Abstract
The GABAergic system in the central amygdala (CeA) plays a major role in ethanol dependence and the anxiogenic-like response to ethanol withdrawal. Alcohol dependence is associated with increased corticotropin releasing factor (CRF) influence on CeA GABA release and CRF type 1 receptor (CRF1) antagonists prevent the excessive alcohol consumption associated with dependence. Genetically-selected Marchigian Sardinian (msP) rats have an overactive extrahypothalamic CRF1 system, are highly sensitive to stress, and display an innate preference for alcohol. The present study examined differences in CeA GABAergic transmission and the effects of ethanol, CRF and a CRF1 antagonist in msP, Sprague-Dawley, and Wistar rats using an electrophysiological approach. We found no significant differences in membrane properties or mean amplitude of evoked GABAA-inhibitory postsynaptic potentials (IPSPs). However, paired-pulse facilitation (PPF) ratios of evoked IPSPs were significantly lower and spontaneous miniature inhibitory postsynaptic current (mIPSC) frequencies were higher in msP rats, suggesting increased CeA GABA release in msP as compared to Sprague-Dawley and Wistar rats. The sensitivity of spontaneous GABAergic transmission to ethanol (44 mM), CRF (200 nM) and CRF1 antagonist (R121919, 1 μM) was comparable in msP, Sprague Dawley, and Wistar rats. However, a history of ethanol drinking significantly increased the baseline mIPSC frequency and decreased the effects of a CRF1 antagonist in msP rats, suggesting increased GABA release and decreased CRF1 sensitivity. These results provide electrophysiological evidence that msP rats display distinct CeA GABAergic activity as compared to Sprague Dawley and Wistar rats. The elevated GABAergic transmission observed in naïve mSP rats is consistent with the neuroadaptations reported in Sprague Dawley rats after the development of ethanol dependence.
Keywords: amygdala, GABA, alcohol, CRF, CRF1 antagonist, electrophysiology
1. Introduction
Alcoholism is an etiologically and clinically heterogeneous disorder in which compulsive alcohol seeking and use represent core symptoms (McLellan et al., 1992). Protracted and excessive exposure to alcohol is a necessary precondition. However, environment and heritability factors play a dramatic role in controlling individual vulnerability to developing alcohol abuse (Bierut et al., 1998; Cloninger et al., 1981; Crabbe, 2002; Lovinger and Crabbe, 2005; Prescott and Kendler, 2000; Sigvardsson et al., 1996). Increasing evidence points to extrahypothalamic corticotropin releasing factor (CRF) neurotransmission and to its interaction with the GABA and the glutamate systems as one of the major neuroadaptive mechanisms in response to alcohol exposure (Koob, 2006).
The significance of the CRF system in shaping genetic predisposition to alcohol abuse has also emerged. For example, in a human study, 14 Crhr1 polymorphisms and 2 haplotype tagging SNPs were identified and analyzed for association with the drinking phenotype (Treutlein et al., 2006). These findings support the hypothesis that genetic variation at the corticotropin releasing factor1 receptor (CRF1) locus, in association with environmental factors, contributes to increased sensitivity to stress and may facilitate the evolution of alcohol dependence in humans (Enoch and Goldman, 1999; Pohorecky, 1991; Treutlein et al., 2006). Of note, comparable mutations at loci encoding for CRF1 receptors has co-segregated with genetic selection for excessive drinking also in genetically selected Marchigian Sardinian (msP) rats (Hansson et al., 2006). These animals drink excessive amounts of ethanol in a binge-type pattern (leading to blood alcohol levels as high as 100–120 mg/dl), are highly sensitive to stress and stress-induced alcohol seeking, show an anxiety-like phenotype, and have depressive-like symptoms that recover following ethanol drinking (Ciccocioppo et al., 2006; Ciccocioppo et al., 1999). Similar to rats with a history of ethanol dependence (Funk et al., 2006; Funk et al., 2007; Roberto et al., 2010) alcohol-preferring msP rats are highly sensitive to administration of CRF1 antagonists. Administration of CRF1 antagonists significantly reduces ethanol drinking and stress-induced reinstatement in msP rats (Gehlert et al., 2007; Hansson et al., 2006). Recent behavioral and genetic studies in msP rats indicate that alteration in the central nucleus of the amygdala (CeA) neurotransmission may play an important role in shaping their innate vulnerability to excessive drinking (Economidou et al., 2008; Hansson et al., 2007; Hansson et al., 2006). The CeA is a major component of the extended amygdala and is implicated in the behavioral responses to stressors and physiological responses associated with alcohol consumption (Funk et al., 2006) and anxiety (Davis et al., 2010; Koob and Volkow, 2010; LeDoux et al., 1988). We have previously demonstrated that ethanol enhances GABAergic transmission in CeA neurons via activation of CRF1 neurotransmission; an effect that is increased following a history of protracted alcohol exposure (Roberto et al., 2010; Roberto et al., 2003). The purpose of the present study was to identify whether there are specific functional electrophysiological differences in the CeA GABAergic system of msP rats compared to Sprague-Dawley and Wistar rats, and how ethanol and CRF regulates GABAergic neurotransmission in the CeA of msP rats with or without a history of alcohol drinking. Here, we observed an increased baseline spontaneous GABAergic transmission in the CeA of msP rats compared to Sprague-Dawley and Wistar rats. We did not observe any strain differences in the effects of ethanol, CRF and a CRF1 antagonist on CeA GABAergic synapses of alcohol naïve rats. Specifically, both ethanol and CRF significantly increased the GABAergic transmission via increased GABA release and the CRF1 antagonist decreased GABAergic transmission. In msP rats voluntary alcohol drinking further increased the baseline spontaneous GABA release resembling previous data in dependent rats (Roberto et al., 2010). More compellingly, in msP rats the ethanol drinking experience reduced the sensitivity of the CeA GABAergic system to CRF1 antagonism.
In summary, the present study provides evidence that the msP rat line, which is characterized by vulnerability to excessive alcohol drinking and innate hypersensitivity to stress, shows distinct CeA GABAergic activity compared to Sprague Dawley and Wistar rats.
2. Methods
2.1. Animals
In the present electrophysiological study, we used 54 adult male msP rats bred for three generations in the Committee on the Neurobiology of Addictive Disorders, at The Scripps Research Institute obtained from the 64th generation of msP rats previously bred at the University of Camerino (Italy). For the strain comparison, adult male Wistar (n = 21) and adult male Sprague-Dawley (n = 16) rats obtained from Charles River (Raleigh, NC) were used. Male Sprague-Dawley, Wistar and msP rats were housed in a temperature- and humidity-controlled room on a 12-h light/dark cycle (lights on at 6:00 am) with food and water available ad libitum. We conducted all care, msP colony breeding and surgical procedures in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the Institutional Animal Care and Use Committee (IACUC) policies of The Scripps Research Institute.
Electrophysiological studies
2.2 Slice preparation
We prepared CeA slices as previously described (Cruz et al., 2012; Roberto et al., 2003; Roberto et al., 2004), from 16 male Wistar rats (435.2 ± 26 g), 20 male Sprague-Dawley rats (395 ± 34 g), and 54 genetically selected Marchigian Sardinian (msP) (425.2 ± 16 g) rats that were anesthetized with isoflurane (1–3%) and decapitated. We cut transverse slices 300–400 μm thick on a Leica 1000S vibratome (Campden, Lafayette, Indiana), incubated them in an interface configuration for ~30 min, and then completely submerged and continuously superfused (flow rate of 2–4 ml/min) them with warm (31° C), equilibrated with 95% O2/5% CO2 artificial cerebrospinal fluid (aCSF) of the following composition (in mM): NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4 · 7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10. Drugs were added to the aCSF from stock solutions to obtain known concentrations in the superfusate.
2.3. Intracellular recording of evoked responses
We recorded from CeA neurons (from the medial subdivision of the CeA) with sharp micropipettes filled with 3M KCl using discontinuous current-clamp mode (Cruz et al., 2012; Haubensak et al., 2010; Roberto et al., 2004). We held most neurons near their resting membrane potential (RMP). Data were acquired with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) and stored for later analysis using pClamp software (Axon Instruments, Foster City, CA). We evoked pharmacologically-isolated GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode while superfusing the slices with the glutamate receptor blockers 6,7-Dinitroquinoxaline-2,3-dione (DNQX; 20 μM) and DL-2-amino-5-phosphonovalerate (APV; 30 μM), and the GABAB receptor antagonist (CGP 55845A; 1 μM). At the end of the recording, to confirm the GABAAergic nature of the IPSP we often superfused 30 μM bicuculline (or 50 μM picrotoxin). To determine the synaptic response parameters for each cell, we performed an input-output (I-O) protocol (Roberto et al., 2003; Roberto et al., 2004) consisting of a range of five current stimulations (50–250 mA; 0.125 Hz), starting at the threshold current required to elicit an IPSP up to the strength required to elicit the maximum amplitude. These stimulus strengths were maintained throughout the entire duration of the experiment. We quantified the synaptic responses by calculating the IPSP amplitude with Clampfit software (Axon Instruments). We examined paired-pulse facilitation (PPF) in each neuron using paired stimuli at 50 msec inter-stimulus interval (Roberto et al., 2004). The stimulus strength was adjusted such that the amplitude of the first IPSP was 50% of maximal, determined from the I-O relationship. We calculated the PPF ratio as the second IPSP amplitude over that of the first IPSP.
2.4. Whole-cell patch-clamp recording of miniature IPSCs
We recorded from CeA neurons visualized in brain slices (300 μm) using infrared differential interference contrast (IR-DIC) optics and CCD camera (EXi Aqua and ROLERA-XR, QImaging) and (Cruz et al., 2012; Gilpin et al., 2011). A w60 water immersion objective (Olympus) was used for identifying and approaching CeA neurons. Whole-cell voltage-clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices), low-pass filtered at 2–5kHz, digitized (Digidata 1440A; Molecular Devices), and stored on a PC using pClamp 10 software (Axon Instruments). All voltage-clamp were performed in a gap-free acquisition mode with a sampling rate per signal of 10 KHz. Patch pipettes (3–5M′Ω) were pulled from borosilicate glass (Warner Instruments) and filled with an internal solution composed of (in mM): 145 KCl; 0.5 EGTA; 2 MgCl2; 10 HEPES; 2 Na-ATP; 0.2 Na-GTP. GABAergic miniature IPSCs (mIPSCs) were recorded in the presence of 20 μM DNQX, 30 μM DL-AP5, 1 μM CGP 55845A and 1 μM tetrodotoxin (TTX). Drugs were constituted in aCSF and applied by bath superfusion. All 124 cells were clamped at −60 mV for the duration of the recording. In all experiments, series resistance (<10 M′Ω) was continuously monitored with a 10 mV hyperpolarizing pulse and experiments with >20% change in series resistance were not included in the final analysis. Frequency, amplitude and kinetics of miniature IPSCs were analyzed and visually confirmed using a semi-automated threshold-based mini detection software (Mini Analysis, Synaptosoft Inc., Fort Lee, NJ). To accurately determine the mIPSC amplitude, only mIPSCs that were > 5 pA were accepted for analysis. The choice of this cutoff amplitude for acceptance of mIPSCs was made to obtain a high signal-to-noise ratio. Averages of mIPSC characteristics were based on a minimum time interval of 3-5 min and a minimum of 50 events. All detected events were used for event frequency analysis, but superimposed events were eliminated for amplitude and decay kinetic analysis. All data are expressed as mean ± SEM.
2.5. Drugs
We purchased CGP 55845A, DL-AP5, picrotoxin and bicuculline from Sigma (St. Louis, MO), CRF from Chempacific Corp (Baltimore, MD), Tetrodotoxin from Biotum (Hayward, CA); DNQX from Tocris (Ellisville, MO) and ethanol from Remet (La Mirada, CA). R121919 was synthesized by Dr. Kenner Rice at the Drug Design and Synthesis Section, Chemical Biology Research Branch, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD.
2.6. Data analysis and statistics
To analyze data acquired from intracellular and whole cell recordings, we used Clampfit 10.2 (Molecular Devices) and Mini Analysis 5.1 software (Synaptosoft, Leonia, NJ), respectively. We used GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA) and Statistica 7 Package (StatSoft) for all statistical analysis of results. The mIPSC results were evaluated with cumulative probability analysis, and statistical significance was determined using the Kolmogorov-Smirnov, non-parametric two-sample test (Van der Kloot, 1991) with p < 0.05 considered significant for each neuron. The pooled data for each experimental condition were then analyzed by paired t-test analyses for individual means comparisons to evaluate single drug (ethanol, CRF, R121919) effect within the same group or within-subject one-way repeated measures ANOVA to evaluate multiple drugs (R121919, R121919 + ethanol) effect. To assess differences resulting from rat strain (Wistar x Sprague-Dawley x msP) and drug interaction between groups, we also used two-way with one factor between (strain) and one factor within (treatment) ANOVA. When appropriate, the Student Newman-Keuls post hoc test was used to assess significance between treatments. We accepted statistical significance at the p< 0.05. All averaged values are presented as mean ± SEM.
2.7. Two bottles choice paradigm
Twenty msP rats, housed two per cage, were divided into two groups (n = 10 subject/group). One group was offered a free choice between water and 10% ethanol (v/v) 24 hours a day for 4 weeks. The second group received only water. Fluids were offered in plastic bottles equipped with metallic drinking spouts. Bottles were weighed daily to measure 10% ethanol and water consumption. For each rat, water and ethanol intake was estimated by dividing the amount of fluids measured from the bottles by two (number of rats per cage) and averaging the body weight of the animals. Alcohol intake was reported in g/kg.
3. Results
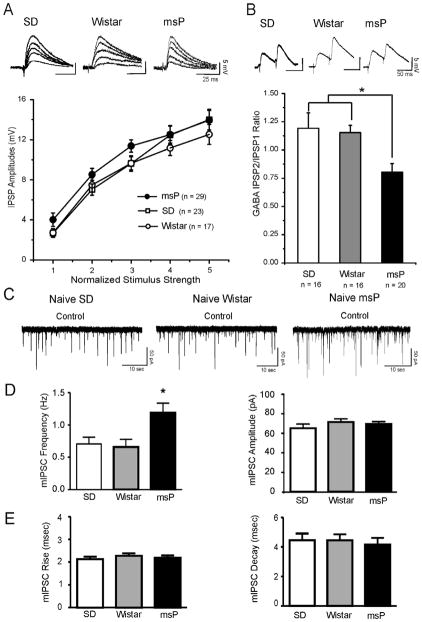
3.1. Baseline spontaneous but not evoked GABAergic responses are elevated in naïve msP compared to Sprague-Dawley and Wistar rats
Using intracellular current-clamp mode, we recorded from 69 CeA neurons from either naïve male Sprague-Dawley (SD), Wistar or msP control rats with a mean RMP of −78 ± 1.0 mV and a mean input resistance of 139 ± 6.6 MΩ. The three groups did not show significant differences in these properties (Table 1), and there were no significant differences in the membrane capacitance (Table 1) or the voltage–current relationship (not shown). We evoked pharmacologically isolated GABAA-IPSPs (IPSPs) by stimulating locally within the CeA. Baseline IPSP input–output curves generated by equivalent stimulus intensities were similar in CeA neurons from msP (n = 29) and in those from Wistar (n = 17) and Sprague Dawley (n = 23) rats (Figure 1A), suggesting no strain difference on evoked GABAergic transmission. Although the data from I/O relationships showed a tendency toward increased amplitude of the evoked IPSPs in CeA neurons from msP rats compared to Wistar and Sprague Dawley, this did not reach statistical significance for any of the intensities (ANOVA, F (2,66)= 2.27, p=0.11 for the middle intensity).
Table 1.
Basal membrane properties of the CeA neurons from Sprague Dawley, Wistar and msP rats.
| Sprague Dawley (n = 23) | Wistar (n = 17) | msP (n = 29) | |
|---|---|---|---|
| Resting membrane potential (mV) | −77.5 ± 1.1 | −78.9 ± 0.8 | −78.1 ± 1.0 |
| Input resistance (MΩ) | 139.5 ± 6.9 | 140.4 ± 6.1 | 138.1 ± 6.8 |
| Membrane Capacitance (pF) | 91.9 ± 15.5 | 96.3 ± 7.5 | 105.8 ± 5.0 |
Figure 1.
A: Top Panel: Representative recordings of evoked GABAA-IPSP amplitudes in CeA neurons from Sprague Dawley (SD), Wistar and msP rats. Bottom Panel: The input-output curves of mean baseline GABAergic transmission are similar in CeA neurons from msP (n = 29), SD (n = 23) and Wistar rats (n = 17). B: Top Panel: Representative recordings of evoked 50 msec PPF of IPSPs in CeA neurons from msP, SD and Wistar rats. Bottom Panel: Histograms plotting the baseline PPF ratio of IPSPs in CeA neurons from msP, SD and Wistar rats. In the msP (n = 20) baseline PPF ratios are significantly (*p< 0.05; ANOVA) lower than SD (n = 16) and Wistar (n = 16) neurons. Error bars represent SEM. C: Representative mIPSC recordings in CeA neurons from SD, Wistar and msP rats. D: Left Panel: Mean ± SEM frequency of mIPSCs from CeA neurons from msP (n = 42), SD (n = 42) and Wistar (n = 16) rats. In msP rats the frequency of mIPSCs is significantly (*p< 0.05, ANOVA) higher than SD and Wistar rats. Right Panel: Mean ± SEM of the mIPSC amplitude in the three rat lines. E: Left Panel: Mean ± SEM rise time of mIPSCs from the CeA neurons from the msP, SD and Wistar rats (same neurons of Panel D). Right Panel: Mean ± SEM of the mIPSC decay time in the three rat lines. There are not significant differences in the three groups.
We then examined PPF of the evoked IPSPs at 50 ms inter-stimulus intervals. Generally, changes in PPF are inversely related to transmitter release (Andreasen and Hablitz, 1994). We found a significant (ANOVA, F(2, 49) 5.674; p = 0.02) difference in the basal PPF ratio of IPSPs from neurons of msP (n = 20) compared to Wistar (n = 16) and Sprague-Dawley rats (n = 16) rats (Fig. 1B), suggesting augmented baseline evoked GABA release in the msP rats. The PPF ratio of IPSPs from neurons of Wistar and Sprague-Dawley rats were not significantly different (Fig. 1B).
To further characterize the increased GABA release, we also examined miniature IPSCs (mIPSCs) using whole-cell recordings in the presence of 1 μM TTX to eliminate action potential-dependent release of neurotransmitter. Notably, in CeA neurons from msP rats, the mean baseline frequency of mIPSCs was significantly (ANOVA, F(2,97)=5.028; p = 0.0084) greater compared with CeA neurons from both Sprague-Dawley and Wistar rats (Fig. 1C and D, left panel), suggesting increased spontaneous basal GABA release in msP rats. The frequency of mIPSCs was not significantly different in Sprague-Dawley and Wistar rats. In contrast, no strain differences were observed in the mean mIPSC amplitude (Sprague-Dawley: 65.3 ± 4.1 pA; Wistar: 71.5 ± 3.34 pA; msP: 69.4 ± 2.6 pA, Fig. 2D, right panel) or in mIPSC rise or decay time (Fig. 2E).
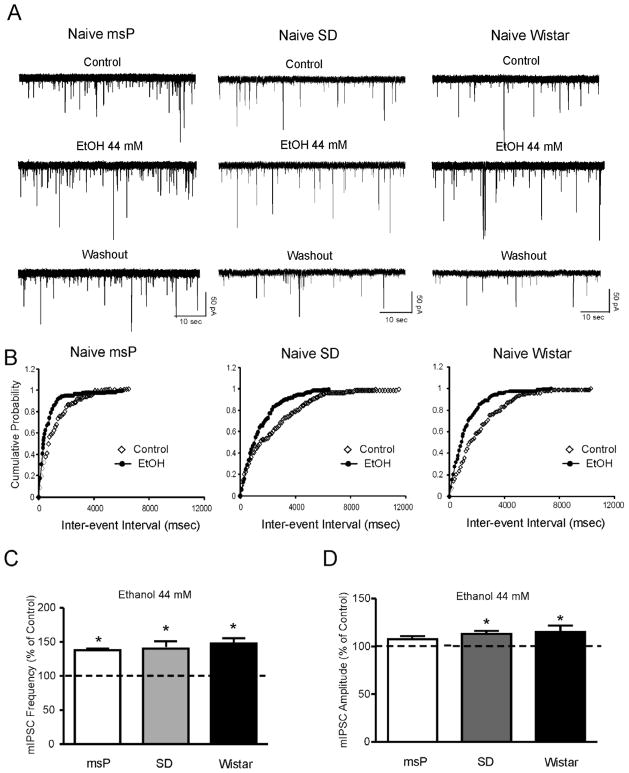
Figure 2.
A: Representative mIPSC recordings in CeA neurons from msP, SD and Wistar rats in control conditions, during application of 44 mM ethanol, and washout. B: Cumulative frequency histogram for the representative neurons of A showing a shift to the left, indicating a shorter inter-event interval (higher frequencies) during the application of 44 mM ethanol. C: Mean ± SEM frequency of mIPSCs in CeA neurons from msP, SD and Wistar rats. Ethanol significantly (*p< 0.05; Student’s t-test) increased the mean mIPSC frequency in each strain, suggesting that ethanol increases presynaptic GABA release. D: Mean ± SEM amplitude of mIPSCs in CeA neurons from msP, SD and Wistar rats. Ethanol does not significantly change the overall mean mIPSC amplitude in mSP rats, but significantly increases mIPSC amplitude in SD and Wistar rats (*p< 0.05).
3.2. Naïve msP, Sprague-Dawley and Wistar rats do not differ in ethanol- and CRF-induced enhancement of CeA GABAergic transmission
Confirming our previous studies, here we found that 44 mM ethanol significantly (p<0.05) increased mIPSC frequencies to 138 ± 2.3% of control in 6/7 CeA neurons of Sprague Dawley rats (Fig. 2A and B). One neuron did not respond to ethanol. The ethanol-induced increase in mIPSC frequency was similar in 6/7 neurons from Wistar rats (140.7 ± 10% of control, p<0.05) and 19/25 msP rats (147.7 ± 7.9% of control, p<0.05), respectively (Fig. 2A, and C). One neuron from Wistar and 6 neurons from msP rats did not respond to ethanol and were not included in the analysis. Overall, the ethanol-induced increase in the mIPSC frequency in the three animal groups was not significantly (F(2,28)=0.0993; p=0.7) different, indicating that ethanol effects on GABA release are equivalent in all three strains. In addition, in each rat strain, 15–20% of the neurons tested did not respond to ethanol. Figure 2B shows that 44 mM ethanol significantly shifted the cumulative frequency distribution to shorter inter-event intervals in CeA neurons of the three rat strains. The increase in mIPSC frequency induced by ethanol returned to control values at about 10–15 minutes of washout (SD: 103.5 ± 9%, Wistar 95.3 ± 8%, msP: 97.2 ± 7%; data not shown). Ethanol also significantly increased the amplitude of mIPSCs in 3/6 CeA neurons from Sprague Dawley and Wistar rats and 8/19 CeA neurons from msP rats (Fig. 2D). The mIPSC kinetics were not altered in any of the animal groups (data not shown).
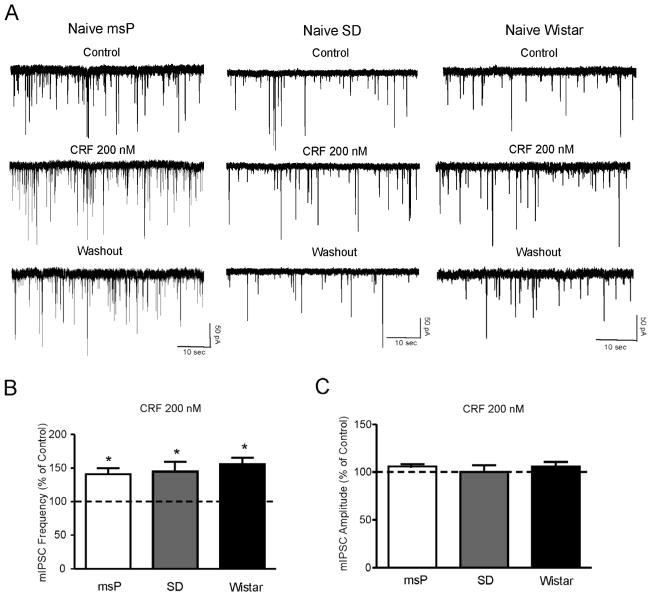
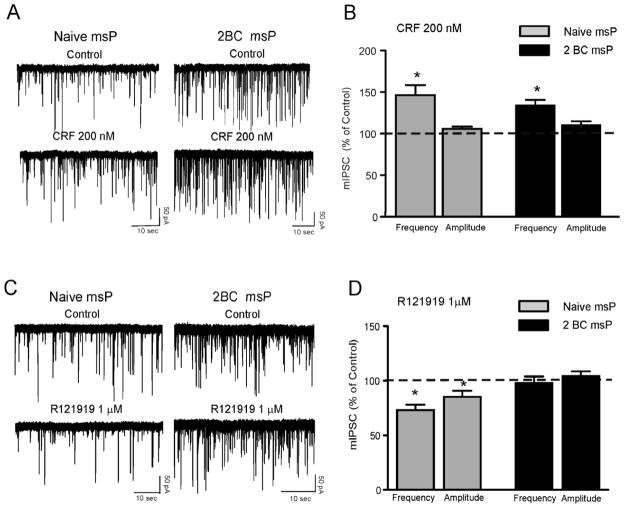
We also tested 200 nM CRF and found that, like ethanol, CRF significantly (p<0.05; n = 9/10) increased mIPSC frequencies to 140.9 ± 9% of control in neurons of msP rats (Fig. 3A and B). Similarly, CRF significantly increased mIPSC frequencies to 155.7 ± 10% of control in neurons from Wistar rats (p<0.05; n = 6/7) and to 144.9 ± 14% of control in neurons from Sprague Dawley rats (p<0.05; n = 7/9) (Fig. 3A and B). Overall, the CRF-induced increase of GABA release in the three strains was not significantly (F(2,20)= 0.0083; p= 0.9) different. The CRF-induced enhancement of mIPSC frequency returned to control values at about 15 minutes of washout (Sprague Dawley: 104.2 ± 8%; Wistar: 106.7 ± 10% and msP: 98.9 ± 9%; data not shown). CRF also significantly enhanced the amplitude of mIPSCs in 2/9 CeA neurons from msP rats, and in 1/6 neurons from both Sprague Dawley and Wistar rats. CRF did not alter the kinetics of mIPSC in any of the animal groups (data not shown).
Figure 3.
A: Representative mIPSC recordings in CeA neurons from msP, SD and Wistar rats in control condition, during application of 200 nM CRF, and washout. B: Mean ± SEM frequency of mIPSCs in CeA neurons from msP, SD and Wistar rats. CRF significantly (*p< 0.05; Student’s t-test) increased the mean mIPSC frequency in all three strains, suggesting increased presynaptic GABA release. C: Mean ± SEM amplitude of mIPSCs in CeA neurons from msP, SD and Wistar rats. CRF does not change the mean mIPSC amplitude.
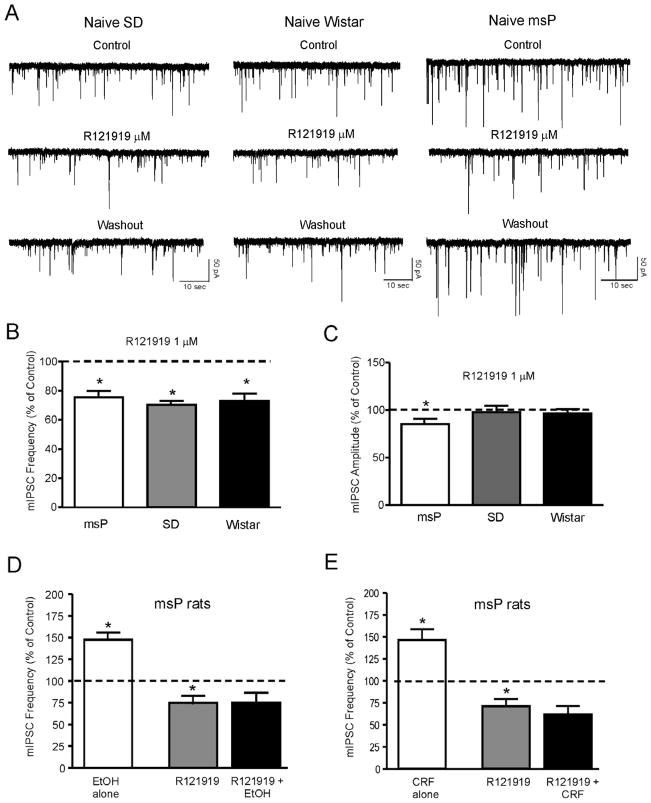
Next we tested the sensitivity of the GABAergic synapses to the CRF1 antagonist, R121919 (Roberto et al., 2010). We found that 1 μM R121919 significantly decreased the frequency of mIPSCs in all three groups of rats. Specifically, R121919 decreased mIPSC frequency to 71.05 ± 5.1% of control (p<0.05; n = 12) in msP rats, to 75.5 ± 4.3% of control (p<0.05; n = 7) in Sprague Dawley rats, and to 70.2 ± 2.8% (p<0.05; n = 6) in Wistar rats, (Fig. 4A and B). R121919 significantly decreased the amplitude of mIPSCs in msP rats but not in Wistar and Sprague Dawley rats (Fig. 4B). We did not observe alteration in the kinetics of mIPSCs in any of the animal groups.
Figure 4.
A: Representative mIPSC recordings in CeA neurons from msP, SD and Wistar rats in control condition, during application of the CRF1 antagonist R121919 (1μM) and washout. B: Mean ± SEM frequency of mIPSCs in CeA neurons from msP, SD and Wistar rats. R121919 significantly (*p< 0.05; Student’s t-test) decreased the mean mIPSC frequency in all three strains, suggesting decreased presynaptic GABA release. C: Mean ± SEM amplitude of mIPSCs in CeA neurons from msP, SD and Wistar rats. In CeA neurons from msP rats, R121919 significantly (*p< 0.05; Student’s t-test) decreased the mean mIPSC amplitude, indicating postsynaptic effects. D: Mean ± SEM frequency of mIPSCs in CeA neurons from msP rats. R121919 significantly (*p< 0.05; within-subject one-way repeated measures ANOVA) decreased the mean mIPSC frequency and blocked the ethanol-induced increase in mIPSC frequency. E: Mean ± SEM frequency of mIPSCs in CeA neurons from msP rats. R121919 significantly (*p< 0.05; within-subject one-way repeated measures ANOVA) decreased the mean mIPSC frequency and blocked the CRF-induced increase in mIPSC frequency.
In the CeA of Sprague Dawley rats (Roberto et al., 2010) and mice (Nie et al., 2004; Nie et al., 2009), CRF1 antagonists blocked the ethanol- and CRF-induced increase in mIPSCs frequencies. Thus, in a separate group of CeA neurons from msP rats, we also tested whether a CRF1 antagonist would block the ethanol and CRF effects on GABA release. In 5/5 CeA neurons from msP rats, R121919 significantly decreased mIPSC frequencies and completely blocked the ethanol- (Fig. 4D) and the CRF-induced (Fig. 4E) increase in mIPSC frequency.
3.3. In msP rats a history of voluntary ethanol drinking increases baseline GABAergic activity and reduces the sensitivity to CRF1 antagonism
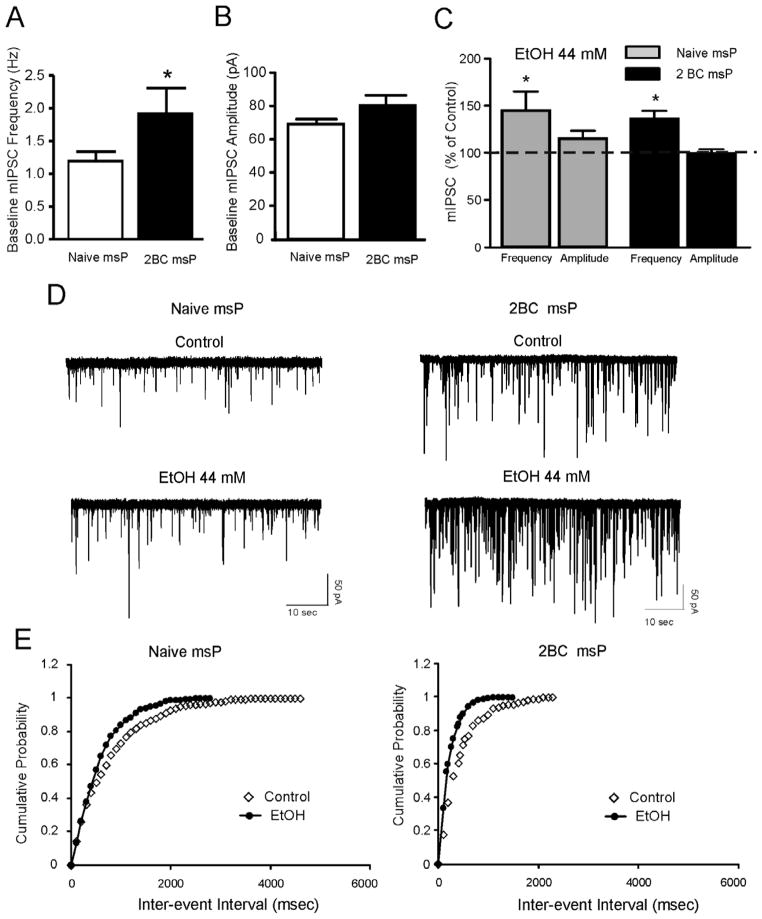
Male msP rats (n=10) were allowed to drink alcohol in a 2 bottle choice paradigm (choice between 10% alcohol and tap water) for 24 hours a day for 4 weeks. A control msP group (n=10) received only water. The msP rats rapidly learned to drink alcohol and baseline intake (7–9 g/kg per day) was reached after the first drinking week. Using these animals we performed whole-cell recordings of mIPSCs in the CeA and found that in msP rats with a history of voluntary ethanol drinking, the frequency of mIPSCs was significantly higher (2.35 ± 0.4 Hz, n=21) compared to naïve age-matched msP rats (1.29 ± 0.1, n = 24) (Fig. 5A and D). In ethanol-exposed msP rats, both acute ethanol (44 mM) (Fig. 5C, D and E) and CRF (200 nM) (Fig. 6A and B) significantly (p< 0.05) increased the frequency of mIPSCs to 129.4 ± 9.6% (n = 6) and to 134.0 ± 6.7% (n = 9) of control respectively. These effects were slightly, but not significantly different from those obtained in naïve msP rats (ethanol: 147.7 ± 7.9%; n = 19; CRF: 146.4 ± 12%; n = 9). This apparent reduction in sensitivity to acute alcohol and CRF application is likely due to the increase in baseline mIPSC frequency following a history of alcohol intake. Notably, in contrast to naïve msP rats, in alcohol-exposed msP rats, R121919 (1 μM) failed to reduce mIPSC frequencies (97.7 ± 6.0; n = 9) and amplitudes (103.8 ± 3.8; n = 9) (Fig. 6C and D).
Figure 5.
A: Mean ± SEM frequency of mIPSCs in CeA neurons from msP rats with or without ethanol history. Basal spontaneous CeA GABAergic transmission is significantly (*p< 0.05) enhanced in msP rats exposed to ethanol drinking via two bottle choice paradigm (designated as 2BC) compared to age-matched alcohol naïve msP (designated as naïve msP) rats. B: Mean ± SEM amplitude of mIPSCs in CeA neurons from naïve msP and 2BC msP rats. C: Ethanol significantly (*p< 0.05; Student’s t-test) increased the mean mIPSC frequency in each group. The ethanol effect was comparable in the two groups (p > 0.05; unpaired t-test). D: Representative mIPSC recordings in CeA neurons from ethanol naive msP (left) and ethanol exposed msP (right) rats. E: Cumulative frequency histogram for the representative CeA neurons of D showing a shift to the left, indicating a shorter inter-event interval (higher frequencies) during the application of 44 mM ethanol in both naïve msP (left panel) and 2BC msP (right panel) rat. Note that in the CeA neuron from the ethanol exposed msP rat, the control inter-event interval is shorter (higher frequencies) than that from naïve msP rat.
Figure 6.
A:Representative mIPSC recordings in CeA neurons from ethanol naive msP (left) and msP rats exposed to ethanol drinking via two bottle choice paradigm (2BC; right) before and during application of CRF.B: CRF (200 nM) significantly (*p< 0.05; Student’s t-test) increased the mean mIPSC frequency but not the mean mIPSC amplitude in naïve and ethanol-exposed rats. The CRF effect was comparable in the two groups (p > 0.05; unpaired t-test). C: Representative mIPSC recordings in CeA neurons from ethanol naive msP (left) and ethanol exposed msP (right) rats before and during application of R121919. D: R121919 (1 μM) significantly (*p< 0.05; Student’s t-test) decreased both the mean frequency and amplitude of mIPSC in ethanol naïve, but not in ethanol exposed msP rats.
4. Discussion
The msP rat line shows excessive daily ethanol drinking (7–9 g/kg) that occurs in binge-like bouts of consumption and that results in blood alcohol levels as high as 100–120 mg/dl (Ciccocioppo et al., 2006). Gene expression studies revealed that msP rats have an innate upregulation of CRF1 mRNA linked to two single nucleotide polymorphisms occurring in the promoter region (position −1836 and −2097) of the gene encoding for the CRF1 receptor in several limbic brain areas though HPA axis activity is comparable to that observed in heterogeneous Wistar rats (Hansson et al., 2007). Voluntary ethanol drinking normalizes extrahypothalamic over-expression of the gene encoding for the CRF1 receptor and reduces stress-induced responses in these animals. Hence, it has been hypothesized that an innate propensity to excessive drinking in this rat line is an attempt to compensate for the negative affect-like effects (i.e., anxiety and depression) linked to overactivity of the extrahypothalamic CRF system. Although a role of CRF signaling in ethanol drinking behaviors in msP rats has been previously reported, a functional analysis of the ethanol and CRF effects in the CeA, an area critical for emotional control and alcohol reinforcement, has until now remained uninvestigated. Here, we hypothesized that GABAergic transmission and CRF signaling in the medial subdivision of the CeA is altered in msP rats compared to Sprague Dawley and Wistar rats. Thus, the purpose of the present study is to characterize the electrophysiological effects of ethanol, CRF and a CRF1 antagonist on GABAergic transmission in msP rats with or without a history of voluntary alcohol intake. Our electrophysiological data show that 1) the evoked baseline CeA GABAergic transmission is not different in msP compared to Sprague Dawley and Wistar rats, 2) the PPF ratio of evoked GABAergic responses is reduced in msP rats compared to Sprague Dawley and Wistar rats suggesting increased evoked GABA release, 3) vesicular action potential-independent GABA release is significantly enhanced in msP compared to Sprague Dawley and Wistar rats, 4) in naive msP, Sprague Dawley and Wistar rats, acute application of ethanol and CRF increases vesicular GABA release to a comparable level, while a CRF1 antagonist reduces it to a comparable level, 5) vesicular GABA release is significantly enhanced in msP rats with a history of ethanol drinking compared to age-matched naïve control msP rats and the inhibitory effect of CRF1 antagonist on vesicular GABA release is lost.
Strain-specific differences in GABAergic transmission in the CeA
Our previous electrophysiological studies of ethanol and CRF effects on GABAergic transmission were obtained using CeA neurons from Sprague Dawley rats (Roberto et al., 2010; Roberto et al., 2003; Roberto et al., 2004), while the Wistar strain is the background strain for msP rats (Ciccocioppo et al., 2006) and for many of the behavioral studies performed in this rat line. Thus, to examine the possibility of strain differences in CeA electrophysiological activity we investigated both evoked and spontaneous GABAergic transmission in CeA neurons from Sprague-Dawley, Wistar and msP rats. Although we did not find significant strain differences in evoked GABAA-IPSP amplitudes, in msP rats the paired pulse stimulation of the evoked IPSPs at inter stimulus intervals of 50 ms resulted in paired-pulse depression rather than paired-pulse facilitation. These data suggest that while the basal probability of evoked transmitter release was not affected, the processes involved in paired pulse modulation were altered. For example, in the evoked recording configuration presynaptic release could be negatively affected by alterations in Ca2+ influx by residual Ca2+, and/or a postsynaptic release-dependent depression due the unavailability of postsynaptic receptors (Atasoy et al., 2008; Fredj and Burrone, 2009; Ramirez and Kavalali, 2011). Paired-pulse depression is a form of synaptic plasticity that plays an important role in neural coding (O’Donovan and Rinzel, 1997) and acts as low-pass filter (Fortune and Rose, 2001). Paired-pulse depression can serve as a gain control mechanism during and after burst activation (Varela and Sherman, 2007) and it may contribute to the generation of synchronous network activity (Tsodyks et al., 2000). Usually, synapses with higher average release probability will be more likely to display depression when activated at short intervals (Thomson, 2000). We found significantly increased baseline mIPSC frequency in the CeA of msP compared to Sprague Dawley and Wistar rats, indicating increased vesicular GABA release. Thus, the enhanced basal spontaneous GABA release observed in CeA of msP rats may cause a decrease in the probability of evoked GABA release and/or depletion of readily-releasable pool of transmitter and/or postsynaptic changes in the quantal amplitude, (i.e., receptor desensitization) mechanisms. Overall our data are in agreement with the growing body of literature suggesting that the processes leading to mIPSCs and evoked ISPCs are not always coordinately regulated (Chung et al., 2010; Fredj and Burrone, 2009; Sara et al., 2005; Wasser et al., 2007; Wasser and Kavalali, 2009).
Ethanol and CRF effects on GABAergic transmission in the CeA
We have previously reported that ethanol increases mIPSC frequency in CeA neurons of Sprague Dawley rats (Roberto et al., 2003; Roberto et al., 2004). These results were confirmed in the present study in which we also demonstrated that ethanol-induced increases of vesicular release of GABA in the CeA of Sprague Dawley rats is equivalent to that of Wistar and msP rats. In addition, as in Sprague Dawley rats (Roberto et al., 2010) and mice (Nie et al., 2004; Nie et al., 2009), CRF robustly increased GABAergic transmission in the CeA of Wistar and msP rats. In the CeA neurons of msP rats we show significantly higher baseline mIPSC frequencies compared to Sprague Dawley and Wistar rats. Despite these higher baseline mIPSC frequencies both ethanol and CRF were able to further increase GABA release at these synapses. Previous studies in our laboratory also showed that CRF1 antagonism and CRF1 receptor deletion blocked alcohol-induced enhancement of CeA GABAergic transmission (Nie et al., 2004; Roberto et al., 2010). Here, we expanded this observation showing that R121919 also decreased mIPSCs frequencies at the CeA synapse of msP rats. Moreover, we showed that the R121919 inhibitory effect on the frequency of the mIPSCs was similar in msP, Wistar, and Sprague Dawley rats. Notably, R121919 also had a significant inhibitory effect on mIPSC amplitude only in CeA neurons from msP rats, suggesting a postsynaptic site of action of the CRF1 antagonist, specific to the msP strain. We speculate that blockade of CRF1 signaling by R121919 may initiate a cascade of the intracellular pathway that alters the postsynaptic function of the GABAA receptors. Both GABAA receptors subunit expression and function can be regulated by protein kinases (Brandon et al., 2002). For example, protein kinase A (PKA) is one of the second messengers engaged by CRF receptor activation (Cruz et al., 2011; Papadopoulou et al., 2004) activated by CRF1 (via Gs and Gq proteins) (Hanoune and Defer, 2001; Papadopoulou et al., 2004). PKA can increase or decrease GABAA receptor function, effects likely contingent on the type of β subunit expressed, the extent of receptor phosphorylation, and the manner of PKA activation (McDonald and Moss, 1997; Nusser et al., 1999; Poisbeau et al., 1999). Specifically, the inhibition of PKA has implications for the uncoupling and internalization of GABAA receptors (Ali and Olsen, 2001; Brown and Bristow, 1996). Hence, the R121919-induced reduction in mIPSC amplitude, which likely reflects a loss of functional receptors at the post-synaptic membrane (Nusser et al., 1998; Poisbeau et al., 1997), could be a result of decreased local PKA activity. More persistent changes than those resulting from receptor internalization might be expected to result from alterations in subunit gene expression.
Here we show that R121919 also blocked ethanol- and CRF-induced enhancement of GABA release in the CeA from msP rats with an efficacy comparable to previous studies carried out in Sprague Dawley rats (Roberto et al., 2010). Taken together, these results suggest that the msP strain-dependent differences in baseline GABAergic synaptic transmission involve presynaptic GABA release in the CeA, and these differences do not directly correlate with any significant differences in the sensitivity of the GABAergic synapses (mainly at the presynaptic site) to either ethanol or CRF. Importantly, CRF1 antagonism may also modulate the GABAergic synapses in the CeA of msP rats at the postsynaptic level.
Neuroplastic changes at GABAergic synapses in the CeA produced by voluntary alcohol drinking
The GABAergic and CRF systems in the CeA have been implicated in the development of ethanol-dependence and withdrawal (Gilpin and Roberto, 2012; Koob and Zorrilla, 2010; Menzaghi et al., 1994; Roberto et al., 2010; Roberto et al., 2004; Roberts et al., 1996). Extracellular CRF levels in the CeA are elevated following exposure to stress and development of alcohol dependence (Merlo Pich et al., 1995; Zorrilla et al., 2001). Alcohol withdrawal increases CRF synthesis and release in the CeA (Funk et al., 2006; Roberto et al., 2010; Sommer et al., 2008) and these increases are normalized by alcohol consumption. At the cellular level, we found increased baseline GABAergic transmission via both presynaptic and postsynaptic mechanisms in the CeA of ethanol-dependent Sprague Dawley rats compared to naïve rats (Roberto et al., 2004). Alcohol dependence also increased sensitivity to the effects of CRF and CRF1 antagonists on CeA GABA transmission and increased CeA CRF and CRF1 mRNA levels (Roberto et al., 2010). Similar to ethanol-dependent Wistar rats, ethanol-naïve msP rats have elevated CRF1 transcript expression in several brain areas linked to stress and addiction and their voluntary alcohol drinking is reduced by CRF1 antagonists (Hansson et al., 2006). Importantly, in the present study we show that naïve msP rats display enhanced CeA GABAergic transmission compared to naïve Sprague Dawley and Wistar rats. Thus, we speculate that the msP line is a phenocopy of ethanol-dependent rats and that their heightened intake of alcohol is triggered by their need to alleviate the negative affect-like state (i.e., anxiety, depression) associated with an overactive CRF system (Ciccocioppo et al., 2006; Hansson et al., 2006). This hypothesis is supported by data showing that in msP rats voluntary alcohol intake reduces CRF1 receptor expression in several brain areas including the CeA, thus reducing the potential detrimental effect of CRF over-activation (Hansson et al., 2007). To some extent this hypothesis is also supported by electrophysiological data from the present study showing that in msP rats after 4 weeks of voluntary alcohol drinking, the baseline spontaneous GABAergic transmission was significantly higher compared to naïve age-matched msP rats. On the other hand, despite the reported down regulation of the CRF1 receptor system in msP rats following alcohol exposure (Hansson et al., 2007), our electrophysiological results show that the increase in GABAergic transmission induced by CRF persisted in msP rats after four weeks of voluntary drinking. Thus, in contrast to Sprague Dawley rats that showed augmented CRF and CRF1 antagonist sensitivity of the CeA GABAergic synapses following ethanol dependence (Roberto et al., 2010), the augmented CRF effects were not observed in msP rats with ethanol history and the CRF1 antagonist effects were completely lost in the CeA from msP rats with alcohol history compared to naïve msP rats. We speculate that ethanol history may alter CRF1 receptor function and/or alter the intracellular signaling affected by CRF1 antagonism at both pre- and postsynaptic sites. Taken together, these data suggest that despite the alterations in the CRF1 signaling and functions induced by ethanol history, CRF is able to increase GABAergic transmission possibly by acting through other receptors (i.e., CRF2) and/or that neuroplastic changes occur at specific synapses within CeA circuitry (medial vs lateral).
In addition, the lack of CRF1 antagonist effect in the CeA from msP with alcohol history compared to alcohol naïve msP rats is in agreement with findings that voluntary alcohol drinking leads to selective down-regulation of CRF1 transcript and function in the CeA (Hansson et al., 2007). Yet, msP rats are highly sensitive to the alcohol drinking and relapse-inhibiting properties of CRF1 antagonists (Gehlert et al., 2007; Hansson et al., 2006) and the CRF peptide antagonist D-Phe 12–41 injected directly into the amygdala decreased dependence-induced drinking (Funk et al., 2006) suggesting that further studies will be required to understand the complex dynamics of CRF1 receptor signaling in msP rats. Furthermore, drinking behavior and relapse may be mediated by overactivity of the CRF1 system in brain areas other than (or not exclusive to) the CeA. For example, current literature supports the possibility that CRF neurotransmission in the BNST and/or the medial raphe nucleus may play an important role in relapse while the VTA could be a critical substrate for CRF effects on alcohol drinking (Huang et al., 2010; Hwa et al., 2012; Le et al., 2002). Other brain regions will have to be examined to fully characterize CRF-related functions in this genetically-selected rat line.
Conclusions
The recruitment of the CRF system had been conceptualized as a mechanism of transition from chronic intermittent alcohol exposure to alcohol dependence (Koob, 2008) and to remain during protracted abstinence after a history of dependence (Rimondini et al., 2002; Valdez et al., 2002). Overall our data support the hypothesis that GABAergic transmission and the recruitment of CRF1 signaling in the CeA play an important role in excessive alcohol intake and that the msP rat line is a relevant model for studying the synaptic changes underlying the development of alcohol dependence. It is also expected that major changes may occur in other nuclei of the amygdala and in the BNST, thus future studies of other brain regions need to be performed for a better understanding of more global CRF-related function in this genetically-selected rat line. In addition, it has been shown that in the CeA, CRF depresses excitatory glutamatergic transmission through activation of CRF1 (Liu et al., 2004). Ongoing studies on CRF-glutamate interaction in msP rats may help clarify how dysregulation of these cellular functions may be linked to the expression of this behavioral phenotype characterized by a high propensity for excessive ethanol drinking. With the growing body of literature demonstrating associations between polymorphisms in human CRHR1 and CRFBP and comorbid stress/anxiety and alcohol use disorders, the present data support a continued focus on CRF1 as a therapeutic target for alcoholism.
GABA release is larger in msP rats compared to Sprague Dawley and Wistar rats
ethanol- and CRF-induced increase in CeA GABA release is comparable in the 3 rat lines
CRF1 antagonist-induced decrease in GABA release is comparable in the 3 rat lines
msP rats with history of ethanol show enhanced GABA release compared to naïve msP
History of ethanol abolishes the CRF1 antagonist-induced decrease in GABA release
Acknowledgments
This is manuscript number 21980 from The Scripps Research Institute. We thank Jenica Tapocik and Sun Hui at The National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, for their technical assistance with genotyping. Supported by grants from NIH: AA17447, AA015566, AA06420, AA01698, F32AA020430 and AA008459. The compound R121919 was synthesized and generously contributed by Dr. Kenner Rice at the Drug Design and Synthesis Section, Chemical Biology Research Branch, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2001;79:1100–1108. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of gamma-aminobutyric acid(A) receptor function and cell surface expression. Pharmacol Ther. 2002;94:113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Bristow DR. Molecular mechanisms of benzodiazepine-induced down-regulation of GABAA receptor alpha 1 subunit protein in rat cerebellar granule cells. Br J Pharmacol. 1996;118:1103–1110. doi: 10.1111/j.1476-5381.1996.tb15512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–1376. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Magnoli EM, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci. 2011;4:207. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatr Clin North Am. 1999;22:289–299. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 2001;24:381–385. doi: 10.1016/s0166-2236(00)01835-x. [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Debold JF, Miczek KA. Alcohol in excess: CRF(1) receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for GABA in alcohol dependence. Adv Pharmacol. 2006;54:205–229. doi: 10.1016/s1054-3589(06)54009-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- McLellan AT, O’Brien CP, Metzger D, Alterman AI, Cornish J, Urschel H. How effective is substance abuse treatment--compared to what? Res Publ Assoc Res Nerv Ment Dis. 1992;70:231–252. [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Scientific World Journal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J Physiol. 1999;521(Pt 2):421–435. doi: 10.1111/j.1469-7793.1999.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MJ, Rinzel J. Synaptic depression: a dynamic regulator of synaptic communication with varied functional roles. Trends Neurosci. 1997;20:431–433. doi: 10.1016/s0166-2236(97)01124-7. [DOI] [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1 alpha receptor-extracellularly regulated kinase signal transduction pathway: The critical role of Ser(301) for signaling switch and selectivity. Molecular Endocrinology. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. Modulation of GABAA receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. J Physiol. 1997;500 (Pt 2):475–485. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Influence of ascertainment strategy on finding sex differences in genetic estimates from twin studies of alcoholism. Am J Med Genet. 2000;96:754–761. [PubMed] [Google Scholar]
- Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. Faseb J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol Psychiatry. 2010:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Uziel A, Markram H. Synchrony generation in recurrent networks with frequency-dependent synapses. J Neurosci. 2000;20:RC50. doi: 10.1523/JNEUROSCI.20-01-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol. 2007;98:3538–3547. doi: 10.1152/jn.00578.2007. [DOI] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser CR, Kavalali ET. Leaky synapses: regulation of spontaneous neurotransmission in central synapses. Neuroscience. 2009;158:177–188. doi: 10.1016/j.neuroscience.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. Epub 2001 Jun 2013. [DOI] [PubMed] [Google Scholar]