Abstract
A new series of unsymmetrical diphenylaminofluorene-based chromophores with various strong π-electron acceptors were synthesized and fully characterized. The systematic alteration of the structural design facilitated the investigation of effects such as molecular symmetry and strength of electron-donating and/or withdrawing termini have on optical nonlinearity. In order to determine the electronic and geometrical properties of the novel compounds, a thorough investigation was carried out by a combination of linear and nonlinear spectroscopic techniques, single crystal X-ray diffraction, and quantum chemical calculations. Finally, on the basis of two-photon absorption (2PA) cross sections, the general trend for π -electron accepting ability, i.e., ability to accept charge transfer from diphenylamine was: 2-pyran-4-ylidene malononitrile (pyranone) > dicyanovinyl > bis(dicyanomethylidene)indane > 1-(thiophen-2-yl)propenone > dicyanoethylenyl > 3-(thiophen-2-yl)propenone. An analog with the 2-pyran-4-ylidene malononitrile acceptor group exhibited a nearly three-fold enhancement of the 2PA< δ (1650 GM at 840 nm), relative to other members of the series.
INTRODUCTION
Conjugated organic molecules with large delocalized π-electron systems continue to be the subject of very active research due to their potential use in nonlinear optics and emerging photonics technologies. Generally, such molecules provide substantial delocalization of π-electrons over the molecule, enhancing polarizability and nonlinear optical properties. Among the various classes of nonlinear optical materials, multiphoton absorbing materials continue to attract increasing interest. Two-photon absorption (2PA) is a nonlinear process that involves the somewhat unusual capability of a molecule to absorb two photons simultaneously in order to populate an energy level within the molecule with energy equal to the sum of the energies of the two photons absorbed.1 2PA-based chromophores are of particular interest because of their utility and potential in numerous applications such as fluorescence bioimaging,2-9 two-photon power-limiting devices,10-12 and two-photon photodynamic therapy.13-15
Dipolar push-pull chromophores perhaps constitute the vast majority of compounds investigated for their nonlinear optical properties.16-20 Push-pull chromophores involve electron-donor and electron-acceptor groups interacting through a π-conjugating spacer. It has been well-established that modulating the degree of ground-state polarization, or the degree of charge separation in the ground state of these molecules, can exert significant influence over their molecular polarizability and hyperpolarizabilities.21, 22 This charge separation in the ground state can be controlled primarily by either modifying the chemical structure, i.e., altering the strength of the donating and accepting substituents, and/or by extending the π-conjugated path.18 The effects of altering the chemical structure in this way were detailed by Oudar in 1977.23
Whereas considerable effort invested in the development of new donor and acceptor groups24-26 has led to major progress in the synthesis of stable and efficient chromophores, the relationships between the structure of the π-conjugated spacer and the nonlinear optical properties remain less clearly understood. Our interest in the control of the electronic properties of fluorene-based π-conjugated systems resulted in various synthetic approaches for enhancement of the 2PA cross section based on linear symmetrical and unsymmetrical fluorene chromophores,27-31 and, recently, two-dimensional branched fluorene-based chromophores.32
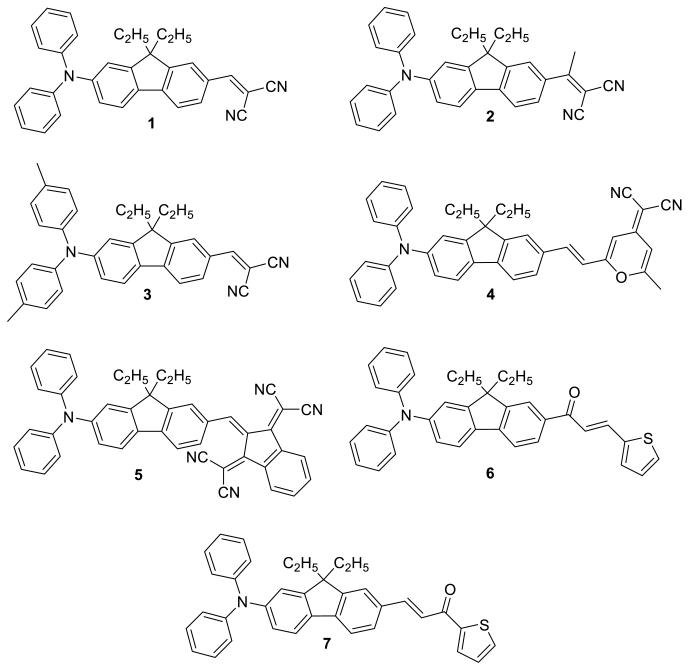
The aromatic character of the fluorene ring can be strongly influenced by the presence of donor or acceptor moieties covalently attached at the 2 and 7 positions. Herein, we have employed this approach in the modification of ground-state polarization by altering the strength of the electron-withdrawing terminal group on a fluorene core using four different electron-acceptor groups that are end-capped with a diphenylamine or di-p-tolylamine donating group. Electron-withdrawing groups include the 2-pyran-4-ylidene malononitrile, dicyanovinyl, bis(dicyanomethylidene)indane, (thiophen-2-yl)propenone, and dicyanoethylenyl (1-7, Chart 1) . In order to determine the electronic and geometrical properties of compounds 1-7, a comprehensive investigation was conducted by a combination of spectroscopic techniques, single crystal X-ray diffraction, and quantum chemical calculations.
Chart 1.
RESULTS AND DISCUSSION
Synthesis
The series of new compounds under discussion are shown in Chart 1. All seven push-pull chromophores consist of an amino functionality as the donor, and four different electron acceptor moieties.
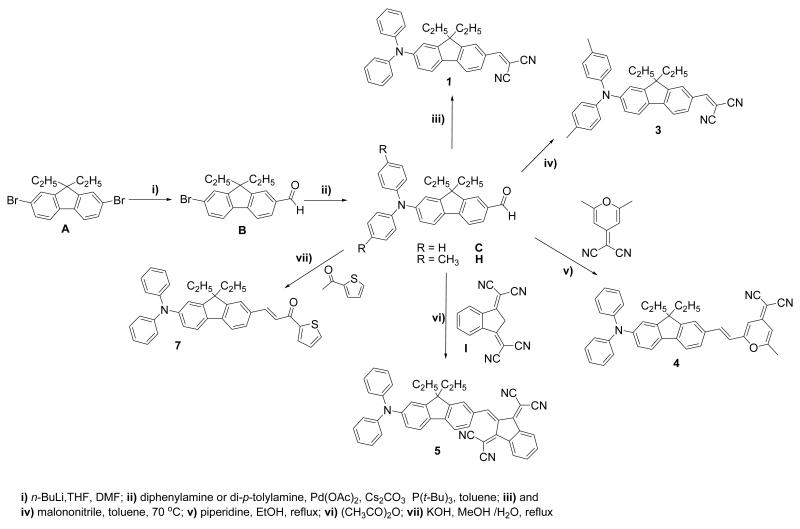
The syntheses of the fluorene derivatives are presented in Schemes 1 and 2. The key step in the synthesis of compounds 1- 3, and 5 was the Knoevenagel condensation of aldehyde derivatives with active methylene group of malonotrile. All these dyes were prepared according to a general synthetic methodology involving lithiation of 2,7-dibromo-9,9-diethyl-9H-fluorene with a stoichiometric amount of n-BuLi, followed by reaction with dimethylformamide (DMF) and subsequent acidic hydrolysis, providing intermediate B. Arylamination of 7- bromo-9, 9-diethylfluorene-2-carbaldehyde B was readily performed using Buchwald-Hartwig amination coupling in the presence of a Pd catalyst and a non nucleophilic base, Cs2CO3, to afford intermediate C.33 Knoevenagel condensation between the resulting aldehyde C with active malonotrile provided compound 1 as a red solid (Scheme 1).
Scheme 1.
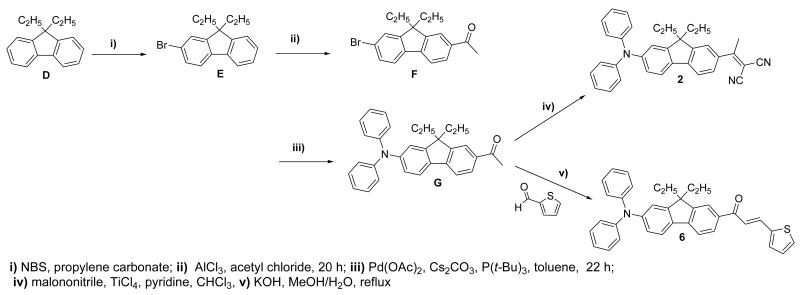
Scheme 2.
Compound F was obtained in a three steps involving C-alkylation of fluorene to give D. Mono bromination of D with NBS provided 2-bromo-9,9-diethyl fluorene (E), which yielded the acetylated derivative F after a Friedel-Crafts acetylation. Dicyanovinyl derivative 2 was synthesized from keto derivative F by condensation with malononitrile in the presence of TiCl4 in pyridine (Scheme 2). After purification, 2 was obtained as orange crystals in 82% yield.
The di-p-tolylamine group was used as an electron-donor group in 3 (Scheme 1). The di-p-tolylamine group was coupled to 7-bromo-9,9-diethylfluorene-2-carbaldehyde B via Buchwald-Hartwig arylamination, producing fluorenyl aldehyde H. Finally, malononitrile was condensed, via Knoevenagel conditions, with H, affording 3 as a red solid. 1H NMR showed the presence of a singlet at 2.34 ppm assigned to the methyl groups of p-tolylamine moiety and no aldehydic proton signal.
The versatility of intermediate C was demonstrated in the synthesis of another chromophore comprised of pyranone as an electron acceptor group. The condensation between C and (2,6-dimethyl-4H-pyran-4-ylidene)malononitrile was carried out in the presence of piperidine in ethanol rendering the trans chromophore. The condensation reaction of the (2,6-dimethyl-4H-pyran-4-ylidene)malononitrile with aldehyde C resulted in 4 (Scheme 1), easily separated via column chromatography as a red solid. The two cyano groups of 2-pyran-4-ylidenemalononitrile are electron-withdrawing group and the electron-deficient pyran ring can act as an auxiliary acceptor.
The indane derivative, 2,2′-(1H-indene-1,3(2H)-diylidene)dimalononitrile (I), was prepared by reacting 1,3-indanedione and malononitrile in ethanol.34 The conditions used for the final Knoevenagel condensation between intermediate C and I was carried out by reflux in acetic acid for 3 h, providing 5 in high yield as a dark blue solid (Scheme 1).
The thiophen-2-yl-prop-2-en-1-one electron-withdrawing group was introduced by Claisen-Schmidt condensation between thiophene-2-carbaldehyde and compound G, affording chromophore 6 as a yellow solid (Scheme 2). Compound H reacted with 1-(thiophen-2-yl)ethanone affording chromophore 7 as a yellow solid (Scheme 1). All compounds were characterized by 1H and 13C NMR, mass spectrometry, and elemental analysis.
Linear Photophysical Properties
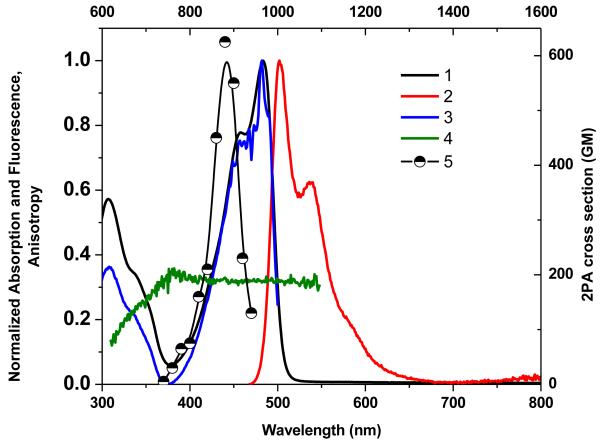
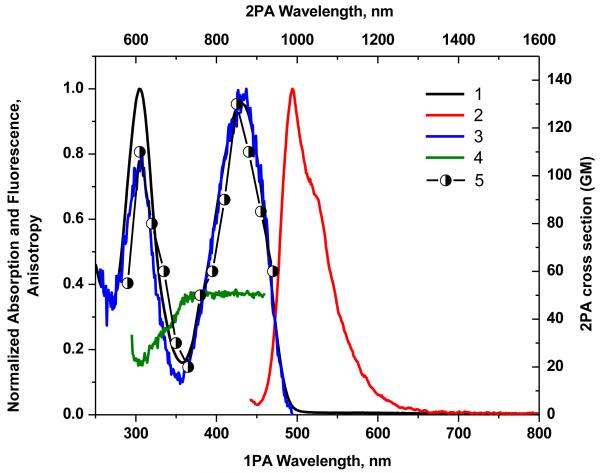
The dipolar molecules 1-7 possess absorption spectra with two distinct peaks in nonpolar solvents, the main band lying within the range 410-630 nm and the second positioned between 305 and 345 nm (Figures 1-7, curve 1). Excitation anisotropy is correlated with the spectral position of various electronic transitions, and can be a very useful tool to estimate the position of 2PA allowed transitions.27, 35 The locations of these electronic transitions over the spectral range 300-550 nm were revealed from excitation anisotropy. The optical properties of the compounds 1-7 are listed in Table 1.
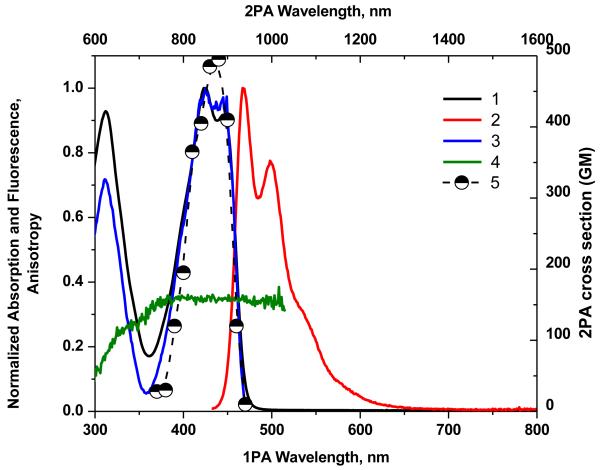
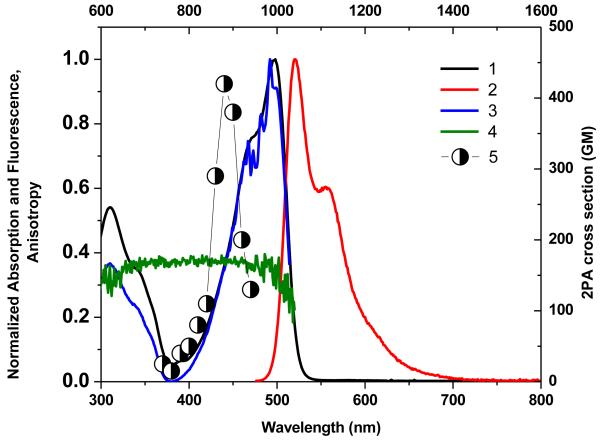
Figure 1.
Normalized absorption (1), emission (2), and excitation spectra (3) of 1 in cyclohexane, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the 2PF method in cyclohexane.
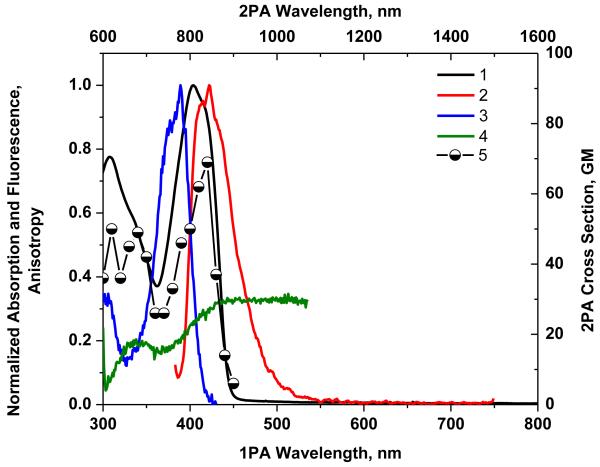
Figure 7.
Normalized absorption (1), emission under λexc = 423 nm (2), and excitation spectra (3) of 7 in cyclohexane, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the 2PF method in cyclohexane.
Table 1.
Main photophysical parameters of compounds 1-7: absorption, λmax.Abs, and fluorescence λmax.Fl, maxima, Stokes shift, Δλst maximum extinction coefficient, εmax, fluorescence quantum yield, Φ, anisotropy, r0, and fluorescence lifetime τ.
| Compound | λ Abs. max | λ FI. max | Δ λ st | εmax. 10− 3·M−1·cm−1 |
Φ |
r0 (p-THF) |
τ (ns) |
|---|---|---|---|---|---|---|---|
| 1 a | 483±1 | 502±1 | 19 | 50 | 0'31±0'05 | 0.32 | 1.00±0.01 |
| 2 a | 430±1 | 495±1 | 65 | 29 | 0'02±0'05 | 0.36 | 0.13±0.08 |
| 3 a | 498±1 | 520±1 | 22 | 54 | 0'66±0'05 | 0.37 | 1.95±0.05 |
| 4 b | 470±1 | 648±1 | 178 | 79 | 0'64±0'05 | 0.32 | 2.8±0.01 |
| 5 b | 345, 634±1 |
500c ±1 | - | 24 | 0'001±0'0 5 |
0.30 | - |
| 6 a | 404±1 | 420±1 | 16 | 40 | 0'01±0'05 | 0.22 | 0.21±0.09 |
| 7 a | 423±1 | 468±1 | 45 | 27 | 0'49±0'05 | 0.35 | 1.3710.02 |
Absorption and emission spectra measured in cyclohexane.
Absorption and emission spectra measured in chloroform.
Excited at 345 nm.
In comparing the linear absorbance spectra of this series of compounds, it is noticeable that incorporation of bisdicyanovinylindane in 5 resulted in the largest red-shift relative to the compounds 1-4 and 6, and 7, indicative of improved charge-transfer characteristics of this donor-acceptor substituted fluorene. To further evaluate the effect of the different electron acceptors on the linear optical properties, 3-(thiophen-2-yl)propenone was used as the benchmark acceptor group, owing to this moiety being the weakest acceptor in the series. Absorption at λ max was found to progressively shift to longer wavelength upon replacing this benchmark by dicyanomethylene and bisdicyanovinylindane (Table 1). A considerable red shift of the main emission bands was observed for chromophores 1-7, (Figures 1-7, curve 2). These results indicate that the fluorescence emission arises from excited state intramolecular charge transfer in these molecules.
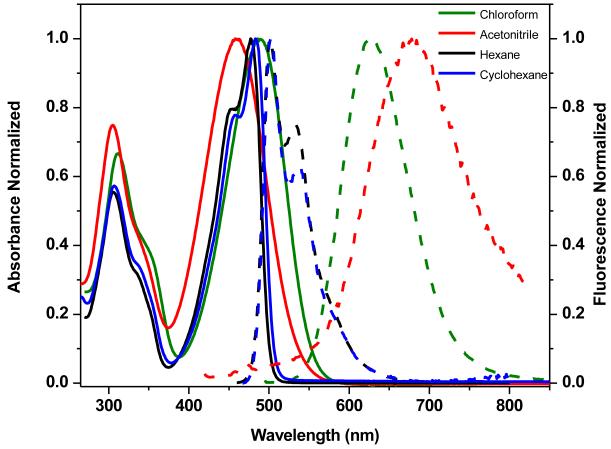
These compounds also displayed pronounced positive solvatochromism (i.e., bathochromic shift with increasing solvent polarity) in their emission spectra, whereas only a slight red shift was observed in the absorption spectra (Figure 8) (representative data are shown for 1 only). This solvatochromic behavior is consistent with the charge-transfer characteristics of donor-acceptor substituted fluorene compounds. A large Stokes shift (Δλst) was observed for 4 in CHCl3 (Figure 4) due to strong solvent-solute dipole-dipole interactions, as a manifestation of the large dipole moment and orientational polarizability in CHCl3 (Δf ≈ 0.152).
Figure 8.
Normalized absorption (solid lines) and emission spectra (dashed lines) for 1 in different solvents.
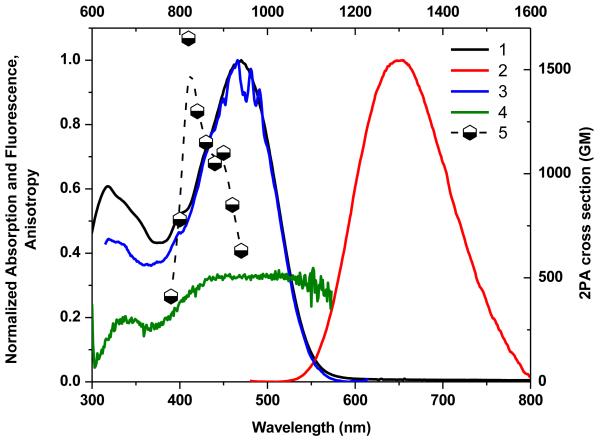
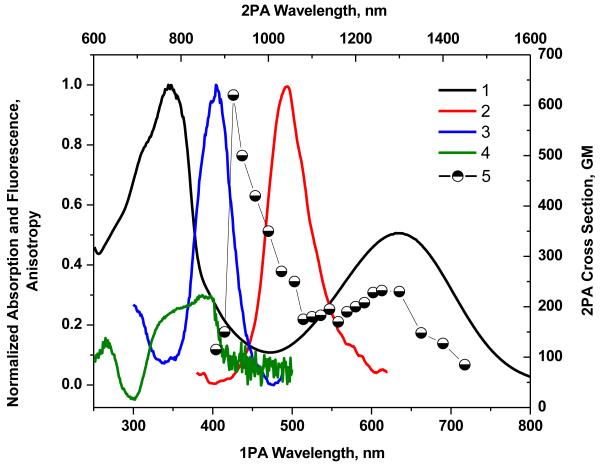
Figure 4.
Normalized absorption (1), emission (2), and excitation spectra (3) of 4 in chloroform and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the 2PF method in chloroform.
Compounds 1-7 exhibited single-exponential fluorescence decay process with lifetime (τ) that was independent of the excitation wavelength over a broad spectral range (280–440 nm). Quantum yields, Φ were determined using a fluorescence method, 35 exciting at the absorption maximum, λmaxabs of each molecule. Derivatives 1, 3, 4, and 7 exhibited good Q in cyclohexane and CHCl3 (0.31-0.66). Significant deviations from planarity of the electron-acceptor group, can explain the largest red shift observed in absorption spectrum of 5 and may explain the lack of any detectable fluorescence upon excitation at the long wavelength maximum.
The anisotropy excitation spectra and anisotropy values, r0, for 1-7 in polyTHF are shown in curve 4 in Figures 1-7. Constant anisotropy values, r0, for 1-7 in the spectral region of excitation wavelength, λexc ≥ 430-495 nm (compounds 1-4, curve 4) and λexc≥ 405-425 nm (compounds 6 and 7), correspond to the main absorption band (S0>S1 transition), while the change in the anisotropy for compound 5 under excitation in the short wavelength absorption band (λexc= 345 nm) exhibited a complicated electronic structure of the main long wavelength absorption band.
Nonlinear Photophysical Properties
The 2PA spectra for compounds 1-7 are shown as curve 5 in Figures 1-7. 2PA spectra for compounds 1, 3, 4, and 7 were acquired using the 2PF technique over a broad spectral region, 740-940 nm, since they possessed high fluorescence quantum yields. 2PA spectra were measured for compounds 2, 5, and 6 by open aperture Z-scan due to their low fluorescence quantum yields. The maxima of 2PA cross section (δ) value for 1-7 were at ca. 880 nm, close to a linear absorption maximum at ca. 440 nm and attributed to the S0® S1 transition (identified by both linear absorption and anisotropy spectra), a formally forbidden transition for 2PA. The peak position of the 2PA spectrum of 2, 6, and 7 correlated quite well with the position of the transition to the first excited state for this system, as indicated by the location of the long wavelength band in the linear absorption spectrum (440 nm). Because these molecules do not possess a center of symmetry, it is not surprising that each of the low-lying transitions (S0® S1 and S0® S2) are observed by two-photon absorption.
As expected, from the linear absorption analysis, the value of λmax did vary significantly as the strength of the electron-withdrawing moiety. There was no strong deviation of the λmax in 2PA spectra with change in the withdrawing group. Compound 4 contained a pyran group and exhibited nearly the three-fold enhancement of δ (1650 GM at 840 nm) relative to the rest of the series (see curves 5 in Figures 1-7). This is particular intriguing considering that in this series the bis(dicyanomethylidene)indane accepting group was assumed to be the strongest electron-withdrawing group compared to the other substituents studied.
Thus, the study of the dipolar fluorene derivatives illustrates that there appears to be an optimum degree of ground-state polarization that maximizes δ. Furthermore, it does not necessarily correspond to the molecule which possesses the strongest ground-state dipole moment (as revealed by quantum calculations (Table S1), as 4 possesses the largest dipole moment in the series). As a result, fluorenyl 4, with its pyranone terminal group, has optimal charge transfer character that maximizes its state and transition dipole moments, the main factors that influence the strength of 2PA.
Single-Crystal X-Ray Structural Analysis
Crystals suitable for single crystal X-ray analysis were obtained for compounds 1, 2, and 6, by the slow diffusion of hexane into a concentrated dichloromethane solution. Solid state structural analysis for the fluorene-containing compounds has generally been rarely reported, due, in part, to difficulties in growing single crystals. Single crystal X-ray structural analysis was completed for compounds 1, 2, and 6 (Table 2). Single crystals could not be obtained for the other compounds in the series.
Table 2.
Summary of the crystal data and structure refinement parameters for 1, 2, and 6.
| Compound | 1 | 2 | 6 |
|---|---|---|---|
| Formula | C33H27N3 | C34H29N3 | C36H31NOS |
| Formula weight | 465.58 | 479.60 | 525.69 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 |
| a /Å | 8.3392(13) | 8.7255(12) | 9.3797(6) |
| b /Å | 10.0974(16) | 10.8750(15) | 10.4703(6) |
| c /Å | 15.684(3) | 15.855(2) | 15.9304(10) |
| α /° | 98.756(2) | 98.469(2) | 79.5150(10) |
| β /° | 101.508(2) | 101.687(2) | 78.4040(10) |
| γ /° | 97.415(2) | 110.096(2) | 67.3940(10) |
| V /Å3 | 1261.5(4) | 1344.6(3) | 1405.30(15) |
| Z | 2 | 2 | 2 |
| Dc /Mg m−3 | 1.226 | 1.185 | 1.242 |
| μ (MoKα)/mm−1 | 0.072 | 0.070 | 0.145 |
| F(000) | 492 | 508 | 556 |
| Reflections collected/ unique |
16761/6046 [R(int)=0.0645] |
14513/4732 [R(int)=0.0434] |
15012/4944 [R(int)=0.0310] |
| Reflections with I>2σ(I) | 3370 | 4732 | 4944 |
| Goodness-of-fit on F2 | 1.001 | 1.012 | 1.043 |
| R, wR (I>2σ(I)) | 0.0585, 0.1293 | 0.0399, 0.0876 | 0.0407, 0.0988 |
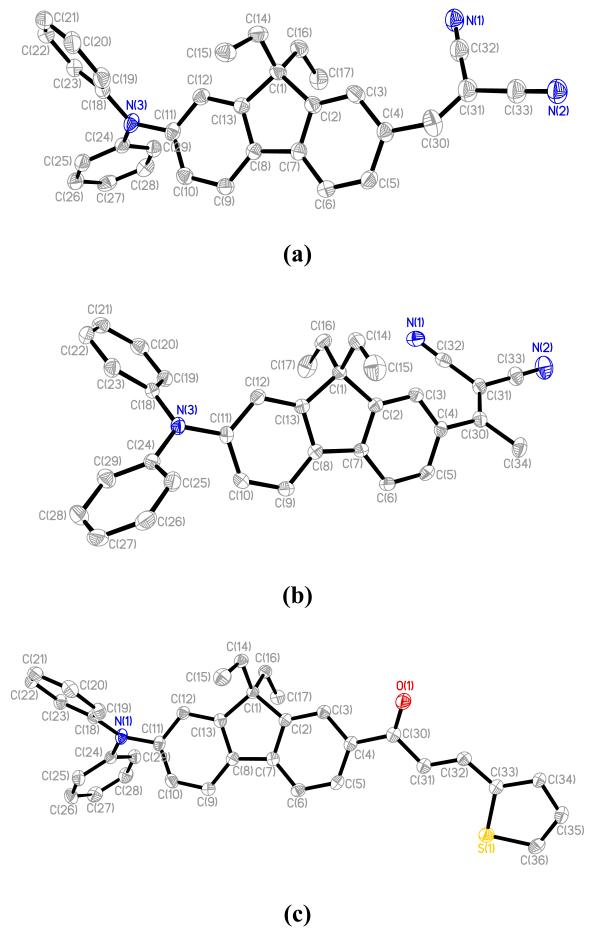
Compounds 1, 2, and 6 crystallize in the same triclinic P-1 space group with a single molecule per asymmetric unit (Figure 9).
Figure 9.
ORTEP drawing for 1 (a), 2 (b), and 6 (c) with the numbering scheme. Thermal ellipsoids are shown with the 50% probability level. Only the major part of the disordered dicyanovinyl fragment in 1 is shown. Hydrogen atoms are omitted for clarity.
The fluorene substructure is essentially planar for all three compounds. Phenyl rings from the diphenylamino donor group are twisted with respect to fluorene bridge, angles range from 60.8(1) to 67.8(1)° (Table 3). Similar values were observed for analogous unsymmetrical fluorene based derivatives.33
Table 3.
Selected structural parameters (Å, °) for experimental (tail x) and calculated (tail c) molecules.
| Parameter/ Molecule |
PA/PB | PA/PC | N* | C(11)-N(3)- C(18) |
C(18)-N(3)- C(24) |
C(24)-N(2)- C(11) |
|---|---|---|---|---|---|---|
| 1c | 60.55 | 82.94 | 0.032 | 119.94 | 121.43 | 118.48 |
| 1x | 60.60(5) | 82.95(5) | 0.032(2) | 119.9(2) | 121.4(2) | 118.5(2) |
| 2c | 69.55 | 67.86 | 0.003 | 120.33 | 119.04 | 120.63 |
| 2x | 63.09(4) | 72.87(4) | 0.118(2) | 119.7(1) | 119.4(1) | 118.8(1) |
| 3c | 68.98 | 66.93 | 0.006 | 120.67 | 118.34 | 120.99 |
| 4c | 70.18 | 68.84 | 0.003 | 120.24 | 119.29 | 120.47 |
| 5c | 69.07 | 67.76 | 0.003 | 120.57 | 118.60 | 120.83 |
| 6c | 70.54 | 68.89 | 0.003 | 120.11 | 119.48 | 120.41 |
| 6x | 67.75(5) | 81.85(5) | 0.119(2) | 119.6(2) | 121.7(2) | 116.7(2) |
| 7c | 70.41 | 68.91 | 0.003 | 120.14 | 119.47 | 120.39 |
Deviation of the central nitrogen atom from its bonded neighboring carbon atoms.
The nitrogen in the diphenylamino group exhibited a trigonal planar environment in 1, showing a slight deviation to a pyramidal configuration in analogs 2 and 6, with C-N-C angles being in the range of 116.7(2) – 121.7(2)°. The largest deviation from the plane formed by C(11), C(18) and C(19) carbons was 0.119(2) Å, suggesting sp2-hybridization of N atom, and, consequently, donation of its pair of non-bonding electrons into the π conjugation chromophore system (Table 3). The molecular geometry of 1 revealed both syn (major disordered part) and trans (minor part) conformations of the dicyanovinyl group (see Experimental section), with the values of torsion angle C(3)-C(4)-C(30)-C(31) being 30.1(4) and 161.1(5)°, respectively. Compound 2 clearly exhibited syn conformation of the dicyanovinyl group, with torsion angle C(3)-C(4)-C(30)-C(31) equal to 42.5(2)°. Compound 6 revealed the largest deviation from planarity for the acceptor group, manifested by torsion angle C(5)-C(4)-C(30)-C(31) of 18.6(3)°, due to H...H repulsion interactions of H atoms attached to C(5) and C(31).
The crystal packing in 1, 2, and 6 was determined by antiparallel dipole-dipole interactions between the molecules related by inversion centers. Bulky diphenylamino group and essentially eclipsed ethyl substituents on the fluorene moiety prevent any close π -π intermolecular interactions (Figure S1).
Quantum Chemical Calculations
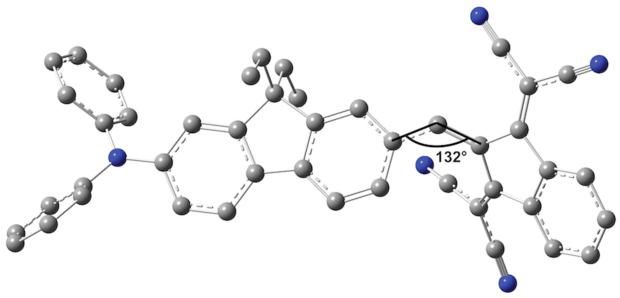
Ground state optimized geometries show only slight differences compared to available crystallographic data. The nitrogen atom in the diarylamine group became essentially coplanar with the bonded carbon atoms, suggesting improved electron donation to the fluorenyl π-system. Torsion angle C(3)-C(4)-C(30)-C(31), describing syn isomerization in 1 and 2, arrived to values 0.2 and 35.9°, respectively. The fluorene core and the acceptor group were positioned essentially in the same plane for optimized geometries of 4 and 7. The bisdicyanovinylindane acceptor in 5 had a distorted geometry due to steric repulsions between dicyanovinyl groups and the fluorene core, with an angle at the bridging C sp2 atom equal to 132.1° (Figure 10) and interplanar angle between the fluorene core and bisdicyanovinylindane group of 38.8°.
Figure 10.
DFT optimized geometry of 5.
The electron acceptor group in 6 deviated from the best plane with a torsion angle C(5)-C(4)-C(30)-C(31) value equal to 8.0°, due to H...H repulsion mentioned in the crystallographic description. Comparison of ground state dipole moment values is an indicator of the donor-acceptor strengths of the studied systems. Both functionals (B3LYP and M06) showed similar tendencies in dipole moments (Table S1). With addition of the methyl group to the terminal phenyl rings, the dipole moment increased (compare compounds 1 and 3). This result can be explained by the hyperconjugation effect. The methylidene malononitrile group (1) showed slightly stronger acceptor properties compared to ethylidene malononitrile (2). Fluorenyl 4, with a pyronone group as an electron-acceptor possessed the largest dipole moment in the series, suggesting enhanced linear and nonlinear absorption properties. Non-planarity observed in 6 led to a decrease in conjugation, and, as a result, a slightly decreased dipole moment when compared to 7.
Singlet excited states
Two main bands in the absorption spectra of 1-7 are well reproduced with time-dependent (TD) DFT calculations (Table 4).
Table 4.
Calculated ground energy and dipole (Eg (eV) and μg (Debye) and excited states (λc (nm), f, μt (Debye) properties at M06/6-311++G** level.
| λ c | f | Transition | μ t | μ g | Eg | |
|---|---|---|---|---|---|---|
| 1 | 346 | 0.3696 | S0->S2 | 4.2122 | 9.9395 | -39110.0516632424 |
| 488 | 0.7821 | S0->S1 | 12.5691 | |||
| 2 | 334 | 0.3583 | S0->S3 | 3.9441 | 8.2282 | -40179.3249837085 |
| 469 | 0.6005 | S0->S1 | 9.2694 | |||
| 3 | 362 | 0.4713 | S0->S2 | 5.6102 | 10.8632 | -41248.562096415 |
| 531 | 0.9627 | S0->S1 | 16.8228 | |||
| 4 | 391 | 0.7373 | S0->S2 | 9.4835 | 13.5913 | -48546.3220781635 |
| 501 | 1.1375 | S0->S1 | 18.7683 | |||
| 5 | 360 | 0.3693 | S0->S7 | 4.3732 | 7.4602 | -54630.8843510249 |
| 652 | 0.5087 | S0->S1 | 10.9133 | |||
| 6 | 352 | 0.5553 | S0->S3 | 6.4272 | 2.3721 | -52188.2893297265 |
| 448 | 0.6647 | S0->S1 | 9.8138 | |||
| 7 | 341 | 0.6301 | S0->S4 | 7.0807 | 4.1917 | -52188.3070881576 |
| 451 | 0.8977 | S0->S1 | 13.3268 |
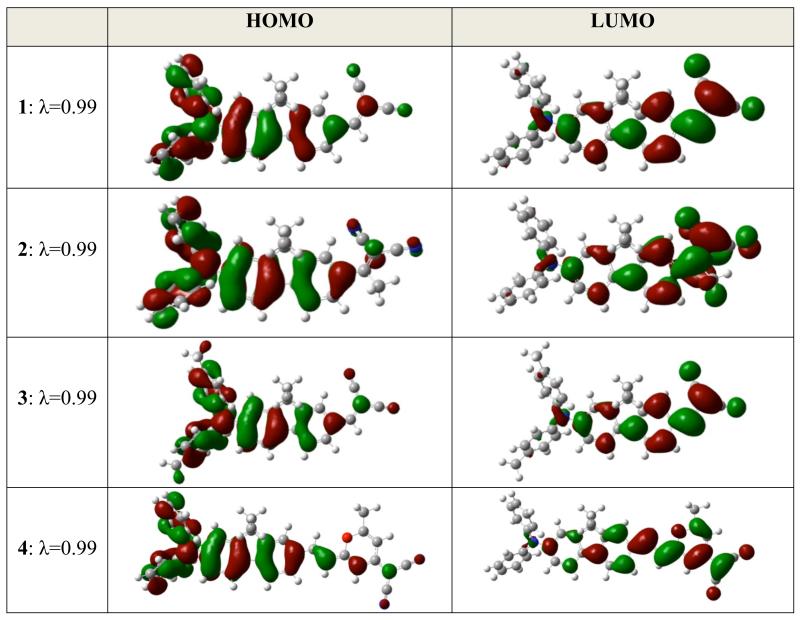
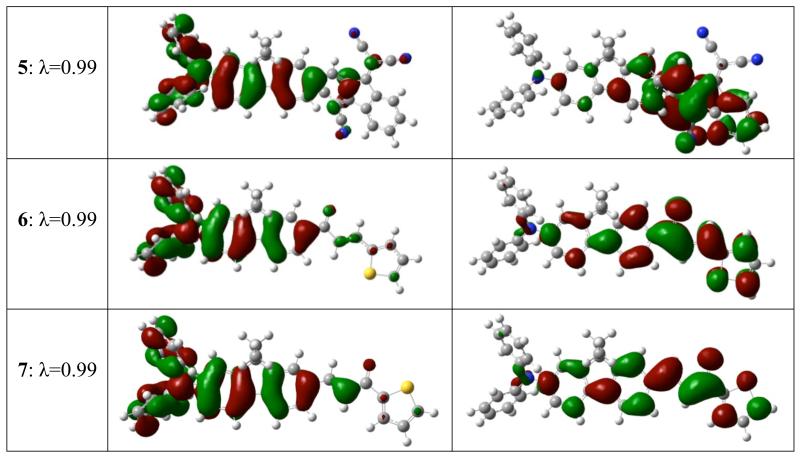
As was recently validated for fluorene-based push-pull chromophores, 2PA in the lowest energy transition correlates with the values of the transition dipole moment and permanent dipole moment differences between the ground and first excited states (S0->S1).36,37 Therefore, only the lowest energy electronic excitation is considered below. The calculated absorption maxima were in good agreement to those experimentally observed, providing credence with the computational method. As revealed by TD DFT calculations, the ground to first excited state transition described the lowest lying energy excitation. Red shifts observed in the absorption spectra, are well reproduced by the calculations, suggesting increased intramolecular charge-transfer (CT) character along the series. However, bisdicyanovinylindane in diphenylaminofluorene 5 resulted in the largest red shift relative to the rest of compounds, but did not lead to improved CT characteristics as revealed by ground and transition dipole moments, as well as, by Natural Transition Orbitals (NTO) analysis. Improvement of CT correlates well with the increase of the both ground and transition dipole moment values. Enhanced hyperconjugation results in slightly improved CT for 3 over 1. Fluorenyl thiophene 6, having the weakest acceptor group (thiophen-2-yl prop-2-en-1-one), revealed the shortest λmax and smallest ground state dipole. NTOs for the S0 -> S1 transition are shown in Figure 11.
Figure 11.
S0 -> S1 Natural Transition Orbitals, λ is fraction of the NTO pair contribution into the given electronic excitation.
We note several common aspects for the chromophores with regard to transition character: (i) as expected, the HOMO wavefunction is more developed on the donor, whereas the LUMO wavefunction is more developed on the acceptor group; (ii) electron density redistribution clearly suggests π -> π* transition character, except the small fraction of σ -> π* coming from donor methyl groups (when present); (iii) ethyl groups located on fluorene are not involved in CT whatsoever; (vi) finally, because of the existence of an overlap of the HOMO and LUMO wavefunctions, transition cannot be attributed to pure CT character. The largest transition dipole moment observed for 4 is mirrored by the LUMO wavefunction, which is even less pronounced on the diphenylamino group-containing in this compound compared to the other molecules. For 5, the LUMO wavefunction is located mainly on the aromatic portion, not on the dicyanovinyl moiety of the acceptor.
Conclusions
In summary, modification of diphenylaminofluorene by the introduction of electron-withdrawing groups resulted in a series of robust two-photon absorbing fluorene based chromophores. It was found that the absorption and emission spectra show significant bathochromic shifts according to the strength of the electron-withdrawing moieties. Compound 4, with its pyranone terminal group, showed the optimal charge transfer character in this series of compounds, which maximizes its state and transition dipole moments (it had largest transition dipole moment), and exhibited the largest 2PA cross section values. Data from linear and nonlinear photophysical studies, crystallographic studies, and quantum mechanical calculations were relatively consistent, and provided insight to the observed behavior of the molecules. It was shown that there is an optimum degree of dipolar molecular character, with the strongest electron-withdrawing group not necessarily providing the largest 2PA. Results from this study provide the rationale to design 2PA materials for applications such as probes for two-photon fluorescence bioimaging applications.
Experimental Section
Materials and Methods
2, 7- Dibromo-9, 9-diethylfluorene (A) 9, 9-diethyl-fluorene (D), and 2-bromo-9,9-diethyl-9H-fluorene (E) and 1,3-bis(dicyanomethylene)indane (I) were prepared as described previously.30, 38, 34 Reactions were carried out under N2 or Ar atmosphere. THF was freshly distilled from Na and benzophenone ketyl. AlCl3 was purified by sublimation before use. All other reagents and solvents were used as received from commercial suppliers. 1H and 13C NMR spectra were recorded on NMR spectrometer at 500 and 125 MHz, respectively and recorded with reference to TMS at 0.00 ppm.
Synthesis of 7- bromo-9, 9-diethylfluorene-2-carbaldehyde (B)
Under nitrogen atmosphere and at −78°C, n-BuLi (3.28 mL, 1.6 M in hexanes) was added dropwise over 20 min to a dry THF solution (15 mL) containing 2,7-dibromo-9,9-diethylfluorene (2 g, 5.26 mmol). After 1 h stirring, 0.58 mL of DMF was added slowly to the reaction solution. After another 2 h stirring, the temperature of the solution was brought back to room temperature and the reaction was quenched with 2 N HCl. The solution was extracted with toluene and subjected to flash column chromatography (silica gel, hexanes/ethyl acetate 4:1). A white solid was obtained. Yield: 89% (1.52 g), m.p. 124-125°C (lit. mp 126-128 °C).38 1H NMR (500 MHz, CDCl3) δ: 9.77 (s, 1H),7.57 (s, 2H), 7.53 (d, J = 7 Hz, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.23-7.21 (m, 2 H), 1.83-1.72 (m, 4H), 0.3 (t, J = 10 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 192.3, 153.4, 150.3, 146.7, 138.9, 135.6, 130.6, 130.3, 126.7, 126.4, 123.2, 123.1, 120.0, 56.6, 32.5, 8.4 ppm.
Synthesis 7-(diphenylamino)-9,9-diethylfluorene-2-carbaldehyde (C)
Under nitrogen atmosphere, a mixture of 7-bromo-9, 9-diethylfluorene-2-carbaldehyde (3 g, 9.11 mmol), diphenylamine (2.31 g, 13.66 mmol), Pd(OAC)2 (0.05 g, 0.22 mmol), P(tBu)3 (0.1 g, 0.49 mmol), and Cs2CO3 (4.45 g, 13.65 mmol) in toluene (20 mL) was stirred and heated at 120 °C for 24 h. After cooling to room temperature, the reaction mixture was passed through short plug (silica gel) and the filtrate was concentrated to give a brow oil. Purification was carried out by flash column chromatography (silica gel, first hexanes, then hexanes/ethyl acetate 4:1). A yellow solid was obtained. Yield: 88% (3.31 g), m.p.156-157°C, 1H NMR (500 MHz, CDCl3) δ: 9.69 (s, 1H), 7.50 (dd, J = 7.5Hz, 2H), 7.40 (dd, J = 8 Hz, 1H), 7.30 (dd, J= 8.5 Hz, 1H), 6.97 - 6.93 (m, 4H), 6.81 (dd, J = 6.5 Hz, 4H), 6.77 (d, J = 2Hz, 1H), 6.74-6.71 (m, 4H), 1.72-1.65 (m, 2H), 1.61-1.53 (m, 2H), 0.02 (t, J = 14.5, 6H). 13C NMR (125 MHz, CDCl3) δ: 192.2, 152.8, 150.5, 148.9, 147.9, 147.6, 134.5, 134.3, 138.9, 129.3, 124.4, 123.1, 122.9, 121.69, 119.1, 118.1, 56.1, 32.4, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C30H28NO 418.2165; Found 418.2176.
Synthesis of 2-((2-(diphenylamino)-9, 9-diethyl-fluoren-7-yl)methylene)malononitrile (1)
7-(Diphenylamino)-9,9-diethyl-fluorene-2-carbaldehyde(0.4 g, 0.96 mmol), malononitrile (0.12 g, 1.92 mmol) and basic aluminum oxide (0.45 g) were stirred in toluene (5 mL) for 24 h at 70 °C. After cooling to room temperature, the reaction solution was filtered. The filtrate was subjected to column chromatography (silica gel, hexanes/ethyl acetate 9:1). A red solid was obtained. Yield: 91% (0.40 g), m.p. 189-190 °C, 1H NMR (500 MHz, CDCl3) δ: 7.89 (d, J = 2 Hz, 1H), 7.83 (dd, J = 8 Hz, 1H), 7.76 (s, 1H), 7.69 (d, J = 8 Hz, 1H), 7.61 (d, J = 8 Hz, 1H), 7.31-7.25 (m, 4H), 7.15 (bd, J = 8 Hz, 4 H), 7.09 (m, 4H), 2.02-1.99 (m, 2H), 1.98-1.85 (m, 2H), 0.38 (t, J = 15 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 159.9, 159.8, 153.3, 151.0, 149.7, 148.6, 147.4, 133.5, 131.6, 129.5, 124.9, 124.7, 124.5, 124.4, 123.6, 123.5, 122.4, 122.3, 122.11, 122.0, 119.6, 119.5, 117.4, 114.6, 113.6, 79.1, 56.3, 32.3, 8.5ppm. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C33H28N3 466.2278; Found 466.2274.
Synthesis of 1-(2-bromo-9,9-diethyl-9H-fluoren-7-yl)ethanone (F)
To a solution of AlCl3 (4.09 g, 30.71 mmol) in 115 mL of CH2Cl2, acetyl chloride (2.08 mL) was added in a nitrogen atmosphere at 0 °C. Then 18 mL of 2-bromo-9,9-diethyl-9H-fluorene (8.1 g, 26.89 mmol) dissolved in 17 mL of CH2Cl2 was added at the same temperature. After stirring at room temperature for 20 h the solution was poured into cold water. The separated organic phase was dried over Na2SO4. The product was purified by column chromatography using a mixture of hexane:ethyl acetate (4:1). White solid was isolated. Yield: 78% (7.2 g), m.p. 120-121 °C, 1H NMR (500 MHz, CDCl3) δ: 7.97 (t, J = 7.5Hz, 2H), 7.74 (d, J = 8 Hz, 1H), 7.63 (d, J = 8Hz, 1H), 7.51-7.49 (m, 2H), 2.66 (s, 3H), 2.11-1.99 (m, 4H), 0.31 (t, J = 15Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 198.0, 153.3, 149.8, 145.3, 139.1, 136.2, 130.5,128.4, 126.6,122.6, 122.4, 121.9, 119.5, 56.7, 32.5, 27.0, 8.4 ppm. HRMS (ESI-TOF) m/z: [M+Na]+ Calcd for C19H19BrONa 365.0511; Found 365.0514.
Synthesis of 1-(2-(diphenylamino)-9,9-diethyl-9H-fluoren-7-yl)ethanone (G)
Under nitrogen atmosphere, a mixture of 1-(2-bromo-9,9-diethyl-9H-fluoren-7-yl)ethanonecarbaldehyde (6 g, 17.47 mmol), diphenylamine (4.43 g, 26.20 mmol), Pd(OAc)2 (0.13 g, 0.59 mmol), P(tBu)3 (0.21 g, 1.06 mmol), and Cs2CO3 (8.33 g, 25.56 mmol) in toluene (55 mL) was stirred and heated at 120 °C for 36 h. After cooling to room temperature, the reaction mixture was passed through short plug (silica gel) and the filtered was concentrated obtaining a yellow-brownish oil. Purification was carried out by column chromatography (silica gel, hexanes/CH2Cl2 3:2). A yellow solid was obtained. Yield: 78 % (5.88 g), m.p. 142-143 °C, 1H NMR (500 MHz, CDCl3) δ: 7.94 (dd, J = 7.5 Hz, 1H), 7.90 (s, 1H), 7.65 (d, J = 8 Hz, 1H), 7.61 (d, J = 8.5 Hz, 1H), 7.28 (t, J = 5.5 Hz, 4H), 7.14 (d, J = 5.5 Hz, 4H), 7.10 (d, J = 2 Hz, 1H), 7.05 (t, J = 7.5 Hz, 3H), 2.64 (s, 3H), 2.03 (m, 2H), 1.91 (m, 2H), 0.34 (t, J = 14.5 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 198.1, 152.6, 150.0, 148.5, 147.7, 146.4, 135.0, 134.7, 129.5, 129.0, 124.5, 124.0, 122.9, 122.3, 121.3, 118.7, 56.2, 32.5, 26.8, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C31H30NO 432.2322; Found 432.2323.
Synthesis of 2-(1-(2-(diphenylamino)-9,9-diethyl-9H-fluoren-7-yl)ethylidene)malononitrile (2)
In a reaction flask, 1-(2-(diphenylamino)-9,9-diethyl-9H-fluoren-7-yl)ethanone (0.8 g, 1.85 mmol) and malononitrile (0.26 g, 4.06 mmol) were added under nitrogen atmosphere, followed by anhydrous chloroform (36 mL) to give a clear yellow solution. It was then added to pyridine (600 mg, 7.58 mmol) and an excess amount of titanium tetrachloride (5.5 mL, 47.65 mmol) with continuous stirring. The reaction mixture turned immediately to deep brown. The solution was stirred for 30 min and subsequently quenched with water (100 mL). The liquid was concentrated in a vacuum and purified using column chromatography (silica gel) first with CH2Cl2, then with a solvent mixture of hexane/CH2Cl2 (1:4) as eluent providing orange crystals. Yield: 73 % (0.65 g), m.p. 200-201 °C. 1H NMR (500 MHz, CDCl3) δ: 7.68 (d, J = 8 Hz, 1H), 7.59 (d, J = 5.5 Hz, 2H), 7.54 (dd, J= 8.5 Hz, 1H), 7.29 (t, J = 5.5 Hz, 4H), 7.14 (d, J = 5.5 Hz, 4H), 7.08-7.03 (m, 4H), 2.69 (s, 3H), 1.99 −1.89 (m, 6H), 0.37 (t, J = 15 Hz). 13C NMR (125 MHz, CDCl3) δ: 175.1, 152.4, 150.4, 148.9, 147.6, 146.2, 134.1, 133.0, 129.8, 129.6, 129.1, 128.8, 127.1, 126.8, 124.8, 124.1, 123.9, 123.5, 123.1, 122.9, 122.5, 122.2, 121.9, 121.4, 119.3, 118.4, 117.7, 113.6, 113.4, 56.5, 32.4, 24.1, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+Na]+ Calcd for C34H29N3Na 502.2254, Found 502.2256.
Synthesis of 7-(di-p-tolylamino)-9,9-diethyl-9H-fluorene-2-carbaldehyde (H)
Under nitrogen atmosphere, a mixture of 7- bromo-9, 9-diethylfluorene-2-carbaldehyde (2 g, 6.07 mmol), di-p-tolylamine (1.77 g, 9.0 mmol), Pd(OAc)2 (0.03g, mmol), P(tBu)3 (0.064g, mmol), and Cs2CO3 (2.96 g, mmol) in toluene (14 mL) was stirred and heated at 120 °C for 36 h. After cooling to room temperature, the reaction mixture was passed through short plug (silica gel) and the filtrate was concentrated to give a yellow-brown oil. Purification was carried out by flash column chromatography (silica gel, hexanes/CH2Cl2 3:2). A fluorescent yellow solid was obtained. Yield: 70% (1.9 g), m.p. 156-157 °C, 1H NMR (500 MHz, CDCl3) δ: 10.01 (s, 1H,), 7.81 (d, J = 8 Hz, 2H); 7.70 (d, J = 8 Hz, 1H); 7.57 (d, J = 8 Hz, 1H), 7.09-6.96 (m, 10H), 2.33 (s, 6H), 2.02-1.98 (m, 2H), 1.90-1.86 (m, 2H), 0.35 (t, J = 15 Hz, 6H) ppm. 13C NMR (125 MHz, CDCl3) δ: 192.2, 152.7, 150.3, 149.3, 148.1, 145.1, 134.2, 133.4, 132.8, 130.9, 130.2, 129.9, 124.7, 122.8, 121.6, 121.5, 118.8, 116.8, 56.1, 32.5, 32.4, 20.8, 20.8, 8.4 ppm. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C32H32NO 446.2478; Found 446.2479.
Synthesis of 2-((2-(di-p-tolylamino)-9,9-diethyl-fluoren-7-yl)methylene)malononitrile (3)
7-(Di-p-tolylamino)-9,9-diethyl-9H-fluorene-2-carbaldehyde (1.5 g, 3.36 mmol), maolononitrile (0.44 g, 6.72 mmol) and basic aluminum oxide (1.58 g) were stirred in toluene (18 mL) for 16 h at 70 °C. After cooling to room temperature, the reaction solution was filtered. The filtrate was subjected to column chromatography (silica gel, toluene). A red solid was obtained. Yield: 86% (1.43 g), m.p. 211-212 °C, 1H NMR (500 MHz, CDCl3)δ: 7.88 (d, J = 2 Hz, 1H); 7.82 (dd, J = 8.5 Hz, 1H); 7.76 (s, 1H); 7.66 (d, J = 8 Hz, 1H); 7.56 (d, J = 8.5 Hz, 1H); 7.10 (d, J = 8 Hz, 4H); 7.04-7.00 (m, 4H); 6.98 (dd, J = 8.5 Hz1H), 2.34 (s, 6H); 1.99-1.95 (m, 2H); 1.89-1.85 (m, 2H); 0.37 (t, J = 15 Hz 6H) ppm. 13C NMR (125 MHz, CDCl3) δ: 159.8, 153.3, 150.8, 150.1, 148.9, 144.8, 133.3, 132.5, 131.7, 130.1, 128.4, 125.2, 124.3, 122.0, 121.8, 119.4, 116.0, 114.7, 113.7, 78.6, 56.2, 32.3, 20.9, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C35H32N3 494.2591; Found 494.2582.
Synthesis of (E)-2-(3-(2-(7-(diphenylamino)-9,9-diethyl-9H-fluoren-2-yl)vinyl)-5-methyl-4-oxocyclohexa-2,5-dienylidene)malononitrile (4)
A mixture of aldehyde C (0.56 g, 1.35 mmol), and 2,6-dimethyl-4-dicyanomethylene-4H-pyran (0.25 g, 1.35 mmol) were dissolved in EtOH (35 mL). After adding piperidine (0.5 mL) slowly through a syringe while stirring, the reaction mixture was refluxed for 72 h. Reddish precipitate was obtained after cooling the reaction to room temperature. Compound 4 was obtained after purifying the product through a silica gel column using hexanes/ethyl acetate (4:1) as eluent. A red solid was obtained. Yield: 35% (0.28 g), m.p. 299-300 °C, 1H NMR (500 MHz, CDCl3) δ:7.64 (d, J = 8 Hz, 1H), 7.58 (d, J = 8 Hz, 1H), 7.54 (s, 1H), 7.51 (d, J = 8 Hz, 1H), 7.46 (s, 1H), 7.28-7.25 (m, 5H), 7.14 (d, J = 8.5 Hz, 4H), 7.09 (d, J =2 Hz, 1H), 7.05-7.02 (m, 3H), 6.76 (d, J = 16 Hz, 1H), 6.70 (d, J = 1.5 Hz, 1H), 6.55 (s, 1H), 2.42 (s, 3H), 2.05-1.88 (m, 4H), 0.38 (t, J = 14.5 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 161.8, 159.5, 156.3, 152.0, 150.7, 148.2, 147.7, 144.2, 138.8, 135.1, 132.4, 129.2, 127.6, 124.2, 123.2, 122.9, 121.8, 120.9, 119.5, 118.6, 116.5, 115.2, 115.1, 106.7, 106.4, 56.1, 32.6, 19.9, 8.5 ppm. HRMS (ESI) m/z: [M+H]+ calcd for C40H34N3O 572.2696; Found 572.2709.
Synthesis of 1,3-bis(dicyanomethylene)indane (I)
A solution of indane-1,3-dione (2.4 g, 16 mmol), malononitrile (2.7 g, 41 mmol) and ammonium acetate (1.25 g, 16 mmol) dissolved in absolute ethanol (30 mL) was heated at reflux for 30 min. After cooling to room temperature, water (25 mL) was added and the solution acidified with concentrated hydrochloric acid. The brown precipitate was filtered off and washed with water. Recrystallization from glacial acetic acid afforded compound as a yellow-brown solid 1. Yield: 41% (1.6 g), m.p. 255-256 °C; 1H NMR (500 MHz, CDCl3) δ: 8.67-8.64 (m, 2H), 7.94-7.90 (m, 2H), and 4.30 (s, 2H). 13C NMR (125 MHz, CDCl3) δ: 165.4, 140.7, 136.4, 127.1, 111.9, 111.7, 79.4, 42.0 ppm. Anal. Calcd. for C15H6N4 (242.23): C, 74.37, H, 2.50; N, 23.13. Found: C, 74.18; H, 2.46; N, 23.28.
Synthesis of 2-[1,3-bis(dicyanomethylidene)indan-2-ylidenemethyl]-7-(N,N-diphenylamino)-9,9-diethylfluorene (5)
7-(Diphenylamino)-9,9-diethylfluorene-2-carbaldehyde (0.56 g, 1.35 mmol) was added to a solution of I (0.3 g, 1.23 mmol) in acetic anhydride (10 mL) at 70°C with vigorous stirring. The mixture was heated at 70-80 °C for 3 h and cooled. The precipitated solid was filtered off, washed with a small amount of acetic anhydride, and recrystallized from the same solvent, affording as blue dark crystals. Yield: 89% (0.70 g), m.p. 291-292 °C, 1H NMR (500 MHz, CDCl3) δ: 8.77 (s, 1H), 8.7 (d, J = 6.3 Hz, 1H), 8.61 (d, J = 6.6 Hz, 1H), 7.87 (m, 2H), 7.73 (d, J = 6 Hz, 1H), 7.62 (d, J = 8.1 Hz, 1H) 7.53 (d, J = 8.1 Hz, 1H), 7.40 (s, 1H), 7.32-7.25 (m, 4H), 7.16-7.02 (m, 8H), 1.98-1.87 (m, 4H), 0.42 (t, J = 14.7 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 161.4, 160.4, 152.8, 151.5, 149.4, 147.5, 147.0, 146.0, 138.1, 136.7, 135.0, 134.8, 134.1, 131.4, 130.0, 129.3, 126.2, 125.4, 124.7, 124.4, 123.4, 122.6, 121.9, 120.2, 117.5, 113.3, 113.1, 112.9, 112.2, 71.9, 56.4, 32.6, 8.4 ppm. Anal. Calcd. for C45H31N5 (641.2679): C, 84.22; H, 4.87; N, 10.91. Found: C, 84.09; H, 4.90; N, 11.10. HRMS (ESI-TOF) m/z: [M+H]+ calcd for C45H32N5 642.2652; Found 642.2630.
Synthesis of (E)-3-(2-(diphenylamino)-9,9-diethyl-9H-fluoren-7-yl)-1-(thiophen-2-yl)prop-2-en-1-one (6)
Thiophene-2-carbaldehyde (0.07 g, 0.69 mmol) was added to the solution of KOH (0.04 g, 0.828 mmol) in MeOH/H2O 5:1 (10 mL). After dissolution, 1-(2-(diphenylamino)-9,9-diethyl-9H-fluoren-7-yl)ethanone (0.30 g, 0.69 mmol) was added to the mixture and stirred for 48 h at reflux. A solid product precipitated was filtered, washed with hexane, and dried. Recrystallization in hexane provided a yellow solid. Yield: 70% (0.25 g), m.p.166-167 °C, 1H NMR (500 MHz, CDCl3) δ: 8.02 (d, J = 8 Hz, 1H); 7.97( d, J = 3 Hz, 1H); 7.70 (d, J = 8 Hz, 1H); 7.63 ( d, J = 8.5 Hz, 1H), 7.45 (s, 1H); 7.43( d, J = 6 Hz, 1H); 7.38 (d, J = 3 Hz, 1H); 7.29 (bt, J = 8 Hz, 5H); 7.14-7.09 (m, 5H); 7.06 (bt, J = 8.5 Hz, 3H); 2.05 (m, 2H), 1.93 (m, 2H,), 0.36 (t, J = 14.5 Hz,6H). 13C NMR (125 MHz, CDCl3) δ: 189.3, 152.7, 150.2, 148.5, 147.7, 146.3, 140.6, 136.6, 135.9, 134.8, 132.0, 131.6, 129.4,129.0,128.7, 128.3, 128.2, 124.5, 124.0, 123.2, 122.7, 121.5, 121.2, 120.9, 118.7, 118.2, 56.3, 32.5, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+H]+ calcd for C36H32NOS 526.2199; Found 526.2193.
Synthesis of (E)-3-(7-(diphenylamino)-9,9-diethyl-9H-fluoren-2-yl)-1-(thiophen-2-yl)prop-2-en-1-one (7)
1-(Thiophen-2-yl)ethanone (0.046 g, 0.37 mmol) was added to the solution of KOH (0.020 g, 0.37 mmol) in MeOH/ H2O 5:1 (10 mL). After dissolution, 7-(diphenylamino)-9,9-diethylfluorene-2-carbaldehyde (0.15 g, 0.37 mmol) was added to the mixture and stirred for 48 h at reflux. A solid product precipitated was filtered, washed with hexane, and dried. Purification was carried out by column chromatography (silica gel, hexanes/ethyl acetate 4:1). A yellow solid was obtained. Yield: 80% (0.15 g), m.p. 231-232 °C, 1H NMR (500 MHz, CDCl3) δ: 7.95 (d, J = 15.5 Hz, 1H), 7.91 (dd, J = 4 Hz, 1H), 7.68 (dd, J = 5Hz, 1H), 7.64-7.60 (m, 2H), 7.58 (d, J = 8.5 Hz, 1H), 7.54 (s, 1H), 7.46 (d, J = 16 Hz, 1H), 7.28-7.24 (m, 4H), 7.20 (t, J = 8.5 Hz, 1H), 7.13-7.09 (m, 5H), 7.04-7.01 (m, 3H), 2.02-1.88 (m, 4H), 0.38 (t, J = 15 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ: 182.0, 152.1, 150.57, 148.1, 147.8, 145.8, 144.9, 144.3, 135.3, 133.5, 132.6, 131.5, 129.2, 128.3, 128.1, 124.1, 123.2, 122.8, 122.6, 120.9, 120.1, 119.4, 118.7, 56.1, 32.6, 8.5 ppm. HRMS (ESI-TOF) m/z: [M+H]+ calcd for C36H32NOS 526.2199; Found 526.2195.
Linear spectral measurements
Linear photophysical properties of all materials were investigated at room temperature in spectroscopic cyclohexane and chloroform. Steady-state absorption spectra were obtained by UV-visible spectrophotometer in 10 mm path length quartz cuvettes with dye concentrations 1.5·10−5 M ≤ C ≤ 3·10−5 M. The steady-state fluorescence, excitation and excitation anisotropy spectra were measured with a spectrofluorimeter in 10 mm spectrofluorometric quartz cuvettes, with dye concentrations ~ (1-1.5)·10−6 M. The values of fluorescence quantum yield Φ, were determined relative to 9, 10-diphenylanthracene in cyclohexane and Rhodamine 6G as a standards (Φ ≈ 0.95).35
Lifetime Measurements
Lifetime measurements were performed by using a Ti:sapphire laser system (pulse duration ~200 fs/pulse (FWHM) and 76 MHz repetition rate) coupled with a second harmonic generator (tuning range 350-440 nm). A broad band-pass filter (D500/200) was placed in front of an avalanche photodiode (APD) detector. Data acquisition was conducted on a time-correlated single photon counting system. The optical density of all the investigated solutions did not exceed 0.12 at the excitation wavelengths to avoid reabsorption in 10 mm path length quartz cuvettes at room temperature.
Two-photon absorption (2PA) measurements
The 2PA spectra of compounds 1-5, and 7 were measured over a broad spectral region by open aperture Z-scan39 and relative 2PF methods40 with Rhodamine B in methanol as a standard.41 Two-photon induced fluorescence spectra were obtained with a spectrofluorimeter coupled with a regenerative amplified laser system that pumped optical parametric generator/amplifiers generating ≈ 140 fs output pulses (FWHM) with repetition rate of 1 kHz. The quadratic dependence of 2PF intensity on the excitation power was verified for every excitation wavelength, λex. The same laser system was used for open aperture Z-scan measurements. A comprehensive description of this experimental methodology was previously reported.42,43
X-ray and Computational Methodology
The X-ray diffraction experiments for 1, 2 and 6 were carried out with a diffractometer, using Mo Kα radiation (λ = 0.71073 Å) at 100 K. The raw data frames were integrated with the SAINT+ program using a narrow-frame algorithm.44 Absorption corrections were applied using the semi-empirical method of the SADABS program.45 The structures were solved by direct methods and refined using the Bruker SHELXTL programs suite 46 by full-matrix least-squares methods on F2 with SHELXL-97 in anisotropic approximation for all non-hydrogen atoms. In 1, the C(31), C(32), N(1), and N(2) atoms of dicyanovinyl group were disordered over two positions with the partial occupancies of 0.64(5) and 0.36(5). All H atoms were placed in idealized positions and refined with constrained C-H distances and Uiso(H) values set to be 1.2Ueq or 1.5Ueq (for methyl group) of the attached C atom. Crystallographic data for all structures have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers: CCDC 831506 - 831508. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or deposit@ccdc.cam.ac.uk).
All calculations were carried out using the Gaussian09 program.47 Starting from crystallographic data (if available), geometry optimizations of molecules 1-7 were performed at the B3LYP/6-311G** level 48-50 for all atoms without any symmetry restriction. The first 7 singlet excited states were obtained using time dependent DFT calculations for isolated molecules starting from optimized geometries at the M06/6-311++G** level of theory.51 Transition densities associated with the electronic excitations are represented via the Natural Transition Orbitals (NTO) formalism.52
Supplementary Material
Figure 2.
Normalized absorption (1), emission (2), and excitation spectra (3) of 2 in cyclohexane, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the open aperture Z-scan method in cyclohexane.
Figure 3.
Normalized absorption (1), emission (2), and excitation spectra (3) of 3 in cyclohexane, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the 2PF method in cyclohexane.
Figure 5.
Normalized absorption (1), emission (2), and excitation spectra (3) of 5 in chloroform, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the open aperture Z-scan method in chloroform.
Figure 6.
Normalized absorption (1), emission under λexc = 404 nm (2), and excitation spectra (3) of 6 in cyclohexane, and fluorescence excitation anisotropy (4) in polyTHF. 2PA spectrum (5) obtained by the open aperture Z-scan method in cyclohexane.
Acknowledgment
The authors wish to acknowledge the National Science Foundation (CHE-0832622 and CHE-0840431), the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (1 R15EB008858-01) and the National Academy of Science (PGA-P210877) for support of this work.
Footnotes
Supporting Information Available: 1H NMR and 13C NMR spectra of B,C, 1,F,G,2, H,3, 4,I, 5, 6 and 7. Crystallographic structural packing data for 1, 2, and 6, along with X-ray diffraction data (CIF). Computational details, including tables on the optimized geometry of molecules 1-7, ground and excited states properties. This material is available free of charge via the Internet at http://pubs.acs.org.at http://pubs.acs.org.
References
- (1).Göppert-Mayer M. Ann. Phys. 1931;9:273–295. [Google Scholar]
- (2).He GS, Markowicz PP, Lin T-C, Prasad PN. Nature. 2002;415:767–770. doi: 10.1038/415767a. [DOI] [PubMed] [Google Scholar]
- (3).Blanchard-Desce M. C. R. Phys. 2002;3:439–448. [Google Scholar]
- (4).Belfield KD, Schafer KJ, Liu Y, Liu J, Ren X, Van Stryland EW. J. Phys. Org. Chem. 2000;13:837–849. [Google Scholar]
- (5).Denk W, Strickler JH, Webb WW. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- (6).Shen Y, Jakubczyk D, Xu F, Swiatkiewicz J, Prasad PN, Reinhardt BA. Appl. Phys. Lett. 2000;76:1–3. [Google Scholar]
- (7).Xu C, Williams RM, Zipfel W, Webb WW. Bioimaging. 1996;4:198–207. [Google Scholar]
- (8).Reinhardt BA, Brott LL, Clarson SJ, Dillard AG, Bhatt JC, Kannan R, Yuan L, He GS, Prasad PN. Chem. Mater. 1998;10:1863–1874. [Google Scholar]
- (9).Baur JW, Alexander MD, Banach M, Jr., Denny LR, Reinhardt BA, Vaia RA, Fleitz PA, Kirkpatrick SM. Chem. Mater. 1999;11:2899–2906. [Google Scholar]
- (10).Lee K-S, Lee J-H, Kim K-S, Woo H-Y, Kim O-K, Choi H, Cha M, He GS, Swiatkiewcz J, Prasad PN, Chung M-A, Jung S-D. MCLC S&T, Sect. B: Nonlinear Opt. 2001;27:133–137. [Google Scholar]
- (11).Morel Y, Irimia A, Najechalski Y, Kervella Y, Stephan O, Baldeck PL, Andraud C. J. Chem. Phys. 2001;114:5391–5396. [Google Scholar]
- (12).Charlot M, Izard N, Mongin O, Riehl D, Blanchard-Desce M. Chem. Phys. Lett. 2006;417:297–302. [Google Scholar]
- (13).Reinhardt BA. Photonics Sci. News. 1999;4:21–34. [Google Scholar]
- (14).Prasad PN, Bhawalkar JD, Kumar ND, Lal M. Macromol. Symp. 1997;118:467–472. [Google Scholar]
- (15).Bhawalkar JD, Kumar ND, Zhao CF, Prasad PN. J. Clin. Laser Med. Surg. 1997;15:201–204. doi: 10.1089/clm.1997.15.201. [DOI] [PubMed] [Google Scholar]
- (16).Verbiest T, Houbrechts S, Kauranen M, Clays K, Persoons A. J. Mater. Chem. 1997;7:2175–2189. [Google Scholar]
- (17).Le Bozec H, Renouard T. Eur. J. Inorg. Chem. 2000;2000:229–239. [Google Scholar]
- (18).Wolf JJ, Wortmann R. Adv. Phys. Org. Chem. 1999;32:121–217. [Google Scholar]
- (19).Long NJ. Angew. Chem. Int. Ed. Engl. 1995;34:21–38. [Google Scholar]
- (20).Steybe F, Effenberger F, Gubler U, Bosshard C, Günter P. Tetrahedron. 1998;54:8469–8480. [Google Scholar]
- (21).Albota M, Beljonne D, Bredas J-L, Ehrlich JE, Fu J-Y, Heikal AA, Hess SE, Kogej T, Levin MD, Marder SR, McCord-Maughon D, Perry JW, Rockel H, Rumi M, Subramaniam G, Webb WW, Wu X-L, Xu C. Science. 1998;281:1653–1656. doi: 10.1126/science.281.5383.1653. [DOI] [PubMed] [Google Scholar]
- (22).Marder SR, Gorman CB, Meyers F, Perry JW, Bourhill G, Bredas JL, Pierce BM. Science. 1994;265:632–635. doi: 10.1126/science.265.5172.632. [DOI] [PubMed] [Google Scholar]
- (23).Oudar JL. J. Chem. Phys. 1977;67:446–457. [Google Scholar]
- (24).Strehmel B, Sarker AM, Detert H. Chem. Phys. Chem. 2003;4:249–259. doi: 10.1002/cphc.200390041. [DOI] [PubMed] [Google Scholar]
- (25).(a) Mignani G, Leising F, Meyruex R, Samson H. Tetrahedron Lett. 1990;31:4743–4746. [Google Scholar]; (b) Jen AK-Y, Rao VP, Drost KJ, Wong KY, Cava MP. J. Chem. Soc. Chem. Commun. 1994;2057:2058. [Google Scholar]; (c) Rao VP, Cai YM, Jen AK-Y. J. Chem. Soc. Chem. Commun. 1994;14:1689–1690. [Google Scholar]
- (26).Wu X, Wu J, Liu Y, Jen AK-Y. J. Am. Chem. Soc. 1999;121:472–473. [Google Scholar]
- (27).Belfield KD, Bondar MV, Przhonska OV, Schafer KJ. J. Fluoresc. 2002;12:449–454. [Google Scholar]
- (28).Belfield KD, Schafer KJ, Mourad W, Reinhardt BA. J. Org. Chem. 2000;65:4475–4481. doi: 10.1021/jo991950+. [DOI] [PubMed] [Google Scholar]
- (29).Belfield KD, Morales AR, Hales JM, Hagan DJ, Van Stryland EW, Chapela VM, Percino J. Chem. Mater. 2004;16:2267–2273. [Google Scholar]
- (30).Belfield KD, Morales AR, Kang B-S, Hales JM, Hagan DJ, Van Stryland EW, Chapela VM, Percino J. Chem. Mater. 2004;16:4634–4641. [Google Scholar]
- (31).Morales AR, Belfield KD, Hales JM, Van Stryland EW, Hagan DJ. Chem. Mater. 2006;18:4972–4980. [Google Scholar]
- (32).Yao S, Belfield KD. J. Org. Chem. 2005;70:5126–5132. doi: 10.1021/jo0503512. [DOI] [PubMed] [Google Scholar]
- (33).Chiang C-L, Wu M-F, Dai D-C, Wen Y-S, Wang J-K, Chen C-T. Adv. Funct. Mater. 2005;15:231–238. [Google Scholar]
- (34).Bello KA, Cheng L, Griffiths J. J. Chem. Soc. Perkin Trans. 1987;6:815–818. [Google Scholar]
- (35).Lakowicz JR. Principles of Fluorescence Spectroscopy. Vol. 648. Kluwer Academic/Plenum; New York: 1999. pp. 52–53.pp. 298–300. [Google Scholar]
- (36).Rebane A, Drobizhev M, Makarov NS, Beuerman E, Haley JE, Krein DM, Burke AR, Flikkema JL, Cooper TM. J. Chem. Phys. A. 2011;115:4255–4262. doi: 10.1021/jp200129h. [DOI] [PubMed] [Google Scholar]
- (37).http://spie.org/x648.html?product_id=796745
- (38).Kannan R, He GS, Yuan L, Xu F, Prasad PN, Dombroskie AG, Reinhardt BA, Baur JW, Vaia RA, Tan L-S. Chem. Mater. 2001;13:1896–1904. [Google Scholar]
- (39).Sheik-Bahae M, Said AA, Wei TH, Hagan DJ, Van Stryland EW. Ieee J. Quantum Elect. 1990;26:760–769. [Google Scholar]
- (40).Xu C, Webb WW. J. Opt. Soc. Am. B. 1996;13:481–491. [Google Scholar]
- (41).Makarov NS, Drobizhev M, Rebane A. Opt. Express. 2008;16:4029–4047. doi: 10.1364/oe.16.004029. [DOI] [PubMed] [Google Scholar]
- (42).Fu J, Padilha LA, Hagan DJ, Van Stryland EW, Przhonska OV, Bondar MV, Slominsky YL, Kachkovski AD. J. Opt. Soc. Am. B. 2007;24:67–76. [Google Scholar]
- (43).Padilha LA, Webster S, Przhonska OV, Hu HH, Peceli D, Rosch JL, Bondar MV, Gerasov AO, Kovtun YP, Shandura MP, Kachkovski AD, Hagan DJ, Van Stryland EW. J. Mater. Chem. 2009;19:7503–7513. [Google Scholar]
- (44).SAINT+, Version 6.2a. Bruker Analytical X-ray System, Inc.; Madison, WI: 2001. [Google Scholar]
- (45).SADABS. Bruker Analytical X-ray System, Inc.; Madison, WI: 1999. [Google Scholar]
- (46).SHELXTL, Version 6.10. Bruker Analytical X-ray System, Inc.; Madison, WI: 1997. [Google Scholar]
- (47).Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg M, Hada M, Ehara K, Toyota R, Fukuda J, Hasegawa M, Ishida T, Nakajima, Honda JLY, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Jr., Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.02. Gaussian, Inc.; Wallingford, CT: 2009. [Google Scholar]
- (48).Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- (49).Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- (50).Vosko SH, Wilk L, Nusair M. Can. J. Phys. 1980;58:1200–1211. [Google Scholar]
- (51).Zhao Y, Truhlar DG. Theor. Chem. Acc. 2008;120:215–241. [Google Scholar]
- (52).Martin RL. J. Chem. Phys. 2003;118:4775–4777. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.