Abstract
The CDP-choline pathway of phosphatidylcholine (PtdCho) biosynthesis was first described more than 50 years ago. Investigation of the CDP-choline pathway in yeast provides a basis for understanding the CDP-choline pathway in mammals. PtdCho is considered as an intermediate in a cycle of synthesis and degradation, and the activity of a CDP-choline cycle is linked to subcellular membrane lipid movement. The components of the mammalian CDP-choline pathway include choline transport, choline kinase, phosphocholine cytidylyltransferase, and choline phosphotransferase activities. The protein isoforms and biochemical mechanisms of regulation of the pathway enzymes are related to their cell and tissue-specific functions. Regulated PtdCho turnover mediated by phospholipases or neuropathy target esterase participates in the mammalian CDP-choline cycle. Knockout mouse models define the biological functions of the CDP-choline cycle in mammalian cells and tissues. This article is part of a Special Issue entitled Phospholipids and Phospholipid Metabolism.
1. Introduction
Phospholipid bilayers maintain the structure and functionality of all unicellular and multicellular membrane systems. Phosphatidylcholine (PtdCho) is the most abundant phospholipid of eukaryotic membranes and is also found in selected prokaryotes. In 1949 Friedkin and Lehninger showed that the synthesis of phospholipids is energy-dependent and enzyme-mediated rather than a random chemi-synthetic process [1]. Eugene P. Kennedy published the first review about phospholipid metabolism in 1957 where the enzymatic synthesis of PtdCho as mediated by a nucleoside intermediate is discussed [2]. The novel intermediate is cytidine diphosphocholine (CDP-Cho) and the biochemical pathway that leads to PtdCho is known as the CDP-choline or Kennedy pathway. Since the first review was published, PtdCho synthesis and the unique regulation exerted by a key enzyme in the pathway, the phosphocholine cytidylyltransferase (CCT), are discussed by numerous articles. Our basic knowledge of the pathway is recently reviewed [3,4], therefore here we highlight advances in molecular and cell biology related to the CDP-choline pathway of PtdCho synthesis, touching on salient historical events along the way (Figure 1). Much of our progress in understanding the relationship between the activity of the CDP-choline pathway and the function of subcellular membranes has been enabled by the molecular cloning of the genes encoding the enzymes in the yeast, Saccharomyces cerevisiae. Basic concepts that are uncovered in yeast are then discussed in the context of the CDP-choline pathway in mammalian systems.
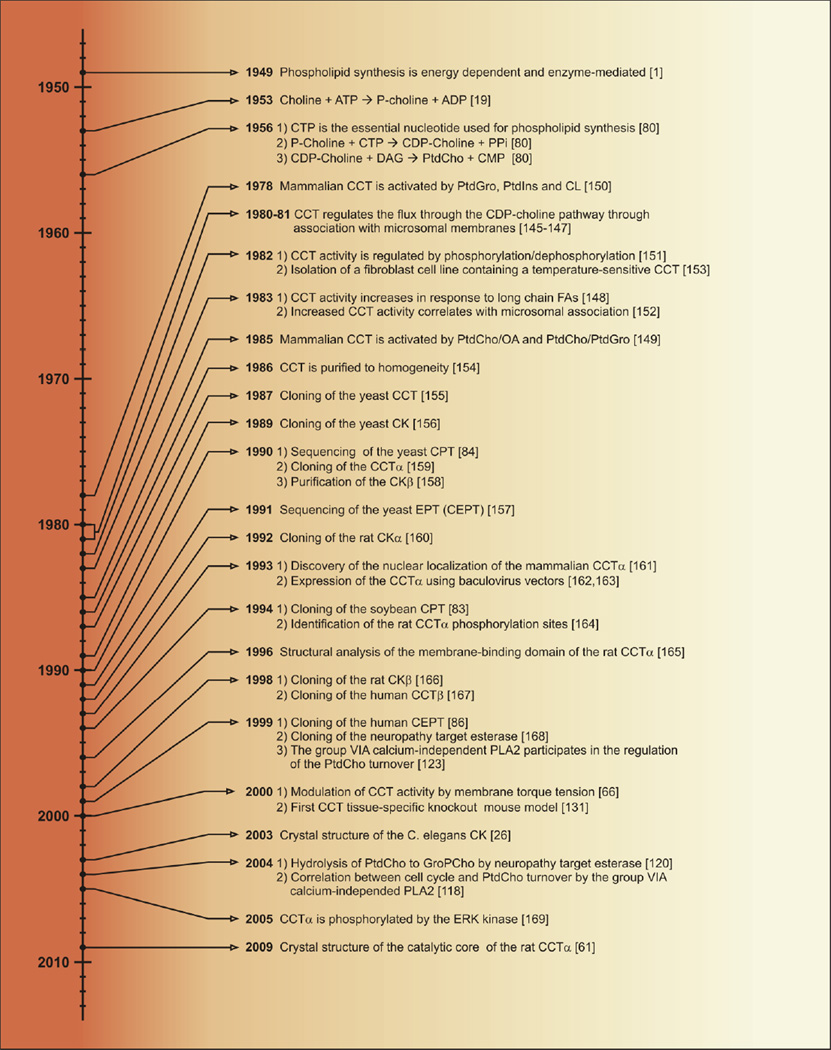
Fig. 1.
Timeline of publications that advanced our ability to interrogate the regulation, the cell biology and the physiological role of the mammalian CDP-choline pathway. The biochemical characterization of the CDP-choline pathway was accomplished in a short period of time, from 1949 to 1956 [1,19,80,81]. Between 1956 and 1986, the unpurified enzymes in the pathway were investigated, with a major focus on CCT. During this time it was recognized that CCT activity regulated the flux through the pathway [145–147], that CCT activity was stimulated by selected lipids [148–150], that CCT was regulated by reversible phosphorylation [151] and that increased CCT activity correlated with greater CCT association with microsomal membranes [145,146,152]. A fibroblast cell line containing temperature-sensitive CCT activity was isolated in 1982 [153]. The rat CCT was purified to homogeneity in 1986 [154]. The genes in the pathway were first cloned and sequenced from yeast between 1987 and 1991 [84,155–157] which enabled the identification and cloning of the mammalian homologs thereafter. The rat CK was purified in 1990 [158]. The CCTα gene was cloned in 1990 [159]. The CKα gene was cloned in 1992 [160]. The nuclear localization of CCTα was discovered in 1993 [161]. The expression of recombinant CCTα was achieved using a baculovirus vector in 1993 [162,163] and the phosphorylation sites of CCT were identified in 1994 [164]. The first CPT clone from higher eukaryotes was obtained from soybean in 1994 [83]. The structure of the CCT membrane-binding domain was described in 1996 [165]. The CKβ gene [166] and the CCTβ gene [167] were cloned in 1998. The gene encoding CEPT was cloned in 1999 [86]. The neuropathy target esterase was cloned in 1999 [168]. The participation of the group VIA calcium-independent phospholipase A2 in PtdCho turnover was recognized in 1999 [123]. The major membrane property that governs CCT association was discovered in 2000 [66]. The first CCT tissue-specific knockout mouse model was derived in 2000 [131]. A CK protein structure from C. elegans was published in 2003 [26]. The neuropathy target esterase [120] and the group VIA calcium-independent phospholipase A2 [118] were identified as regulating PtdCho turnover in 2004. CCTα was found to be phosphorylated by the ERK kinase [169]. A structure of the CCTα catalytic domain was described in 2009 [61].
2. Lessons learned from yeast - PtdCho is an intermediate, not an end point
PtdCho is the most abundant phospholipid in S. cerevisiae and can be synthesized by three pathways in yeast: (1) the phosphatidylethanolamine methyltransferase (PEMT), which is the primary route, (2) the CDP-choline pathway, which is non-essential, and (3) reacylation of glycerophosphocholine (GroPCho) [5]. The rate-limiting step in the synthesis of PtdCho via the CDP-choline pathway is catalyzed by Pct1, the yeast CCT homolog, which is regulated by interaction with membrane lipids [6], similar to the mammalian CCT. Pct1 can be found in the nucleoplasm, and at endoplasmic reticular (ER) and nuclear membranes [7]. Pct1 access to the nucleus is mediated by the importin proteins Kap60 and Kap95 and, in particular, the interaction with Kap95 is necessary to maintain PtdCho synthesis [7].
The PtdCho generated by the CDP-choline pathway undergoes turnover in yeast when the culture temperature is elevated or in response to choline supplementation to the culture medium [8]. Turnover is mediated by Nte1, a phospholipase B that deacylates PtdCho producing glycerophosphocholine (GroPCho) [9]. Nte1 is localized at the ER membrane and is proposed to regulate PtdCho abundance and ER topology, which in turn, modulates the activity of Opi1 [10], the inositol-responsive transcription factor that governs expression of many genes in the phospholipid biosynthetic pathway. Yeast genetics indicates that the Nte1-mediated deacylation of PtdCho and GroPCho production is linked to the CDP-choline biosynthetic pathway through the function of Sec14 [11]. Sec14 is a phospholipid transfer protein that is a component of the secretory machinery that buds secretory vesicles from the Golgi apparatus and traffics them to the plasma membrane. Recent data suggest that a cycle of PtdCho synthesis and degradation is a driver for vesicular traffic, and that Sec14 may buffer the activity of the cycle [12]. Inactivation of Sec14 function decreases Golgi-mediated vesicular trafficking; however, inactivation of the CDP-choline pathway, together with inactivation of Sec14, leads to activation of alternate phospholipases A and D which restore Golgi-mediated vesicular transport capability. The amount of diacylglycerol (DAG) appears to be crucial for maintaining vesicle secretion [13,14] and so changing the rate of the CDP-choline biosynthetic pathway or inducing phospholipase activity would modulate the secretory process. The PtdCho product of the CDP-choline pathway is an intermediate in the cycle of synthesis and degradation, not a metabolic end point. The most recently identified biochemical activity in the CDP-choline cycle is a novel acyltransferase which converts GroPCho to PtdCho [5]. The gene encoding the GroPCho acyltransferase has not yet been identified in yeast, and so its specific involvement in PtdCho resynthesis has not been genetically confirmed. Such an enzyme would possibly modulate GroPCho levels together with Gde1, a glycerophosphodiesterase, which catalyzes the breakdown of GroPCho to choline plus glycerol phosphate [9]. Altogether, these studies indicate that the CDP-choline cycle is specifically linked to membrane lipid movement within cells and PtdCho is a key intermediate in the cycle.
3. The CDP-choline pathway in mammals
3.1. Choline Transport
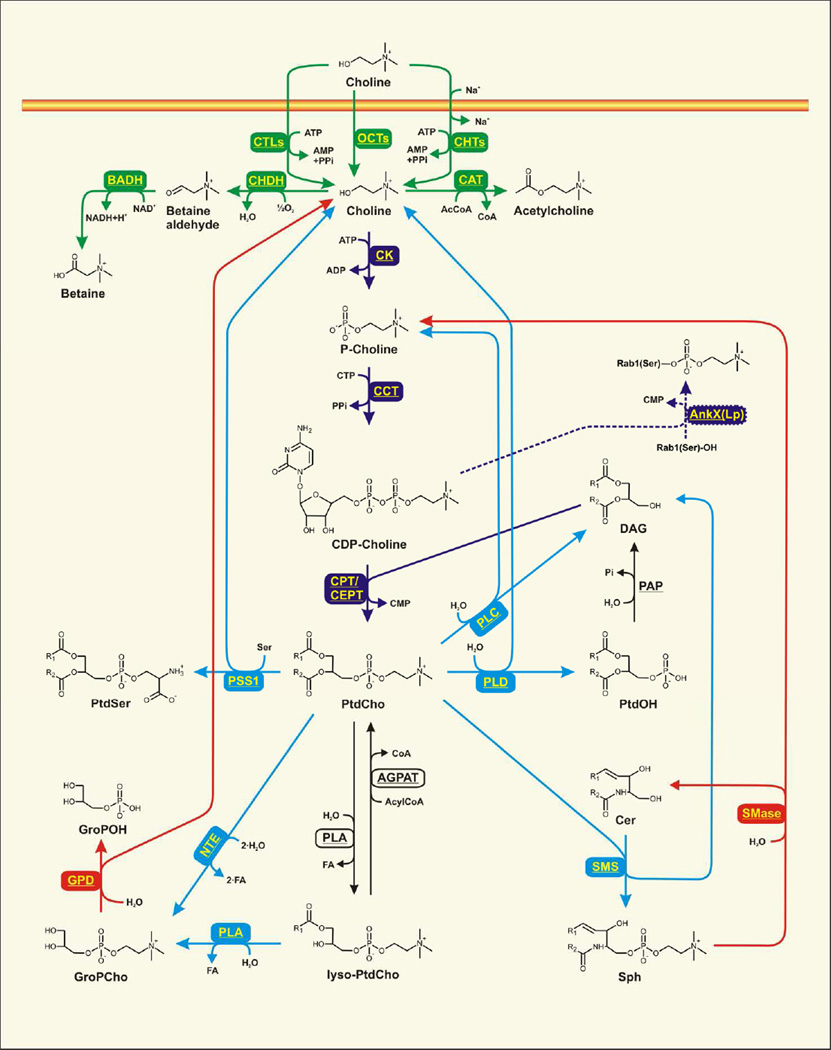
Choline is an essential nutrient and is primarily provided by the diet. The transport of choline in and out of the cell is an important process that is tightly regulated and linked to both the CDP-choline pathway and to choline metabolism (Figure 2). Choline transport is recently reviewed, and the readers are referred to these manuscripts for details [15–17]. Although the major role of the choline transporter is choline uptake from the extracellular space, choline transporters are also important for directing choline to mitochondria for degradation and production of betaine, and for cellular exit of choline in mammals. Three types of transporters mediate choline movement across membranes in higher eukaryotes: the organic cation transporters, high-affinity choline transporters, which are tightly associated with the synthesis of acetylcholine in neurons, and choline transporter-like proteins. The enzymes responsible for the conversion of choline to betaine are selectively expressed in the kidney and in the liver. Betaine is an osmolyte and the kidney utilizes betaine to control physiological osmotic pressure. Betaine in the liver is used in methyl group metabolism which, in turn, is linked to PtdCho synthesis via the PEMT reaction [18].
Fig. 2.
Biochemical relationships between the CDP-choline pathway of PtdCho synthesis, the degradation of PtdCho, and general phospholipid metabolism in mammals. Choline uptake is mediated by the organic cation transporters (OCTs) which rely on facilitated diffusion governed by the choline concentration gradient and the electrical potential across the membrane. Active transport of choline is mediated by the choline transporter-like proteins (CTLs) that are energized by adenosine triphosphate (ATP) hydrolysis. The high-affinity choline transporters (CHTs) are Na+-dependent and require ATP hydrolysis. Intracellular choline can be acted upon by three different enzymes: the choline dehydrogenase (CHDH), choline acetyltransferase (CAT) and choline kinase (CK). CHDH is localized inside the mitochondria and oxidizes choline to betaine aldehyde, with oxygen being the final electron acceptor. The betaine aldehyde is oxidized to betaine by the betaine aldehyde dehydrogenase (BADH) in concert with the conversion of oxidized nicotinamide adenine nucleotide (NAD+) into the reduced nicotinamide adenine nucleotide (NADH). Choline acetylation is catalyzed by the choline acetyltransferase (CAT) that transfers the acetyl group from acetyl-coenzyme A (AcCoA) to choline, yielding free coenzyme A (CoA) and acetylcholine. CK catalyzes the esterification of the choline hydroxyl group with the γ-phosphate of the ATP to produce phosphocholine (P-choline) plus adenosine diphosphate (ADP). The phosphocholine cytidylyltransferase (CCT) uses cytidine triphosphate (CTP) to convert P-choline into CDP-choline, with the release of pyrophosphate (PPi). The CDP-choline is esterified with diacylglycerol (DAG) by the cholinephosphotransferase (CPT) or the choline/ethanolaminephosphotransferase (CEPT) to produce 1,2-diacyl-glycerophosphocholine (PtdCho) and cytidine monophosphate (CMP). The Legionella pneumophila-encoded AnkX(Lp) can hijack CDP-choline to esterify a serine hydroxyl group of Rab1 protein. PtdCho can be hydrolyzed into choline and phosphatidic acid (PtdOH) by phospholipase D (PLD); in turn, PtdOH can be hydrolyzed into DAG and Pi. PtdCho can also be hydrolyzed into DAG and P-choline by phospholipase C (PLC). Neuropathy target esterase (NTE) hydrolyzes the two acyl chains (FA) of PtdCho to yield glycerophosphocholine (GroPCho), which can then be further hydrolyzed into glycerophosphate (GroP) and choline by the glycerophosphodiesterase (GPD). Phosphatidylserine synthase 1 (PSS1) catalyzes a base-exchange reaction with PtdCho substituting the choline group with serine (Ser) to produce phosphatidylserine (PtdSer). The sphingomyeline synthase (SMS) substitutes DAG for ceramide (Cer) to yield sphingomyelin (Sph). Sph can be hydrolyzed into P-choline and Cer by sphingomyelinase (SMase). Phospholipases A (PLA) can degrade PtdCho to yield lysophosphatidylcholine (lyso-PtdCho) and further hydrolysis of lyso-PtdCho yields GroPCho. The free hydroxyl group in lyso-PtdCho can be esterified by the acylglycerophosphate acyltransferase (AGPAT) using acylCoA as the donor of the acyl-group to yield PtdCho.
3.2. Choline Kinase (CK)
CK catalyzes the first committed step in PtdCho synthesis through the CDP-choline pathway (Figure 2). Choline is phosphorylated to phosphocholine with consumption of one molecule of ATP. In 1953 Wittenberg and Kornberg first reported the isolation and biochemical characterization of CK [19] and showed that the enzymatic activity is commonly distributed in different tissues, including liver, brain, kidney and intestinal mucosa. Extensive reviews on the genetics, biochemistry and physiological roles of CK [20–23] are available. In brief, CK exists in mammalian cells as at least three isoforms that are encoded by two separate genes. The active enzyme consists of a dimer made up of subunits encoded by either gene, and, due to the different kinetic characteristics of the CK isoforms, the balance between homo- or hetero-dimers has been proposed as a mechanism for the regulation of the enzymatic activity [24]. By virtue of the CK’s micromolar affinity for choline, choline taken up into cells is readily trapped as phosphocholine, which constitutes the largest pool of intermediates in the CDP-choline pathway. To avoid the complete conversion of choline into phosphocholine and to be able to have sufficient acetylcholine, neuronal cells express the high-affinity choline transporters which are associated with the choline acetyltransferase and together these activities enable the rapid conversion of newly imported choline into acetylcholine. Phosphocholine for PtdCho synthesis can also be supplied from sources other than CK. For example, phosphocholine arises from sphingomyelin degradation by a lysosomal sphingomyelinase [25].
All the CKs from different species share the same phosphotransferase consensus sequence and the crystal structure of CK from C. elegans shows that the protein is a member of the eukaryotic protein kinase folding family [26]. The biochemical characterization of CK from different organisms has been the subject of some controversy due largely to the fact that different degrees of ethanolamine kinase activity are associated with CK. The differences may be due to purification procedures, or the fact that molecules structurally similar to choline act as phosphate acceptors, although with specificity lower than choline: choline (trimethylethanolamine) > dimethylethanolamine > monomethylethanolamine > ethanolamine [19]. However, two plant proteins possessing CK activity show no measurable ethanolamine kinase activity [27]. Also, the subsequent discovery of two ethanolamine-specific kinases [28] argues strongly that the CDP-ethanolamine pathway is distinct from the CDP-choline pathway of phospholipid synthesis in mammalian cells, as indicated by the existence of kinases that are specific for either choline or ethanolamine.
The CK is the first committed step in the CDP-choline pathway of PtdCho synthesis and its regulation has been investigated in cultured mammalian cells. Regulation of CK activity by phosphorylation [20], as well as signaling by its product, phosphocholine [29], are related to mitogenesis and tumor growth. Accordingly, CK inhibitors effectively reduce the growth of tumors [16,30–33]. The two CK gene promoter regions are similar to each other and contain the recognition sequences for typical housekeeping transcriptional activity as well as tissue-specific elements. In addition, the CKα promoter has xenobiotic response elements that activate expression of this isoform in response to drugs and toxins [24,34]. The CKα knockout mouse model demonstrates that the CKα isoform is essential during embryogenesis, as homozygotes do not survive beyond the time of uterine implantation of the embryos [35]. On the other hand, loss of CKβ expression results in viable mice but the knockouts exhibit rostrocaudal muscular dystrophy [36]. The CKβ is expressed at a higher level in hindlimb muscle, suggesting that this isoform has a specialized role in this tissue.
3.3. Phosphocholine Cytidylyltransferase (CCT)
CCT catalyzes the conversion of phosphocholine plus CTP to CDP-choline plus pyrophosphate (Figure 2). The importance of mammalian CCT has made it the topic of numerous reviews that cover the details of CCT biochemistry and regulation that are summarized below [37–43]. CCT is the key regulatory enzyme in the CDP-choline pathway because it is the slowest step and thereby determines the biosynthetic flux from choline to PtdCho. Many studies reveal that the CDP-choline product of the CCT enzyme is present in extremely small amounts in cells, despite high phosphocholine levels, indicating that the CCT step constitutes a bottleneck in the pathway and that newly synthesized CDP-choline is readily incorporated into PtdCho. Thus, the term "ratelimiting" is used to describe the CCT step. The CCT governs its activity in response to the lipid composition of the cell membranes that are in close proximity to the enzyme. The CCT may or may not associate with the membranes, or associate to various extents, and membrane association stimulates catalytic activity. Although PtdCho is thought to be the sole metabolic fate for the CDP-choline intermediate, recent data show that a bacterial enzyme from Legionella pneumophila can hijack CDP-choline for use in the post-translational modification of host cell protein(s) during infection [44]. This maneuver allows the bacteria to control membrane transport and escape lysosome-mediated clearance [45]. The host Rab1 GTPase is modified by phosphocholination which, in turn, modulates membrane transport through both the endocytic and exocytic pathways of the cell [45]. The CDP-choline intended for PtdCho synthesis would be reduced by this mechanism, and the metabolic sequelae such as reduction of PtdCho or sphingomyelin, or elevation of DAG (Figure 2) may also contribute to the alterations in host membrane movement in favor of L. pneumophila.
Key transcription factors involved in cholesterol or fatty acid metabolism, such as sterol response element binding protein (SREBP), do not have a major role in activating CCT gene expression. One report indicates the CCT gene promoter to be responsive to SREBP [46]. However, subsequent experiments show only a modest increment in CCT transcript expression upon enforced SREBP expression, accompanied by a large increase in DAG [47]. DAG, a co-substrate for PtdCho synthesis together with CDP-choline, is a metabolic repository for increased fatty acid synthase activity along with triacylglycerol. On the other hand, CCT transcriptional regulation is linked to the cell cycle, cell growth and differentiation. Information about the transcriptional regulation of CCT expression is reviewed recently [41]. Two genes encode CCT in mammals and the protein isoforms, called CCTα and CCTβ, have highly conserved catalytic and regulatory domains but differ significantly at both termini. CCTα has a nuclear localization signal in its amino-terminal sequence whereas CCTβ does not [37]. Cell cycle progression from stages G0 to G1 is reported to be accompanied by translocation of CCT from the nucleus to the cytoplasm [48], but this observation is countered by an extensive study that shows no CCT translocation at any cell cycle stage in a number of synchronized cell lines [49]. Years later, investigation of lipid droplet formation in Drosophila S2 cells reveals that the fly homolog of CCTα rapidly shuttles between the nucleus and cytoplasm, although the steady state level of the protein in the nucleus is higher [50]. CCTα exits from the nucleus and associates with the surfaces of developing lipid droplets, where it becomes active and stimulates PtdCho synthesis to provide an amphiphilic barrier between the droplet and the cytosol [50]. These recent data support the idea that CCTα protein expression is often in excess of what is required to maintain PtdCho synthesis, and the protein in the nucleus is available for a rapid response to the physiological demands for PtdCho either within or outside of the nucleus. Nuclear export and stable association with extranuclear membranes requires the membrane binding domain of CCTα [51]. If the protein is in the cytosol during an apoptotic crisis, it can undergo caspase-mediated proteolysis [52]. CCTα is subject to degradation by other proteolytic enzymes as well, such as calpain [53], and interaction between CCTα and calmodulin is one mechanism that can protect CCTα from calpain-mediated proteolysis [54]. Another mechanism of protection may be the association of CCT with cell membranes, as segments of the CCT protein are shielded from proteolysis when associated with vesicles in vitro [55,56]. These data suggest that proteolytic inactivation of CCT is a common mechanism for interrupting PtdCho synthesis.
The CCTβ isoform lacks the nuclear localization signal in its sequence and so is found exclusively outside of the nucleus and is usually expressed at much lower levels compared to CCTα. CCTβ is highly expressed in brain [57] and in some, but not all, neuronal cell lines [58,59]. CCTβ transcripts and protein are abundant in the distal axons of primary neurons, whereas expression of CCTα is evident in the cell body and proximal axon. During nerve-growth factor-induced differentiation of cultured rodent neuronal cells, CCTβ translocates from cytosol to membranes [58]. Although deletion of the gene encoding CCTβ dramatically reduces PtdCho synthesis in distal axons [60], the amounts of PtdCho in the cell body, proximal and distal axon regions are not reduced in CCTβ-deficient neurons, pointing out the plasticity of CCTα and the capacity of the CDP-choline pathway in supplying PtdCho throughout the cell as needed. CCTβ- deficiency in primary cells reduces the branching of neurons, not the number of axons or the rate of axon growth [58,60]. These data indicate that the interaction between CCTβ protein and the membranes of the distal axon may impact the mode of neuronal extension or the membrane curvature.
A crystal structure of the CCT catalytic domain shows that it is a member of the nucleotidyltransferase superfamily [61]. The regulatory carboxy-terminus, which also contains the amphipathic helix of the membrane binding domain, is not included in the structure. The amphipathic helix mediates membrane association similar to other proteins that contain the same structure [62], including the HDL lipoproteins [63], and the core protein of hepatitis C virus [64,65]. The helix lies parallel to the membrane bilayer and the hydrophobic half of the helix inserts into the bilayer to different extents, depending on the membrane curvature elastic stress, a biophysical property of the bilayer dictated by the lipid composition [66–68]. The positive curvature elastic stress of the membrane is a feedback inhibitor of the CDP-choline pathway because CCT does not strongly associate with stressed lamellar vesicles that are made up of a large fraction of PtdCho. Introduction of DAG or free fatty acid into the vesicles introduces negative elastic stress and enables CCT association and activity. This unique biochemical regulation enables CCT to respond immediately to local changes in membrane lipid composition and to increase PtdCho synthesis when PtdCho is depleted. Although the amphipathic helix, also called the M domain, mediates membrane binding and cellular distribution of the CCT protein [51], this domain alone, together with the catalytic domain, is not sufficient to enhance CCT activity in vivo [69]. Thus the interplay between the M domain and the other domains in the protein are the subject of continued study. Both the amino-terminal and distal carboxy-terminal sequences of CCT can also bind select lipid species and influence the molecular architecture and activity of the protein [70–72], thereby fine-tuning the CCT response to the membrane environment. The distal carboxy-terminus can be phosphorylated on multiple serine residues, which also can affect the affinity of the membrane binding domain for lipids. The amphipathic helix of CCT mediates not only the binding to membrane lipids, but also changes the shape of the membrane, similar to a growing family of proteins [73]. Overexpression of CCTα, which is localized primarily in the nucleus in cultured cells, promotes development of the nucleoplasmic reticulum [74]. Prelamin A works together with CCT [75,76] to alter the nuclear membrane structure, but without affecting PtdCho synthesis [77]. CCT can also change the physical properties of lipid vesicles causing their remodeling into tubules in vitro [62,78].
CCTα is expressed ubiquitously and deletion of the gene encoding CCTα is embryonic lethal in mice [79]. The embryos undergo aberrant cell division soon after fertilization, indicating that expression of this isoform is essential during early development. The CCTβ isoform is less abundant, but highest levels are found in gonads and brain [57]. The CCTβ-deficient mouse model is viable but the gonads undergo premature senescence and the ovaries exhibit reduced oocyte development, indicating that CCTβ is important to supply PtdCho in the ovarian follicle during oogenesis.
3.4. Choline Phosphotransferase (CPT)
CPT catalyzes the final step in the synthesis of PtdCho via the Kennedy pathway by the transfer of phosphocholine from CDP-choline to DAG (Figure 2). In 1956, Kennedy and Weiss used a microsomal fraction to investigate the properties of enzymatic activities that used both CDP-choline and CDP-ethanolamine as substrates [80]. In 1958, Weiss, Smith and Kennedy published a detailed study of CPT biochemistry where they confirmed that CDP-choline was the energetically active substrate for CPT and that the reaction was reversible [81]. The early biochemistry of CPT is reviewed [82]. The first CPT gene from higher eukaryotes was cloned in 1994 from soybean [83] and it encodes a protein that is about 30% identical, and almost 60% similar, to the yeast CPT1 which was sequenced in 1990 [84]. Forty years after the first work in Kennedy's lab, the two human CPT isoforms expressed in yeast revealed that one isoform, called CPT, was specific for CDP-choline while the other, called choline-ethanolamine phosphotransferase (CEPT), had dual specificity for both CDP-choline and CDP-ethanolamine [85,86]. Despite about 60% identity between the amino acid sequences of these proteins, only the CEPT is able to complement loss of the yeast CPT1 in its interaction with Sec14 and affect cell growth [85,86]. Similar to what is reported for CK and ethanolamine kinase, a distinct human ethanolamine phosphotransferase (EPT) is identified more recently [87], clarifying that the CDP-choline and CDP-ethanolamine pathways are indeed separate routes of phospholipid biosynthesis in mammalian cells, although the CEPT is probably a participant in both pathways. The CPT activity responsible for the synthesis of platelet-activating factor (PAF) is different from those responsible for PtdCho synthesis on the basis of the enzyme's sensitivity to dithiothreitol [88]. The gene encoding this dithiothreitolsensitive CPT in the PAF biosynthetic pathway has not yet been identified.
The CPT and CEPT enzymes are integral membrane proteins and have not been purified to homogeneity. The activities of these proteins are affected by the composition of the membranes or micelles in which they are embedded [89]. The assay of CPT activity in microsomes or mixed micelles is not straightforward, and detergent micelles that are used to deliver the DAG substrate for the assay can inhibit the reaction. The DAG substrate is lipophilic whereas the CDP-choline cosubstrate is water-soluble, and the reaction occurs at the hydrophobic-hydrophilic interface of membranes which impacts kinetic behavior [89]. Fractionation of subcellular organelles reveals that the endoplasmic reticulum (ER) is the site with highest CPT activity per milligram protein (defined as 100%), followed by the Golgi apparatus (37%), crude mitochondria (14%), plasma membrane (1%), and nucleus (<1%) [90–92]. CPT activity is also associated with synaptic membranes [93]. The putative mitochondrial activity is likely associated with an ER-like fraction called the mitochondrial-associated membrane (MAM) that co-purifies with crude mitochondria during subcellular fractionation of organelles [94]. The MAM is in contact with the Golgi apparatus and is involved in lipid trafficking [95]. Microscopic imaging of the human CPT and CEPT proteins has refined their subcellular localizations. CPT is primarily associated with the Golgi apparatus, and its localization is not altered upon treatment of cells with brefeldin A, which disrupts the ER [96]. The CEPT is found in both the ER and nuclear membranes [96]. Altogether, the data indicate that the CPT/CEPT enzymes reside in multiple subcellular membranes that can move among organelles.
Inhibition of the CPT activity reduces phospholipid synthesis and results in accumulation of CPT substrates. Reduction of PtdCho synthesis is one event that follows infection of mammalian host cells by a bacterial pathogen, resulting in apoptosis [97]. CPT was thought to be the direct target of farnesol-induced apoptosis [98,99], but further analysis implicates reduction of the DAG substrate for CPT as the culprit mechanism for inhibiton of PtdCho synthesis by farnesol [52,100]. Activities that can modify the DAG level in the Golgi apparatus also modulate Golgi secretory function [101,102], implicating the CPT, among other activities such as phospholipases, in membrane lipid movement. However, the levels of CPT and CEPT expression do not determine the amount of membrane phospholipid that is made. Overexpression studies show that despite high levels of CPT activity, the rate of phospholipid synthesis is determined by the amount(s) of CDP-choline and/or DAG co-substrate available from enzymatic activities upstream of the CPT step [100,103]. Expression of the gene encoding CPT, but not CEPT, increases during LPSinduced ER biogenesis in B lymphocytes [104]. Since CPT expression is associated with elaboration of new ER membrane [103,105], it may be useful as a marker protein for ER expansion. Similar to the hijacking of CDP-choline by L. pneumophila, reduction of PtdCho synthesis is one mechanism that follows infection of mammalian host cells by the bacterial pathogen Streptococcus pneumoniae, resulting in apoptosis [97].
4. The CDP-choline Pathway Provides PtdCho on Demand
Through the years, our understanding of the molecular regulation of the CDP-choline pathway has been largely obtained using immortalized mammalian cell lines, where inhibition of either CK or CCT leads to cell death, often by apoptotic mechanisms [30,33,106–111]. These experiments show that the CDP-choline pathway of PtdCho synthesis is essential in proliferating cells. The activity of CCTα and the rate of PtdCho synthesis increase dramatically when synchronized or quiescent cells are stimulated to enter the G1 phase of the cell cycle [37,48,112–115]. Depending on the cell line, the increased activity is associated with increased CCT gene expression [112,116], CCTα dephosphorylation and enhanced activity [117], enhanced membrane association [113] and/or CCTα translocation out of the nucleus [48]. The PtdCho pool is dynamic and the amount of PtdCho represents a balance between synthesis and degradation. PtdCho synthesis during G1 is accompanied by PtdCho turnover mediated by the calcium-independent phospholipase A [118] and the neuropathy target esterase [119,120]. The net accumulation of PtdCho is due to reduction of turnover, together with reduction of CCT activity, as cells approach S phase [113,117]. Cells that are arrested in S phase upon treatment with inhibitors of DNA synthesis continue to synthesize phospholipids, demonstrating that DNA synthesis and PtdCho synthesis are not linked [117]. A fibroblast cell line with temperature-sensitive CCT activity does not arrest at a particular cell cycle stage prior to apoptosis after temperature-shift [106], supporting the view that DNA synthesis and PtdCho synthesis are not linked. Although later reports indicate that PtdCho and other phospholipids are synthesized in the G2/M phase, rather than S phase, those investigators are not aware of the inability to arrest phospholipid synthesis using inhibitors of DNA synthesis to synchronize cells [121,122], and so these latest conclusions are questionable. Curiously, limitation of free choline in the culture medium does not readily result in cell death in cultured cells, if at all, arguing that the CDP-choline pathway is not essential. Delipidation of serum prior to addition to the culture medium is required to demonstrate choline dependence in a fibroblast cell line [115]. Thus, serum PtdCho or lysoPtdCho provide substantial amounts of phospholipid for cells and can provide the choline in serum-based cell culture models to buffer against depletion of free choline. The incorporation of serum lipid into cells is quantitative [123], but the amount may be insufficient, or the process may be too slow to support an immediate PtdCho response to stimulation. For example, differentiation of cultured neuronal cells induced by retinoic-acid requires elevated PtdCho that is supplied by activation of the CDP-choline pathway. Addition of excess PtdCho supplement in the culture medium can initiate the differentiation program as well [59].
The CDP-choline pathway is thought to be the only source of PtdCho in mammalian tissues except liver, where the PEMT is expressed at very high levels [57]. The phenotypes of the global knockout mouse models for the CKs and CCTs support this idea. (There are no knockout mouse models for the CPT or CEPT.) Deficiencies in the ubiquitous and most abundant CKα or CCTα isoforms result in a lethal phenotype very early in embryogenesis [35,79]. Deficiencies in the less abundant, more selectively expressed of CKβ or CCTβ isoforms result in viable mice with tissuespecific impairments in differentiated functions [36,57]. The CKβ knockout mouse displays hindlimb muscular dystrophy and CKβ is the major isoform in this tissue [124]. The CCTβ knockout mouse displays premature gonadal senescence and CCTβ is expressed at its highest levels in the gonads and brain [57]. Interestingly, the CCTβ phenotype is prevented by probucol therapy [125]. Probucol is a cholesterol-lowering drug that also rescues the gonadal dysfunction in high density lipoprotein (HDL)-receptor knockout mice [126]. PtdCho is a quantitatively significant component of HDL [127]. By increasing HDL uptake by tissues, probucol increases the incorporation of both PtdCho and cholesterol. The incorporation of PtdCho from serum lipoproteins into tissues is an underappreciated source of phospholipid in mammals. Tissue-specific CCTα-deficiencies in mice do not result in reduction of differentiated tissue mass, but rather, result in reduction of differentiated tissue function. The CCTα-deficient liver produces less plasma HDL and very low density lipoproteins [128]. The CCTα-deficient lung epithelial type II cells produce significantly less surfactant PtdCho [129]. The CCTα-deficient macrophages produce less cytokine after stimulation [130] and are unable to sustain their viability upon cholesterol loading [131]. CCTα-deficient B lymphocytes secrete immature, rather than mature, immunoglobulins in response to stimulation [132]. Defective or miscued secretion, which results from impaired or aberrant vesicular trafficking, is common to all of these mouse models, indicating that the CDP-choline pathway has an essential role in stimulus-secretion coupling.
5. PtdCho Turnover
The CDP-choline cycle (Figure 2) provides not only PtdCho, but also provides the important breakdown products of PtdCho. PtdCho turnover has an integral role in PtdCho homeostasis in proliferating cells [133] and major products of PtdCho degradation are GroPCho and phosphocholine [123]. Neuropathy target esterase catalyzes the degradation of PtdCho to GroPCho and this activity is stimulated in response to high NaCl [134]. GroPCho is an organic osmolyte that is protective against high osmolarity insult and is particularly important in renal [135] and neural [136] cells. GroPCho can also inhibit the phospholipid insertion and extraction process of the mammalian phosphatidylinositol transfer protein α [137], which is an ortholog of yeast Sec14. A GroPCho phosphodiesterase regulates the level of GroPCho [138], together with neuropathy target esterase, and the proteins co-localize with each other in the perinuclear ER region of the cell [138,139], indicating an important site of PtdCho turnover. The GroPCho phosphodiesterase produces choline which is re-incorporated into PtdCho, or acetylated in neural cells to make the neurotransmitter acetyl-choline in distal axons [137], or oxidized to betaine which is an organic osmolyte [140]. Axon maintenance requires PtdCho turnover via neuropathy target esterase and loss of esterase expression results in neurodegeneration [139,141,142], indicating that PtdCho is an intermediate in a fundamental neural process. The CDP-choline pathway is not the only source of PtdCho in mammalian cells and PtdCho derived from PEMT [143,144] or extracellular sources [123] is incorporated into the PtdCho pool which then is subject to degradation by the catabolic aspect of the CDP-choline cycle.
6. Conclusion
PtdCho is the most abundant bilayer-forming phospholipid in mammalian cells and perturbation of its synthesis or metabolism has far-reaching effects on membrane movement throughout the cell. The CDP-choline cycle is integrated into a larger metabolic network and interruption of the CDP-choline cycle can affect the distribution of lipid-related metabolites in several other pathways (Figure 2). Inhibition of the synthetic aspect of the cycle results in elevated DAG levels and/or reduced sphingomyelin levels and can have profound effects on ER and Golgi-mediated functions and cell survival. Interruption of the catabolic aspect of the cycle results in higher PtdCho levels to support cell proliferation and differentiation, but also results in reduced GroPCho levels, which functions in the osmotic regulation of cell volume. The activity of the CDP-choline cycle is integral to the dynamics of membrane movement.
Highlights.
The yeast CDP-choline pathway provides a basis for the mammalian pathway.
The elements of the CDP-choline pathway are related to their cellular functions.
The CDP-choline cycle is regulated and linked to membrane movement.
Acknowledgements
This work was supported by National Institutes of Health grant GM062896, Cancer Center (CORE) Support Grant CA21765 and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedkin M, Lehninger AL. Oxidation-coupled incorporation of inorganic radiophosphate into phospholipide and nucleic acid in a cell-free system. J. Biol. Chem. 1949;177:775–788. [PubMed] [Google Scholar]
- 2.Kennedy EP. Metabolism of lipides. Annu. Rev. Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- 3.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem. Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 4.Gibellini F, Smith TK. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB. Life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 5.Stalberg K, Neal AC, Ronne H, Stahl U. Identification of a novel GPCAT activity and a new pathway for phosphatidylcholine biosynthesis in S. cerevisiae. J. Lipid Res. 2008;49:1794–1806. doi: 10.1194/jlr.M800129-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Friesen JA, Park YS, Kent C. Purification and kinetic characterization of CTP:phosphocholine cytidylyltransferase from Saccharomyces cerevisiae. Protein Expr. Purif. 2001;21:141–148. doi: 10.1006/prep.2000.1354. [DOI] [PubMed] [Google Scholar]
- 7.MacKinnon MA, Curwin AJ, Gaspard GJ, Suraci AB, Fernandez-Murray JP, McMaster CR. The Kap60-Kap95 karyopherin complex directly regulates phosphatidylcholine synthesis. J. Biol. Chem. 2009;284:7376–7384. doi: 10.1074/jbc.M809117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowd SR, Bier ME, Patton-Vogt JL. Turnover of phosphatidylcholine in Saccharomyces cerevisiae : The role of the CDP-choline pathway. J. Biol. Chem. 2000;276:3756–3763. doi: 10.1074/jbc.M003694200. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Murray JP, McMaster CR. Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway. J. Biol. Chem. 2005;280:38290–38296. doi: 10.1074/jbc.M507700200. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Murray JP, Gaspard GJ, Jesch SA, McMaster CR. NTE1-encoded phosphatidylcholine phospholipase B regulates transcription of phospholipid biosynthetic genes. J. Biol. Chem. 2009;284:36034–36046. doi: 10.1074/jbc.M109.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh R, Bankaitis VA. Phosphatidylinositol transfer proteins: negotiating the regulatory interface between lipid metabolism and lipid signaling in diverse cellular processes. Biofactors. 2011;37:290–308. doi: 10.1002/biof.180. [DOI] [PubMed] [Google Scholar]
- 12.McMaster CR. Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem. Cell Biol. 2001;79:681–692. doi: 10.1139/o01-139. [DOI] [PubMed] [Google Scholar]
- 13.Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature (London) 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Phosphatidylcholine synthesis influences the diacylglycerol homeostasis required for Sec14p-dependent golgi function and cell growth. Mol. Biol. Cell. 2001;12:511–520. doi: 10.1091/mbc.12.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp. Biol. Med. (Maywood.) 2006;231:490–504. doi: 10.1177/153537020623100503. [DOI] [PubMed] [Google Scholar]
- 16.Janardhan S, Srivani P, Sastry GN. Choline kinase: an important target for cancer. Curr. Med. Chem. 2006;13:1169–1186. doi: 10.2174/092986706776360923. [DOI] [PubMed] [Google Scholar]
- 17.Michel V, Bakovic M. The Ubiquitous Choline Transporter SLC44A1. Cent. Nerv. Syst. Agents Med. Chem. 2012 doi: 10.2174/187152412800792733. [DOI] [PubMed] [Google Scholar]
- 18.Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010;43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Wittenberg J, Kornberg A. Choline phosphokinase. J. Biol. Chem. 1953;202:431–444. [PubMed] [Google Scholar]
- 20.Aoyama C, Liao H, Ishidate K. Structure and function of choline kinase isoforms in mammalian cells. Prog. Lipid Res. 2004;43:266–281. doi: 10.1016/j.plipres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Aoyama C, Yamazaki N, Terada H, Ishidate K. Structure and characterization of the genes for murine choline/ethanolamine kinase isozymes α and β. J. Lipid Res. 2000;41:452–464. [PubMed] [Google Scholar]
- 22.Wu G, Vance DE. Choline kinase and its function. Biochem. Cell Biol. 2010;88:559–564. doi: 10.1139/O09-160. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama C, Ohtani A, Ishidate K. Expression and characterization of the active molecular forms of choline/ethanolamine kinase-α and -β in mouse tissues, including carbon tetrachloride-induced liver. Biochem. J. 2002;363:777–784. doi: 10.1042/0264-6021:3630777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen SM, Groener JE, Bax W, Suter A, Saftig P, Somerharju P, Poorthuis BJ. Biosynthesis of phosphatidylcholine from a phosphocholine precursor pool derived from the late endosomal/lysosomal degradation of sphingomyelin. J. Biol. Chem. 2001;276:18722–18727. doi: 10.1074/jbc.M101817200. [DOI] [PubMed] [Google Scholar]
- 26.Peisach D, Gee P, Kent C, Xu Z. The crystal structure of choline kinase reveals a eukaryotic protein kinase fold. Structure. 2003;11:703–713. doi: 10.1016/s0969-2126(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 27.Monks DE, Goode JH, Dewey RE. Characterization of soybean choline kinase cDNAs and their expression in yeast and Escherichia coli. Plant Physiol. 1996;110:1197–1205. doi: 10.1104/pp.110.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lykidis A, Wang J, Karim M, Jackowski S. Overexpression of a mammalian ethanolamine-specific kinase accelerates the CDP-ethanolamine pathway. J. Biol. Chem. 2001;276:2174–2179. doi: 10.1074/jbc.M008794200. [DOI] [PubMed] [Google Scholar]
- 29.Kiss Z. Regulation of mitogenesis by water-soluble phospholipid intermediates. Cell Signal. 1999;11:149–157. doi: 10.1016/s0898-6568(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 30.Lacal JC. Choline kinase: a novel target for antitumor drugs. IDrugs. 2001;4:419–426. [PubMed] [Google Scholar]
- 31.Gallego-Ortega D, Gomez del PT, Valdes-Mora F, Cebrian A, Lacal JC. Involvement of human choline kinase α and β in carcinogenesis: a different role in lipid metabolism and biological functions. Adv. Enzyme Regul. 2011;51:183–194. doi: 10.1016/j.advenzreg.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–1123. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez de MA, Rodriguez-Gonzalez A, Lacal JC. From Ras signalling to ChoK inhibitors: a further advance in anticancer drug design. Cancer Lett. 2004;206:137–148. doi: 10.1016/j.canlet.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Ishidate K, Tsuruoka M, Nakazawa Y. Induction of choline kinase by polycyclic aromatic hydrocarbon carcinogens in rat liver. Biochem. Biophys. Res. Commun. 1980;96:946–952. doi: 10.1016/0006-291x(80)91446-1. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Aoyama C, Young SG, Vance DE. Early embryonic lethality caused by disruption of the gene for choline kinase α, the first enzyme in phosphatidylcholine biosynthesis. J. Biol. Chem. 2008;283:1456–1462. doi: 10.1074/jbc.M708766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sher RB, Aoyama C, Huebsch KA, Ji S, Kerner J, Yang Y, Frankel WN, Hoppel CL, Wood PA, Vance DE, Cox GA. A rostrocaudal muscular dystrophy caused by a defect in choline kinase β, the first enzyme in phosphatidylcholine biosynthesis. J. Biol. Chem. 2006;281:4938–4948. doi: 10.1074/jbc.M512578200. [DOI] [PubMed] [Google Scholar]
- 37.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J. Biol. Chem. 2005;280:853–856. doi: 10.1074/jbc.R400031200. [DOI] [PubMed] [Google Scholar]
- 38.Cornell RB. How cytidylyltransferase uses an amphipathic helix to sense membrane phospholipid composition. Biochem. Soc. Trans. 1998;26:539–544. doi: 10.1042/bst0260539. [DOI] [PubMed] [Google Scholar]
- 39.Cornell RB, Northwood IC. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem. Sci. 2000;25:441–447. doi: 10.1016/s0968-0004(00)01625-x. [DOI] [PubMed] [Google Scholar]
- 40.Clement JM, Kent C. CTP:Phosphocholine cytidylyltransferase: insights into regulatory mechanisms and novel functions. Biochem. Biophys. Res. Commun. 1999;257:643–650. doi: 10.1006/bbrc.1999.0512. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto H, Banchio C, Vance DE. Transcriptional regulation of phosphatidylcholine biosynthesis. Prog. Lipid Res. 2008 doi: 10.1016/j.plipres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Kent C. Regulatory enzymes of phosphatidylcholine biosynthesis: a personal perspective. Biochim. Biophys. Acta. 2005;1733:53–66. doi: 10.1016/j.bbalip.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Weinhold PA, Feldman DA. Choline-phosphate cytidylyltransferase. Methods Enzymol. 1992;29:248–258. doi: 10.1016/0076-6879(92)09031-w. [DOI] [PubMed] [Google Scholar]
- 44.Itzen A, Goody RS. Covalent coercion by Legionella pneumophila. Cell Host. Microbe. 2011;10:89–91. doi: 10.1016/j.chom.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature (London) 2011;477:103–106. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kast HR, Nguyen CM, Anisfeld AM, Ericsson J, Edwards PA. CTP:phosphocholine cytidylyltransferase, a new sterol- and SREBP-responsive gene. J. Lipid Res. 2001;42:1266–1272. [PubMed] [Google Scholar]
- 47.Ridgway ND, Lagace TA. Regulation of the CDP-choline pathway by sterol regulatory element binding proteins involves transcriptional and post-transcriptional mechanisms. Biochem. J. 2003;372:811–819. doi: 10.1042/BJ20030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Northwood IC, Tong AH, Crawford B, Drobnies AE, Cornell RB. Shuttling of CTP:phosphocholine cytidylyltransferase between the nucleus and endoplasmic reticulum accompanies the wave of phosphatidylcholine synthesis during the G0 -->G1 transition. J. Biol. Chem. 1999;274:26240–26248. doi: 10.1074/jbc.274.37.26240. [DOI] [PubMed] [Google Scholar]
- 49.DeLong CJ, Qin L, Cui Z. Nuclear localization of enzymatically active green fluorescent protein- CTP:phosphocholine cytidylyltransferase alpha fusion protein is independent of cell cycle conditions and cell types. J. Biol. Chem. 2000;275:32325–32330. doi: 10.1074/jbc.M004644200. [DOI] [PubMed] [Google Scholar]
- 50.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, Farese RV, Jr., Walther TC. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gehrig K, Morton CC, Ridgway ND. Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain. J. Lipid Res. 2009;50:966–976. doi: 10.1194/jlr.M800632-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagace TA, Miller JR, Ridgway ND. Caspase processing and nuclear export of CTP:phosphocholine cytidylyltransferase α during farnesol-induced apoptosis. Mol. Cell. Biol. 2002;22:4851–4862. doi: 10.1128/MCB.22.13.4851-4862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallampalli RK, Ryan AJ, Salome RG, Jackowski S. Tumor necrosis factor-α inhibits expression of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 2000;275:9699–9708. doi: 10.1074/jbc.275.13.9699. [DOI] [PubMed] [Google Scholar]
- 54.Chen BB, Mallampalli RK. Calmodulin binds and stabilizes the regulatory enzyme, CTP: phosphocholine cytidylyltransferase. J. Biol. Chem. 2007;282:33494–33506. doi: 10.1074/jbc.M706472200. [DOI] [PubMed] [Google Scholar]
- 55.Bogan MJ, Agnes GR, Pio F, Cornell RB. Interdomain and membrane interactions of CTP:phosphocholine cytidylyltransferase revealed via limited proteolysis and mass spectrometry. J. Biol. Chem. 2005;280:19613–19624. doi: 10.1074/jbc.M414028200. [DOI] [PubMed] [Google Scholar]
- 56.Craig L, Johnson JE, Cornell RB. Identification of the membrane-binding domain of rat liver CTP:phosphocholine cytidylyltransferase using chymotrypsin proteolysis. J. Biol. Chem. 1994;269:3311–3317. [PubMed] [Google Scholar]
- 57.Jackowski S, Rehg JE, Zhang Y-M, Wang J, Miller K, Jackson P, Karim MA. Disruption of CCTβ2 expression leads to gonadal dysfunction. Mol. Cell. Biol. 2004;24:4720–4733. doi: 10.1128/MCB.24.11.4720-4733.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter JM, Waite KA, Campenot RB, Vance JE, Vance DE. Enhanced expression and activation of CTP:phosphocholine cytidylyltransferase β2 during neurite outgrowth. J. Biol. Chem. 2003;278:44988–44994. doi: 10.1074/jbc.M307336200. [DOI] [PubMed] [Google Scholar]
- 59.Marcucci H, Paoletti L, Jackowski S, Banchio C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010;285:25382–25393. doi: 10.1074/jbc.M110.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strakova J, Demizieux L, Campenot RB, Vance DE, Vance JE. Involvement of CTP:phosphocholine cytidylyltransferase-β2 in axonal phosphatidylcholine synthesis and branching of neurons. Biochim. Biophys. Acta. 2011;1811:617–625. doi: 10.1016/j.bbalip.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Johnson J, Ding Z, Paetzel M, Cornell RB. Crystal structure of a mammalian CTP: phosphocholine cytidylyltransferase catalytic domain reveals novel active site residues within a highly conserved nucleotidyltransferase fold. J. Biol. Chem. 2009;284:33535–33548. doi: 10.1074/jbc.M109.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornell RB, Taneva SG. Amphipathic helices as mediators of the membrane interaction of amphitropic proteins, and as modulators of bilayer physical properties. Curr. Protein Pept. Sci. 2006;7:539–552. doi: 10.2174/138920306779025675. [DOI] [PubMed] [Google Scholar]
- 63.Rothblat GH, Mahlberg FH, Johnson WJ, Phillips MC. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J. Lipid Res. 1992;33:1091–1097. [PubMed] [Google Scholar]
- 64.Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauchlan J. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J. Biol. Chem. 2006;281:22236–22247. doi: 10.1074/jbc.M601031200. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki R, Sakamoto S, Tsutsumi T, Rikimaru A, Tanaka K, Shimoike T, Moriishi K, Iwasaki T, Mizumoto K, Matsuura Y, Miyamura T, Suzuki T. Molecular determinants for subcellular localization of hepatitis C virus core protein. J. Virol. 2005;79:1271–1281. doi: 10.1128/JVI.79.2.1271-1281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attard GS, Templer RH, Smith WS, Hunt AN, Jackowski S. Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9032–9036. doi: 10.1073/pnas.160260697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shearman GC, Attard GS, Hunt AN, Jackowski S, Baciu M, Sebai SC, Mulet X, Clarke JA, Law RV, Plisson C, Parker CA, Gee A, Ces O, Templer RH. Using membrane stress to our advantage. Biochem. Soc. Trans. 2007;35:498–501. doi: 10.1042/BST0350498. [DOI] [PubMed] [Google Scholar]
- 68.Davies SM, Epand RM, Kraayenhof R, Cornell RB. Regulation of CTP: phosphocholine cytidylyltransferase activity by the physical properties of lipid membranes: An important role for stored curvature strain energy. Biochemistry. 2001;40:10522–10531. doi: 10.1021/bi010904c. [DOI] [PubMed] [Google Scholar]
- 69.Ridsdale R, Tseu I, Wang J, Post M. Functions of membrane binding domain of CTP:phosphocholine cytidylyltransferase in alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 2010;43:74–97. doi: 10.1165/rcmb.2009-0231OC. [DOI] [PubMed] [Google Scholar]
- 70.Dennis MK, Taneva SG, Cornell RB. The intrinsically disordered nuclear localization signal and phosphorylation segments distinguish the membrane affinity of two cytidylyltransferase isoforms. J. Biol. Chem. 2011;286:12349–12360. doi: 10.1074/jbc.M110.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lykidis A, Jackson P, Jackowski S. Lipid activation of CTP:phosphocholine cytidylyltransferase α: Characterization and identification of a second activation domain. Biochemistry. 2001;40:494–503. doi: 10.1021/bi002140r. [DOI] [PubMed] [Google Scholar]
- 72.Taneva S, Dennis MK, Ding Z, Smith JL, Cornell RB. Contribution of each membrane binding domain of the CTP:phosphocholine cytidylyltransferase-α dimer to its activation, membrane binding, and membrane cross-bridging. J. Biol. Chem. 2008;283:28137–28148. doi: 10.1074/jbc.M802595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoh T, Takenawa T. Mechanisms of membrane deformation by lipid-binding domains. Prog. Lipid Res. 2009;48:298–305. doi: 10.1016/j.plipres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Lagace TA, Ridgway ND. The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum. Mol. Biol. Cell. 2005;16:1120–1130. doi: 10.1091/mbc.E04-10-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goulbourne CN, Malhas AN, Vaux DJ. The induction of a nucleoplasmic reticulum by prelamin A accumulation requires CTP:phosphocholine cytidylyltransferase-α. J. Cell Sci. 2011;124:4253–4266. doi: 10.1242/jcs.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol. 2011;21:362–373. doi: 10.1016/j.tcb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Gehrig K, Ridgway ND. CTP:phosphocholine cytidylyltransferase α (CCTα) and lamins alter nuclear membrane structure without affecting phosphatidylcholine synthesis. Biochim. Biophys. Acta. 2011;1811:377–385. doi: 10.1016/j.bbalip.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Taneva SG, Lee JM, Cornell RB. The amphipathic helix of an enzyme that regulates phosphatidylcholine synthesis remodels membranes into highly curved nanotubules. Biochim. Biophys. Acta. 2012;1818:1173–1186. doi: 10.1016/j.bbamem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, Magdaleno S, Tabas I, Jackowski S. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase α gene (Pcyt1a) Mol. Cell. Biol. 2005;25:3357–3363. doi: 10.1128/MCB.25.8.3357-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kennedy EP, Weiss SB. The function of cytidine coenzyme in the biosynthesis of phospholipids. J. Biol. Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 81.Weiss SB, Smith SW, Kennedy EP. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J. Biol. Chem. 1958;231:53–64. [PubMed] [Google Scholar]
- 82.Cornell RB. Cholinephosphotransferase from mammalian sources. Methods Enzymol. 1992;209:267–272. doi: 10.1016/0076-6879(92)09033-y. [DOI] [PubMed] [Google Scholar]
- 83.Dewey RE, Wilson RF, Novitzky WP, Goode JH. The AAPT1 gene of soybean complements a cholinephosphotransferase-deficient mutant of yeast. Plant Cell. 1994;6:1495–1507. doi: 10.1105/tpc.6.10.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hjelmstad RH, Bell RM. The sn-1,2-diacylglycerol choline phosphotransferases of Saccharomyces cerevisiae : Nucleotide sequence, transcriptional mapping, and gene product analysis of the CPT1 gene. J. Biol. Chem. 1990;265:1755–1764. [PubMed] [Google Scholar]
- 85.Henneberry AL, Wistow G, McMaster CR. Cloning, genomic organization, and characterization of a human cholinephosphotransferase. J. Biol. Chem. 2000;275:29808–29815. doi: 10.1074/jbc.M005786200. [DOI] [PubMed] [Google Scholar]
- 86.Henneberry AL, McMaster CR. Cloning and expression of a human choline/ethanolaminephosphotransferase: synthesis of phosphatidylcholine and phosphatidylethanolamine. Biochem. J. 1999;339:291–298. [PMC free article] [PubMed] [Google Scholar]
- 87.Horibata Y, Hirabayashi Y. Identification and characterization of a human ethanolaminephosphotransferase1 (hEPT1) J. Lipid Res. 2006;48:503–508. doi: 10.1194/jlr.C600019-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Woodard DS, Lee TC, Snyder F. The final step in the de novo biosynthesis of platelet-activating factor. Properties of a unique CDP-choline, 1-alkyl-2-acetyl-sn-glycerol choline-phosphotransferase in microsomes from the renal inner medulla of rats. J. Biol. Chem. 1987;262:2520–2527. [PubMed] [Google Scholar]
- 89.Wright MM, McMaster CR. PC and PE synthesis: mixed micellar analysis of the cholinephosphotransferase and ethanolaminephosphotransferase activities of human choline/ethanolamine phosphotransferase 1 (CEPT1) Lipids. 2002;37:663–672. doi: 10.1007/s11745-002-0947-6. [DOI] [PubMed] [Google Scholar]
- 90.Moreau P, Morre DJ. Cell-free transfer of membrane lipids. Evidence for lipid processing. J. Biol. Chem. 1991;266:4329–4333. [PubMed] [Google Scholar]
- 91.Sparace SA, Moore TS. Phospholipid Metabolism in Plant Mitochondria: II. Submitochondrial sites of synthesis of phosphatdylcholine and phosphadylethanolamine. Plant Physiol. 1981;67:261–265. doi: 10.1104/pp.67.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stith IE, Das SK. Development of cholinephosphotransferase in guinea pig lung mitochondria and microsomes. Biochim. Biophys. Acta. 1982;714:250–256. doi: 10.1016/0304-4165(82)90331-2. [DOI] [PubMed] [Google Scholar]
- 93.Hargreaves KM, Clandinin MT. Phosphocholinetransferase activity in plasma membrane: effect of diet. Biochem. Biophys. Res. Commun. 1987;145:309–315. doi: 10.1016/0006-291x(87)91322-2. [DOI] [PubMed] [Google Scholar]
- 94.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 95.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 96.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol. Biol. Cell. 2002;13:3148–3161. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zweigner J, Jackowski S, Smith SH, Van Der MM, Weber JR, Tuomanen EI. Bacterial inhibition of phosphatidylcholine synthesis triggers apoptosis in the brain. J. Exp. Med. 2004;200:99–106. doi: 10.1084/jem.20032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams SN, Anthony ML, Brindle KM. Induction of apoptosis in two mammalian cell lines results in increased levels of fructose-1,6-bisphosphate and CDP-choline as determined by 31P MRS. Magn. Reson. Med. 1998;40:411–420. doi: 10.1002/mrm.1910400311. [DOI] [PubMed] [Google Scholar]
- 99.Anthony ML, Zhao M, Brindle KM. Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J. Biol. Chem. 1999;274:19686–19692. doi: 10.1074/jbc.274.28.19686. [DOI] [PubMed] [Google Scholar]
- 100.Wright MM, Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. J. Biol. Chem. 2001;276:25254–25261. doi: 10.1074/jbc.M011552200. [DOI] [PubMed] [Google Scholar]
- 101.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 102.Lev S. Lipid homoeostasis and Golgi secretory function. Biochem. Soc. Trans. 2006;34:363–366. doi: 10.1042/BST0340363. [DOI] [PubMed] [Google Scholar]
- 103.Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J. Biol. Chem. 2007;282:7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- 104.Fagone P, Sriburi R, Ward-Chapman C, Frank M, Wang J, Gunter C, Brewer JW, Jackowski S. Phospholipid biosynthesis program underlying membrane expansion during B lymphocyte differentiation. J. Biol. Chem. 2007;282:7591–7605. doi: 10.1074/jbc.M608175200. [DOI] [PubMed] [Google Scholar]
- 105.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cui Z, Houweling M, Chen MH, Record M, Chap H, Vance DE, Tercé F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in chinese hamster ovary cells. J. Biol. Chem. 1996;271:14668–14671. doi: 10.1074/jbc.271.25.14668. [DOI] [PubMed] [Google Scholar]
- 107.Van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr. Pharm. Des. 2008;14:2061–2074. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- 108.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim. Biophys. Acta. 2002;1585:87–96. doi: 10.1016/s1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 109.Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010;287:123–135. doi: 10.1016/j.canlet.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Gonzalez A, Ramirez de Molina A, Benitez-Rajal J, Lacal JC. Phospholipase D and choline kinase: their role in cancer development and their potential as drug targets. Prog. Cell Cycle Res. 2003;5:191–201. [PubMed] [Google Scholar]
- 111.Rodriguez-González A, Ramirez de Molina A, Fernandez F, Ramos MA, del Carmen Núñez M, Campos J, Lacal JC. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene. 2003;22:8803–8812. doi: 10.1038/sj.onc.1207062. [DOI] [PubMed] [Google Scholar]
- 112.Tessner TG, Rock CO, Kalmar GB, Cornell RB, Jackowski S. Colony-stimulating factor-1, regulates CTP: phosphocholine cytidylyltransferase mRNA levels. J. Biol. Chem. 1991;266:16261–16264. [PubMed] [Google Scholar]
- 113.Tseu I, Ridsdale R, Liu J, Wang J, Post M. Cell cycle regulation of pulmonary phosphatidylcholine synthesis. Am. J. Respir. Cell Mol. Biol. 2002;26:506–515. doi: 10.1165/ajrcmb.26.4.4702. [DOI] [PubMed] [Google Scholar]
- 114.Kitos TE, Drobnies A, Ng MN, Wen Y, Cornell RB. Contribution of lipid mediators to the regulation of phosphatidylcholine synthesis by angiotensin. Biochim. Biophys. Acta. 2006;1761:261–271. doi: 10.1016/j.bbalip.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 115.Terce F, Brun H, Vance DE. Requirement of phosphatidylcholine for normal progression through the cell cycle in C3H/10T1/2 fibroblasts. J. Lipid Res. 1994;35:2130–2142. [PubMed] [Google Scholar]
- 116.Hogan M, Kuliszewski M, Lee W, Post M. Regulation of phosphatidylcholine synthesis in maturing type II cells: increased mRNA stability of CTP:phosphocholine cytidylyltransferase. Biochem. J. 1996;314:799–803. doi: 10.1042/bj3140799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 1994;269:3858–3867. [PubMed] [Google Scholar]
- 118.Manguikian AD, Barbour SE. Cell cycle dependence of group VIA calcium independent phospholipase A2 activity. J. Biol. Chem. 2004;279:52881–52892. doi: 10.1074/jbc.M410659200. [DOI] [PubMed] [Google Scholar]
- 119.Glynn P. Neuropathy target esterase and phospholipid deacylation. Biochim. Biophys. Acta. 2005;1736:87–93. doi: 10.1016/j.bbalip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 120.Zaccheo O, Dinsdale D, Meacock PA, Glynn P. Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J. Biol. Chem. 2004;279:24024–24033. doi: 10.1074/jbc.M400830200. [DOI] [PubMed] [Google Scholar]
- 121.Lin W, Arthur G. Phospholipids are synthesized in the G2/M phase of the cell cycle. Int. J. Biochem. Cell Biol. 2007;39:597–605. doi: 10.1016/j.biocel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 122.Lin W, Arthur G. Phosphatidylcholine catabolism in the MCF-7 cell cycle. Biochem. Cell Biol. 2006;84:737–744. doi: 10.1139/o06-046. [DOI] [PubMed] [Google Scholar]
- 123.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J. Biol. Chem. 1999;274:9400–9408. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 124.Wu G, Sher RB, Cox GA, Vance DE. Differential expression of choline kinase isoforms in skeletal muscle explains the phenotypic variability in the rostrocaudal muscular dystrophy mouse. Biochim. Biophys. Acta. 2010;1801:446–454. doi: 10.1016/j.bbalip.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 125.Gunter C, Frank M, Tian Y, Murti KG, Rehg JE, Jackowski S. Probucol therapy overcomes the reproductive defect in CTP: phosphocholine cytidylyltransferase β2 knockout mice. Biochim. Biophys. Acta. 2007;1771:845–852. doi: 10.1016/j.bbalip.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J. Clin. Invest. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Veen JN, Lingrell S, Vance DE. The membrane lipid phosphatidylcholine is an unexpected source of triacylglycerol in the liver. J. Biol. Chem. 2012;287:23418–23426. doi: 10.1074/jbc.M112.381723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacobs RL, Devlin C, Tabas I, Vance DE. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase α in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004;279:47402–47410. doi: 10.1074/jbc.M404027200. [DOI] [PubMed] [Google Scholar]
- 129.Tian Y, Zhou R, Rehg JE, Jackowski S. Role of phosphocholine cytidylyltransferase α in lung development. Mol. Cell. Biol. 2007;27:975–982. doi: 10.1128/MCB.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian Y, Pate C, Andreolotti A, Wang L, Tuomanen E, Boyd K, Claro E, Jackowski S. Cytokine secretion requires phosphatidylcholine synthesis. J. Cell Biol. 2008;181:945–957. doi: 10.1083/jcb.200706152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang D, Tang W, Yao PM, Yang C, Xie B, Jackowski S, Tabas I. Macrophages deficient in CTP:phosphocholine cytidylyltransferase-α are viable under normal culture conditions but are highly susceptible to free cholesterol-induced death. Molecular genetic evidence that the induction of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response. J. Biol. Chem. 2000;275:35368–35376. doi: 10.1074/jbc.M007099200. [DOI] [PubMed] [Google Scholar]
- 132.Fagone P, Gunter C, Sage CR, Gunn KE, Brewer JW, Jackowski S. CTP:phosphocholine cytidylyltransferase α is required for B-cell proliferation and class switch recombination. J. Biol. Chem. 2009;284:6847–6854. doi: 10.1074/jbc.M807338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lykidis A, Jackowski S. Regulation of mammalian cell membrane biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 2000;65:361–393. doi: 10.1016/s0079-6603(00)65010-9. [DOI] [PubMed] [Google Scholar]
- 134.Gallazzini M, Ferraris JD, Kunin M, Morris RG, Burg MB. Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15260–15265. doi: 10.1073/pnas.0607133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gallazzini M, Burg MB. What's new about osmotic regulation of glycerophosphocholine. Physiology (Bethesda. ) 2009;24:245–249. doi: 10.1152/physiol.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fisher SK, Heacock AM, Keep RF, Foster DJ. Receptor regulation of osmolyte homeostasis in neural cells. J. Physiol. 2010;588:3355–3364. doi: 10.1113/jphysiol.2010.190777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Komatsu H, Westerman J, Snoek GT, Taraschi TF, Janes N. L-α- glycerylphosphorylcholine inhibits the transfer function of phosphatidylinositol transfer protein α. Biochim. Biophys. Acta. 2003;1635:67–74. doi: 10.1016/j.bbalip.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 138.Gallazzini M, Ferraris JD, Burg MB. GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11026–11031. doi: 10.1073/pnas.0805496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang PA, Wu YJ. Neuropathy target esterase: an essential enzyme for neural development and axonal maintenance. Int. J. Biochem. Cell Biol. 2010;42:573–575. doi: 10.1016/j.biocel.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Kempson SA, Montrose MH. Osmotic regulation of renal betaine transport: transcription and beyond. Pflugers Arch. 2004;449:227–234. doi: 10.1007/s00424-004-1338-6. [DOI] [PubMed] [Google Scholar]
- 141.Read DJ, Li Y, Chao MV, Cavanagh JB, Glynn P. Neuropathy target esterase is required for adult vertebrate axon maintenance. J. Neurosci. 2009;29:11594–11600. doi: 10.1523/JNEUROSCI.3007-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Glynn P. Axonal degeneration and neuropathy target esterase. Arh. Hig. Rada Toksikol. 2007;58:355–358. doi: 10.2478/v10004-007-0029-z. [DOI] [PubMed] [Google Scholar]
- 143.Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, Kennedy BP, Dyck JR, Vance DE. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 2010;285:22403–22413. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem. J. 2003;370:987–993. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weinhold PA, Feldman DA, Quade MM, Miller JC, Brooks RL. Evidence for a regulatory role of CTP:choline phosphate cytidylyltransferase in the synthesis of phosphatidylcholine in fetal lung following premature birth. Biochim. Biophys. Acta. 1981;665:134–144. doi: 10.1016/0005-2760(81)90241-1. [DOI] [PubMed] [Google Scholar]
- 146.Sleight R, Kent C. Regulation of phosphatidylcholine biosynthesis in cultured chick embryonic muscle treated with phospholipase C. J. Biol. Chem. 1980;255:10644–10650. [PubMed] [Google Scholar]
- 147.Pritchard PH, Vance DE. Choline metabolism and phosphatidylcholine biosynthesis in cultured rat hepatocytes. Biochem. J. 1981;196:261–267. doi: 10.1042/bj1960261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pelech SL, Pritchard PH, Brindley DN, Vance DE. Fatty acids promote translocation of CTP:phosphocholine cytidylyltransferase to the endoplasmic reticulum and stimulate rat hepatic phosphatidylcholine synthesis. J. Biol. Chem. 1983;258:6782–6788. [PubMed] [Google Scholar]
- 149.Feldman DA, Rounsifer ME, Weinhold PA. The stimulation and binding of CTP: phosphorylcholine cytidylyltransferase by phosphatidylcholine-oleic acid vesicles. Biochim. Biophys. Acta. 1985;833:429–437. doi: 10.1016/0005-2760(85)90100-6. [DOI] [PubMed] [Google Scholar]
- 150.Feldman DA, Kovac CR, Dranginis PL, Weinhold PA. The role of phosphatidylglycerol in the activation of CTP:phosphocholine cytidylyltransferase from rat lung. J. Biol. Chem. 1978;253:4980–4986. [PubMed] [Google Scholar]
- 151.Pelech SL, Vance DE. Regulation of rat liver cytosolic CTP:phosphocholine cytidylyltranserase by phosphorylation and dephosphorylation. J. Biol. Chem. 1982;257:14198–14202. [PubMed] [Google Scholar]
- 152.Lim PH, Pritchard PH, Paddon HB, Vance DE. Stimulation of hepatic phosphatidylcholine biosynthesis in rats fed a high cholesterol and cholate diet correlates with translocation of CTP: phosphocholine cytidylyltransferase from cytosol to microsomes. Biochim. Biophys. Acta. 1983;753:74–82. doi: 10.1016/0005-2760(83)90100-5. [DOI] [PubMed] [Google Scholar]
- 153.Esko JD, Nishijima M, Raetz CRH. Animal cells dependent on exogenous phosphatidylcholine for membrane biogenesis. Proc. Natl. Acad. Sci. U. S. A. 1982;79:1698–1702. doi: 10.1073/pnas.79.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weinhold PA, Rounsifer ME, Feldman DA. The purification and characterization of CTP:phosphorylcholine cytidylyltransferase from rat liver. J. Biol. Chem. 1986;261:5104–5110. [PubMed] [Google Scholar]
- 155.Tsukagoski Y, Nikawa J, Yamashita S. Molecular cloning and characterization of the gene encoding cholinephosphate cytidylyltransferase in Saccharomyces cerevisiae. Eur. J. Biochem. 1987;169:477–486. doi: 10.1111/j.1432-1033.1987.tb13635.x. [DOI] [PubMed] [Google Scholar]
- 156.Hosaka K, Kodaki T, Yamashita S. Cloning and characterization of the yeast CKI gene encoding choline kinase and its expression in Escherichia coli. J. Biol. Chem. 1989;264:2053–2059. [PubMed] [Google Scholar]
- 157.Hjelmstad RH, Bell RM. sn-1,2-Diacylglycerol choline- and ethanolaminephosphotransferases in Saccharomyces cerevisiae: Nucleotide seqeunce of the EPT1 gene and comparison of the CPT1 and EPT1 gene products. J. Biol. Chem. 1991;266:5094–5103. [PubMed] [Google Scholar]
- 158.Porter TJ, Kent C. Purification and characterization of choline/ethanolamine kinase from rat liver. J. Biol. Chem. 1990;265:414–422. [PubMed] [Google Scholar]
- 159.Kalmar GB, Kay RJ, Lachance A, Aebersold R, Cornell RB. Cloning and expression of rat liver CTP:phosphocholine cytidylyltransferase: an amphipathic protein that controls phosphatidylcholine synthesis. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6029–6033. doi: 10.1073/pnas.87.16.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Uchida T, Yamashita S. Molecular cloning, characterization, and expression in Escherichia coli of a cDNA encoding mammalian choline kinase. J. Biol. Chem. 1992;267:10156–10162. [PubMed] [Google Scholar]
- 161.Wang Y, Sweitzer TD, Weinhold PA, Kent C. Nuclear localization of soluble CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 1993;268:5899–5904. [PubMed] [Google Scholar]
- 162.MacDonald JI, Kent C. Baculovirus-mediated expression of rat liver CTP:phosphocholine cytidylyltransferase. Protein Expr. Purif. 1993;4:1–7. doi: 10.1006/prep.1993.1001. [DOI] [PubMed] [Google Scholar]
- 163.Luche MM, Rock CO, Jackowski S. Expression of rat CTP:phosphocholine cytidylyltransferase in insect cells using a baculovirus vector. Arch. Biochem. Biophys. 1993;301:114–118. doi: 10.1006/abbi.1993.1122. [DOI] [PubMed] [Google Scholar]
- 164.MacDonald JIS, Kent C. Identification of phosphorylation sites in rat liver CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 1994;269:10529–10537. [PubMed] [Google Scholar]
- 165.Dunne SJ, Cornell RB, Johnson JE, Glover NR, Tracey AS. Structure of the membrane binding domain of CTP:phosphocholine cytidylytransferase. Biochemistry. 1996;35:11975–11984. doi: 10.1021/bi960821+. [DOI] [PubMed] [Google Scholar]
- 166.Aoyama C, Nakashima K, Matsui M, Ishidate K. Complementary DNA sequence for a 42 kDa rat kidney choline/ethanolamine kinase. Biochim. Biophys. Acta. 1998;1390:1–7. doi: 10.1016/s0005-2760(97)00177-x. [DOI] [PubMed] [Google Scholar]
- 167.Lykidis A, Murti KG, Jackowski S. Cloning and characterization of a second human CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 1998;273:14022–14029. doi: 10.1074/jbc.273.22.14022. [DOI] [PubMed] [Google Scholar]
- 168.Glynn P, Read DJ, Lush MJ, Li Y, Atkins J. Molecular cloning of neuropathy target esterase (NTE) Chem. Biol. Interact. 1999;119–120:513–517. doi: 10.1016/s0009-2797(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 169.Agassandian M, Zhou J, Tephly LA, Ryan AJ, Carter AB, Mallampalli RK. Oxysterols inhibit phosphatidylcholine synthesis via ERK docking and phosphorylation of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 2005;280:21577–21587. doi: 10.1074/jbc.M412409200. [DOI] [PubMed] [Google Scholar]