Abstract
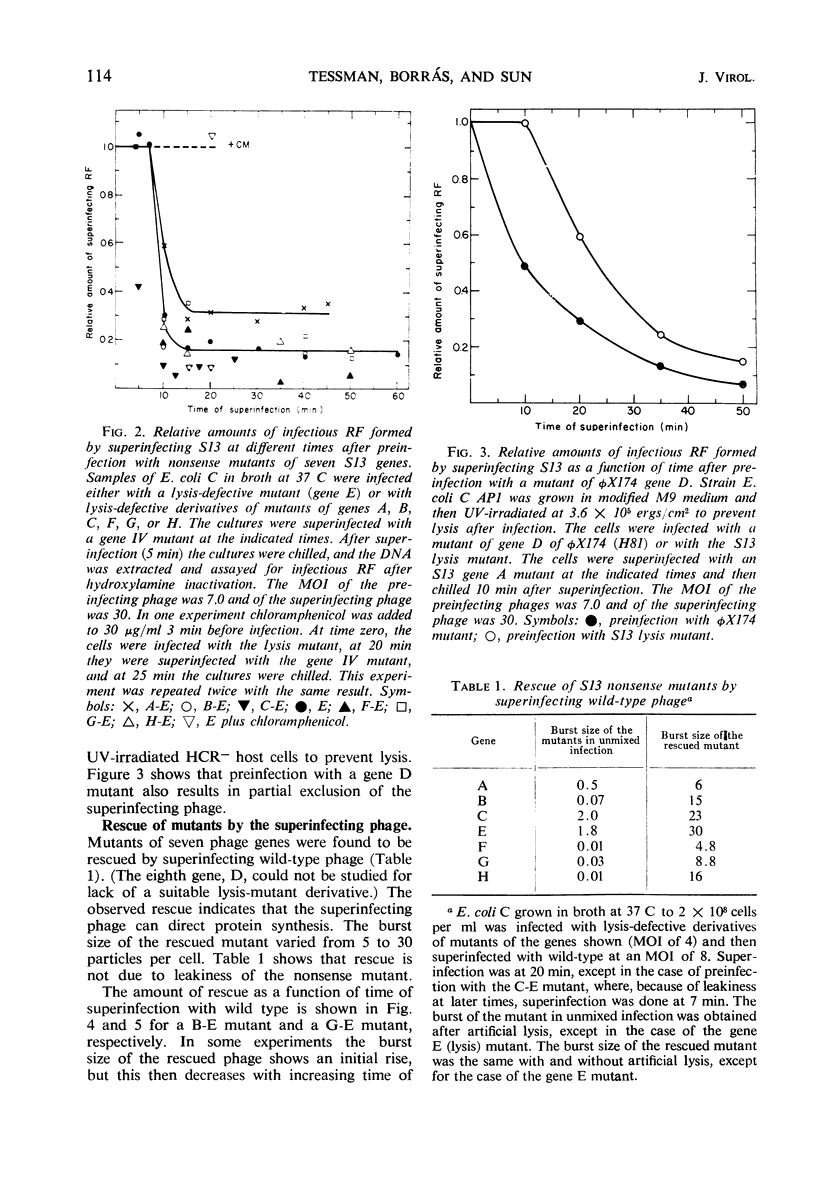
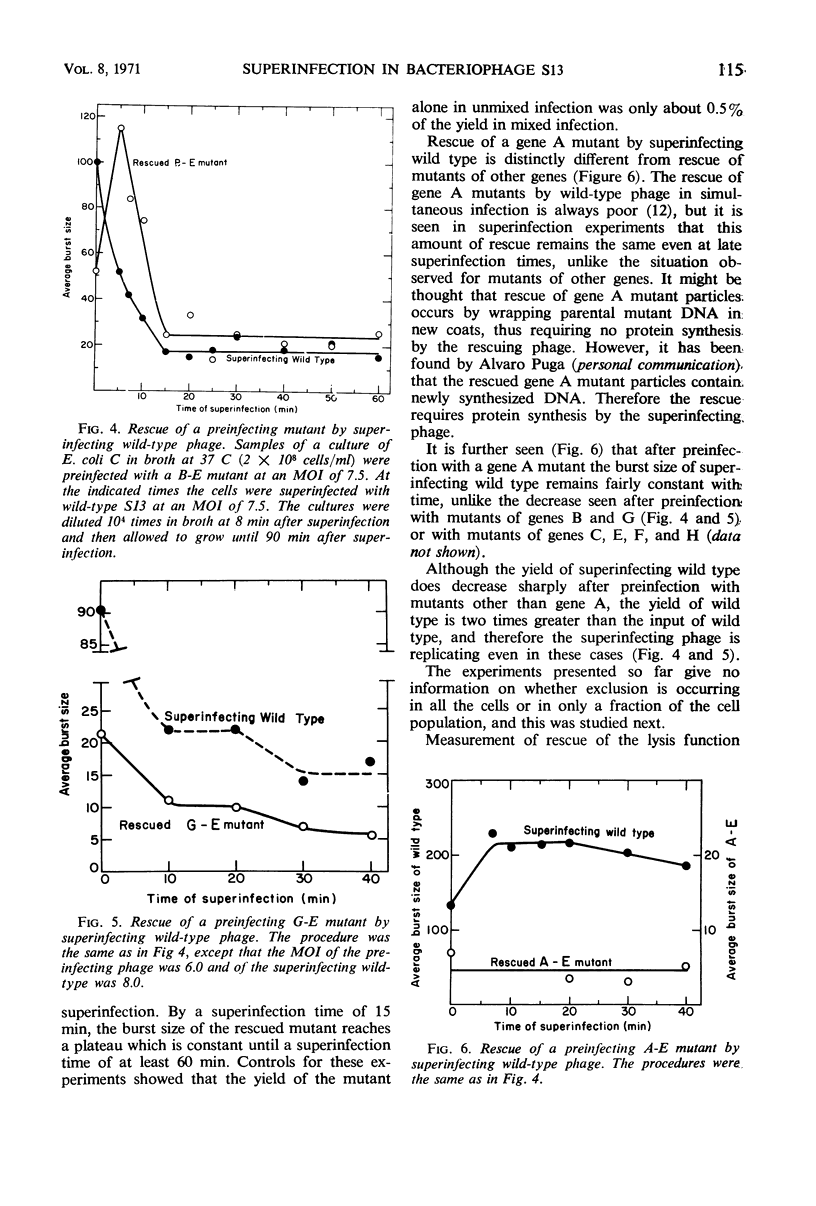
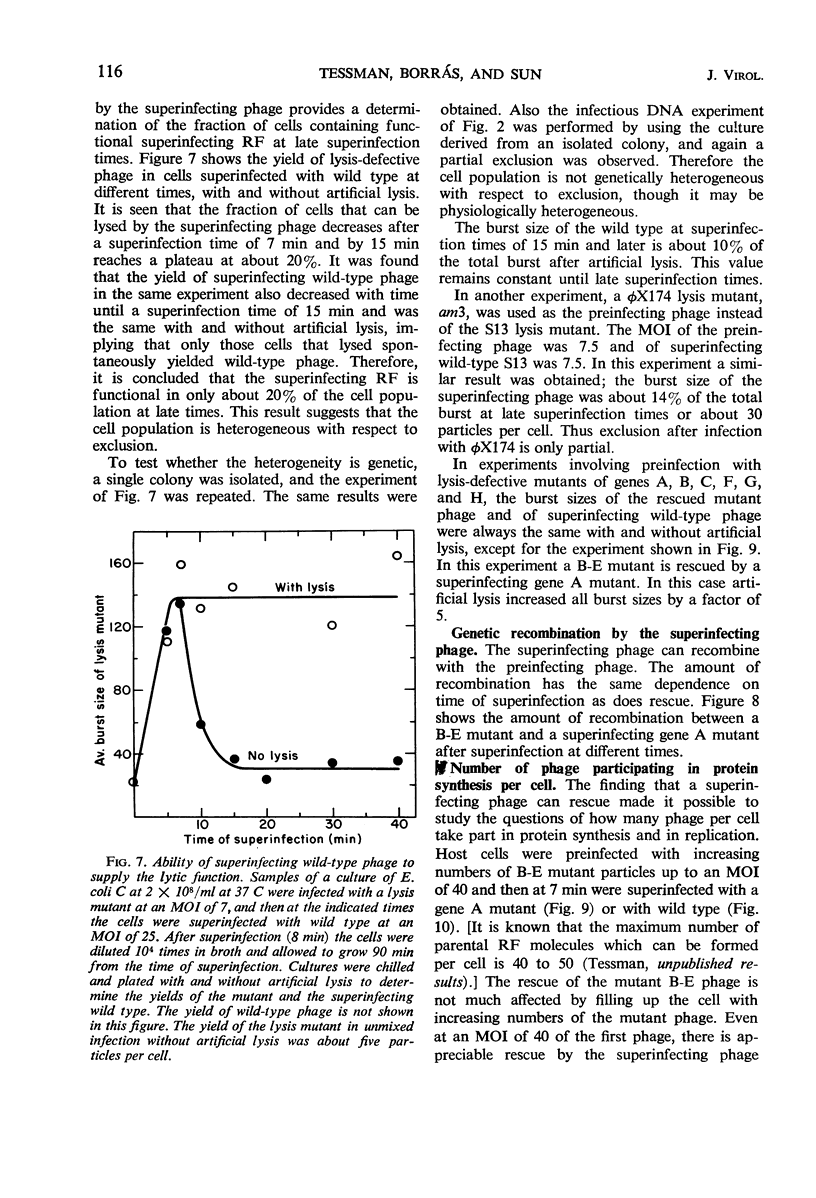
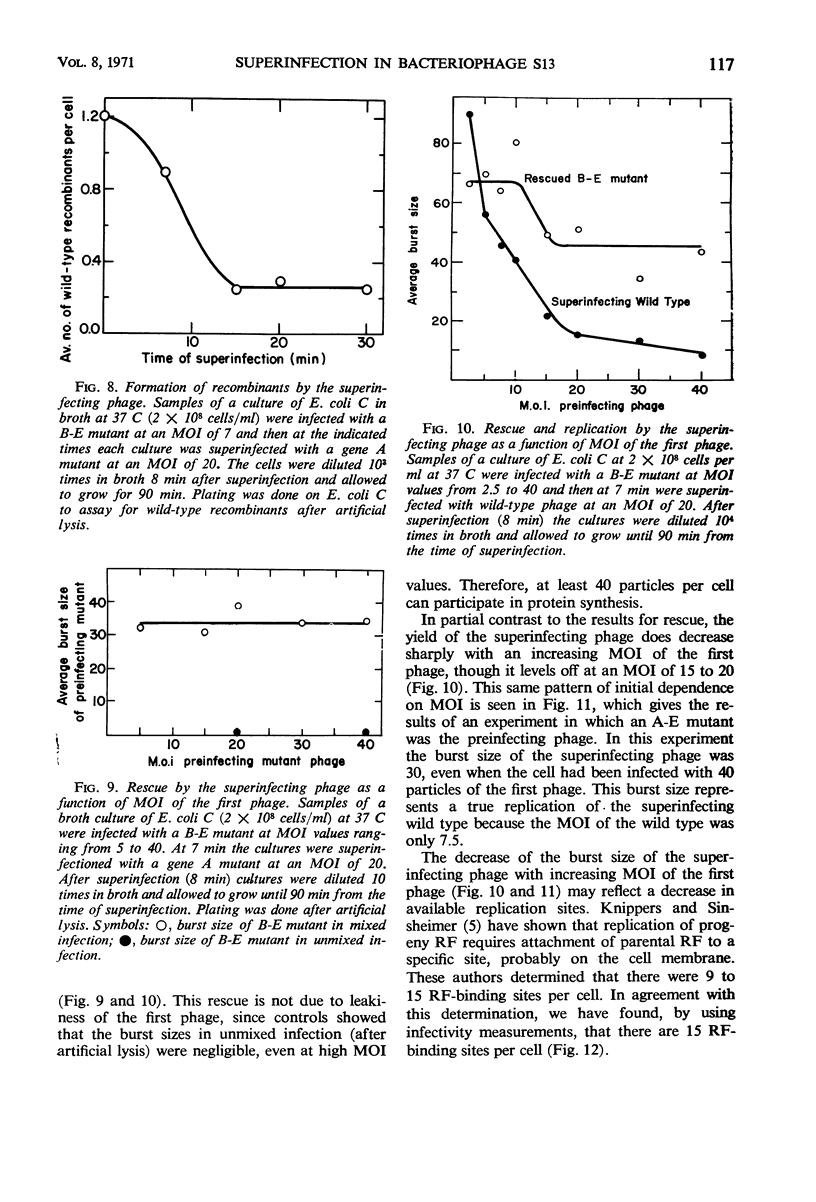
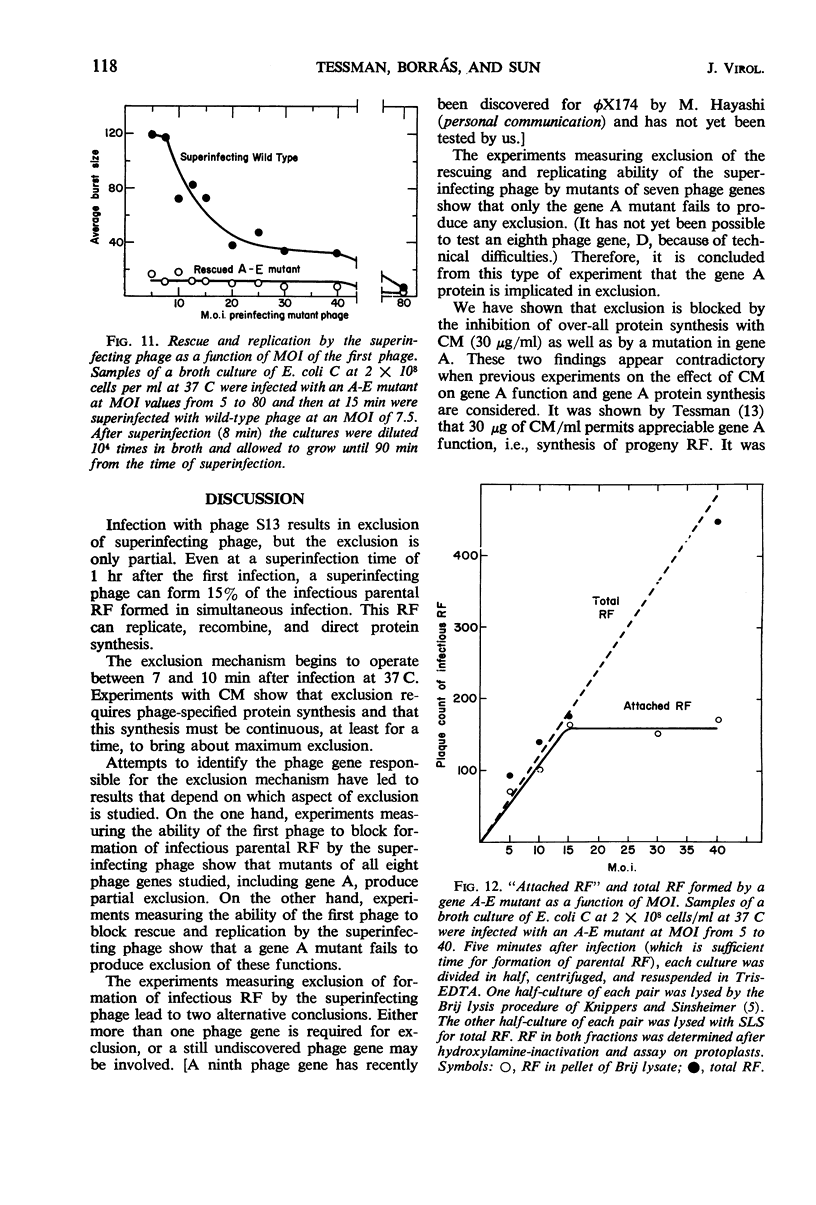
Bacteriophage S13 shows exclusion of superinfecting homologous phage, but the exclusion is only partial. The superinfecting phage can form infectious replicative form deoxyribonucleic acid (RF), can direct protein synthesis, and can form progeny particles even at a superinfection time as late as 60 min after the first infection. Exclusion is also only partial for the closely related phage φX174. Seven min after the first infection, the exclusion mechanism begins to operate, requiring continuous phage-specified protein synthesis. The gene A protein (required for synthesis of progeny RF) appears to be involved in the exclusion mechanism. In superinfection experiments, it was found that at least 40 phage particles per cell can replicate and can carry out protein synthesis, though the number of sites for binding of RF to the membrane is only about 15 per cell. The results suggest that attachment of RF to a binding site is not required for protein synthesis. Evidence is presented that non-attached parental RF can serve as a template for single-stranded deoxyribonucleic acid synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gelfand D. H., Hayashi M. Electrophoretic characterization of phiX174-specific proteins. J Mol Biol. 1969 Sep 28;44(3):501–516. doi: 10.1016/0022-2836(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Jeng Y., Gelfand D., Hayashi M., Shleser R., Tessman E. S. The eight genes of bacteriophages phi X174 and S13 and comparison of the phage-specified proteins. J Mol Biol. 1970 Apr 28;49(2):521–526. doi: 10.1016/0022-2836(70)90262-7. [DOI] [PubMed] [Google Scholar]
- Knippers R., Salivar W. O., Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXVI. Transfer of the parental DNA of bacteriophage phiX174 into progeny bacteriophage particles. J Mol Biol. 1969 Feb 14;39(3):641–654. doi: 10.1016/0022-2836(69)90150-8. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Komano T., Knippers R., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XXII. Synthesis of progeny single-stranded DNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):911–916. doi: 10.1073/pnas.59.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXVII. Synthesis of a viral-specific chloramphenicol-resistant protein in phiX174-infected cells. J Mol Biol. 1969 Feb 14;39(3):655–668. doi: 10.1016/0022-2836(69)90151-x. [DOI] [PubMed] [Google Scholar]
- Shleser R., Ishiwa H., Mannes B., Tessman E. S. Early replicative form DNA of bacteriophage S13. I. Sucrose gradient analysis of replicative form made by gene IV mutants. J Mol Biol. 1968 May 28;34(1):121–129. doi: 10.1016/0022-2836(68)90238-6. [DOI] [PubMed] [Google Scholar]
- Shleser R., Puga A., Tessman E. S. Synthesis of replicative form deoxyribonucleic acid and messenger ribonucleic acid by gene IV mutants of bacteriophage S13. J Virol. 1969 Oct;4(4):394–399. doi: 10.1128/jvi.4.4.394-399.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. B. General inhibition of Escherichia coli macromolecular synthesis by high multiplicites of bacteriophage phiX174. J Mol Biol. 1970 Jan 28;47(2):215–229. doi: 10.1016/0022-2836(70)90341-4. [DOI] [PubMed] [Google Scholar]
- Stone A. B. Some factors which influence the replication of the replicative form of bacteriophage OX174. Biochem Biophys Res Commun. 1967 Feb 8;26(3):247–254. doi: 10.1016/0006-291x(67)90113-1. [DOI] [PubMed] [Google Scholar]
- TESSMAN E. S. COMPLEMENTATION GROUPS IN PHAGE S13. Virology. 1965 Feb;25:303–321. doi: 10.1016/0042-6822(65)90208-4. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Yarus M. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. 8. Evidence for an essential bacterial "site". J Virol. 1967 Feb;1(1):135–144. doi: 10.1128/jvi.1.1.135-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


