Abstract
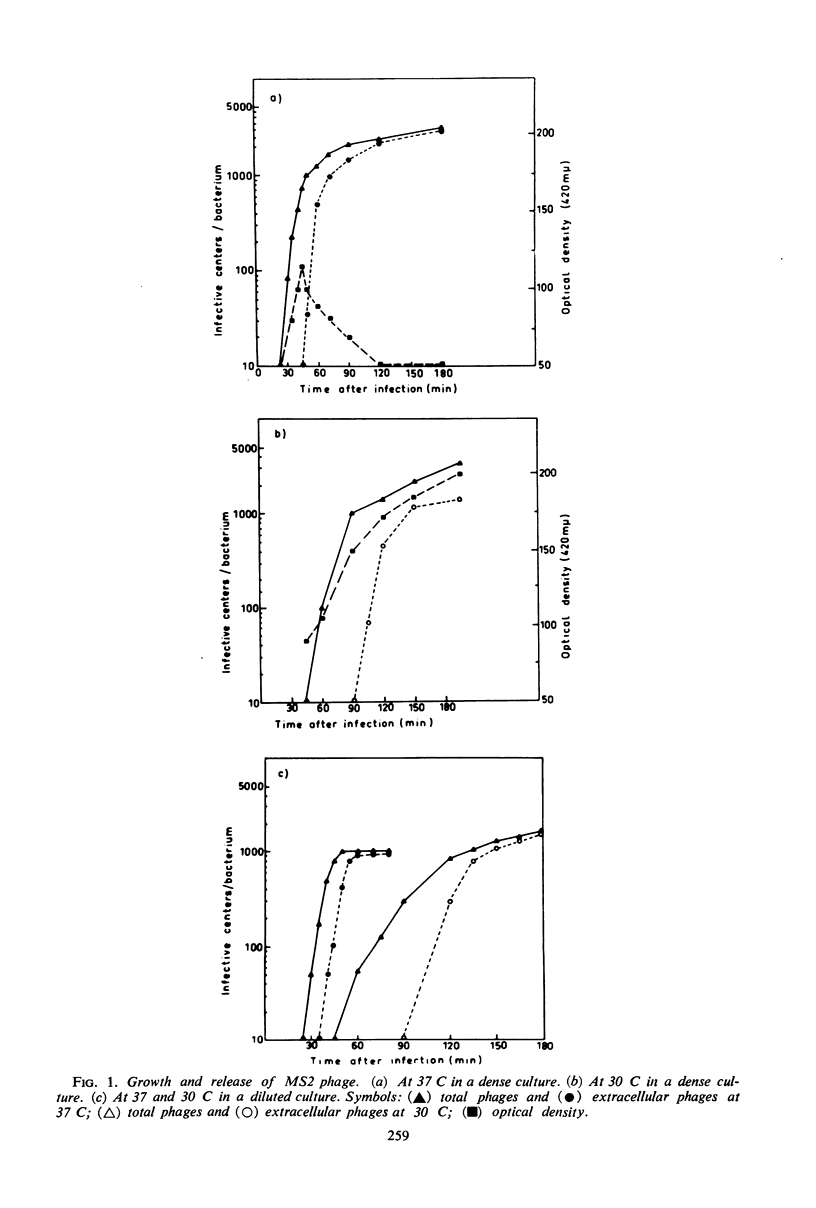
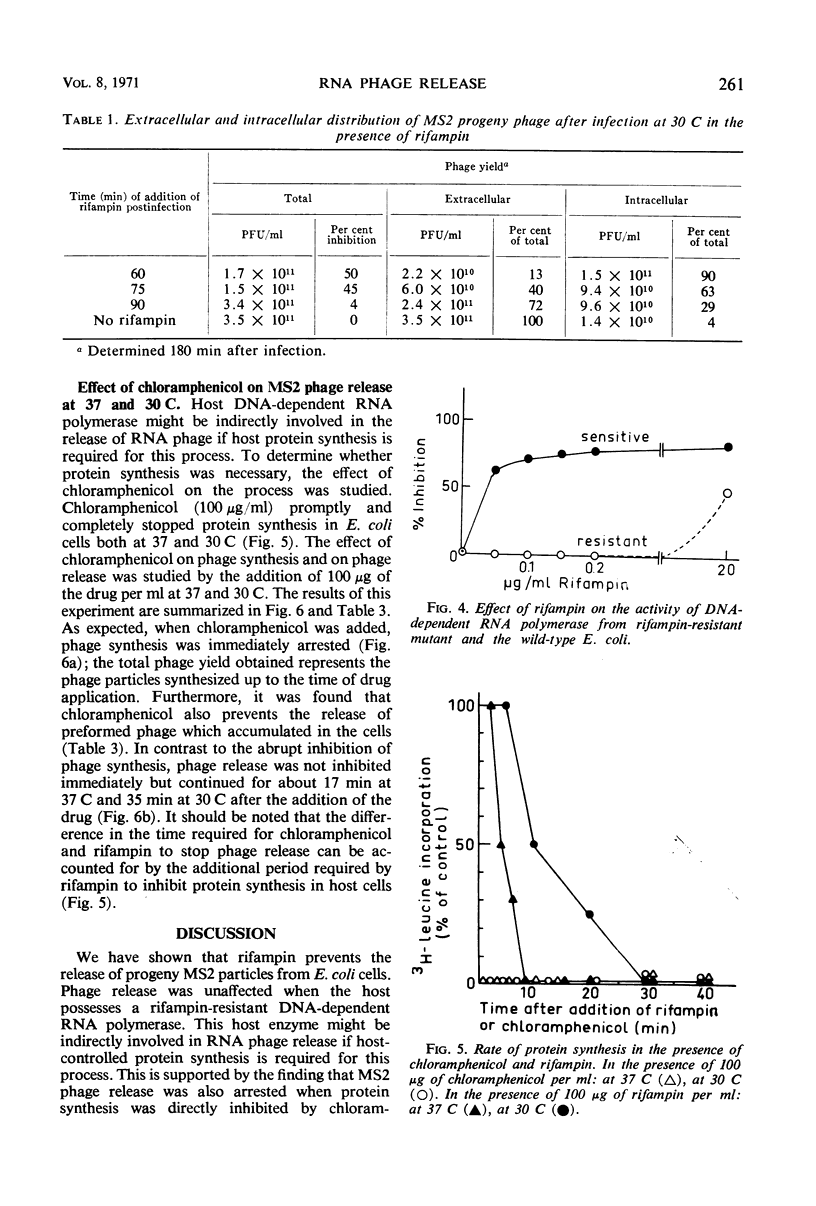
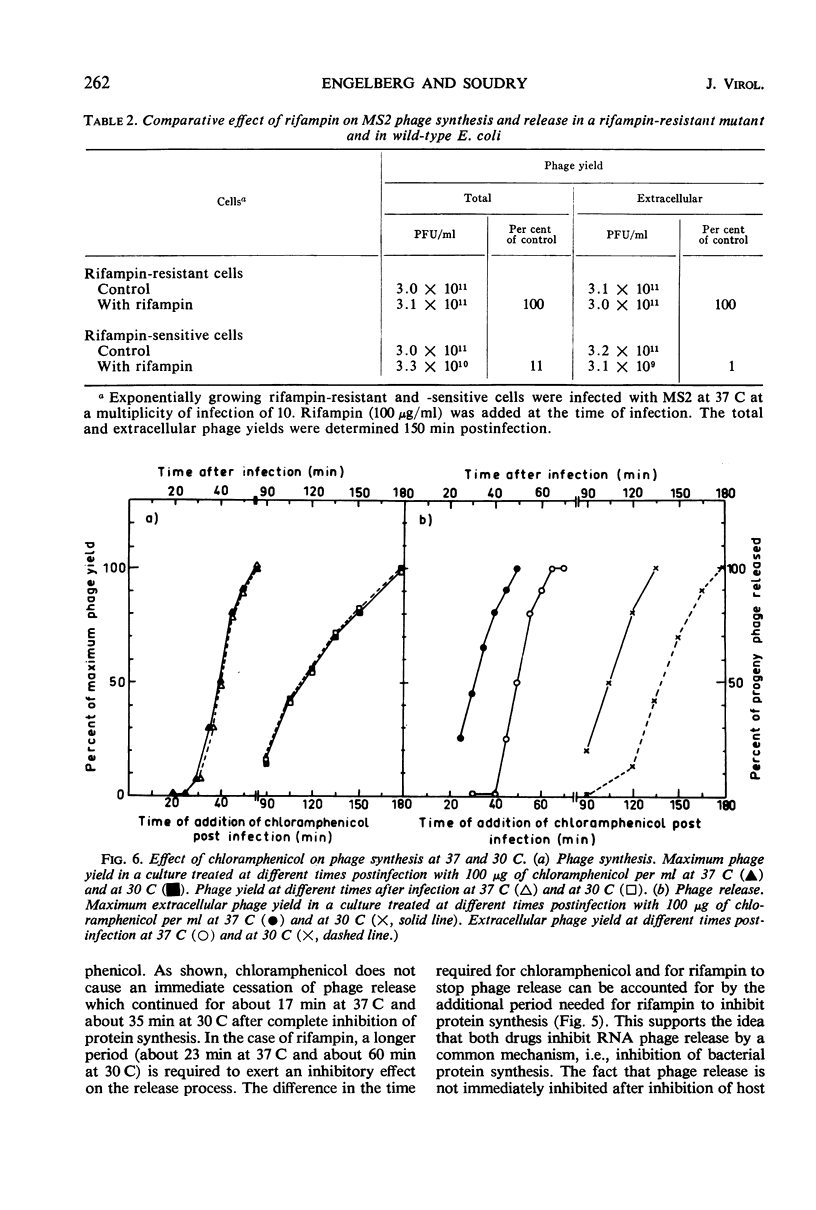
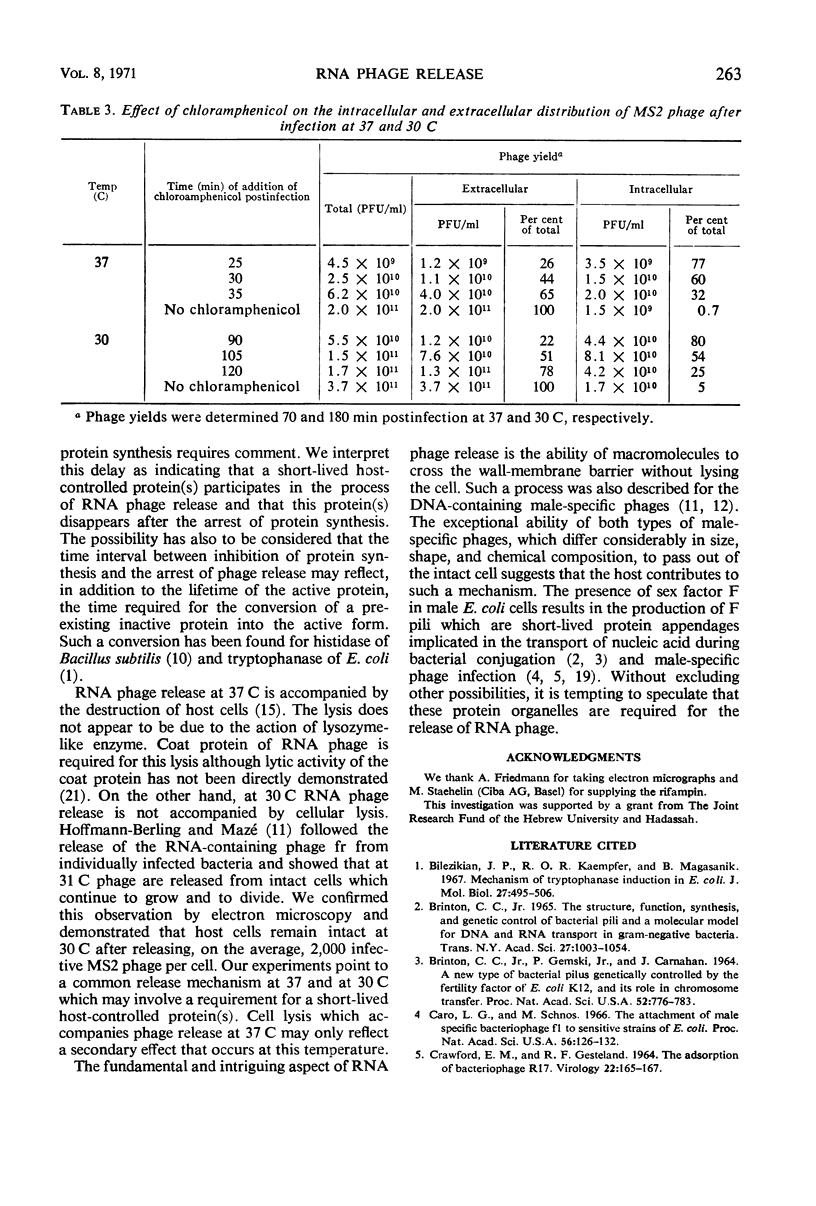
The release of the ribonucleic acid (RNA)-containing phage MS2 from Escherichia coli is accompanied by cellular lysis at 37 C, whereas at 30 C phage are released from intact cells. Chloramphenicol or rifampin prevents the release of progeny phage particles at both temperatures. Neither drug causes an immediate cessation of phage release and after inhibition of protein synthesis by chloramphenicol phage release proceeds for about 17 min at 37 C and about 35 min at 30 C. Rifampin does not inhibit phage release from mutant cells possessing a rifampin-resistant deoxyribonucleic acid-dependent RNA polymerase. The results indicate that a short-lived host-controlled protein(s) is essential for the release of RNA phage particles at both temperatures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J. P., Kaempfer R. O., Magasanik B. Mechanism of tryptophanase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):495–506. doi: 10.1016/0022-2836(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromageot H. P., Zinder N. D. Growth of bacteriophage f2 in E. coli treated with rifampicin. Proc Natl Acad Sci U S A. 1968 Sep;61(1):184–191. doi: 10.1073/pnas.61.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MOLECULAR BASIS OF HISTIDASE INDUCTION IN BACILLUS SUBTILIS. J Mol Biol. 1963 Oct;7:401–420. doi: 10.1016/s0022-2836(63)80033-9. [DOI] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancini G. C., Sartori G. Rifamycins LXI: in vivo inhibition of RNA synthesis of rifamycins. Experientia. 1968 Nov 15;24(11):1105–1106. doi: 10.1007/BF02147783. [DOI] [PubMed] [Google Scholar]
- Marino P., Baldi M. I., Tocchini-Valentini G. P. Effect of rifampicin on DNA-dependent RNA polymerase and on RNA phage growth. Cold Spring Harb Symp Quant Biol. 1968;33:125–127. doi: 10.1101/sqb.1968.033.01.016. [DOI] [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., STRAND M. COMPLEXES OF F-PILI AND RNA BACTERIOPHAGE. Science. 1965 Apr 23;148(3669):511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D., Lyons L. B. Cell Lysis: Another Function of the Coat Protein of the Bacteriophage f2. Science. 1968 Jan 5;159(3810):84–86. doi: 10.1126/science.159.3810.84. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]



