruvate carboxykinase gene promoter, in which serum concentrations of soluble hIL6R were described to range between 4 and 8 μg/mL9,20, several hundred times higher than those in human. Moreover, these hIL6R transgenic mice express only the soluble type of hIL6R, not the membrane-bound type. Another line of hIL6R transgenic mice was established to express the membrane bound type of hIL6R, driven by a strong promoter cassette pCAGGS21, in which serum concentrations of soluble hIL6R were described to range between 80 and 100 ng/mL22, about 4–5 times higher than those in human. Taken together, for the first time we have succeeded in genetically humanizing IL6R in mice to produce blood levels of soluble hIL6R similar to those in human, with plasma concentrations of soluble hIL6R ranging between 15 and 30 ng/mL (Fig. 1d).
No apparent abnormalities were observed in human IL6R knock-in mice, a fact which shows that human IL6R expression does not affect animal health under normal breeding conditions. Histological observation revealed that the spleens of Il6rahIL6R/hIL6R mice did not show any abnormalities (Fig. 3a). Homozygous Il6rahIL6R/hIL6R mice deplete the downstream signal from IL6R because human IL6R cannot bind to mouse endogenous Il619,23, but this lack of IL6 signaling would not have apparent effects on animal health in normal conditions, as described in several reports that either Il6 or Il6ra gene-disrupted mice are viable and have normal appearance24,25,26,27. We have detected elevated plasma SAA levels that confirm the species-specific ligand responses after intraperitoneal injection of mouse Il6 or human IL6 to the hIL6R knock-in mice. Homozygous Il6rahIL6R/hIL6R mice exclusively respond to human IL6 only, and not to mouse Il6 (Fig. 1e). These responses are compatible with the fact that mouse Il6ra can respond to both mouse Il6 and human IL6, whereas hIL6R can respond to human IL6 only19,23. These results strongly suggest that our hIL6R knock-in mouse expresses not mouse endogenous Il6ra, but a functional hIL6R molecule that can transduce the downstream signals normally.
Recently, either albumin-expressing hepatocyte-specific or lysozyme M-expressing macrophage/granulocyte-specific Il6ra gene knockout mice have been established by crossing mice having floxed alleles of Il6ra (Il6rafl/fl) with mice expressing Cre recombinase under the control of albumin (AlbCre) or lysozyme M promoter (LysCre)27. McFarland-Mancini et al. demonstrated that soluble Il6ra level in plasma was more dependent on immune cell secretion than hepatic production by showing that AlbCre+/+/Il6rafl/fl mice had higher levels of soluble Il6ra (67.95% of Cre−/−/Il6rafl/fl) than LysCre+/−/Il6rafl/fl mice (39.95%)27. However, SAA production after challenge with turpentine in AlbCre+/+/Il6rafl/fl mice was severely inhibited, whereas plasma SAA level in LysCre+/−/Il6rafl/fl mice was similar to that of wild-type mice. Consequently, membrane-bound Il6ra on the hepatocytes makes a critical contribution to hepatic SAA production, meaning that trans-signaling by soluble Il6ra may not have a significant role in SAA production. We have demonstrated that Il6rahIL6R/hIL6R mice can respond to exogenous hIL6 to produce SAA, which strongly suggests that Il6rahIL6R/hIL6R mice express intact membrane-bound hIL6R, at least on the hepatocytes.
The Il6rahIL6R/hIL6R-hIL6 transgenic mouse established in this study showed basically typical Castleman's disease symptoms (enlargement of systemic lymph nodes and splenomegaly) similar to those previously reported in Il6ra+/+-hIL6 transgenic mice7,8. Histological observation revealed that the number of white pulps is also increased in Il6rahIL6R/hIL6R-hIL6 transgenic mice. White pulp consists of an accumulation of lymphocytes, mostly B-cells; therefore, these results indicate that the knocked-in hIL6R can respond normally to human IL6 to cause B-cell differentiation and proliferation in white pulp in vivo in the same way as endogenous mouse Il6ra in wild-type mice. Extramedullary hematopoiesis was also observed in the spleen of Il6rahIL6R/hIL6R-hIL6 transgenic mice (data not shown) as previously reported in Il6ra+/+-hIL6 transgenic mice7,8.
We have also examined the therapeutic efficacy of an hIL6R-specific neutralizing antibody on the Castleman's disease-like symptoms in this mouse model. As far as we know, this humanized Castleman's disease mouse model is the first small rodent that can be used to evaluate in vivo efficacy of a therapeutic antibody specific to human IL6R. Our results suggest that sufficient efficacy was observed at a low dose, 0.1 mg/body of tocilizumab, when administered to this mouse model in our dosing scheme. Even when we increased the dose of tocilizumab to 0.25 or 0.5 mg/body, further reduction of spleen weights was not observed; therefore, it may be possible to decrease the dose of tocilizumab further to find the minimal dose level. We would like to define the therapeutic window of tocilizumab, as well as the improved antibodies28 described below, in a future study. Marked elevation of plasma soluble hIL6R and human IL6 levels was also observed in our mouse model similar to that reported by Nishimoto et al. in the patients with Castleman's disease or rheumatoid arthritis that had been treated with tocilizumab14. Nishimoto et al. concluded that it was likely that soluble hIL6R increased because the formation of a tocilizumab/soluble hIL6R immune complex prolonged its elimination half-life, and that free serum IL6 increased because IL6R-mediated consumption of IL6 was inhibited by the lack of tocilizumab-free IL6R14. We consider that increased plasma levels of soluble hIL6R and human IL6 in tocilizumab-treated Il6rahIL6R/hIL6R-hIL6 transgenic mice could be caused by a mechanism similar to that in humans, and that our humanized Castleman's disease model would substantially reflect the clinical outcomes seen in the tocilizumab-treated patients.
Nowadays various technologies for optimizing therapeutic antibodies (in other words, antibody-engineering technologies) have been intensively developed by leading researchers, and improving the pharmacokinetics of these expensive therapeutic antibodies to reduce the dose or dosing frequency will be an increasingly important issue28. It is necessary to determine the therapeutic window, dosing frequency and route of administration while fully understanding the binding affinity to antigen, the pharmacokinetics and the biodistribution of each antibody modified with various sorts of functions28. We propose that our mouse model, expressing a physiological level of hIL6R, will be well-suited for preclinical studies assessing a modified function added to the backbone of new therapeutic antibodies. Our mouse model also has the merit of being smaller in body size than other animal species, such as primates, so smaller amounts of candidate agents would be sufficient for evaluation.
Antibody titers to the drug tocilizumab were only minimally detected, despite the repeated and frequent subcutaneous administration (Fig. 5), so that evaluation of in vivo efficacy of humanized hIL6R-neutralizing antibody was possible after 4-week treatment in this novel Castleman's disease mouse model. Although the cause of these low titer levels remains to be investigated, we are currently considering two possibilities. The first is that tolerance might be successfully induced by relatively higher first dosing (2 mg/body) of humanized antibody intravenously. This possibility is suggested by two recent reports using MR16-1, a rat antibody to mouse Il6ra. Yoshida et al. reported that first intravenous dosing (2 mg/body) inhibited the production of antibodies to MR16-1 after repetitive intraperitoneal or subcutaneous injections of MR16-1 in NZB/NZW F1 mice29. Sakurai et al. also suggested the possibility that tolerance induction would inhibit the production of antibody to drug after finding that antibodies to MR16-1 were detected in some mice treated with 15 mg/kg of MR16-1 intravenously every 3 days but not detected in 50 mg/kg groups30. In our study, however, in Il6ra+/+-hIL6 transgenic mice expressing only mouse Il6ra, the same doses of tocilizumab produced extremely high titers of anti-tocilizumab antibodies, suggesting that there might be some other mechanism than tolerance induction from a first higher dosing. Therefore we would like to propose a second possibility: that IL6 signal blockade by tocilizumab itself might also suppress the production of antibodies to tocilizumab. In Il6ra+/+-hIL6 transgenic mice, which express mouse Il6ra but not human IL6R, tocilizumab cannot inhibit IL6 signaling. Therefore, tocilizumab would be treated as nothing more than a foreign substance, not as a therapeutic agent, and might stimulate systemic inflammation induced by hIL6 as well as a strong immune response. As a result, extremely high titers of antibodies to tocilizumab were detected in tocilizumab-treated Il6ra+/+-hIL6 transgenic mice (Fig. 5). We also speculate that there would be considerable interindividual variability in the exacerbation of systemic inflammatory response, which could cause the large interindividual variation of spleen weights seen in Il6ra+/+-hIL6 transgenic mice (Fig. 2). In summary, at least two mechanisms, namely tolerance induction (with relatively higher first dosing) and IL6 signal blockade, might be necessary to inhibit the production of antibodies to tocilizumab.
IL6 is a multifunctional cytokine that has a wide range of biological activities in various target cells. Therefore not only Castleman's disease and rheumatoid arthritis but many other diseases and disorders, such as multiple myeloma, sepsis, mesangial proliferative glomerulonephritis, and cancer cachexia, may also be associated with IL6 over-production and subsequent uncontrolled IL6 signaling18,19,31. It is predicted that an increasing number of researchers in the future will continue to develop many therapeutic agents molecularly-designed to target hIL6R. Finally, we expect that our mouse model provides a novel system for evaluating in vivo efficacy of the next generation of hIL6R-specific therapeutic agents and also for assessing antibody-engineering technologies to treat patients with Castleman's disease, rheumatoid arthritis, and other diseases caused by abnormalities in IL6 signaling.
Methods
Generation of human IL6R gene knock-in mice
All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co. Ltd.
A line of human IL6R gene knock-in mice was established basically by the protocol we reported previously12,32,33,34. The methods are briefly described as follows. The targeting vector, constructed by the seamless insertion of human IL6R gene cDNA (GenBank # NM_000565) into the mouse Il6ra genomic locus on the BAC clone with pRed/ET system (Quick and Easy BAC Modification kit, GeneBridges GmbH, Heidelberg) as shown in Fig. 1a, was introduced by electroporation to the 129/SvEv mouse ES cells. The ES cells were selected in a culture medium containing G418. Homologous recombinant ES cell clones were injected into C57BL/6J (B6) mouse (CLEA Japan, Inc., Tokyo) blastocysts to produce chimera mice. Chimera mice were bred with B6 females to generate offspring. After confirmation of germline transmission, neo gene cassette was removed from the knock-in allele by pronuclear microinjection of the Cre recombinase expression vector12. Removal of the neo gene cassette was confirmed by PCR using the primers m10355 (5′-TCTGCAGTAGCCTTCAAAGAGC-3′) and m11166R (5′-AACCAGACAGTGTCACATTCC-3′). Neo-deleted allele was determined at 2.7 kb, whereas neo-intact allele and wild-type allele were detected at 4.2 kb and 0.8 kb, respectively (Fig. 1b). Heterozygous mice were intercrossed to produce homozygous mice.
RT-PCR analysis was performed to determine tissue distribution of human and mouse IL-6R expression. Total RNA samples extracted from tissue samples with Isogen reagents (Nippon Gene Co. Ltd., Tokyo, Japan) were reverse-transcribed with SuperScript III reverse-transcriptase (Invitrogen) to synthesize cDNA. PCRs were performed with a common forward primer 6RIK-s1 (5′-CCCGGCTGCGGAGCCGCTCTGC -3′) set in 5′ untranslated region and species-specific reverse primers in the coding sequences: 6RLIcA2 (5′-AGCAACACCGTGAACTCCTTTG-3′) for mouse Il6ra and RLI6-a1 (5′-ACAGTGATGCTGGAGGTCCTT-3′) for human IL6R, respectively. Serum concentrations of soluble-type receptors were determined as described below.
Species-specific ligand-receptor reaction was examined by plasma levels of SAA after intraperitoneal injection of human and mouse IL-6. In this experiment Il6rahIL6R/hIL6R mice and Il6ra+/+ mice were used. Plasma SAA levels were determined by the commercially available ELISA kit (Invitrogen), according to the manufacturer's protocol.
Establishment of humanized Castleman's disease model mice
The hIL6R knock-in mice were crossed with the H-2Ld-hIL6 transgenic mice to establish double transgenic mice, that is, Il6rahIL6R/hIL6R-hIL6 transgenic mice, which have homozygous alleles for the knocked-in hIL6R and the H-2Ld-hIL6 transgene. These mice were maintained under specific pathogen-free conditions and fed standard laboratory chow (CE-2, CLEA Japan, Inc.) ad libitum. Genotypes for these mice were determined by PCRs for knocked-in hIL6R allele and H-2Ld-hIL6 transgene. Genotyping for knocked-in hIL6R allele was performed by the PCR analysis mentioned above. H-2Ld-hIL6 transgene was detected by PCR with the forward primer (5′- ACCTCTTCAGAACGAATTGACAAA -3′) and the reverse primer (5′- AGCTGCGCAGAATGAGATGAGTTGT -3′). After an initial denaturation at 94 degrees C for 4 min, 35 cycles of 94 degrees C for 30 sec, 65 degrees C for 30 sec and 72 degrees C for 30 sec were run with TaKaRa Ex Taq (TaKaRa). H-2Ld-hIL6 transgene is detected as a signal at approximately 450 bp.
Treatment with an hIL6R-neutralizing antibody in humanized Castleman's disease model mice
These humanized Castleman's disease model mice were injected with 2 mg/body of humanized mAb to human IL6R (tocilizumab), rat mAb to mouse Il6ra (MR16-1) or physiological saline used for vehicle, intravenously, once at 4 weeks of age. Then from the week after the first injection, mice were given 0.1, 0.25 or 0.5 mg/body of tocilizumab or 0.1 mg/body of MR16-1 subcutaneously twice weekly. In the treatment regimen, first dosing was set at a relatively high amount to attempt to induce tolerance in mice to mAbs originating from other species29. Under isoflurane anesthesia, whole blood samples were collected from the inferior vena cava. Spleens were removed, weighed and fixed with 10% neutral buffered formalin for histological examination. Il6ra+/+-hIL6 transgenic mice, previously reported by Katsume et al. as a Castleman's disease model8, were used as a disease control. Additionally Il6ra+/+ and Il6rahIL6R/hIL6R mice were used for healthy control.
Soluble human and mouse IL-6R-specific ELISA
The plasma levels of soluble human and mouse IL-6R were determined by using a commercially available kit (R&D Systems) according to the manufacturer's protocols.
Human and mouse IL-6-specific ELISA
The blood levels of human and mouse IL-6 were determined using a commercially available IL-6-specific ELISA kit (Invitrogen) according to the manufacturer's instruction.
Measurement of antibody titer to drug
Plasma samples were incubated with biotin-labeled tocilizumab and SULFO-TAG-labeled tocilizumab overnight. These mixtures were placed in the wells of MSD SA plates and incubated for 2 hours. After washing and addition of the read buffer, chemiluminescence was determined immediately by SECTOR PR 400 (Meso Scale Discovery, Maryland).
Statistical analysis
Statistical analysis was performed using JMP 9.02 (SAS Institute Japan, Tokyo, Japan). Statistical significance in spleen weights was determined by nonparametric comparisons with control using Dunn method for joint ranking. P < 0.05 was regarded as statistically significant.
Author Contributions
K.J. and O.U. conceived and designed the experiments and coordinated the work presented. O.U., H.T., Y.H., E.F., A.K., Y.K., N.A.W., T.T., M.Kakefuda, C.G. and M.Kawaharada performed the experiments. S.S. and K.H. commented on the manuscript. O.U. and K.J. wrote the manuscript.
Supplementary Material
Supplementary Figure S1
Acknowledgments
We wish to thank Dr. A. Kawamura for critical discussion, Dr. J. Amano for giving biotin-labeled TCZ and MR16-1, Dr. T. Igawa for giving hIL6R, Dr. K. Kamei for important suggestions for determining anti-drug antibody, Mr. A. Takakura for measuring plasma soluble hIL6R, Mr. K. Matsumoto and Mr. K. Satoh for reproduction of the humanized Castleman's disease mouse model, and Dr. S. Shiota, Mrs. S. Uchida and Mrs. Y. Nakajima for skillful technical assistance. We also thank Mrs. S. Matsuura and Editing Services at Chugai Pharmaceutical Co., Ltd. for reviewing the manuscript.
References
- Loisel S. et al. Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment. Crit Rev Oncol Hematol. 62, 34–42 (2007). [DOI] [PubMed] [Google Scholar]
- Chapman K., Pullen N., Graham M. & Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nature Rev. Drud Discov. 6, 120–126 (2007). [DOI] [PubMed] [Google Scholar]
- Hansel T. T., Kropshofer H., Singer T., Mitchell J. A. & George A. J. The safety and side effects of monoclonal antibodies. Nature Rev. Drug Discov. 9, 325–338 (2010). [DOI] [PubMed] [Google Scholar]
- Palestro G., Turrini F., Pagano M. & Chiusa L. Castleman's disease. Adv. Clin. Path. 3, 11–22 (1999). [PubMed] [Google Scholar]
- Cronin D. M. P. & Warnke R. A. Castleman disease: an update on classification and the spectrum of associated lesions. Adv. Anat. Pathol. 16, 236–246 (2009). [DOI] [PubMed] [Google Scholar]
- Yoshizaki K. et al. Pathogenic significance of interleukine-6 (IL-6/BSF-2) in Castleman's disease. Blood. 74, 1360–1367 (1989). [PubMed] [Google Scholar]
- Kitamura H. et al. Bone marrow neutrophilia and suppressed bone turnover in human interleukin-6 transgenic mice. Am. J. Pathol. 147, 1682–1692 (1995). [PMC free article] [PubMed] [Google Scholar]
- Katsume A. et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman's disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 20, 304–311 (2002). [DOI] [PubMed] [Google Scholar]
- Peters M. et al. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 183, 1399–1406 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N. et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 182, 1461–1468 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 53, 851–856 (1993). [PubMed] [Google Scholar]
- Jishage K. et al. Role of Lkb1, the causative gene of Peutz-Jegher's syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci U S A. 99, 8903–8908 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 106, 2627–2632 (2005). [DOI] [PubMed] [Google Scholar]
- Nishimoto N. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 112, 3959–3969 (2008). [DOI] [PubMed] [Google Scholar]
- Galicia J. C. et al. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 5, 513–516 (2004). [DOI] [PubMed] [Google Scholar]
- Jensen L. E. & Whitehead A. S. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem. J. 334, 489–503 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki M., Yamada Y., Nishimoto N., Yoshizaki K. & Mihara M. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett. 84, 231–240 (2002). [DOI] [PubMed] [Google Scholar]
- Jones S. A., Horiuchi S., Topley N., Yamamoto N. & Fuller G. M. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 15, 43–58 (2001). [DOI] [PubMed] [Google Scholar]
- Kallen K. J. The role of transsignaling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 1592, 323–343 (2002). [DOI] [PubMed] [Google Scholar]
- Peters M. et al. Extramedullary expansion of hematopoietic progenitor cells in interleukin (IL)-6-sIL-6R double transgenic mice. J Exp Med. 185, 755–766 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Yamamura K. & Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 108, 193–199 (1991). [DOI] [PubMed] [Google Scholar]
- Horita H., Yoshida K., Kishimoto T. & Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A. 92, 4862–4866 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. et al. Identification of two novel regions of human IL-6 responsible for receptor binding and signal transduction. J Immunol. 153, 1744–1753 (1994). [PubMed] [Google Scholar]
- Poli V. et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 13, 1189–1196 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich F. T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010). [DOI] [PubMed] [Google Scholar]
- Jones G. W. et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol. 184, 2130–2139 (2010). [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini M. M. et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 184, 7219–7228 (2010). [DOI] [PubMed] [Google Scholar]
- Igawa T. et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 28, 1203–1207 (2010). [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hashizume M., Suzuki M. & Mihara M. Induction of high-dose tolerance to the rat anti-mouse IL-6 receptor antibody in NZB/NZW F1 mice. Rheumatol Int. 31, 1445–1449 (2011). [DOI] [PubMed] [Google Scholar]
- Sakurai T. et al. The Effects of Interleukin-6 Signal Blockade on Fertility, Embryo-fetal Development, and Immunization In vivo. Birth Defects Res B Dev Reprod Toxicol. 95, 304–317 (2012). [DOI] [PubMed] [Google Scholar]
- Nishimoto N. & Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2, 619–626 (2006). [DOI] [PubMed] [Google Scholar]
- Kurihara Y. et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368, 703–710 (1994). [DOI] [PubMed] [Google Scholar]
- Suzuki H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 (1997). [DOI] [PubMed] [Google Scholar]
- Ohi A. et al. Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b+/− mice. Am J Physiol Renal Physiol. 301, F1105–1113 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1