Abstract
Amacrine cells represent the most diverse class of retinal neuron, comprising dozens of distinct cell types. Each type exhibits a unique morphology and generates specific visual computations through its synapses with a subset of excitatory interneurons (bipolar cells), other amacrine cells, and output neurons (ganglion cells). Here, we review the intrinsic and network properties that underlie the function of the most common amacrine cell in the mammalian retina, the AII amacrine cell. The AII connects rod and cone photoreceptor pathways, forming an essential link in the circuit for rod-mediated (scotopic) vision. As such, the AII has become known as the rod-amacrine cell. We, however, now understand that AII function extends to cone-mediated (photopic) vision, and AII function in scotopic and photopic conditions utilizes the same underlying circuit: AIIs are electrically coupled to each other and to the terminals of some types of ON cone bipolar cells. The direction of signal flow, however, varies with illumination. Under photopic conditions, the AII network constitutes a cross-over inhibition pathway that allows ON signals to inhibit OFF ganglion cells and contributes to motion sensitivity in certain ganglion cell types. We discuss how the AII’s combination of intrinsic and network properties accounts for its unique role in visual processing.
Keywords: A2 amacrine cell, gap junction, glycinergic synapse, mouse, guinea pig, primate
The rod bipolar cell pathway in the mammalian retina
Psychophysical experiments in human subjects (Stiles, 1959; Alpern, 1965) and electrophysiological recordings from mammalian ganglion cells (Barlow et al., 1957; Gouras & Link, 1966) demonstrated that threshold responses during rod- and cone-mediated vision are independent. This independence suggested that the outputs of the two photoreceptor types are conveyed to ganglion cells by distinct neural pathways. Such an arrangement was confirmed by the identification of rod- and cone-specific bipolar cells (Dowling & Boycott, 1966; Boycott & Dowling, 1969; Kolb, 1970; Boycott & Kolb, 1973). Though it was assumed initially that both rod and cone bipolar cells contacted ganglion cells directly (Boycott & Dowling, 1969), subsequent electron-microscopic (EM) analysis revealed that rod bipolar cells do not make synapses with ganglion cells; rather, rod bipolars provide synaptic input to a narrow-field, bistratified amacrine called the AII amacrine cell, which is the most common amacrine cell in the retina (Kolb & Famiglietti, 1974; Famiglietti & Kolb, 1975; Strettoi & Masland, 1996).
Today, the so-called rod bipolar cell pathway of the mammalian retina is well-described (Figure 1) (Bloomfield & Dacheux, 2001): rods make synapses onto rod bipolar cells, which depolarize in response to light. Rod bipolar cells make excitatory dyad synapses with two postsynaptic elements from separate neurons: AII and A17 amacrine cells (Famiglietti & Kolb, 1975; Dacheux & Raviola, 1986; Raviola & Dacheux, 1987). The A17 returns a GABAergic feedback synapse onto the rod bipolar terminal (Kolb & Nelson, 1983; Dacheux & Raviola, 1986; Raviola & Dacheux, 1987; Protti & Llano, 1998; Hartveit, 1999; Zhang et al., 2002; Singer & Diamond, 2003; Chavez et al., 2006). The AII, which is depolarized by input from the rod bipolar cell, is coupled by gap junctions to other AIIs and to the axon terminals of ON cone bipolar cells (Famiglietti & Kolb, 1975; Strettoi et al., 1992; Strettoi et al., 1994; Mills & Massey, 1995; Veruki & Hartveit, 2002b, a; Petrides & Trexler, 2008). The AII also makes inhibitory synapses onto the terminals of some OFF cone bipolar cells and onto the dendrites of some OFF ganglion cells (Pourcho & Goebel, 1985; Sterling et al., 1988; Strettoi et al., 1992; Tsukamoto et al., 2001; Manookin et al., 2008; Murphy & Rieke, 2008; Münch et al., 2009). Thus, the AII distributes the rod signal to multiple parallel cone bipolar pathways. In effect, then, the rod bipolar pathway “piggybacks” on the cone pathways (Strettoi et al., 1992). This arrangement likely arose to integrate rods, which are evolutionarily newer than cones, into existing retinal circuitry (Lamb, 2009).
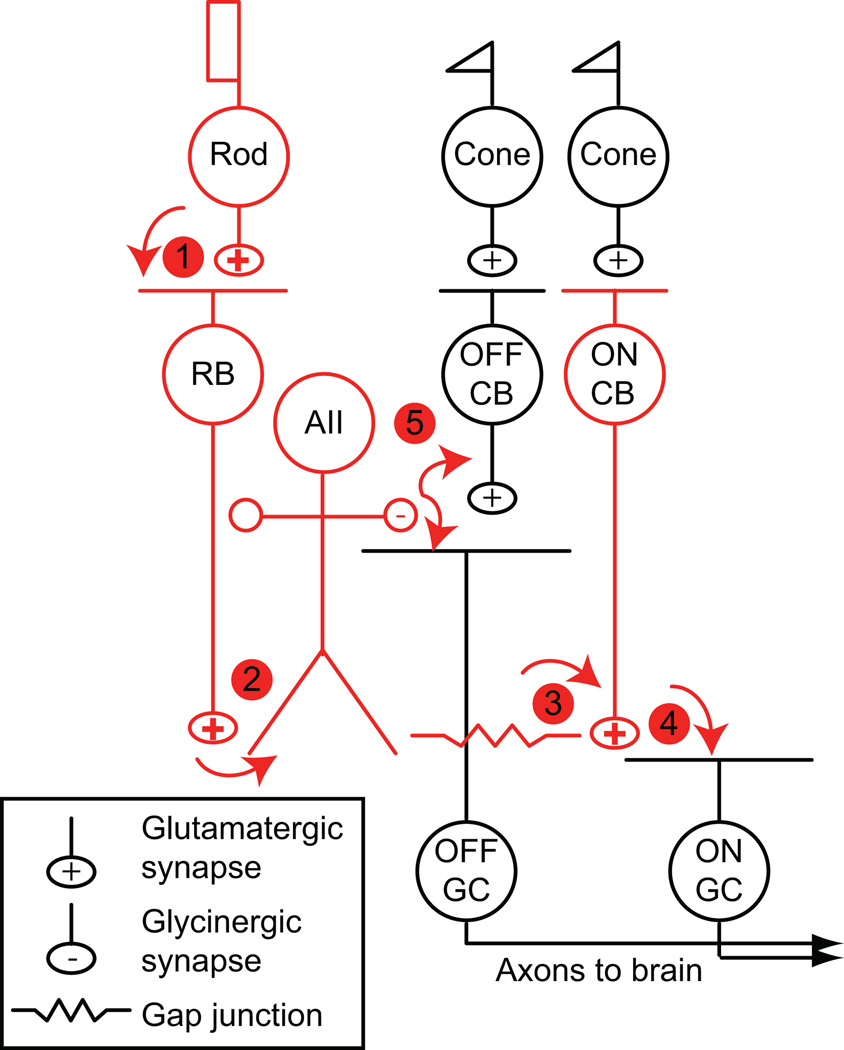
Figure 1. The rod bipolar pathway of the mammalian retina.
Principal neurons are colored red and synapses are numbered. Cones make synapses with two classes of cone bipolar cell (CB): ON and OFF CBs. ON and OFF CBs make excitatory synapses with ganglion cells (GCs), the output cells of the retina.
(1) Rods make synapses with an ON bipolar cell, the rod bipolar (RB), and with a subset of OFF CBs (not shown). Gap junctions between rods and cones are not illustrated (Raviola & Gilula, 1973; DeVries & Baylor, 1995).
(2) RBs contact AII amacrine cells.
(3) AIIs are coupled by gap junctions to some types of ON CBs and to other AIIs.
(4) ON CBs make synapses with ON GCs.
(5) AIIs make inhibitory glycinergic synapses with some OFF CBs and OFF GCs.
Anatomy underlies the sensitivity of the rod bipolar pathway
Signaling in the rod bipolar cell pathway is remarkably sensitive and reliable. The circuit is capable of encoding a rod’s response to a single absorbed photon: the firing of a ganglion cell can be modulated by the absorption of a single photon within its receptive field, and the absorption of ~10 photons within the retina as a whole can generate a visual percept (Hecht et al., 1942; Barlow et al., 1971; Sakitt, 1972; Mastronarde, 1983; Field et al., 2005). The sensitivity of ganglion cells to light arises from the convergence of thousands of rod outputs onto single ganglion cells: each rod bipolar cell pools the output of 20–100 rods, each AII receives input from 10–20 rod bipolars and makes electrical synapses with ~5 ON cone bipolar terminals (numbers vary with species and retinal location) (Dacheux & Raviola, 1986; Sterling et al., 1988; Vaney et al., 1991; Young & Vaney, 1991; Strettoi et al., 1992; Tsukamoto et al., 2001). Additionally, the divergence of a rod’s output to multiple neurons (1 rod → 2 rod bipolars → 5 AIIs → 8 ON cone bipolars) may increase the reliability with which the rod signal is transferred through the retina (Sterling et al., 1988; Vaney et al., 1991; Young & Vaney, 1991; Tsukamoto et al., 2001). As noted bySterling et al. (1988), divergence generates multiple copies of a rod’s signal that are transferred to a single ganglion cell by hundreds of ON cone bipolar cell synapses.
AIIs themselves are remarkably sensitive to light: in the dark-adapted retina, AIIs respond reliably to dim flashes of light sufficient to isomerize single rhodopsin molecules in fewer than 1 in 3 to 1 in 100 rods (varying by species) (Pang et al., 2004; Trexler et al., 2005; Dunn et al., 2006). This sensitivity arises in part from the convergence of hundreds of rod outputs onto a single AII. Furthermore, electrical coupling between AIIs enhances the effective convergence of rods onto AIIs by permitting AIIs to be depolarized by rod bipolars that are not directly presynaptic (Sterling et al., 1988; Bloomfield et al., 1997; Pang et al., 2004).
Gap junction-mediated coupling between AIIs can be demonstrated with the diffusion of tracer molecules. For example, Neurobiotin injected into a single AII spreads to neighboring AIIs, demonstrating cytoplasmic coupling (Vaney, 1991; Mills & Massey, 1995); this coupling, studied by light microscopy, confirms EM evidence for gap junctions between AII dendrites and somata (Kolb & Famiglietti, 1974; Famiglietti & Kolb, 1975; Strettoi et al., 1992; Strettoi et al., 1994; Vardi & Smith, 1996). These gap junctions are homomeric protein complexes composed of connexin (Cx) 36 (Feigenspan et al., 2001; Mills et al., 2001; Deans et al., 2002). The first evidence for functional electrical coupling between AIIs came from the work of Bloomfield et al. (Bloomfield et al., 1997), who demonstrated that light-mediated modulation of receptive field size varied directly with light-mediated modulation of tracer coupling. Later, Veruki and Hartveit (Veruki & Hartveit, 2002a) recorded direct electrical coupling between pairs of AIIs in a retinal slice preparation [see also (Tian et al., 2010)].
Gap junctions and signal processing
Though the convergence within the rod bipolar pathway in part underlies its sensitivity, convergence also increases the noise of neural responses by the square root of the number of inputs (Baylor et al., 1984; Sterling et al., 1988; Smith & Vardi, 1995). Gap junctions, by acting as low-pass filters, can average uncorrelated noise and improve the signal-to-noise ratio (S/N) in instances in which coupled cells receive correlated input (Lamb & Simon, 1976; Tessier-Lavigne & Attwell, 1988). Thus, it is thought that the AII network serves a noise-reducing role in rod-mediated vision.
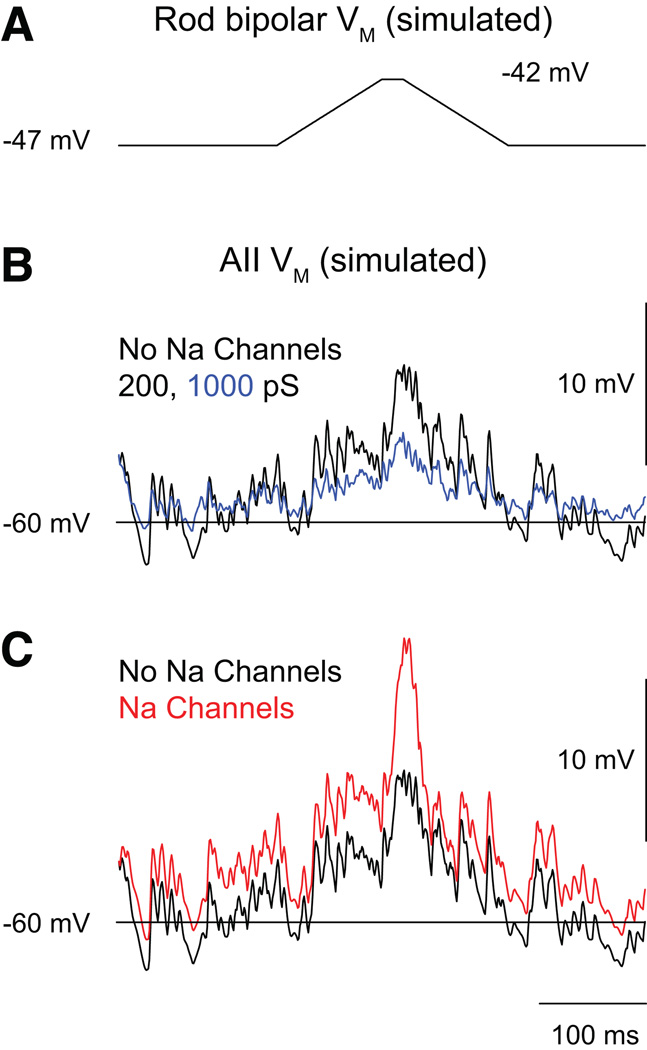
A computational model of the AII network suggested that the network reduces noise in the rod bipolar pathway by filtering noise arising from spontaneous release at rod bipolar cell synapses and amplifying selectively light-evoked rod bipolar cell outputs that diverge to multiple coupled AIIs (Smith & Vardi, 1995). When the AIIs in the model lacked voltage-gated conductances (i.e., when they were passive neurons), their simulated responses to single photons could be differentiated from noise throughout a range of junctional coupling strength. Inclusion of voltage-gated conductances within the AIIs [noted first by (Boos et al., 1993); also see (Tian et al., 2010)] improved considerably the S/N of their simulated responses. Thus, the model indicated that a coupled network of AII amacrine cells with voltage-gated conductances might provide a mechanism for non-linear amplification of small, correlated signals during rod vision (Figure 2) (Smith & Vardi, 1995).
Figure 2. A computational model of the AII network predicts effects of coupling strength and Na channel expression on postsynaptic responses to divergent rod bipolar output.
(A) The voltage change in a single rod bipolar cell elicited by a “single-photon” response. The cell’s synaptic output diverges to five coupled AII amacrines. (B) the postsynaptic responses elicited when coupling strength (black = junctional conductance of 200 pS; blue = junctional conductance of 1000 pS) is altered. (C) the postsynaptic responses elicited when Na channel expression (black = no Na channels; red = Na channels present; junctional conductance = 200 pS) is varied. It is evident that the postsynaptic response is larger when the coupling conductance is low and Na channels are present. Figure modified from (Smith and Vardi, 1995).
Experimental evidence has supported the first point made by the aforementioned study, that coupling within the AII network improves the sensitivity of AIIs: measured light-independent (i.e., background) noise was higher, and the S/N of light responses lower, in AIIs from mice that lack AII-AII gap junctions (Cx36 −/− mice) relative to wild-type mice (Dunn et al., 2006). Further, it is worth noting that direct measurements from AIIs indicated that the coupling conductances used in the modeling study were within the physiological range (albeit on the low end) (Veruki & Hartveit, 2002a; Veruki et al., 2008).
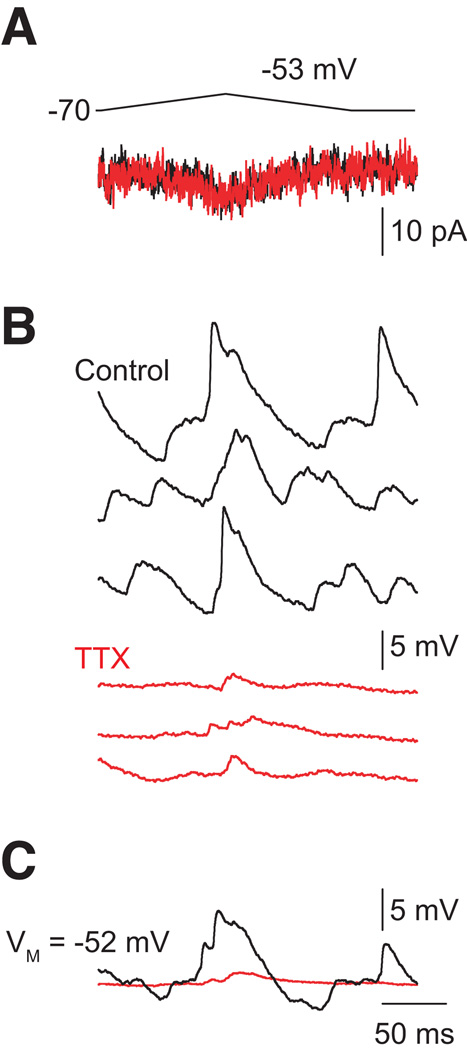
Experiments, however, have not provided evidence for active amplification of correlated light-evoked signals by AIIs. In a light-adapted retinal slice preparation,Tian et al. (2010) found that voltage-gated Na channels can accelerate and, under some circumstances, boost the amplitudes of EPSPs recorded in AIIs and evoked by depolarizing single rod bipolar cells (Figure 3). But, in the same study, blocking voltage-gated Na channels with TTX did not reduce the amplitudes of light-evoked currents recorded in ganglion cells (though the light-evoked currents were slowed considerably). Thus, Na channels may shape the time course of signal transfer through the AII network but apparently do not influence the S/N of such signals. Indeed, Dunn et al. demonstrated that the gain of dim light-evoked AII voltage and current responses (current- and voltage-clamp recordings, respectively) scaled identically with background, indicating that postsynaptic amplification of RB output, at least at the level of individual AIIs, does not play a role in increasing S/N (Dunn et al., 2006).
Figure 3. Representative AII responses to rod bipolar output.
Minimal presynaptic depolarization (to −53 mV at left) elicits presynaptic Ca currents (A) and EPSPs (B: individual responses; C: averaged responses). EPSPs evoked by minimal stimulation are reduced substantially by TTX. TTX has no effect on the presynaptic Ca current. TTX also eliminates fluctuations in VM. Figure modified from (Tian et al., 2010).
Modulation of coupling
AII-AII coupling is modulated by background light. In the dark-adapted condition, the AII’s receptive field is small (~50 µm, 2–3 times the diameter of its dendritic tree) (Bloomfield et al., 1997; Xin & Bloomfield, 1999; Bloomfield & Xin, 2000). The receptive field size increases (up to several hundred µm) with background illumination through the scotopic regime before the size diminishes again at photopic intensities (Bloomfield et al., 1997). Parallel changes in tracer coupling between AIIs are observed with changes in background illumination. Hence, the dependence of receptive field size on background apparently reflects a background-dependent change in electrical coupling between AIIs, which has been postulated to gate signal transfer between the rod and cone pathways (Bloomfield et al., 1997). The reduced coupling between AIIs at photopic intensities is thought to limit the lateral spread of neural signals in the inner retina, thereby improving the spatial acuity of bipolar, amacrine and ganglion cell receptive fields.
Light is thought to affect AII-AII coupling by modulating the release of the neuromodulator dopamine (DA) from dopaminergic amacrine cells (Witkovsky, 2004). Although it was observed that DA, acting through D1 receptors (D1Rs), reduces tracer coupling between AIIs (Hampson et al., 1992), the mechanism by which this occurs is uncertain. It is well-known that activation of D1Rs induces cAMP production by activating PKA (Schorderet & Nowak, 1990; Neve et al., 2004), and predictably, direct activation of PKA and application of membrane-permeant cAMP analogs mimic the DA effect (Mills & Massey, 1995; Xia & Mills, 2004; Urschel et al., 2006; Kothmann et al., 2009). Additionally, the DA- and cAMP- regulated phosphoprotein 32 kDa (DARPP-32) is expressed by AIIs (Partida et al., 2004; Witkovsky et al., 2007). DARPP-32 is phosphorylated at multiple sites by a number of signaling pathways that are activated by D1R activation, and DARPP-32 phosphorylation can either promote or inhibit phosphorylation of other proteins by modulating the activity of PP1 and PKA (Svenningsson et al., 2004).
Initial studies suggested that DA does not modulate AII-ON cone bipolar tracer coupling (Mills & Massey, 1995), which appears to be mediated by heteromeric Cx36 / Cx45 gap junctions (Han & Massey, 2005; Maxeiner et al., 2005; Dedek et al., 2006). Further quantitative analysis indicated that tracer coupling between these two cell types indeed is suppressed by DA, and that the strength of AII-ON cone bipolar coupling should therefore be reduced in daylight (Xia & Mills, 2004). Physiological evidence, however, suggests that the AII-ON cone bipolar gap junction continues to function as an electrical synapse in the light-adapted retina (Xin and Bloomfield, 1999; Manookin et al., 2008; Münch et al., 2009). Therefore, it will be necessary to measure directly whether the conductance of the AII-ON cone bipolar gap junction in the intact retina is modulated by light.
Two competing and conflicting hypotheses have been advanced to link D1R activation to the modulation of gap junctions: 1) PKA induced phosphorylation of Cx36, likely at serines 110 and 293, decreases gap junction conductance (Urschel et al., 2006), and 2) DA-stimulated dephosphorylation of Cx36 at serine-293 decreases gap junction conductance (Kothmann et al., 2009). This dephosphorylation is mediated by the activation of protein phosphatase 2A (PP2A) activation by PKA; this signaling pathway is likely complex, as PP2A also dephosphorylates DARPP-32 at Thr75, thereby promoting inhibition of PKA (Svenningsson et al., 2004).
Adding to the uncertainty surrounding the relationship between D1R activation and the modulation of AII-AII coupling is the lack of evidence for D1R expression by AIIs: antibody labeling finds no D1R in AIIs (Veruki & Wässle, 1996) [but see (Nguyen-Legros et al., 1997)], and AIIs do not express GFP (green fluorescent protein) in BAC (bacterial artificial chromosome) transgenic mice in which the D1R promoter drives GFP expression (J. Singer, unpublished observations). Further, direct application of either dopamine or D1R agonists and antagonists does not affect junctional conductances recorded in pairs of AIIs in an in vitro retinal slice preparation (J. Singer, unpublished observations). Thus, additional work is required to elucidate the localization and function of D1Rs as they relate to modulation of AII-AII coupling.
Selective network connections: ON cone bipolar cells
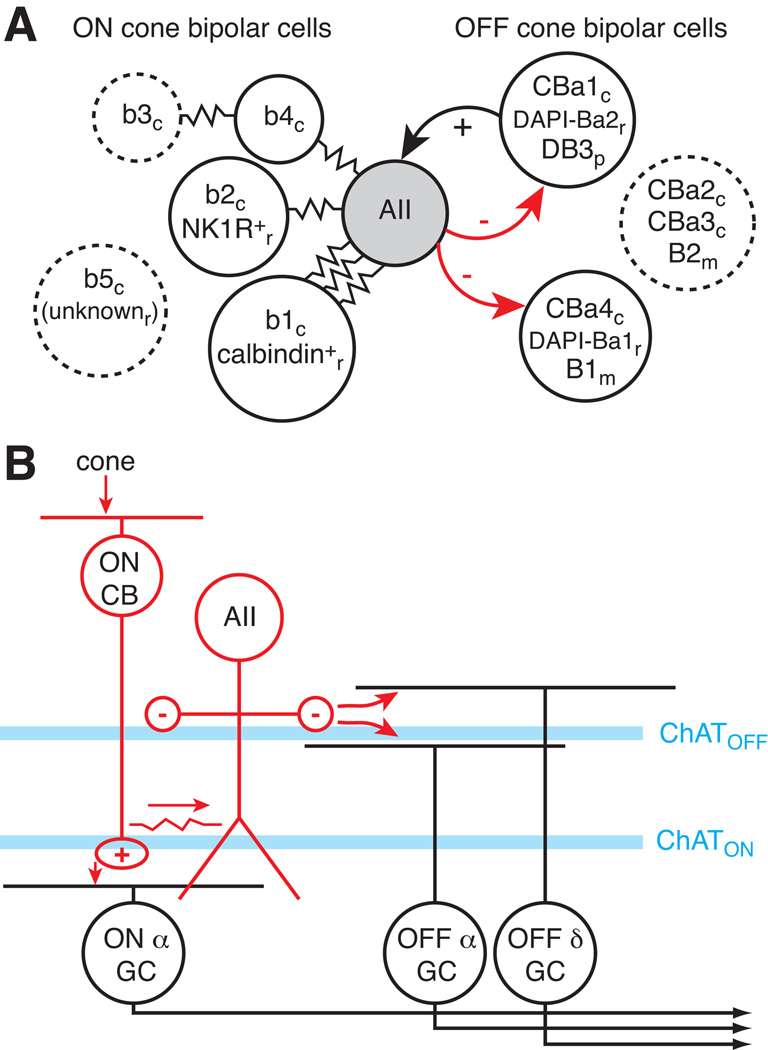
The AII’s dendrites span the entire depth of the inner plexiform layer enabling potential contact with numerous types of bipolar, amacrine and ganglion cell. Only a subset of each cell class, however, actually connects with the AII. High resolution analysis of the cat retina by serial EM revealed that the AII avoids making gap junctions with two types of ON cone bipolar cell (b3, b5) and forms unequal numbers of gap junctions with the remaining types (McGuire et al., 1984; Cohen & Sterling, 1990). The AII made a large number of gap junctions with the b1 type and 7–8-fold fewer contacts (based on surface area) with b2 and b4 types. Furthermore, the b3 type, which lacked a direct connection, was connected effectively to the AII through b3-b4 gap junctions (Cohen & Sterling, 1990) (Figure 4A).
Figure 4. Selective connections of the AII amacrine cell.
(A) Schematic diagram indicates the gap junction coupling (resistor symbols) for different bipolar cell types. In most cases, connections were identified by EM and cell type names are from the original studies (see text). Species are indicated by subscript letter (c=cat; r=rabbit; m=mouse; p=primate). Cell types that were not directly connected to the AII are outlined with a dashed line.
(B) Photopic circuit for AII signaling. When the rod bipolar cell (not shown) saturates in bright light, cones stimulate ON cone bipolar cells, which excite the AII through the gap junction. The AII can then inhibit, via a glycine synapse, two OFF ganglion cell types: Alpha and Delta, which stratify on different sides of the cholinergic band (immunopositive for choline acetyltransferase; ChAT) in the outer half of the inner plexiform layer (i.e., the OFF ChAT layer). The AII could also inhibit certain types of OFF cone bipolar cell (not shown).
Complementary light-microscopic studies have been performed in the rabbit retina using tracer coupling and anti-glycine immunohistochemistry. Glycine labeling implies coupling because glycine only enters ON cone bipolar cells through their gap junctions with AIIs (i.e., the bipolar cells do not express glycine transporters; Vaney et al., 1998). These experiments suggested that one (unknown) bipolar type lacked any connection to the AII, while one of the remaining bipolar types (calbindin-immunoreactive; calbindin+) showed a dominant connection (Petrides & Trexler, 2008; Massey & Mills, 1999). EM analysis confirmed that the calbindin+ type makes numerous gap junctions with AIIs; whereas another type (neurokinin 1 receptor-immunopositive; NK1R+) makes fewer junctions (Massey & Mills, 1999; Kim et al., 2005; 2010). An electrophysiological assessment of AII-ON cone bipolar coupling in rat retina found that four types of ON cone bipolar cell were coupled to AIIs, but it was not possible to rank-order the connection strengths by cell type (Veruki & Hartveit, 2002b).
The ON cone bipolar cell with the strongest connection to the AII is that type presynaptic to the ON Alpha ganglion cell: the b1 type in cat (Freed & Sterling, 1988; Cohen & Sterling, 1990) and the calbindin+ type in rabbit (Massey & Mills, 1996; 1999; Strettoi et al., 1994; Zhang et al., 2005) (Figure 4A). ON Alpha ganglion cells are highly sensitive under scotopic conditions; presumably, this can be attributed to the strong coupling between their presynaptic bipolar cells and the AII network (Müller et al., 1988; Dunn et al., 2006; Murphy & Rieke, 2006).
In the cat retina, the ON cone bipolar cell with the weakest connection to the AII was the b5 cell presumed to be a specialized ‘S-cone bipolar cell,’ with selective input from S-cones: the 5% of cones with peak sensitivity to short-wavelength (S) light. Thus, highly sensitive scotopic signals would apparently not reach those ganglion cells postsynaptic to S-cone bipolar cells. Primate small-bistratified ganglion cells, however, receive input from S-cone bipolar cells and display responses mediated by the rod bipolar/AII amacrine pathway (Crook et al., 2009; Field et al., 2009). Furthermore, primate S-cone bipolar cells showed apparent contacts with AII cells, as determined by immunohistochemistry and confocal microscopic analysis (Field et al., 2009). Thus, the AII cell connections to the ON cone bipolar cells apparently are weighted differently by cell type with the specific pattern of connections differing between species.
Selective network connections: OFF cone bipolar cells
As with their connections to the ON pathway, AIIs make synapses with only a subset of OFF bipolar cells. In the mouse retina, for example, EM analysis showed that AII lobular appendages, the sites of glycine release, are presynaptic to a B1 OFF bipolar cell type but not a B2 type (Tsukomoto et al., 2001). Interestingly, the B1 type received input at its dendrites only from cone photoreceptors, whereas the B2 type, despite its label as a ‘cone bipolar cell,’ received input from both cones and rods (Figure 4A). This combined connectivity with both cones and rods was identified as a ‘tertiary rod pathway’ through certain bipolar cell types (Soucy et al., 1998; Hack et al., 1999; Tsukamoto et al., 2001; Li et al., 2004; Tsukamoto et al., 2007). The differences between B1 and B2 types suggest that some OFF bipolars may be connected to either the ‘primary rod pathway’ (i.e., the AII circuit) or the tertiary rod pathway (i.e., direct rod input) but not both. All bipolar types presumably would receive signals through the ‘secondary rod pathway,’ whereby rods signal through gap junctions with cones and then through chemical synaptic input to cone bipolar cells (Nelson, 1977; Smith et al., 1986; Schneeweis & Schnapf, 1995).
In cat, AIIs avoid contacting certain OFF cone bipolar cell types (CBa2, CBa3) but do contact others (CBa1, CBa4; McGuire et al., 1984). The CBa1 cell seemed to be specialized among the OFF bipolars, similar to the cat’s ON b1 bipolar, to receive dominant input from the AII. Not only does the CBa1 cell receive input from the AII, it also makes reciprocal ribbon synapses onto the AII; these reciprocal synapses provide a mechanism for OFF bipolar cells to excite the AII at light offset (McGuire et al., 1984). Indeed, similar reciprocal synapses were observed in rabbit (DAPI-Ba2 cell type; Strettoi et al., 1992; Merighi et al., 1996), rat (Chun et al., 1993) and primate (DB3 cell type; Jacoby & Marshak, 2000; Wässle et al., 1995) (Figure 4A).
The function of reciprocal AII-OFF bipolar cell synapses, however, requires further study: although the AII’s light response shows an OFF response component at an early stage of light adaptation (rabbit; Xin & Bloomfield, 1999), its ON response component dominates under most conditions (Nelson, 1982; Dacheux & Raviola, 1986; Pang et al., 2007; Murphy & Rieke, 2008). Thus, although synaptic transmission between OFF cone bipolars and AIIs has been demonstrated electrophysiologically (Veruki et al., 2003), the relevance of such transmission during physiological responses to light is uncertain.
Selective network connections: OFF ganglion cells
The AII could, in theory, signal to the ON and OFF pathways exclusively via its contacts with bipolar cell terminals. The AII, however, also makes direct chemical synapses onto the dendrites of a few OFF ganglion cell types (Manookin et al., 2008; Murphy & Rieke, 2008; Münch et al., 2009; Anderson et al., 2011). For example, OFF Alpha/Transient and OFF Delta/Sustained cells in both mouse and guinea pig appear to receive direct inputs from the AII because firing in scotopic conditions is driven primarily by the modulation of inhibitory glycinergic synapses (Murphy & Rieke, 2006; Manookin et al., 2008; Beaudoin et al., 2008; van Wyk et al., 2009).
Paired-cell recordings demonstrated directly a synapse between AIIs and OFF Alpha cells in the mouse (Murphy & Rieke, 2008; Münch et al., 2009). In the guinea pig, pharmacological manipulations confirmed the role for the AII in modulating Alpha and Delta cell responses (Manookin et al., 2008; Beaudoin & Demb, unpublished observations). In cat, EM analysis showed that both OFF Alpha and Beta cells receive direct AII input, and for Alpha cells, the number of AII contacts about equaled the number of OFF cone bipolar contacts (Kolb & Nelson, 1993; Owczarzak & Pourcho, 1999). Similar contacts with a select number of ganglion cells were also observed in rabbit (Strettoi et al., 1992; Anderson et al., 2011) and rat (Chun et al., 1993).
The AII contributes to network function under photopic conditions
Under photopic conditions, when rod bipolar signaling is saturated, light stimulation of cones depolarizes ON cone bipolar cells, which then can depolarize coupled AIIs. The direction of signaling (ON cone bipolar → AII; Figure 4B) is reversed relative to that under scotopic conditions (AII → ON cone bipolar; Figure 1). Thus, depolarization of the AII under photopic conditions should not require a conventional ionotropic glutamate receptor (iGluR; i.e., AMPA, kainate or NMDA channel), in contrast to all other known circuits in the retina. Indeed, photopic AII light responses persist in the presence of iGluR antagonists that block the conventional retinal pathways (Xin & Bloomfield, 1999; Trexler et al., 2005; Pang et al., 2007). Furthermore, AII inhibition of OFF ganglion cells (Alpha, Beta and Delta types) persists in bright light in the absence of iGluR function through the following circuit: cone→ON cone bipolar→ AII amacrine→ OFF ganglion cell (Figure 4B) (Cohen, 1998; Cohen & Miller, 1999; Beaudoin et al., 2008; Manookin et al., 2008; Münch et al., 2009). These results show that the ON cone bipolar-AII gap junction functions in bright light allowing AII cells to contribute to vision under photopic conditions.
Computational role in photopic vision
In the ON pathway, the functioning gap junction between ON cone bipolar cells and AIIs should degrade spatial acuity in bright light (Mills & Massey, 1995). Since certain bipolar pathways show little or even no contact with the AII network (Figure 4A), the impact of electrical coupling on spatial acuity should vary between parallel cone bipolar cell pathways.
In the OFF pathway, the AII plays several roles in vision under photopic conditions. Tonic release of glycine by the AII helps to set the resting potential of both OFF bipolar and ganglion cells as well as the basal release rate of glutamate from OFF bipolar terminals (Zaghloul et al., 2003; Margolis & Detwiler, 2007; Liang & Freed, 2010). Glycinergic modulation of OFF bipolar terminals increases the operating range of glutamate release (Liang & Freed, 2010). Additionally, certain OFF ganglion cell types integrate AII-mediated inhibition and OFF bipolar cell-mediated excitation (Figure 5). Thus, the AII generates cross-over inhibition from the ON pathway to the OFF pathway that competes with OFF pathway excitation. At light onset, the AII compensates for the rectifying OFF bipolar cell synapses by generating hyperpolarizing responses in the ganglion cell (Demb et al., 2001a; Zaghloul et al., 2003; Molnar et al., 2009; Werblin, 2010). At light offset, tonic release of glycine from the AII is suppressed, and the resulting ‘disinhibition’ of the ganglion cell combines with conventional excitation from OFF bipolar cells to extend the operating range for encoding negative contrasts (Manookin et al., 2008). Thus, the AII signals OFF bipolar terminals and OFF ganglion cells through both increases and decreases from a tonic level of glycine release.
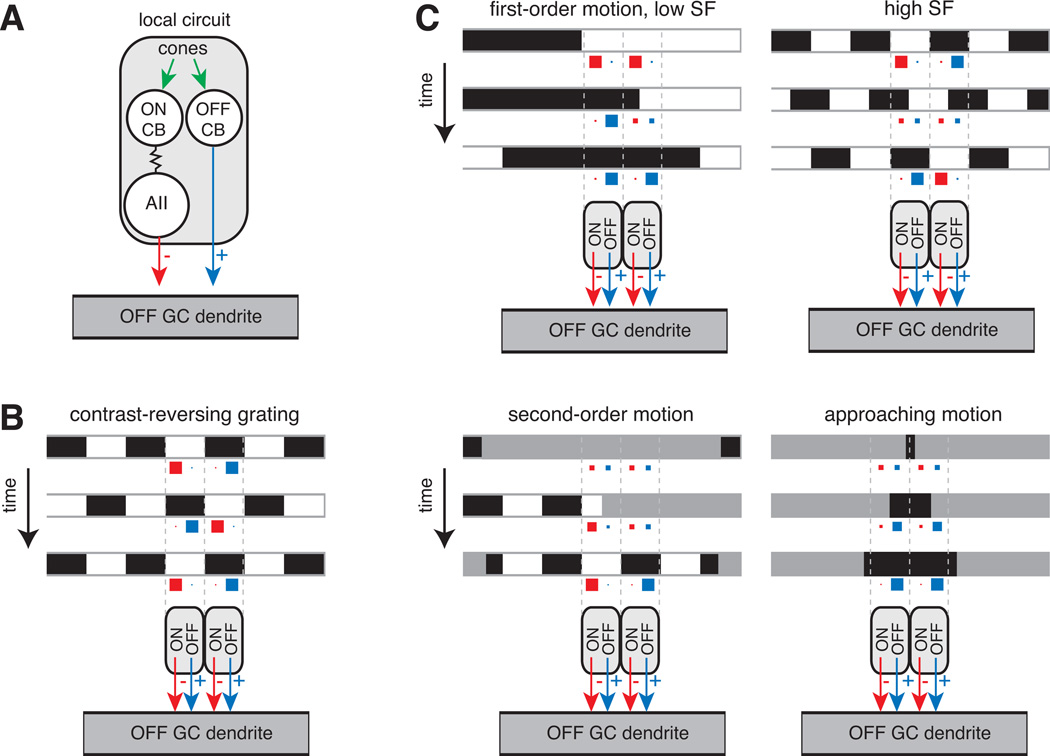
Figure 5. Function of the AII in motion detection.
(A) A local circuit is formed by an OFF cone bipolar cell paired with an ON cone bipolar cell that signals through the AII. Both the OFF bipolar and AII converge on the ganglion cell dendrite. This local circuit is summarized in parts (B) and (C) as an OFF-pathway excitatory input (black arrow) paired with an ON-pathway inhibitory input (red arrow).
(B) Contrast-reversing grating: a high spatial-frequency grating reverses contrast over time. Image frames are shown at three time points. The level of inhibition (red) and excitation (blue) in each of two local circuits is illustrated by the size of squares: larger squares represent higher release rates. On each frame, excitation in one region combines with inhibition in the neighboring region. The summed activity typically results in a burst of firing at each grating reversal showing that excitation dominates (see text).
(C) First-order motion: a drifting grating is shown for both low and high spatial frequency (SF) stimuli. Bars move rightward and stimulate both OFF bipolar and AII amacrine cell release. At the low spatial frequency, excitation and inhibition alternate; whereas at the high spatial frequency, excitation and inhibition co-occur. In different experiments (see text), the high spatial frequency stimulus generates either a net excitation or a cancellation at the ganglion cell level.
Second-order motion: a stationary grating’s contrast is modulated by a moving contrast boundary. The OFF bipolar and AII cell are stimulated as the low contrast boundary moves rightward, revealing the underlying stationary grating. The combination of these inputs generates a modulation of membrane potential around rest and drives firing (see text).
Approaching motion: a small dark stimulus expands over time. The stimulus drives increased release from OFF cone bipolars while suppressing release from AIIs leading to high firing rates in the ganglion cell (see text).
There are two possible computational advantages to combining inhibition and excitation at the ganglion cell. First, the AII’s inhibition can compete with the OFF bipolar cell’s excitation at the scale of the ganglion cell’s wide dendritic tree (hundreds of microns). By contrast, AII inhibition of OFF bipolar cell terminals will have little effect if the adjacent OFF bipolars are not active (i.e., because the OFF bipolar cell release rate is commonly rectified and cannot be decreased below zero); therefore, AII inhibition at OFF bipolar terminals is limited to local interactions where simultaneous excitation and inhibition compete over a narrow spatial scale (i.e., tens of microns). Second, postsynaptic inhibition acts in the presence of high release rates from bipolar and amacrine cell terminals. The high release rates convey signals with high S/N to the ganglion cell (assuming release rates are governed by Poisson statistics). This contrasts with the presynaptic locus of inhibition (AII→OFF cone bipolar terminal), where the release onto the ganglion cell is only excitatory and is suppressed in the presence of inhibition, resulting in signals with low S/N.
The competition between OFF pathway bipolar cell-mediated excitation and ON pathway AII-mediated inhibition tunes the OFF ganglion cell response to different forms of motion. A drifting grating represents first-order motion, defined by spatio-temporal changes in luminance. A low spatial-frequency grating will alternately stimulate OFF pathway excitation when dark bars pass over OFF bipolar cells and ON-pathway inhibition when light bars pass over ON bipolar cells and their coupled AII amacrine cells (Figure 5C). A high spatial-frequency grating will simultaneously excite both OFF excitatory and ON inhibitory inputs. This simultaneous stimulation of the two pathways leads, in some cases, to a tonic elevation in firing when the excitatory inputs are relatively stronger (Cleland et al., 1971; Manookin & Demb, 2006). Likewise, a contrast-reversing grating activates both pathways simultaneously, but the excitation dominates, resulting in a burst of firing at each contrast reversal (Figure 5B) (Hochstein and Shapley, 1976; Demb et al., 2001a). Thus, in these cases the AII-mediated inhibition attenuates the effect of excitation, preventing excessive depolarization, but does not cancel it. The recordings mentioned above were made in cat or guinea pig cells. In the mouse retina, however, the response to a drifting high spatial frequency grating showed that the AII and OFF bipolar inputs canceled one another and the ganglion cell did not fire (Münch et al., 2009). Thus, the combined OFF bipolar/AII circuit has the potential to cancel responses selectively at high spatial frequencies. The degree of cancellation may depend on certain stimulus conditions (i.e., level of light adaptation) or may differ between species.
A second-order motion stimulus is defined by spatio-temporal changes in contrast (Figure 5C). As a low contrast window moves and reveals a stationary, high contrast grating pattern, the combined OFF excitatory and ON inhibitory inputs modulate the membrane potential around rest, driving bursts of firing (Demb et al., 2001b; Rosenberg & Issa, 2010) (the source of inhibition in the earlier study was not identified as the AII, but we now understand this to be the case [Manookin et al., 2008]). Finally, an expanding dark stimulus yields a strong response in OFF Alpha ganglion cells, because local OFF bipolar cells are excited as AII cells are simultaneously suppressed (Münch et al., 2009) (Figure 5C). It has been proposed that this response to approaching motion might be important for certain behaviors (e.g., avoiding an approaching back-lit object or predator).
Concluding remarks
The existence of gap junction-mediated coupling between AIIs is well-established, as is the modulation of this coupling by ambient light and DA. However, it is not immediately apparent why coupling between AIIs is necessary for ganglion cell processing of rod signals. Because AIIs are coupled to ON cone bipolar terminals, rod signals arising in the AIIs’ dendrites will be integrated by the ganglion cells postsynaptic to the ON cone bipolar terminals even if AIIs are not coupled to each other. The AII-ON cone bipolar cell gap junctions would serve to filter noise, and the voltage-gated Ca channels in the bipolar cell terminals would amplify rod signals nonlinearly. Similarly, OFF ganglion and bipolar cells postsynaptic to AIIs will receive glycinergic input even in the absence of AII-AII coupling, and chemical synaptic transmission is an inherently nonlinear process owing to the dependence of exocytosis on two nonlinear processes: voltage-dependent Ca channel gating and Ca2+ activation of the vesicular release machinery. To fully appreciate the roles of these processes, we need to better understand the dynamics of transmission at the AII’s glycinergic synapses. Furthermore, it is possible that there are undiscovered advantages to having two sites of coupling that could be modulated independently.
Additionally, the primary site of noise reduction in the rod bipolar pathway occurs upstream of the AII, at the the rod → rod bipolar synapse (Dunn et al., 2006). A postsynaptic thresholding mechanism arising from saturation in the second messenger cascade linking glutamate receptor activation with membrane conductance serves to eliminate the majority of rod noise from rod bipolar cell signals (van Rossum & Smith, 1998; Field & Rieke, 2002; Sampath & Rieke, 2004). Indeed, noise measured in AIIs is actually much greater than that in rod bipolar cells (Dunn et al., 2006). This noise arises at the rod bipolar → AII synapse, which operates at high gain (Dunn et al., 2006), likely owing to the high release probability of vesicles (Singer et al., 2004; Jarsky et al., 2010). Thus, noise is a consequence of this high gain. Both the gain and noise in ganglion cell responses match that of the AIIs and are 4–6 times that which would arise from noiseless summation of rod output in the rod bipolar pathway. Hence, the noise in ganglion cell responses is apparently inherited from noise in AIIs (Dunn et al., 2006). This noise in the ganglion cells might be even higher if AIIs were uncoupled.
It will be informative to determine whether the pattern of AII synapses, investigated by EM, can predict the function of parallel pathways during night vision. We might then understand why certain ganglion cell types are more sensitive than others under scotopic conditions (Purpura et al., 1988; Lee et al., 1997; Deans et al., 2002; Völgyi et al., 2004). Understanding the role of AII cells in specific perceptions and behaviors will ultimately require methods for reversibly inactivating these cells. For example, one could test whether avoidance of approaching dark stimuli is lost following inactivation of AII cells (Münch et al., 2009). There are several types of narrow-field glycinergic amacrine cell other than the AII (Wässle et al., 2009), and so it will be interesting to learn whether there is enough redundancy in retinal circuitry such that certain behaviors survive inactivation of single amacrine cell types.
References
- Alpern M. Rod-Cone Independence in the after-Flash Effect. Journal of Physiology. 1965;176:462–472. doi: 10.1113/jphysiol.1965.sp007561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, Koshevoy P, Grimm B, Tasdizen T, Whitaker R, Marc RE. Exploring the retinal connectome. Molecular Vision. 2011;17:355–379. [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. Journal of Physiology. 1957;137:327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Research. 1971;(Suppl 3):87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. Journal of Physiology. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. Journal of Physiology. 2008;586:5487–5502. doi: 10.1113/jphysiol.2008.156224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Progress in Retinal & Eye Research. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. Journal of Physiology. 2000;523(Pt 3):771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual Neuroscience. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wässle H. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. Journal of Neuroscience. 1993;13:2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1969;255:105. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. Journal of Comparative Neurology. 1973;148:91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. Journal of Comparitve Neurology. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurons in the cat's retina and lateral geniculate nucleus. Journal of Physiology. 1971;217:473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Convergence and divergence of cones onto bipolar cells in the central area of cat retina. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1990;330:323–328. doi: 10.1098/rstb.1990.0202. [DOI] [PubMed] [Google Scholar]
- Cohen ED. Interactions of inhibition and excitation in the light-evoked currents of X type retinal ganglion cells. Journal of Neurophysiology. 1998;80:2975–2990. doi: 10.1152/jn.1998.80.6.2975. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The network-selective actions of quinoxalines on the neurocircuitry operations of the rabbit retina. Brain Research. 1999;831:206–228. doi: 10.1016/s0006-8993(99)01448-1. [DOI] [PubMed] [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. Journal of Neuroscience. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. Journal of Neuroscience. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Völgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. European Journal of Neuroscience. 2006;24:1675–1686. doi: 10.1111/j.1460-9568.2006.05052.x. [DOI] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. Journal of Neuroscience. 2001a;21:7447–7454. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Sterling P. Cellular basis for the response to second-order motion cues in Y retinal ganglion cells. Neuron. 2001b;32:711–721. doi: 10.1016/s0896-6273(01)00484-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proceedings of the National Academy of Science of the United States of America. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proceedings of the Royal Society of London B: Biological Sciences. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. Journal of Neuroscience. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Jr, Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Research. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. Journal of Neuroscience. 2001;21:230–239. doi: 10.1523/JNEUROSCI.21-01-00230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Greschner M, Gauthier JL, Rangel C, Shlens J, Sher A, Marshak DW, Litke AM, Chichilnisky EJ. High-sensitivity rod photoreceptor input to the blue-yellow color opponent pathway in macaque retina. Nature Neuroscience. 2009;12:1159–1164. doi: 10.1038/nn.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annual Review of Physiology. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. Journal of Neuroscience. 1988;8:2303–2320. doi: 10.1523/JNEUROSCI.08-07-02303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. Journal of Physiology. 1966;184:499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proceedings of the National Academy of Sciences: USA. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. Journal of Neuroscience. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Massey SC. Electrical synapses in retinal ON cone bipolar cells: subtype-specific expression of connexins. Proceedings of the National Academy of Science of the United States of America. 2005;102:13313–13318. doi: 10.1073/pnas.0505067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. Journal of Neurophysiology. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hecht S, Shlaer S, Pirenne MH. Energy, Quanta, and Vision. Journal of General Physiology. 1942;25:819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S, Shapley RM. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. Journal of Physiology. 1976;262:265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. Journal of Comparative Neurology. 2000;416:19–29. doi: 10.1002/(sici)1096-9861(20000103)416:1<19::aid-cne3>3.0.co;2-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. Journal of Neuroscience. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Jung CK, Kang TH, Jeon JH, Cha J, Kim IB, Chun MH. Synaptic connections of calbindin-immunoreactive cone bipolar cells in the inner plexiform layer of rabbit retina. Cell & Tissue Research. 2010;339:311–320. doi: 10.1007/s00441-009-0895-6. [DOI] [PubMed] [Google Scholar]
- Kim IB, Park MR, Kang TH, Kim HJ, Lee EJ, Ahn MD, Chun MH. Synaptic connections of cone bipolar cells that express the neurokinin 1 receptor in the rabbit retina. Cell & Tissue Research. 2005;321:1–8. doi: 10.1007/s00441-005-1122-8. [DOI] [PubMed] [Google Scholar]
- Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1970;258:22. doi: 10.1098/rstb.1970.0036. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. Rod pathways in the retina of the cat. Vision Research. 1983;23:301–312. doi: 10.1016/0042-6989(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. Journal of Comparative Neurology. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O'Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. Journal of Neuroscience. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD. Evolution of vertebrate retinal photoreception. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364:23. doi: 10.1098/rstb.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Simon EJ. The relation between intercellular coupling and electrical noise in turtle photoreceptors. Journal of Physiology. 1976;263:257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Smith VC, Pokorny J, Kremers J. Rod inputs to macaque ganglion cells. Vision Research. 1997;37:2813–2828. doi: 10.1016/s0042-6989(97)00108-9. [DOI] [PubMed] [Google Scholar]
- Li W, Keung JW, Massey SC. Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina. Journal of Comparative Neurology. 2004;474:1–12. doi: 10.1002/cne.20075. [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed MA. The ON pathway rectifies the OFF pathway of the mammalian retina. Journal of Neuroscience. 2010;30:5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. Journal of Neuroscience. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. Journal of Neuroscience. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. Journal of Comparative Neurology. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina. Visual Neuroscience. 1999;16:1181–1189. doi: 10.1017/s0952523899166173. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. Journal of Neurophysiology. 1983;49:325–349. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, AmmerMüller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. Journal of Neuroscience. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. Journal of Neuroscience. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Raviola E, Dacheux RF. Connections of two types of flat cone bipolars in the rabbit retina. Journal of Comparative Neurology. 1996;371:164–178. doi: 10.1002/(SICI)1096-9861(19960715)371:1<164::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Mills SL, O'Brien JJ, Li W, O'Brien J, Massey SC. Rod pathways in the mammalian retina use connexin 36. Journal of Comparative Neurology. 2001;436:336–350. [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. Journal of Computational Neuroscience. 2009;37:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Wässle H, Voigt T. Pharmacological modulation of the rod pathway in the cat retina. Journal of Neurophysiology. 1988;59:1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nature Neuroscience. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52:511–524. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nature Neuroscience. 2008;11:318–326. doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. Journal of Comparative Neurology. 1977;172:109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. Journal of Neurophysiology. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal of Receptors and Signal Transduction Research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caille I, Bloch B. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Visual Neuroscience. 1997;14:545–551. doi: 10.1017/s0952523800012207. [DOI] [PubMed] [Google Scholar]
- Owczarzak MT, Pourcho RG. Transmitter-specific input to OFF-alpha ganglion cells in the cat retina. Anatomical Record. 1999;255:363–373. doi: 10.1002/(SICI)1097-0185(19990801)255:4<363::AID-AR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light response in the mouse retina. Journal of Physiology. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. Journal of Physiology. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida GJ, Lee SC, Haft-Candell L, Nichols GS, Ishida AT. DARPP-32-like immunoreactivity in AII amacrine cells of rat retina. Journal of Comparative Neurology. 2004;480:251–263. doi: 10.1002/cne.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides A, Trexler EB. Differential output of the high-sensitivity rod photoreceptor: AII amacrine pathway. Journal of Comparative Neurology. 2008;507:1653–1662. doi: 10.1002/cne.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. Journal of Comparative Neurology. 1985;233:473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- Protti DA, Llano I. Calcium currents and calcium signaling in rod bipolar cells of rat retinal slices. Journal of Neuroscience. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Dacheux RF. Excitatory dyad synapse in rabbit retina. Proceedings of the National Academy of Science of the United States of America. 1987;84:7324–7328. doi: 10.1073/pnas.84.20.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proceedings of the National Academy of Science of the United States of America. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Husson TR, Issa NP. Subcortical representation of non-Fourier image features. Journal of Neuroscience. 2010;30:1985–1993. doi: 10.1523/JNEUROSCI.3258-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakitt B. Counting every quantum. Journal of Physiology. 1972;223:131–150. doi: 10.1113/jphysiol.1972.sp009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Schorderet M, Nowak JZ. Retinal dopamine D1 and D2 receptors: characterization by binding or pharmacological studies and physiological functions. Cellular and Molecular Neurobiology. 1990;10:303–325. doi: 10.1007/BF00711177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. Photovoltage of rods and cones in the macaque retina. Science. 1995;268:1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. Journal of Neuroscience. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nature Neuroscience. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. Journal of Neuroscience. 1986;6:3505–3517. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Visual Neuroscience. 1995;12:851–860. doi: 10.1017/s095252380000941x. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. Journal of Neuroscience. 1988;8:623–642. doi: 10.1523/JNEUROSCI.08-02-00623.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles WS. Color vision: the approach through increment-threshold sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 1959;45:14. [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. Journal of Comparative Neurology. 1994;347:139–149. doi: 10.1002/cne.903470111. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Masland RH. The number of unidentified amacrine cells in the mammalian retina. Proceedings of the National Academy of Sciences: USA. 1996;93:14906–14911. doi: 10.1073/pnas.93.25.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. Journal of Comparative Neurology. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annual Review of Pharmacology and Toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Attwell D. The effect of photoreceptor coupling and synapse nonlinearity on signal:noise ratio in early visual processing. Proceedings of the Royal Society of London B: Biological Sciences. 1988;234:171–197. doi: 10.1098/rspb.1988.0043. [DOI] [PubMed] [Google Scholar]
- Tian M, Jarsky T, Murphy GJ, Rieke F, Singer JH. Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. Journal of Neuroscience. 2010;30:4650–4659. doi: 10.1523/JNEUROSCI.4212-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. Journal of Neurophysiology. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. Journal of Neuroscience. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. Journal of Neuroscience. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. Journal of Biological Chemistry. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- van Rossum MC, Smith RG. Noise removal at the rod synapse of mammalian retina. Visual Neuroscience. 1998;15:809–821. doi: 10.1017/s0952523898155037. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Wässle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Visual Neuroscience. 2009;26:297–308. doi: 10.1017/S0952523809990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neuroscience Letters. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Gynther IC, Young HM. Rod-signal interneurons in the rabbit retina: 2. AII amacrine cells. Journal of Comparative Neurology. 1991;310:154–169. doi: 10.1002/cne.903100203. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. Journal of Neuroscience. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Smith RG. The AII amacrine network: coupling can increase correlated activity. Vision Research. 1996;36:3743–3757. doi: 10.1016/0042-6989(96)00098-3. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002a;33:935–946. doi: 10.1016/s0896-6273(02)00609-8. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. Journal of Neuroscience. 2002b;22:10558–10566. doi: 10.1523/JNEUROSCI.22-24-10558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. Journal of Physiology. 2003;549:759–774. doi: 10.1113/jphysiol.2003.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Oltedal L, Hartveit E. Electrical synapses between AII amacrine cells: dynamic range and functional consequences of variation in junctional conductance. Journal of Neurophysiology. 2008;100:3305–3322. doi: 10.1152/jn.90957.2008. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Wässle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. European Journal of Neuroscience. 1996;8:2286–2297. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. Journal of Neuroscience. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. Journal of Comparative Neurology. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, Haverkamp S. Glycinergic transmission in the Mammalian retina. Frontiers in Molecular Neuroscience. 2009;2:6. doi: 10.3389/neuro.02.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Visual Neuroscience. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Documenta Ophthalmologica. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Svenningsson P, Yan L, Bateup H, Silver R. Cellular localization and function of DARPP-32 in the rodent retina. European Journal of Neuroscience. 2007;25:3233–3242. doi: 10.1111/j.1460-9568.2007.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Visual Neuroscience. 2004;21:791–805. doi: 10.1017/S0952523804045122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Visual Neuroscience. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- Young HM, Vaney DI. Rod-signal interneurons in the rabbit retina: 1. Rod bipolar cells. Journal of Comparative Neurology. 1991;310:139–153. doi: 10.1002/cne.903100202. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. Journal of Neuroscience. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Hoshi H, Mills SL, Massey SC. Stratification of alpha ganglion cells and ON/OFF directionally selective ganglion cells in the rabbit retina. Visual Neuroscience. 2005;22:535–549. doi: 10.1017/S0952523805224148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Trexler EB, Massey SC. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. Journal of Neuroscience. 2002;22:10871–10882. doi: 10.1523/JNEUROSCI.22-24-10871.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]