Abstract
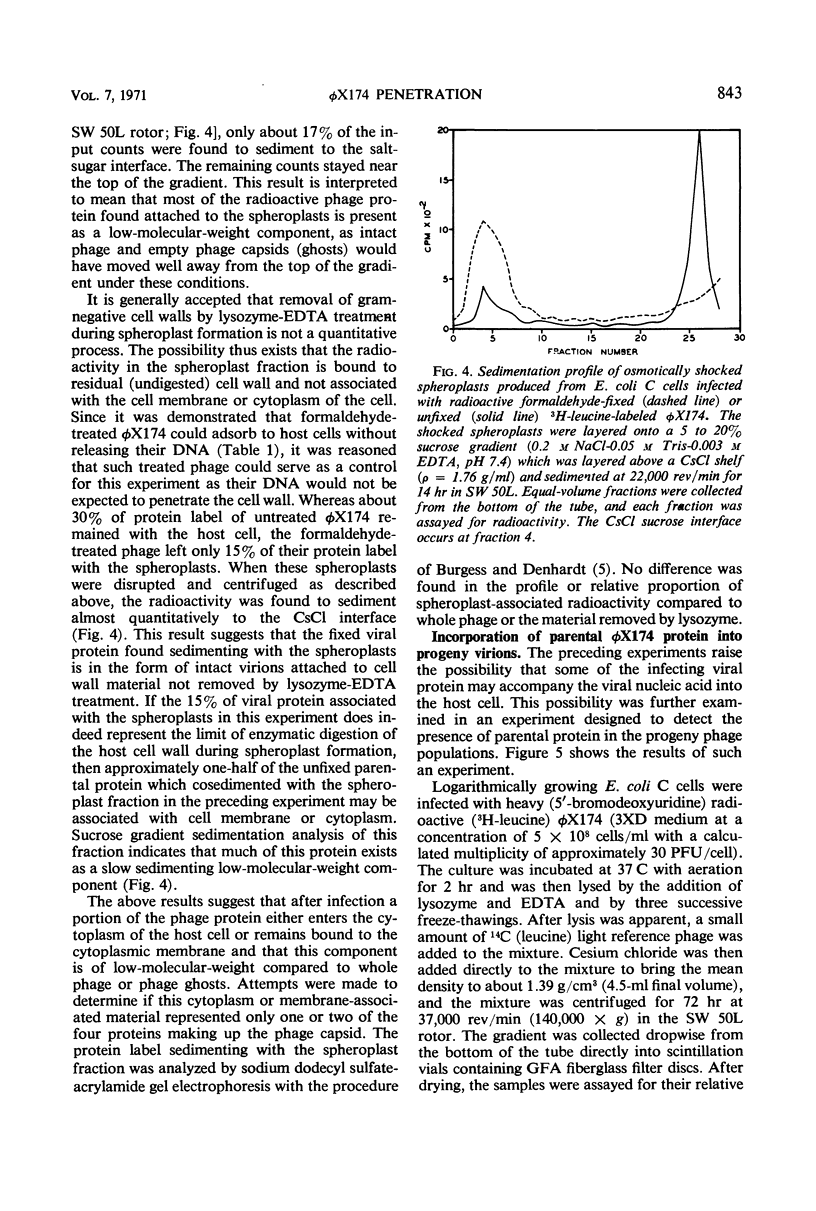
Bacteriophage φX174 is an icosahedral phage which attaches to host cells without the aid of a complex tail assembly. When φX174 was mixed with cell walls isolated from the bacterial host, the virions attached to the wall fragments and the phage deoxyribonucleic acid (DNA) was released. Attachment was prevented if the cell walls were treated with chloroform. Release of phage DNA, but not viral attachment, was prevented if the cell walls were incubated with lysozyme or if the virions were inactivated with formaldehyde. Treatment of the cell walls with lysozyme released structures which were of uniform size (6.5 by 25 nm). These structures attached φX174 at the tip of one of its 12 vertices, but the viral DNA was not released. The virions attached to these structures were oriented with their fivefold axis of symmetry normal to the long axis of the structure. No virions were attached to these structures by more than one vertex. Freeze-etch preparations of φX174 adsorbed to intact bacteria showed that the virions were submerged to one half their diameter into the host cell wall, and the fivefold axis of symmetry was normal to the cell surface. A second cell could not be attached to the outwardly facing vertex of the adsorbed phage and thus the phage could not cross-link two cells. When the virions were labeled with 3H-leucine, purified, and adsorbed to Escherichia coli cells, about 15% of the radioactivity was recovered as low-molecular-weight material from spheroplasts formed by lysozyme-ethylenediaminetetraacetic acid. Other experiments revealed that about 7% of the total parental virus protein label could be recovered in newly formed progeny virus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Burgess A. B., Denhardt D. T. Studies on phiX174 proteins. I. Phage-specific proteins synthesized after infection of Escherichia coli. J Mol Biol. 1969 Sep 28;44(3):377–386. doi: 10.1016/0022-2836(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Burgess A. B. Studies on the proteins of phi X174. II. The protein composition of the phi X coat. Proc Natl Acad Sci U S A. 1969 Oct;64(2):613–617. doi: 10.1073/pnas.64.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- FUJIMURA R., KAESBERG P. The adsorption of bacteriophage phi-X174 to its host. Biophys J. 1962 Nov;2:433–449. doi: 10.1016/s0006-3495(62)86866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B. Early intracellular events in the replication T4 phage DNA. II. Partially replicated DNA. Proc Natl Acad Sci U S A. 1965 Aug;54(2):634–640. doi: 10.1073/pnas.54.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- MAASS D., WEIDEL W. FINAL PROOF FOR THE IDENTITY OF ENZYMIC SPECIFICITIES OF EGG-WHITE LYSOZYME AND PHAGE T2 ENZYME. Biochim Biophys Acta. 1963 Oct 29;78:369–370. doi: 10.1016/0006-3002(63)91648-2. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXXI. Abortive infection at low temperatures. J Mol Biol. 1970 Apr 14;49(1):23–47. doi: 10.1016/0022-2836(70)90374-8. [DOI] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXXII. Early steps in the infection process: attachment, eclipse and DNA penetration. J Mol Biol. 1970 Apr 14;49(1):49–66. doi: 10.1016/0022-2836(70)90375-x. [DOI] [PubMed] [Google Scholar]
- STOUTHAMER A. H., DAEMS W. T., EIGNER J. Electron microscope studies of bacteriophage adsorption with negative and positive staining. Virology. 1963 Jun;20:246–250. doi: 10.1016/0042-6822(63)90112-0. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., KELLENBERGER E. The E. coli B-receptor for the phage T5. II. Electron microscopic studies. Biochim Biophys Acta. 1955 May;17(1):1–9. doi: 10.1016/0006-3002(55)90314-0. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]





