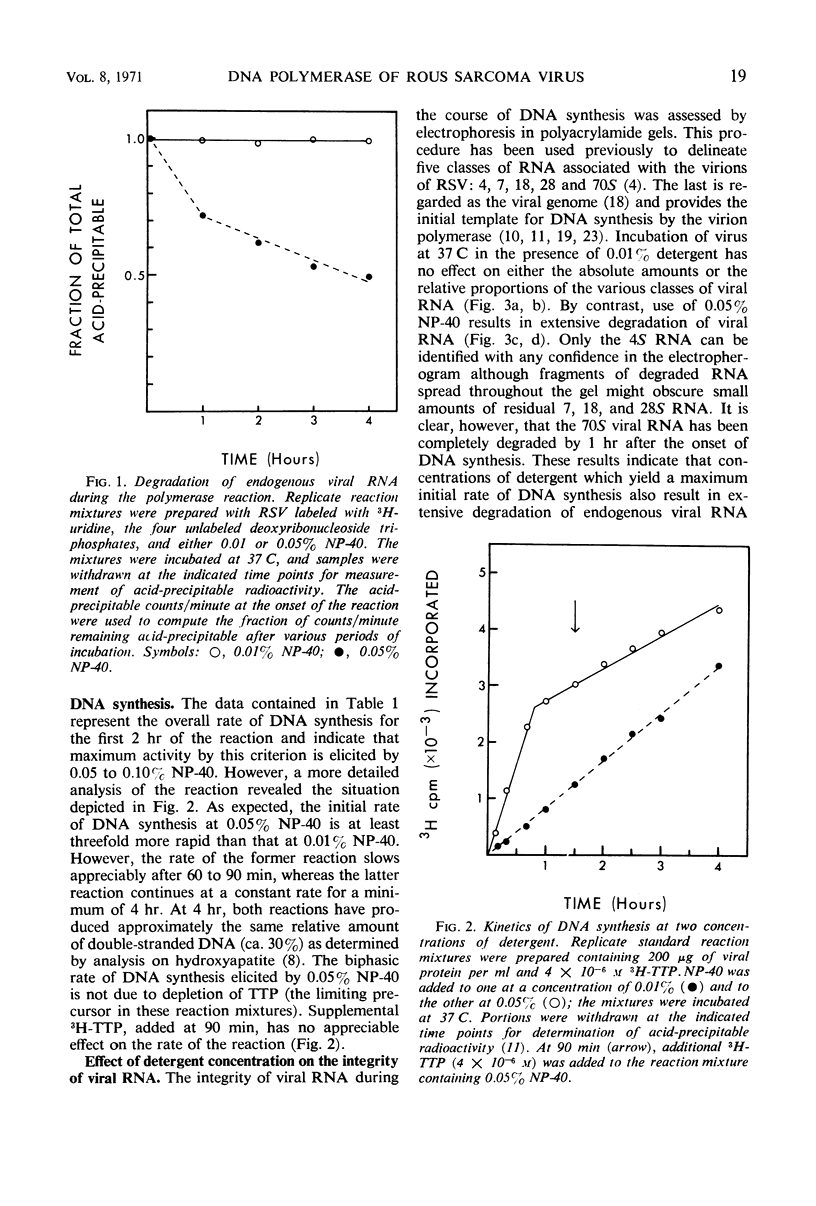
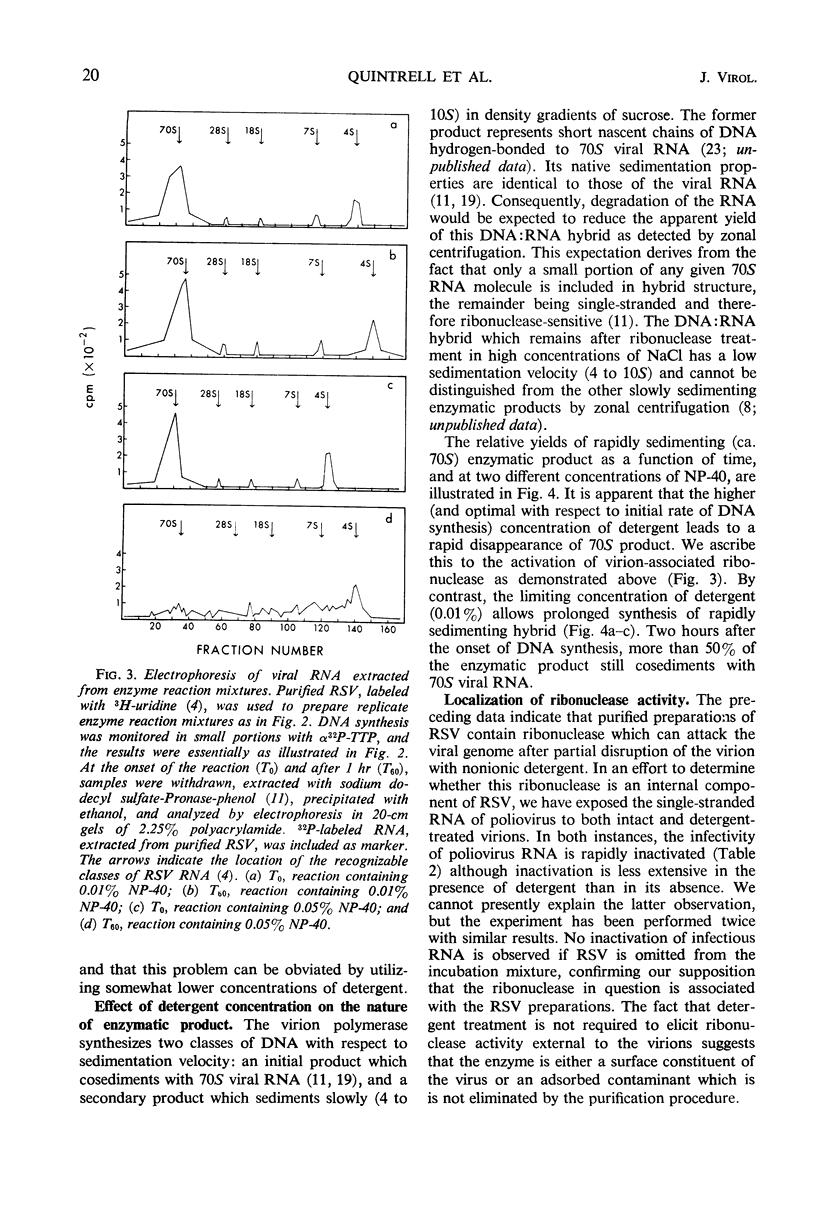
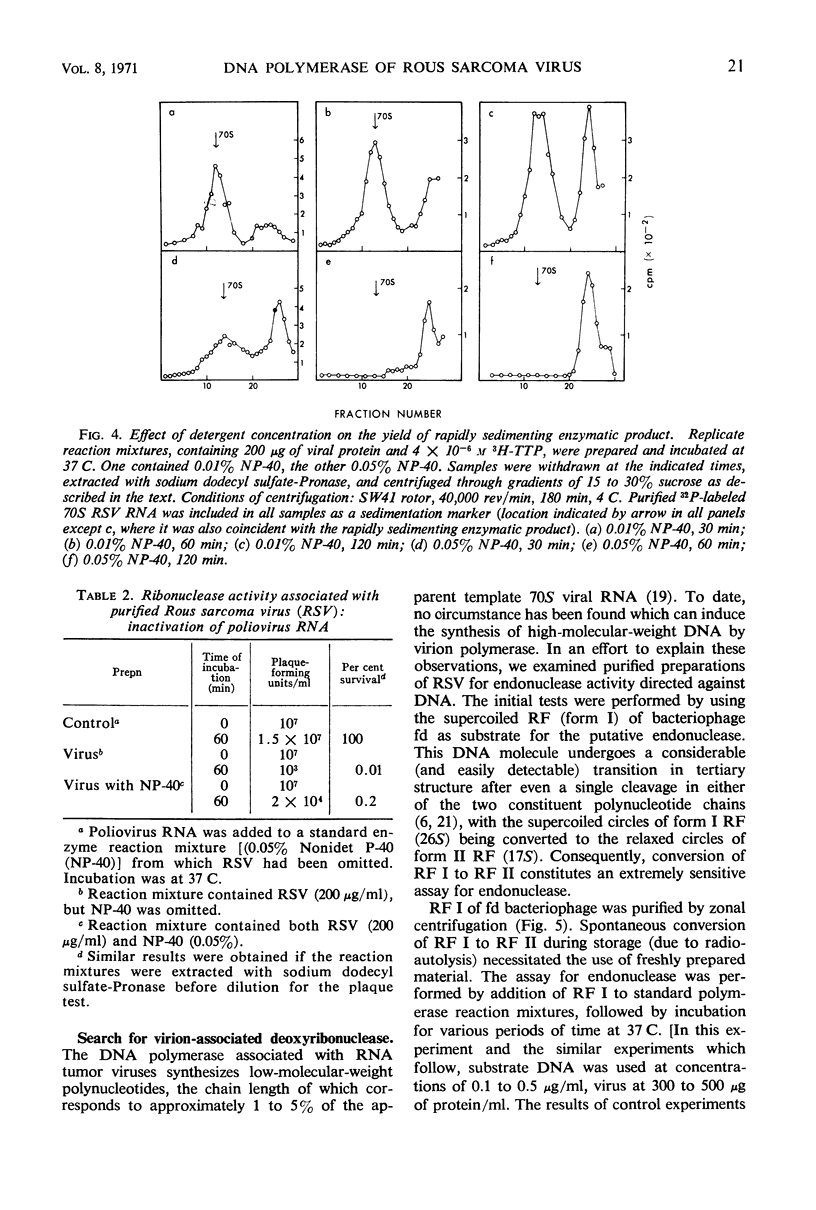
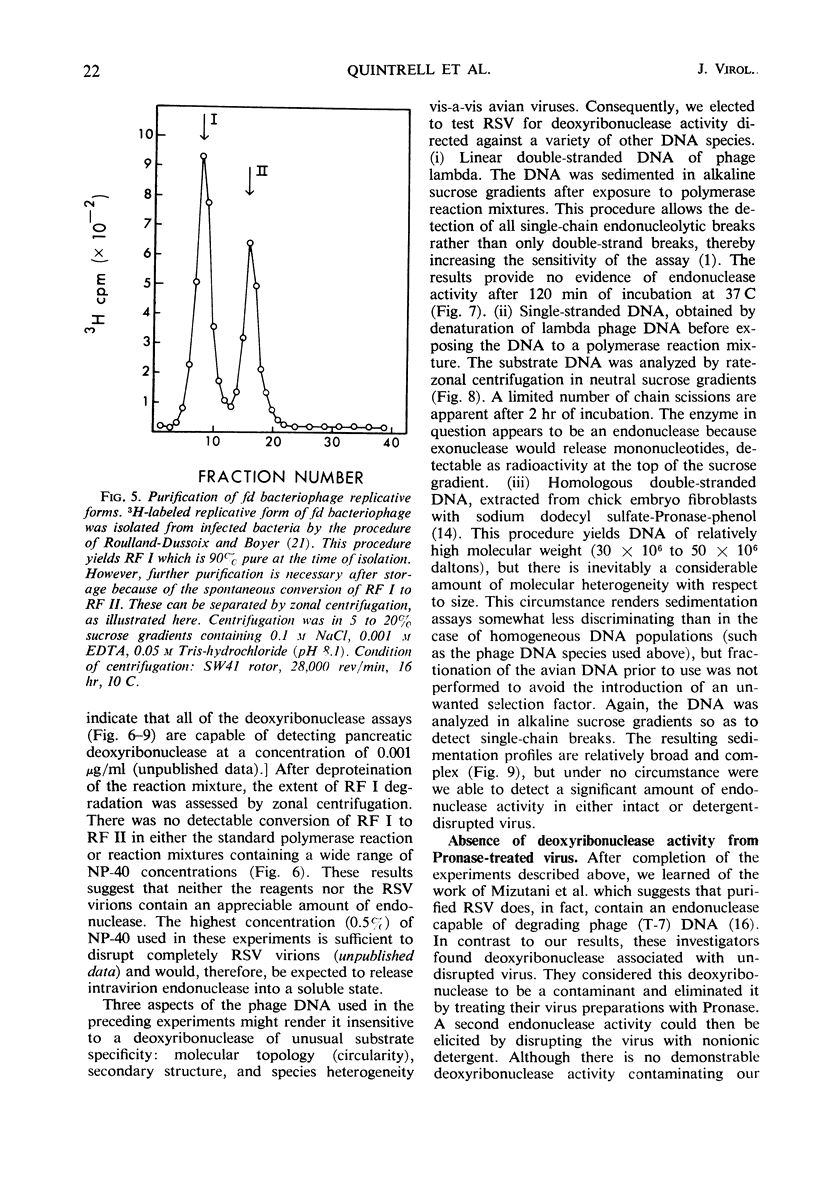
Abstract
Purified preparations of Rous sarcoma virus (RSV) contain ribonuclease which is either a constituent of the virion surface or an adsorbed contaminant. Treatment of the virus with nonionic detergent to activate ribonucleic acid (RNA)-dependent deoxyribonucleic acid (DNA) polymerase renders the viral genome susceptible to hydrolysis by the external ribonuclease. The extent of this susceptibility can be substantially reduced by the use of limited amounts of detergent. At a concentration of detergent which provides a maximum initial rate of DNA synthesis, the degradation of endogenous viral RNA results in a reduced yield of high molecular weight DNA: RNA hybrid from the polymerase reaction. Attempts to detect virion-associated deoxyribonuclease, by using a variety of double helical DNA species as substrates, have been unsuccessful, but small amounts of nuclease activity directed against single-stranded DNA may be present in purified virus.
Full text
PDF

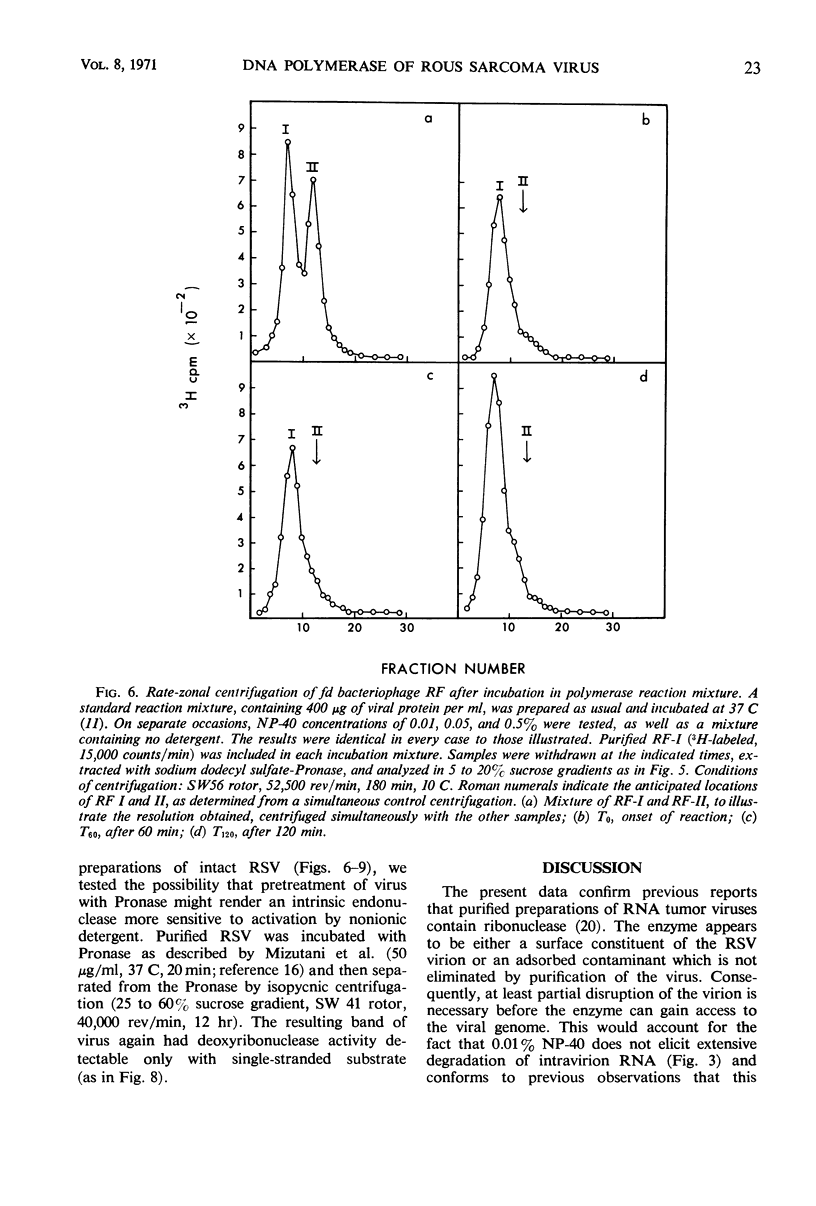
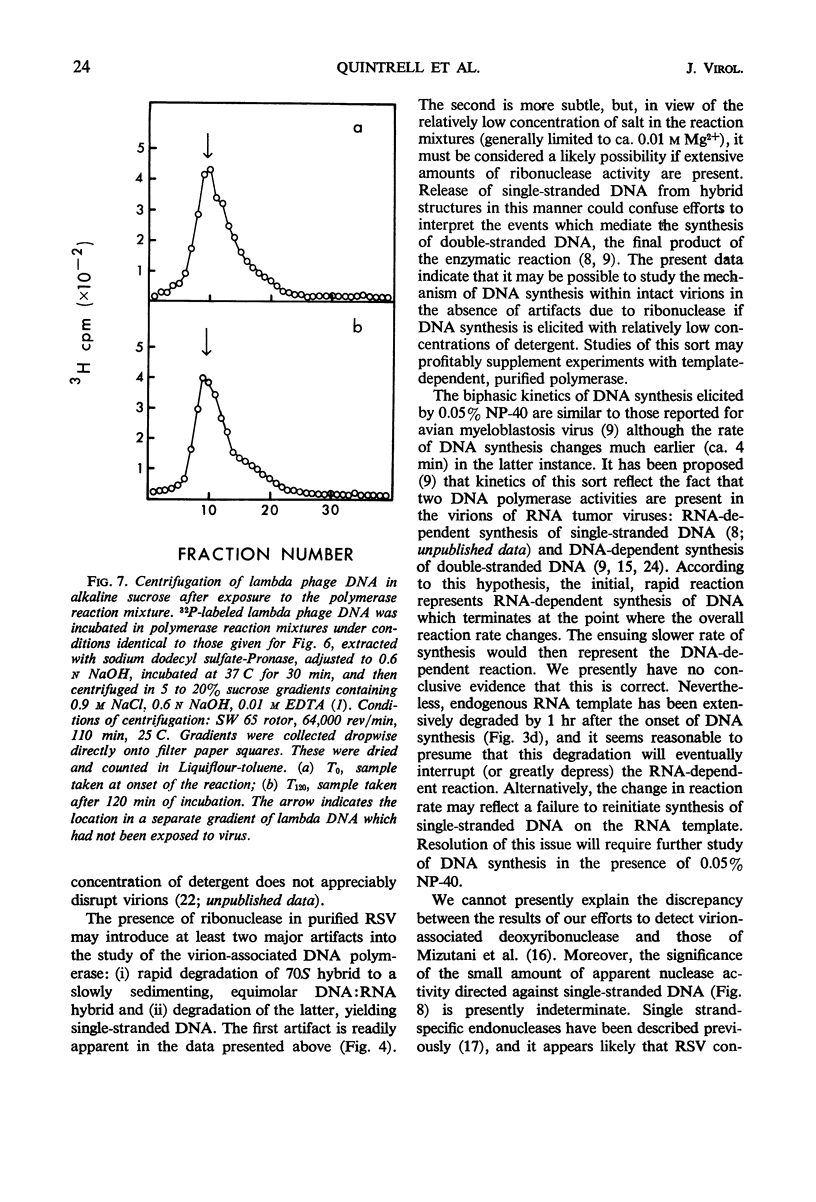
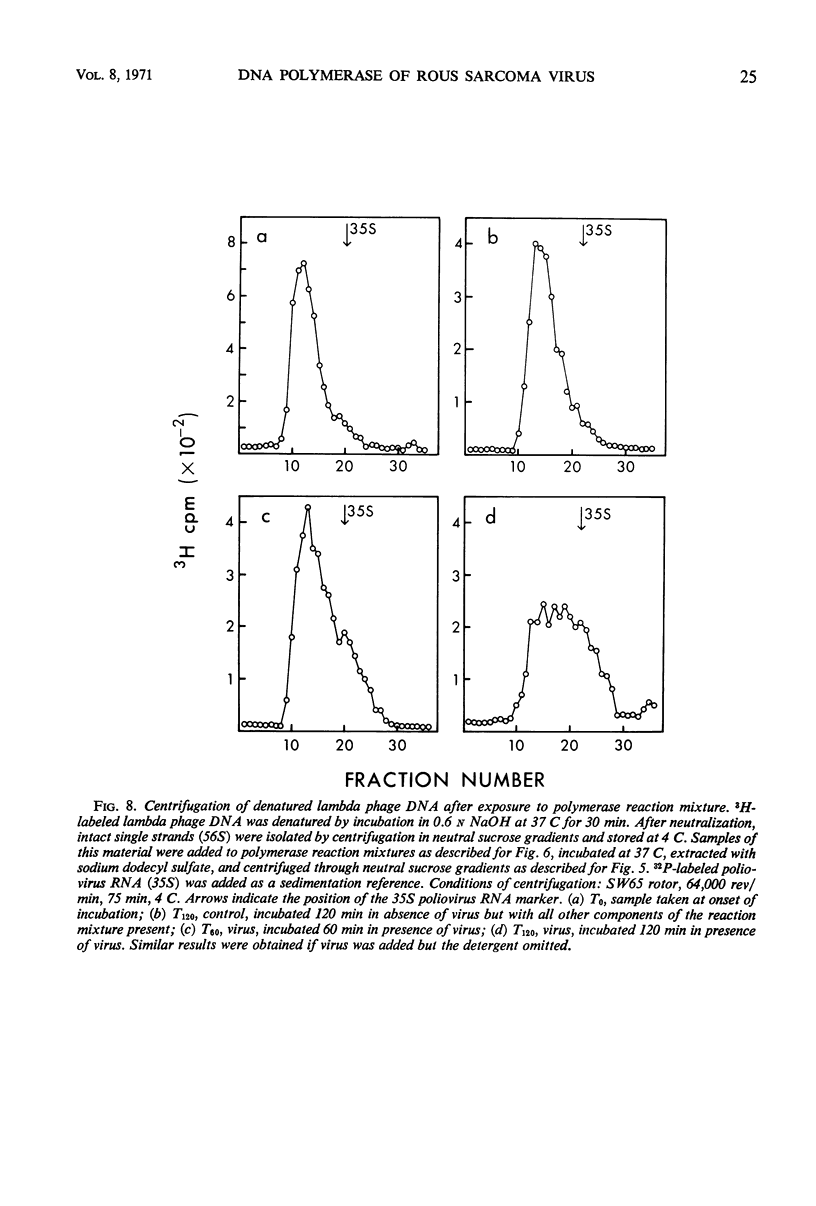
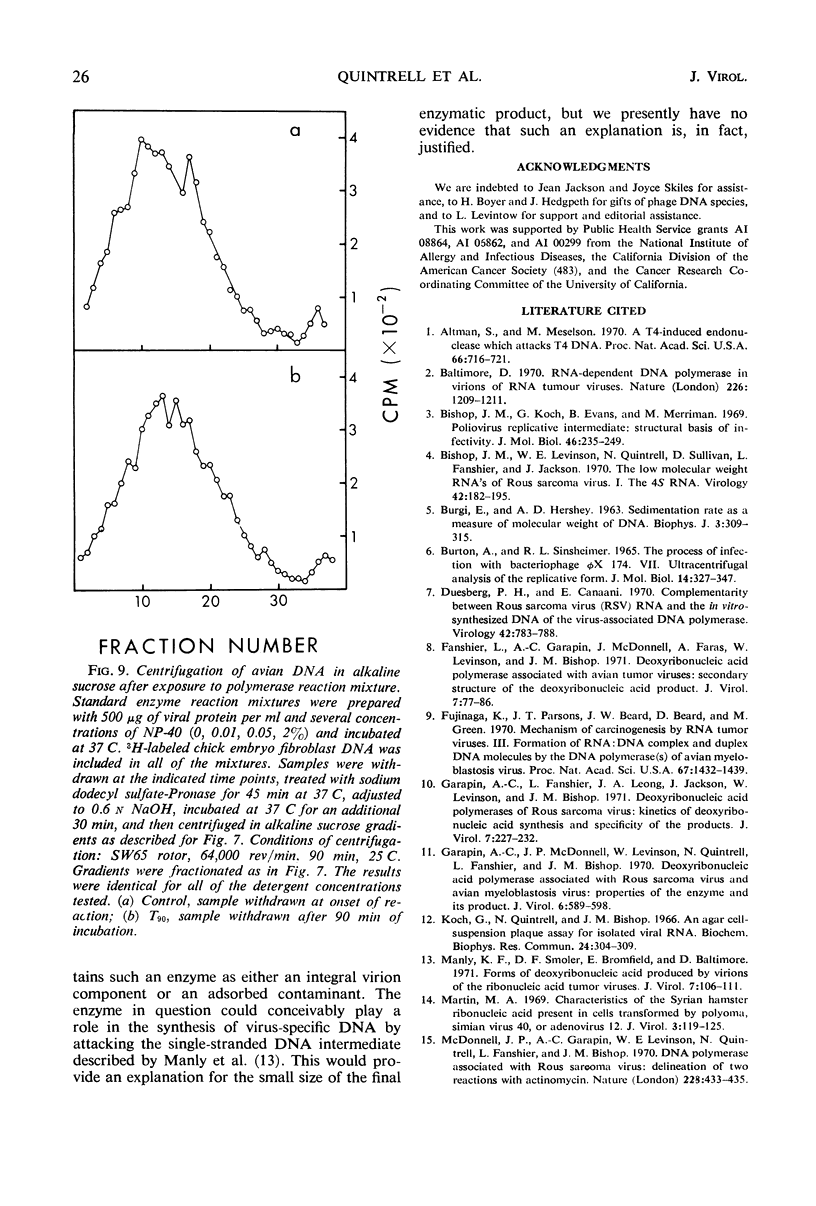

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Meselson M. A T4-induced endonuclease which attacks T4 DNA. Proc Natl Acad Sci U S A. 1970 Jul;66(3):716–721. doi: 10.1073/pnas.66.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G., Evans B., Merriman M. Poliovirus replicative intermediate: structural basis of infectivity. J Mol Biol. 1969 Dec 14;46(2):235–249. doi: 10.1016/0022-2836(69)90419-7. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Burton A., Sinsheimer R. L. The process of infection with bacteriophage phi-X174 VII. Ultracentrifugal analysis of the replicative form. J Mol Biol. 1965 Dec;14(2):327–347. doi: 10.1016/s0022-2836(65)80185-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Parsons J. T., Beard J. W., Beard D., Green M. Mechanism of carcinogenesis by RNA tumor viruses. 3. Formation of RNA, DNA complex and duplex DNA molecules by the DNA polymerase (s) of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1432–1439. doi: 10.1073/pnas.67.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Fanshier L., Leong J. A., Jackson J., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerases of Rous sarcoma virus: kinetics of deoxyribonucleic acid synthesis and specificity of the products. J Virol. 1971 Feb;7(2):227–232. doi: 10.1128/jvi.7.2.227-232.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A. Characteristics of the syrian hamster ribonucleic acid present in cells transformed by polyoma, simian virus 40, or adenovirus 12. J Virol. 1969 Feb;3(2):119–125. doi: 10.1128/jvi.3.2.119-125.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Boettiger D., Temin H. M. A DNA-depenent DNA polymerase and a DNA endonuclease in virions of Rous sarcoma virus. Nature. 1970 Oct 31;228(5270):424–427. doi: 10.1038/228424a0. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- Rokutanda M., Rokutanda H., Green M., Fujinaga K., Ray R. K., Gurgo C. Formation of viral RNA-DNA hybrid molecules by the DNA polymerase of sarcoma-leukaemia viruses. Nature. 1970 Sep 5;227(5262):1026–1028. doi: 10.1038/2271026a0. [DOI] [PubMed] [Google Scholar]
- Rosenbergova M., Lacour F., Huppert J. Mise en évidence d'une activité nucléasique associée au virus de la myéloblastose aviaire, lors de tentatives de purification de ce virus et de son acide ribonucléique. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 10;260(19):5145–5148. [PubMed] [Google Scholar]
- Roulland-Dussoix D., Boyer H. W. The Escherichia coli B restriction endonuclease. Biochim Biophys Acta. 1969 Nov 19;195(1):219–229. doi: 10.1016/0005-2787(69)90618-2. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J. DNA synthesis by RNA-containing tumor viruses. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1034–1041. doi: 10.1073/pnas.67.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- Takanami M. Analysis of the 5'-terminal nucleotide sequences of ribonucleic acids 1. the 5'-termini of Excherichia coli ribosomal RNA. J Mol Biol. 1967 Jan 28;23(2):135–148. doi: 10.1016/s0022-2836(67)80022-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]


