Abstract
The development of novel therapeutic agents for disorders of cognition such as Alzheimer’s disease (AD) is of paramount importance given the ever-increasing elderly population, however; there is also considerable interest in any strategy that might enhance the clinical efficacy of currently available treatments. The purpose of this study was to evaluate an adjunctive treatment strategy to memory enhancement, namely combining the commonly prescribed acetylcholinesterase inhibitor (AChEI) donepezil, with a positive allosteric modulator (PAM) of α7 nicotinic-acetylcholine receptors (α7-nAChRs), PNU-120596. The treatment strategy was evaluated in a (non-spatial) spontaneous novel object recognition (NOR) task in young rats; a water maze spatial learning and recall procedure in aged, cognitively-impaired rats, and a delayed match to sample (working/short term memory) task in aged rhesus monkeys. In all three experiments a similar drug response was observed, namely that donepezil administered alone improved task performance in a dose-dependent manner; that PNU-120596 administered alone was without significant effect, but that the combination of PNU-120596 with a subthreshold dose of donepezil was effective. The positive effect of the drug combination appeared to be α7-nAChR mediated given that it was blocked in the NOR task by the selective α7-nAChR antagonist methyllycaconitine (MLA). Collectively, these data indicate that PNU-120596 increases the effective dose range of donepezil in learning/memory-related tasks in young and age-impaired animal models. The results suggest that α7-nAChR-selective PAMs like PNU-120596 have potential as adjunctive treatments with acetylcholinesterase inhibitors (e.g., donepezil) for age-related illnesses such as AD as well memory disorders not necessarily associated with advanced age.
Keywords: Alzheimer’s Disease, cholinergic, memory, pro-cognitive, Mild Cognitive Impairment, aging
1. Introduction
The aging of the world’s population (World Health Organization 2012) is expected to result in a dramatic rise in the incidence of neurodegenerative illnesses such as Alzheimer’s disease over the next several decades (CDC, 2007; Kinsella et al., 2009). Accordingly, the risk of developing AD now represents one of the greatest fears of older adults (Riordan et al., 2011), a concern that appears well justified given the profound cognitive deficits and the relentless progression of the illness toward functional incapacity and death. For nearly two decades the primary therapeutic strategy for treating the cognitive dysfunction in AD has been one of a cholinergic replacement strategy. This approach is based on more than 35 years of basic and clinical research which indicates that cholinergic neurons in the forebrain support information processing and cognition, that they become compromised with age (and especially in the setting of AD), and that the cholinergic deficits generally correlate well with the degree of cognitive decline (reviewed, Bartus, 2000; Terry and Buccafusco, 2003). The pathophysiologic results of the early studies which primarily relied on histologic examination of post mortem tissues, enzymes assays, etc. are now supported by modern imaging techniques in living AD patients (e.g., positron emission tomography for measurements of acetylcholinesterase, magnetic resonance imaging of cholinergic structures in the brain such as the nucleus basalis of Meynert, see Sabbagh and Cummings, 2011 for review).
While a number of cholinergic-based approaches (e.g., muscarinic agonists, nicotinic agonists) have been pursued for the treatment of AD, to date, only the clinical data derived from studies with acetylcholinestease inhibitors (AChEIs) have convinced regulatory agencies (e.g., the United States Food and Drug Administration, FDA) that their efficacy/side effect ratio warranted approval for use in human patients. The original FDA approvals of the currently prescribed AChEIs in the United States, donepezil, rivastigmine, and galantamine were based on several large clinical studies in patients diagnosed with mild to moderate AD (Boada-Rovira et al., 2004; Mintzer and Kershaw, 2003; Raskind et al., 2000; Winblad et al., 2001). Additional neuropsychiatric/behavioral evaluations and measures of daily functioning have typically indicated that AD patients administered a AChEI performed better than those administered a placebo (Burns et al., 1999, Birks et al., 2000, Lanctôt et al., 2003, Thompson et al., 2004, Birks, 2006, Takeda et al., 2006 and Hansen et al., 2008). The extent of the improvement was generally modest and not sustained over time, however, and some have questioned the clinical significance of the aforementioned study results (Giacobini, 2000; Birks, 2006; Hansen et al., 2008).
An additional limitation of AChEIs in AD is the variety of dose-limiting side effects that may prevent the administration of doses that are high enough for optimal effects on cognition. While both nicotinic and muscarinic acetylcholine receptors (nAChRs and mAChRs, respectively) are considered important therapeutic targets for improving cognition in AD, doses of AChEIs high enough to significantly improve nAChR signaling (via the increase in synaptic acetylcholine levels) are accompanied by adverse reactions (e.g., nausea, vomiting, and diarrhea) that likely result from muscarinic overstimulation (see Maelicke and Albuquerque, 2000). Accordingly, an alternative (nAChR-based) treatment strategy that would theoretically be less prone to adverse reactions would be to selectively activate or “sensitize” nAChRs to acetylcholine. The later approach to drug development via compounds now known as nicotinic “positive allosteric modulators” (PAMs) is currently being pursued in multiple laboratories. These compounds interact with nAChRs via binding sites that are distinct from those for acetylcholine and traditional nAChR agonists (i.e., orthosteric sites). Interestingly, among the AChEIs that are prescribed clinically, galantamine has also been described as a PAM of α7 homomeric and α4β2 heteromeric nAChRs (Harvey, 1995; Maelicke and Albuquerque, 2000; Samochocki et al, 2003. However, the effects of galantamine as a nicotinic PAM are relatively modest compared to the more recently developed PAMs (Bertrand and Gopalakrishnan, 2007) and further it is unclear if the PAM activity of galantamine confers any advantages over the other currently prescribed AChEIs (e.g., donepezil), that do not possess this activity (see Hernandez et al., 2006)
The purpose of the study described here was to evaluate an adjunctive treatment strategy to memory enhancement, namely combining the most commonly prescribed AChEI in the US, donepezil, with a PAM of α7-nAChRs, PNU-120596, to determine if the PAM might improve the effective dose range of donepezil. The treatment strategy was evaluated in young and aged rats (classified as cognitively impaired, see below) as well as aged non-human primates for effects on memory-related task performance.
2. Material and Methods
All procedures employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
2.1 Drugs
All drug doses were calculated based on the weight of the salt, except where noted. Donepezil hydrochloride was purchased from A&A Pharmachem Inc, Ottawa Ontario Canada and methyllycaconitine citrate was purchased from Tocris Bioscience, Ellisville MO. PNU-120596 was synthesized utilizing the reaction of 5-chloro-2,4-dimethoxyphenylisocyanate and 3-amino-5-methyl isoxazole via an adaptation of the procedure of Williams et. al., 2011 yielding a white crystalline solid, mp = 219–220°C; lit. mp =219.5–220.5 °C. The purity and composition of PNU-120596 was further confirmed with NMR spectroscopy.
2.2 Rodent Behavioral Studies
All rat behavioral experiments were conducted in rooms with ambient lighting of approximately 25–30 Lux (lumen/m2). Animals were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments.
2.2.1 Subjects
Experimentally naive, male Sprague-Dawley (young, 3–4 months old) and Fischer 344 (young, 3–4 months old and aged 24–25 months old) rats were obtained from Hilltop Lab Animals (Scottdale, PA). Animals were housed 2–3 per cage (45 × 30 × 18 cm polycarbonate cage with corncob bedding) in a vivarium of constant temperature (21–23°C) and humidity (40–50%) for at least 1 week prior to behavioral testing. Lighting was maintained on a 12-hr light-dark cycle (7:00 a.m.–7:00 p.m.) and food and water were available ad libitum.
2.2.2 Spontaneous Novel Object Recognition
The novel object recognition memory procedure was adapted from Ennaceur and Delacour (1988). Briefly, male Sprague-Dawley rats were transported in their home cages from the colony room to a holding room outside the laboratory and acclimated to laboratory conditions (i.e., tail marking, daily handling and weighing) for at least 3 days prior to the start of behavioral experimentation. During experimentation, animals were acclimated for at least 30 min prior to the beginning of each experimental phase and remained in the holding room for approximately 15 min at the end of testing before being returned to the colony room.
Habituation- animals were acclimated, weighed and individually placed the training/testing environment (an opaque plastic chamber, 78.74 cm × 39.37 cm × 31.75 cm with bedding on the floor) for 10 minutes of chamber exploration.
Training trial- 24 hrs after the habituation session, animals were acclimated, weighed and injected with the test compound (drug or vehicle) and after an appropriate pretreatment interval placed in the chamber with its nose facing the center of a long wall and allowed to explore two identical objects for 15 min. The animal’s behavior was observed and recorded on videotape via a camera located 69 cm above the chamber.
Test trial- after a retention delay interval of 48 hrs (a delay interval that produced complete forgetting), animals were returned to the lab, acclimated and then tested for object novelty (i.e, recognition memory). Two objects, one object similar to training (familiar) and a new (novel) object were placed in the chamber and the animal was allowed to explore the objects during a 5 min trial. The experimental objects to be discriminated were a plastic multi-colored Duplo-Lego block configured tower (12 cm in height, 6 cm in width) paired with a ceramic conical-shaped green Christmas tree salt/pepper shaker (12 cm in height, 5 cm in diameter); all objects existed in duplicate. The objects were placed 19.3 cm from the sides of the two short walls and 19.3 cm from the sides of the long walls of the chamber; distance between the two objects was approximately 40 cm. The role of familiar and novel object as well as chamber position of object were randomly assigned across subjects and treatments and objects were cleaned between sessions with a dilute 50% ETOH solution to eliminate olfactory cues. Object exploration occurred when the animal directed its nose to the object at a distance of ≤ 2 cm and/or touching it with its nose; rearing up against the object to investigate the object was also considered exploration, whereas physically climbing on the object, using the object to support itself while rearing and investigating the chamber arena or digging at the base of the object was not considered appropriate object exploratory behavior. The primary behavioral measure was time (sec) spent exploring both objects separately and calculated as the difference of exploration time between the novel object and familiar object during the test trial and defined as the recognition index (RI). For data inclusion, the test subject had to explore each individual object for a minimum of 4 sec and at least 12 sec with both objects combined. Animals were tested only once in this behavioral task.
2.2.3 Water-maze
The learning and memory capabilities of the young (3–4 month old) and aged (24–25 month old) male F344 rats were evaluated using a water maze task as described previously (Rowe et al., 2007).
Test Apparatus
The maze consisted of a circular pool (160 cm in diameter, 60.9 cm in depth) filled to within 15 cm of the rim with water (22°C) made opaque by the addition of nontoxic white latex paint (Createx Colors, East Granby, CT). A retractable pneumatic-driven circular escape platform (Plexiglas; 15.24 cm in diameter) was located 2 cm below the water’s surface in a constant position in one of four imaginary quadrants of the pool. The animal could use only distal visual cues (colored posters, 3-D objects and a blue colored curtain) from within the testing room to locate the submerged platform. Latencies to locate the hidden platform during training, drug test trials, and probe test (see procedures below) were recorded and analyzed using a computer-based tracking system (San Diego Instruments, San Diego, CA).
Hidden Platform Task
The animals were given 15 trials over 5 consecutive days (3 trials per day; 20–30 min inter-trial interval) with the platform submerged. Trial duration was maximally 120 seconds; if the platform was not located the animal was guided to and had to remain on the platform for 20 seconds. The circular pool was divided into 8 equally divided sections which served as animal start positions; the start position associated with the hidden platform location was not used. Start positions were randomized within and across subjects over the 5 days of training and 3 days of pharmacological testing. Animals were placed into the pool facing the side wall.
Probe Trial
On the last training trial, a 30 sec probe test was performed in which the platform was pneumatically lowered out of reach of the animal. After 30 sec, the platform was raised to its original position for an additional 30 sec allowing time for the subject to find the escape platform. Spatial reference memory was assessed by measuring the latency to first entry into the annulus-40 as well as total dwell time in the annulus-40. The annulus-40 was defined as a 40 cm diameter target zone centered around the hidden platform. Additionally, % time in target quadrant, and path length were assessed.
Visible Platform Test
On day 6, animals not selected to receive additional behavioral testing were given 4 successive 60 sec trials in which the platform was raised 2.5 cm above the water’s surface (visual cued condition) to assess any possible visual, motivational or motor deficits that may have influenced water-maze performance. Animals selected for pharmacological testing received the visible platform test at the conclusion of testing (day 9). Animals that required >40 sec to reach the visible platform on any trial were excluded from analysis.
Identification of Age-Impaired Rats
The behavioral status (i.e., the designation as age-impaired or unimpaired in the water maze) of the aged animals was defined on the basis of their average escape latencies to find the submerged platform on days 3, 4 and 5 of acquisition training relative to the mean escape latency of young controls. Rats were defined as age-impaired (AI) if their average escape latency was ≥ 3 S.D.s from the average latency of the young controls. Aged rats with escape latencies ≤ 0.5 S.D.s from the young controls were considered aged-unimpaired (AU), whereas aged animals with escape latencies that fell between those values were considered aged-other (AO) and were not used for additional behavioral testing. Animals that were classified as AI were weighed, injected with test compound (drug or vehicle) and after an appropriate pretreatment interval given additional water-maze training (3 trials/day for 3 consecutive days). On the third test day, a probe trial followed the two drug-test trials (as described above). Following the drug treatment experiment, AI animals were assessed in the visible platform test (as described above).
2.2.4 Drug administration
Donepezil was dissolved in physiological saline (0.9% NaCl) and administered either orally (po; 2ml/kg) with a 60 min pretreatment interval or intraperitoneally (ip; 1 ml/kg)) with a 30 min pretreatment interval. Methyllcaconitine was dissolved in saline and injected ip (1 ml/kg) with a 60 min pretreatment interval. PNU 120596 was dissolved in 10% DMA and 45% β-cyclodextrin and injected ip (2 ml/kg) with a 30 min pretreatment interval. The doses and pretreatment times for donepezil in rats were based on previously published studies where brain penetration, AChE inhibition, and brain acetylcholine levels were assessed (Kosasa et al., 1999; Geerts et al., 2005). For PNU 120596, the doses and pretreatment times were based on Hurst et al., 2005; Ng et al., 2007, as well as pharmacokinetic studies performed in rats at Memory Pharmaceuticals, Inc. The results of these studies indicated the following: PNU 120596 (3 mg/kg, po; 1 mg/kg iv) had an oral bioavailability of 9% with a plasma t ½ of 90 min (iv dosing). Following oral dosing of 3 mg/kg, PNU 120596 had a AUC of 147 ng.h/ml; Tmax was 25 min and Cmax was 181 ng/ml. Brain concentrations 1 hr after oral dosing 3 mg/kg was 50.8 ng/g; plasma levels were 43.1 ng/ml. The brain/plasma ratio was 1.18. In addition, in young animals in the NOR task the oral (gavage) route of administration was used for translational purposes (see the NHP section below) while the i.p. route was used in the aged rats in the water maze task. The latter approach was preferred due to the negative effects stress we have previously observed with oral gavage used in aged rats.
After drug administration visual inspection was used to monitor for potential cholinergic-based side effects (e.g., salivation, tremors, flattened body posture and loss of motor coordination).
2.3 Non-human Primate Studies
2.3.1 Test Subjects
Non-human primate subjects included 4 aged male and 3 aged female rhesus macaques (Macaca mulatta, see Table 1 for additional details). Each animal was well trained (>100 individual sessions) in the delayed matching-to-sample (DMTS) task. The animals were maintained on tap water (unlimited) and standard laboratory monkey chow (Harlan Teklad Laboratory monkey diet, Madison, WI) supplemented with fruits and vegetables. Food was removed from cages at about 0630 hours, and replaced after the completion of testing of all subjects for the day (at about 1630 hours). Additional nourishment was derived from 300 mg reinforcement food pellets (commercial composition of standard monkey chow and banana flakes, Noyes Precision food pellets, P.J. Noyes Co., Lancaster, NH) obtained during experimental sessions. On weekends animals were fed without time restrictions. Room temperature and humidity were maintained at 22 ± 0.6°C and 52 ± 2%, respectively.
Table 1.
Monkey Subject Information
| Subject ID | Gender | Age | Wt (kg) | Delay Intervals (sec) | |
|---|---|---|---|---|---|
| Short | Long | ||||
| 23 | M | 26 | 8.2 | 5 | 10 |
| 226 | M | 25 | 6.8 | 10 | 30 |
| 979 | F | 32 | 11.2 | 5 | 45 |
| 7nv | M | 34 | 8.6 | 5 | 60 |
| dp3 | F | 31 | 8.2 | 5 | 90 |
| dp5 | F | 23 | 8.0 | 5 | 75 |
| h1v | F | 28 | 6.9 | 5 | 45 |
|
|
|||||
| Mean | 28.4 | 8.3 | 5.7 | 50.7 | |
| SEM | 1.5 | 0.6 | 0.7 | 10.2 | |
Each test subject had previously participated in one or more short-term studies assessing the effects of reversible drugs on DMTS performance. Prior drug experience produced no observable untoward effects in the animals, and each subject received at least a 4-week washout period (with continued weekday DMTS testing) prior to the start of this study.
2.3.2 Delayed Match to Sample (DMTS) Testing
DMTS testing was conducted using a modification of the procedure we have described previously (Terry et al., 2005; Terry et al., 2011). Test panels attached to each animal’s home cage presented the task by using a computer-automated system. A touch-sensitive screen (15 in. AccuTouch LCD Panelmount TouchMonitor)/pellet dispenser units (Med Associates) mounted in light-weight aluminum chasses was attached to the home cage. The stimuli included rectangles of various colors (e.g., red, blue, yellow). A trial was initiated by presentation of a sample square composed of one of three colors. The sample rectangle remained in view until the monkey touched within its borders to initiate a pre-programmed delay (retention) interval. Following the delay interval, the two choice rectangles located below where the sample had been were presented. One of the two choice colors was presented with the color matching the stimulus, whereas the other (incorrect) color was presented as one of the two remaining colors. A correct (matching) choice was reinforced. Non-matching choices were neither reinforced nor punished. The inter-trial interval was 5 sec and each session consisted of 96 trials. The presentation of stimulus color, choice colors, and choice position were fully counterbalanced so as to relegate non-matching strategies to chance levels of accuracy. Three different presentation sequences were rotated through each daily session to prevent the subjects from memorizing the first several trials. Delay intervals were established during numerous non-drug or vehicle sessions prior to initiating the study.
Training sessions
The duration for each delay interval was adjusted for each subject until three levels of group performance accuracy were approximated: zero delay (85–100% of trials answered correctly); short delay interval (70–84% correct); and long delay interval (50–65% correct). The assignment of retention intervals based upon an individual’s baseline task accuracy is necessary to avoid ceiling effects in the most proficient animals during drug studies, while also serving to insure that each animal begins testing at relatively the same level of task difficulty. In addition to session accuracy, two response latencies also were measured: the “sample latency”, which is the time between presentation of the sample color and the animal pressing in sample rectangle; and the “choice latency”, which is the time between presentation of the choice colors and the animal pressing one of the choice rectangles.
Drug Administration
Once the duration for each delay interval was adjusted and stabilized so that performances of the monkeys were within the ranges of accuracy described above, pharmacological studies were initiated. The standard weekly regimen for each drug dose included, Monday: the administration of vehicle followed by DMTS testing; Tuesday: drug administration followed by DMTS test session; Wednesday: DMTS testing (in the absence of drug or vehicle) initiated 24 hours after Tuesday’s dosing; Thursday and Friday: same as Tuesday and Wednesday. This timing of administration (i.e., the particular day of drug and/or placebo administration) could be modified in response to some husbandry or housing-related constraints and adapted to each monkey. Donepezil, PNU-120596 (and the drug combinations) were administered orally (in a solid chocolate formulation) in an ascending dose series one hour before DMTS testing according to guidelines specified above. Given that neither PNU-120596 nor the Donepezil-PNU-120596 combination had previously been evaluated in aged monkeys, a non-randomized (ascending order) approach to dosing was conducted due to the potential for adverse reactions especially at the higher doses. The rationale for the doses selected for donepezil was based on previous behavioral and functional brain imaging data (dose range 0.05–0.250 mg/kg) in rhesus monkeys (Tsukada et al., 2004) as well as to approximate the typical (oral) dose range used in human patients (5–10 mg/day). Thus, for a 70 kg patient this would correspond to a range of 0.07–0.14 mg/kg. Our dosing approach was designed to cover this range as well as to include lower (subthreshold) doses. In the absence of available pharmacokinetic data for PNU-120596 in monkeys, we used the aforementioned studies in rodents as a guide.
In addition to behavioral analysis, each subject was observed just prior to and immediately after testing to ascertain and record any adverse reactions (e.g., salivation, diarrhea, failure to eat the normal daily rations or to perform the appetitively-motivated DMTS task).
2.4 Statistical Analyses
For one and two factor comparisons, analysis of variance (with repeated measures when indicated) was used followed by the Student Newman Keuls or Dunnett’s method (for comparisons to vehicle controls only) for post hoc analysis (SigmaPlot 11.2). For each figure presented error values denoted by ± indicates the standard error of the mean. Differences between means from experimental groups were considered significant at the p<0.05 level. Trends toward significance were considered at the p<0.10.
3. Results
3.1 Rodent Behavioral Studies
3.1.1 Object Recognition Memory
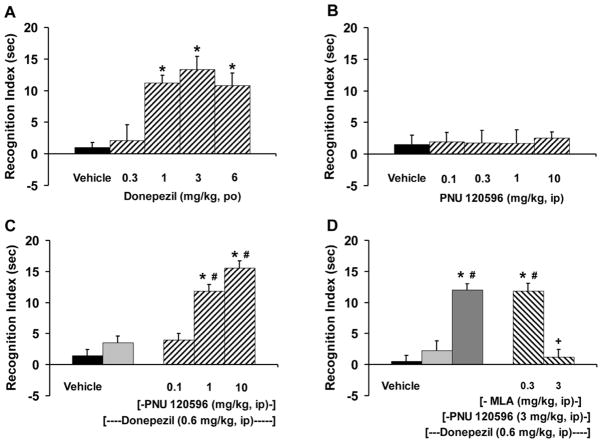
Administration of donepezil (0.3–6 mg/kg, po) to young adult Sprague-Dawely rats prior to object recognition training produced a significant and dose-dependent increase in object recognition memory when tested at the 48 hr retention delay interval [F(4,35)=13.39, p<0.0001]. Test doses of 1, 3 and 6 mg/kg of donepezil were significantly different from control and produced RI times of 11 to 13 sec (Fig. 1A). Assessment of the total object exploration time (i.e., for both objects) during the 5 min test session indicated such that all pharmacological test groups spent approximately the same amount of time exploring the objects [F(4,35)=0.155, p=0.959]. The average object exploration time across the treatment groups for donepezil was 41.9 ± 4.1 sec (data not shown). In contrast to donepezil, PNU 120596 (0.1–10 mg/kg, ip) administered prior to object recognition training failed to significantly enhance object recognition memory when tested at the 48 hr retention delay interval [F(4,35)=0.055, p=0.994]. RI times following PNU 120596 ranged from 1.7 to 2.6 sec and were not different from that observed following vehicle control (1.6 sec; Fig. 1B). Assessment of the total object exploration time during the 5 min test session indicated no significant effects of treatment [F(4,35)=0.473, p=0.755] with an average object exploration time of 70.2 ± 5.6 sec (data not shown).
Figure 1.
Assessment of recognition memory using the object recognition task in Sprague-Dawley rats. (A) Donepezil (0.3–6 mg/kg, po) given prior to training enhanced performance when tested at a 48 hr retention delay interval; (N=8 rats/treatment). (B) PNU 120596 (0.1–10 mg/kg, ip) given prior to training and tested 48 hrs later failed to enhance performance; (N=8 rats/treatment). (C) Co-administration of donepezil (0.6 mg/kg, ip) plus PNU 120596 (0.1–10 mg/kg, ip) given prior to training enhanced performance at the 48 hr retention delay interval; (N=8 rats/treatment). (D) Pretreatment with the α7-nicotinic receptor antagonist MLA (0.3 & 3 mg/kg, ip) reversed the recognition memory enhancement produced by the donepezil (0.6 mg/kg, ip)-PNU 120596 (3 mg/kg, ip) drug combination. Bars represent the mean (± S.E.M.) values for each treatment; (N=6–8 rats/treatment). * = significantly different (p<0.05) from vehicle response. # = significantly different (p<0.05) from donepezil response. + = significantly different (p<0.05) from the donepezil-PNU 120596 drug combination.
In the next series of experiments, we administered a subthreshold dose of donepezil (0.6 mg/kg, ip) in combination with various test doses of PNU 120596 (0.1–10 mg/kg, ip) and assessed this drug combination on recognition memory (Fig. 1C). Co-administration of donepezil with PNU 120596 prior to training resulted in a significant and dose-dependent enhancement of object recognition memory when tested at the 48 hr delay interval [F(4,35)=50.06, p<0.0001]. Test doses of 1 and 10 mg/kg of donepezil-PNU 120596 drug combination were significantly different from control and donepezil alone performance and produced RI times of 12 to 16 sec. Total object exploration time during the 5 min test session was not significantly different across the treatment groups [F(4,35)=1.57, p=0.201]. The average object exploration time across the treatment groups was 49.4 ± 2.5 sec (data not shown).
To assess the pharmacological specificity of α7-nAChRs in mediating the pro-cognitive effects of the donepezil-PNU 120596 drug combination, we pretreated the test subjects with the α7-nAChRs antagonist MLA (0.3 and 3 mg/kg, ip) immediately before the donepezil (0.6 mg/kg, ip) plus PNU 120596 (3 mg/kg, ip) administration (Fig. 1D). The test dose combination of donepezil (0.6 mg/kg, ip) plus PNU 120596 (3 mg/kg, ip) resulted in a significant enhancement of recognition memory at the 48 hr delay interval. This test dose combination produced an RI time of 12 sec. Co-administration of MLA plus the donepezil-PNU 120596 drug combination resulted in a significant and dose-dependent blockade of recognition memory enhancement [F(4,32)=25.07, p<0.0001]. The 3 mg/kg dose of MLA completely antagonized the pro-cognitive effect produced by the donepezil-PNU 120596 drug combination. Total object exploration time during the 5 min test session was not significantly different across the treatment groups [F(4,32)=1.308, p=0.288]. The average exploration time across the treatment groups was 38.2 ± 4.3 (data not shown).
3.1.2 Water-maze
Task Acquisition
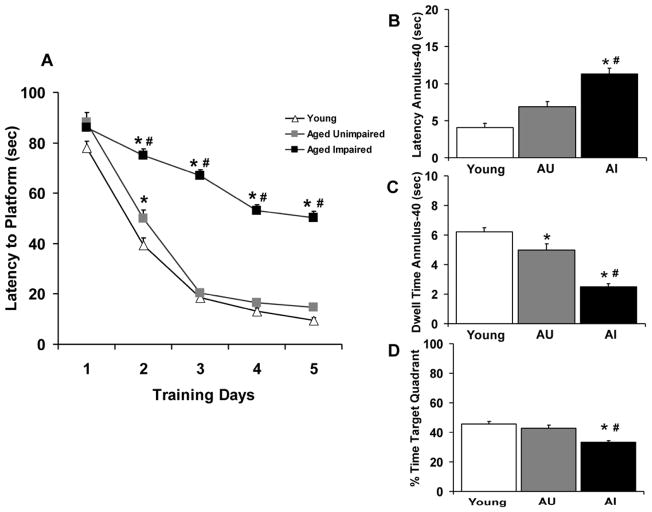
Aged (24–25 month old) male F344 rats were characterized as either aged cognitively impaired (AI) or aged cognitively unimpaired (AU) based on their mean latency to hidden platform performance to young rat performance on training days 3–5 in the water-maze task (Fig. 2A). ANOVA indicated a significant overall group effect on acquisition (escape latency), [F(2,223)=269.73, p<0.0001] and a significant platform latency by day effect (F(4,892)=223.4, p<0.0001]. ANOVA also indicated a significant platform latency by day by group interaction [F(8, 892)= 15.88, p<0.0001]. Post hoc comparisons indicated that the AI rats were significantly impaired at finding the hidden platform on Days 2, 3, 4 and 5 of acquisition training compared to the AU and young rats; AU animals performed the task as proficiently as the young adults. Of the 250 aged rats screened in the water maze task for the present study, 121 (48 %) were classified as AI and 42 (17 %) were classified as AU. The performance of the remaining aged animals (87 rats, 35%) did not fall clearly into either defined category and were excluded from further study. Swim speeds (27.1. ± 1.7, 25.3 ± 1.6 and 23.6 ± 1.1 cm/sec) for the young, AU and AI animals, respectively, were not significantly different [F(2,223)= 1.34, p=0.278] despite the observation that the young animals swam slightly faster than the aged animals. Performance during the 30 sec probe trial (Fig. 2B, C and D) indicated that the AI rats took longer to reach the annulus-40 and spent less time in the annulus-40 area than either the AU or young animals. Additionally, AI animals spent less overall time in the platform target quadrant than the AU and young animals. Path length performance was not significantly different across the 3 cohorts ([F(2,223)=1.22, p=0.297]; data not shown).
Figure 2.
Assessment of spatial reference memory using the Morris water-maze task in young (6 month old) and aged (24–25 month old) Fischer rats. (A) Acquisition curve across 5 days of training showing latencies (sec) to the hidden platform by young, aged cognitively unimpaired (AU) and aged cognitively impaired (AI) rats.(B) Assessment of latency (sec) to first entry into the annulus-40 area for each subgroup of rats during the 30 sec probe trial. (C) Total dwell time (sec) in the annulus-40 area for each subgroup of rats during the probe trial. (D) Percentage (%) of time spent in the target platform quadrant for each group of rats during the probe trial. Symbols or bars represent the mean (± S.E.M.) values for each subgroup of rats; (N=63 young rats, 42 AU rats and 121 AI rats). * = significantly different (p<0.05) from young rat performance. # = significantly different (p<0.05) from AU rat performance.
Pharmacological Effects
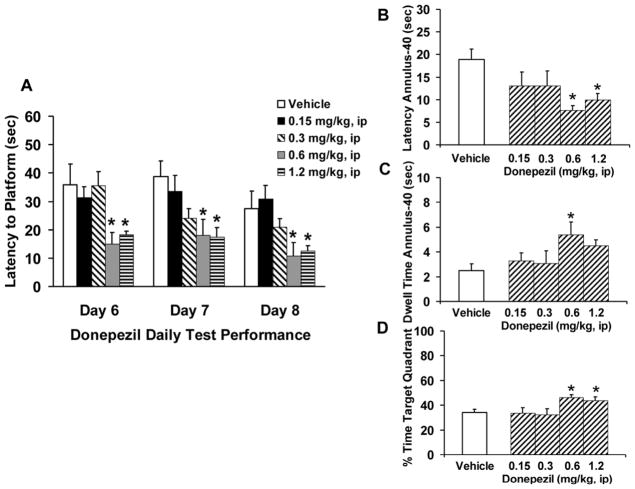
Pharmacological testing occurred in AI rats that were dosed once a day for 3 consecutive days with water-maze assessment following each drug dosing. Acquisition latencies across the donepezil and vehicle treatment groups were not significantly different from each other [F(4,40)= 0.192, p=0.941]. The mean overall escape latency was 54.9 ± 3.7 sec. Administration of donepezil (0.15–1.2 mg/kg, ip) dose-dependently reversed the AI rat water-maze performance deficit (Fig. 3). Repeated measures ANOVA indicated a significant effect of drug treatment [F(4,40)=7.63, p=0.0001] as well as a significant treatment by day effect [F(2,80)=5.75, p=0.0047]. Test doses of 0.6 and 1.2 mg/kg of donepezil decreased the swim latency to find the hidden platform compared to that of vehicle treated controls (Fig. 3A). Assessment of spatial recall via a 30 sec probe trial (Fig. 3B, C and D) showed that donepezil significantly improved AI rat performance on latency to annulus-40 [F(4,40)=3.23, p=0.021], dwell time in annulus-40 [F(4,40)=3.59, p=0.013] and % time in target quadrant [F(4,40)=4.68, p=0.003]. Path length [F(4,40)=2.04, p=0.106] was not significantly different from vehicle control (data not shown).
Figure 3.
Effects of donepezil on spatial reference memory in aged cognitively impaired (AI) Fischer rats. (A) Donepezil (0.15–1.2 mg/kg, ip) given on three days of water-maze testing decreased the latency (sec) to find the hidden platform compared to control performance. (B) Latency (sec) to first entry into the annulus-40 area following donepezil administration during the 30 sec probe trial. (C) Total dwell time (sec) in the annulus-40 area. (D) Percentage (%) of time spent in the target platform quadrant. Bars represent the mean (± S.E.M.) values for each treatment group; (N=9 AI rats/treatment). * = significantly different (p<0.05) from AI rat control performance.
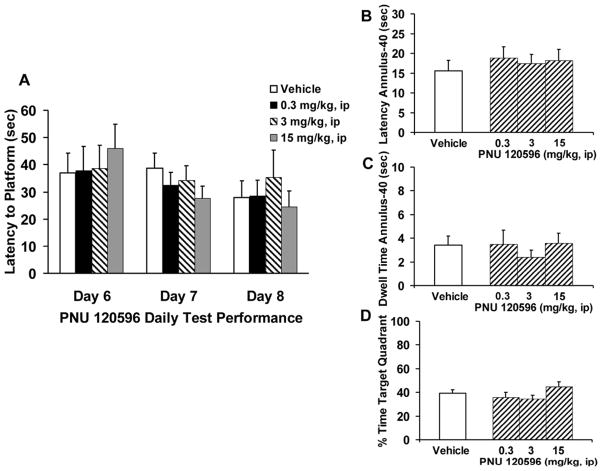
Pharmacological treatment with PNU 120596 alone (0.3–15 mg/kg, ip) failed to reverse the AI rat water-maze performance deficit (Fig. 4). Escape latency to the hidden platform across the three days of PNU 120596 administration (Fig. 4A) were not significantly different from vehicle control performance [F(3,32)=0.101, p=0.958]. Although, there was a significant treatment by day interaction [F(2,64)=3.79, p=0.027], this statistical effect was due to the gradual improvement on acquisition performance over time as post hoc assessments revealed no statistical treatment effect for the individual test days (days 6 thru 8). Assessment of spatial recall during the probe trial (Fig. 4B, C and D) indicated that PNU 120596 failed to significantly alter AI rat performance compared to vehicle control on any of the behavioral measures; latency to annulus-40 [F(3,32)=0.289, p=0.832], dwell time in annulus-40 [F(3,32)=0.412, p=0.745], % time in target quadrant [F(3,32)=1.64, p=0.198] or path length [F(3,32)=0.155, p=0.925; data no shown]. Statistical analysis of the acquisition latencies for the PNU 120596 and vehicle treatment groups were not significantly different from one another [F(3,32)= 0.768, p=0.521]. The mean overall escape latency was 55.9 ± 3.4 sec.
Figure 4.
Effects of PNU 120596 on spatial reference memory in aged cognitively impaired (AI) Fischer rats. (A) PNU 120596 (0.3–15 mg/kg, ip) given on three days of water-maze testing failed to alter latency (sec) to the hidden platform. (B) Latency (sec) to first entry into the annulus-40 area following PNU 120596 administration during the 30 sec probe trial. (C) Total dwell time (sec) in the annulus-40 area. (D) Percentage (%) of time spent in the target platform quadrant. Bars represent the mean (± S.E.M.) values for each treatment group; (N=9 AI rats/treatment). * = significantly different (p<0.05) from AI rat control performance.
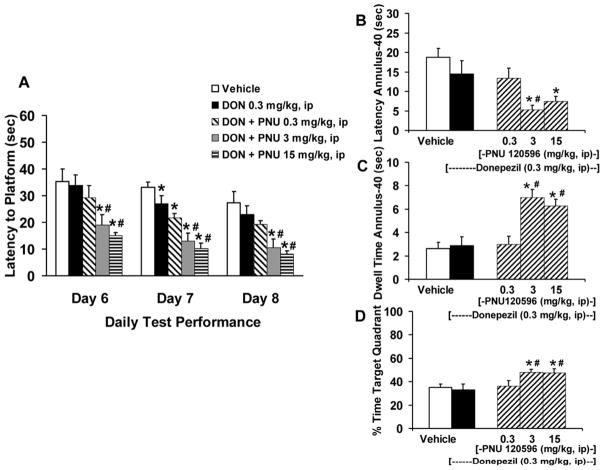
To assess the pharmacological effect of donepezil combined with PNU 120596 in AI animals in the water-maze task, we co-administered a subthreshold dose of donepezil (0.3 mg/kg, ip) with various test doses of PNU 120596 (0.3–15 mg/kg, ip). Analysis of the drug combination across each of the 3 days of testing revealed a significant and dose-dependent decrease in latency to the hidden platform in AI rats (Fig. 5A), main effect of drug treatment [F(4,40)=19.25, p<0.0001], treatment by day interaction [F(2,80)=17.71, p<0.0001]. Assessment of spatial recall during the probe trial (Fig. 5B, C and D) indicated that the donepezil-PNU 120596 drug combination significantly improved AI rat performance on latency to annulus-40 [F(4,40)=5.89, p=0.0008], dwell time in annulus-40 [F(4,40)=11.00, p<0.0001] and % time in target quadrant [F(4,40)=4.33, p=0.005]. Path length [F(4,40)=1.633, p=0.185] was not significantly altered (data not shown). Acquisition latencies for the treatment groups were also not significantly different from one another [F(4,40)= 1.02, p=0.411] and the mean overall acquisition escape latency was 57.7 ± 3.4 sec.
Figure 5.
Effects of co-administration of donepezil and PNU 120596 on spatial reference memory in aged cognitively impaired (AI) Fischer rats. (A) Administration of a fixed low dose of donepezil (0.3 mg/kg, ip) in combination with PNU 120596 (0.3–15 mg/kg, ip) given on three days of water-maze testing significantly decreased latency (sec) to the hidden platform. (B) Latency (sec) to first entry into the annulus-40 area following donepezil plus PNU 120596 administration during the 30 sec probe trial. (C) Total dwell time (sec) in the annulus-40 area. (D) Percentage (%) of time spent in the target platform quadrant. Bars represent the mean (± S.E.M.) values for each treatment group; (N=9 AI rats/treatment). * = significantly different (p<0.05) from AI rat control performance; # = significantly different (p<0.05) from AI rat donepezil performance.
3.2 Non-Human Primate Studies
3.2.1 Delayed Match to Sample Accuracy
Donepezil
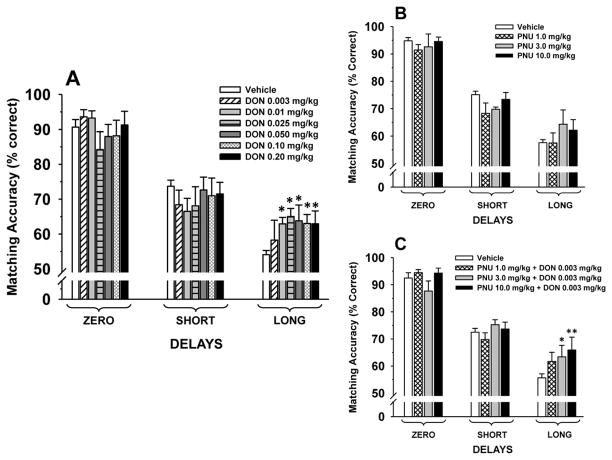
Vehicle (peanut butter & Prima-Burger®) was administered on 6–7 occasions during the donepezil study. The data obtained from these sessions were averaged and used for comparison with donepezil treatment sessions. As a group (N=7) the mean performance accuracies in the DMTS task after vehicle administration met the performance criteria set forth above (see Methods): Zero delay, 90.7%, Short delay 73.7%, and Long delay, 54.1% trials correct and always followed a significant (p<0.001) delay-dependent decline in accuracy. As indicated in Fig. 6A oral administration of donepezil was associated with dose-dependent improvements in DMTS accuracy; main effect of dose [F(6,36)=0.36, p=0.90]; delay [F(2,72)=51.5, p<0.001]; dose × delay interaction [F(12,72)=2.11, p=0.027]. Post hoc analysis indicated that all but the lowest dose of donepezil (0.003 mg/kg) were associated with significant (p<0.05) improvement at the long delay intervals compared to vehicle-associated performance.
Figure 6.
(A) Dose-effect relationship for each delay in the Delayed Match to Sample (DMTS) task by 7 aged rhesus monkeys, 1 hr after the oral administration of donepezil. The baseline was determined from the average of 6–7 vehicle sessions conducted during the study. (B) Dose-effect relationship for each delay in the DMTS task by 6 aged rhesus monkeys, 1 hr after the oral administration of PNU 120596. The baseline was determined from the average of 2–3 vehicle sessions conducted during the study. (C) Effect of a subthreshhold dose of donepezil combined with PNU 120596 (across 3 doses). The baseline was determined from the average of 3–6 vehicle sessions conducted during the study. Each bar represents the mean (% correct) ± S.E.M. over 96 trials per session. *p<0.05, ** p<0.01= significant difference from vehicle response.
PNU-120596
In the second phase of the monkey DMTS studies, monkey 7nv was not reliably performing the DMTS task (i.e., the subject was often not accepting oral formulations and/or finishing all of the DMTS task trials.) and was removed from the study. In the remaining six subjects the mean accuracies in the DMTS task after vehicle administration again met the predetermined performance criteria and followed a significant (p<0.001) delay-dependent decline in accuracy, (see Fig. 6B). PNU-120596 was not associated with significant effects on DMTS accuracy; main effect of dose [F(3,15)=1.19, p=0.35; delay [F(2,30)=180.7, p<0.001]; dose × delay interaction [F(6,30)=0.78, p=0.59].
Donepezil + PNU-120596
In the third phase of the study a subthreshold dose of donepezil (0.003 mg/kg) was combined with PNU 120596 (N=6). Under vehicle conditions the mean accuracies in the DMTS task after vehicle administration again met the predetermined performance criteria and followed a significant (p<0.001) delay-dependent decline in accuracy (see Fig. 6C). As indicated in Fig. 6C oral administration of two of the drug combinations was associated with improvements in DMTS accuracy, main effect of dose [F(3,15=2.01), p=0.15]; delay [F(2,29)=81.9, p<0.001]; dose × delay interaction [F(6,29)=2.64, p=0.037]. Post hoc analysis indicated that the combination of donepezil 0.003 mg/kg with either PNU 120596 3.0 mg/kg or 10.0 mg/kg was associated with significant (p<0.03 and p=0.001, respectively) improvements in DMTS accuracy at the long delay intervals compared to vehicle-associated performance.
3.2.2 Delayed Match to Sample Latencies
Median sample (trial initiator) and choice (color selection) latencies on trials completed correctly or incorrectly under baseline (vehicle) conditions and after drug administration were also evaluated. Sample latencies typically ranged between 1.4 to 1.9 seconds whereas choice latencies typically ranged between 1.7 and 2.7 seconds. Moreover, choice latencies were (in general) slightly longer when an incorrect response was made. None of the DMTS latencies were altered by donepezil, PNU 120596, or the drug combinations, however.
4. Discussion
While there are a number of areas of research underway for developing novel therapies for AD there is also great interest in any strategy that might enhance the clinical efficacy of the currently available agents (see Riordan et al., 2011). As noted in the Introduction, the purpose of this study was to evaluate the combination of the clinically prescribed AChEI, donepezil, with a selective PAM of α7-nAChRs as an adjunctive treatment strategy to memory enhancement. The results can be summarized as follows: 1) in young rats, donepezil improved the performance of an NOR (recognition memory) task in a dose-dependent manner, 2) PNU 120596 did not affect NOR performance when administered alone, however, the combination of PNU 120596 with a subthreshold dose of donepezil was associated with improved NOR performance, 3) the positive effect of the combination of PNU 120596 and donepezil on NOR performance in young rats appeared to be α7-nAChR mediated given that it was blocked by the selective α7-nAChR antagonist MLA. 4) in aged (learning and memory-impaired) rats, donepezil improved the performance of a water maze (spatial learning) task in a dose-dependent manner, 5) PNU 120596 did not affect water maze performance when administered alone in age-impaired rats, however, the combination of PNU-120596 with a subthreshold dose of donepezil was associated with improved task performance, 6) in a similar fashion to the rodent behavioral studies, donepezil improved the performance of a DMTS (working/short term memory) task in aged monkeys in a dose-dependent manner; PNU 120596 alone did not result in significant effects on DMTS performance, however, the combination of PNU 120596 with a subthreshold dose of donepezil did. Collectively, these data indicate that PNU 120596 increases the effective dose range of donepezil in learning and memory-related tasks in young and age-impaired rodents, as well as in aged non-human primates.
In the rodent behavioral experiments the tasks that were utilized (NOR and water maze) were selected for several reasons. NOR (Ennaceur and Delacour 1988) is a rodent model of (non-spatial) recognition memory, which is assumed to consist of two components, a recollective (episodic) component and a familiarity component (Squire et al., 2004). Recognition memory is demonstrated in the NOR task when subjects explore a novel object more than a familiar one. While debated, there is considerable evidence that the hippocampus is involved in object recognition memory in both rodents (Myhrer, 1988; Rampon et al., 2000; Broadbent et al., 2004) and humans (Reed and Squire, 1997; Squire, 1992) and further, object recognition memory has also been observed to be negatively affected in non-demented, aged individuals as well as in patients with AD (Flicker et al., 1987, Purdy et al., 2002 and Schiavetto et al., 2002). The water maze (spatial learning and recall) procedure was employed in this study since it also requires the hippocampus (which is well documented to be adversely affected in aging and AD), important components of human learning and memory such as information acquisition and encoding, consolidation, retention, and retrieval (McNamara and Skelton, 1993; McDonald and White, 1995). Furthermore, while it is relatively well established that water maze task performance declines with increasing age in animals (reviewed, Brandeis et al., 1989), the ability to classify aged learning and memory status (i.e., unimpaired vs. impaired) relative to young animals provides a preclinical translational model reflective of the human aging process (Rowe et al., 2007; Quirion et al., 1995). Notably, aged rats classified as cognitively impaired (when compared to unimpaired subjects) have been shown to possess decreased hippocampal acetylcholine levels (Rowe et al., 1999; Quirion et al., 1995), impaired synaptic plasticity (i.e., long-term potentiation; Bach et al., 1999; Tombaugh et al., 2002) and alterations in calcium mediated slow afterhyperpolarizations (Landfield and Pitler, 1984; Tombaugh et al., 2005).
Aged non-human primates (NHPs) were selected for the final phase of these studies due to their unique translational value (see reviews, King et al, 1988; Bantrop, 2001; Sibal and Samson KJ. 2001; Goodman and Check, 2002; Capitanio and Emborg 2008; Nelson and Winslow, 2009; Shively and Clarkson, 2009), especially their neurobiological and behavioral similarities to humans. Within the multiple species of NHPs, the genetic homology (~95%), brain anatomy, and behavioral repertoire of old world macaques (e.g., rhesus monkeys) resemble humans more than that of any other laboratory animal, except higher apes (Jackson et al., 1969; Rumbaugh 1973; Rice 1987; Magness et al., 2005). Moreover, as macaques age, like humans, their performance across multiple domains of cognition (sustained attention, visuospatial learning, working memory, recognition memory, cognitive flexibility, etc.) declines significantly (see Bachevalier et al., 1991; Buccafusco and Jackson, 1991; Bartus, 2000; Nagahara et al., 2010; Zeamer et al., 2011). Macaques also exhibit multiple age-related changes in the brain that are similar to humans including the deposition of amyloid plaques, the increase in reactive astrocytes and microglia, decreased neurotransmitters and their receptors, the loss of neocortical neurons, etc. (Walker et al., 1988; Price and Sisodia, 1994; Summers et al., 1997; Smith et al., 1999; Smith et al., 2004)
The DMTS test (often described as a working/short term memory task) in NHPs allows for the assessment of mnemonic processes (i.e. discrimination, encoding and retention) that are vital to human cognition and executive function, such as attention, strategy formation, reaction time in complex situations, and memory for recent events (see review, Paule et al., 1998). Moreover, variations in the DMTS procedure have been used in humans to assess the effects of aging as well as a variety of illnesses on cognitive function including Alzheimer’s Disease, Lewy Body Dementia, Korsakoff’s Disease, unipolar depression, and alcoholism, (Oscar-Berman and Bonner, 1985; Irle et al. 1987; Aggleton et al., 1988; Sahgal et al., 1992; Perryman and Fitten 1993; Elliott et al., 1996)
The findings reported here compliment previous animal studies which have suggested that selective PAMS of α7-nAChRs have significant potential as therapeutic agents for disorders of cognition. The α7-nAChR has long been considered a therapeutic target in disorders like AD and schizophrenia given the deficits in α7-nAChR protein that have been observed in the brains of patients who suffered from these disorders (Freedman et al., 1995; Burghaus et al., 2000; Guan et al., 2000). At the time of the preparation of this manuscript the early results of Phase 2 clinical trials in Alzheimer’s Disease patients administered α7-nAChRs agonists, EVP-6124, RG3487 (also referred to as MEM3454) or TC 5619 appear promising (unpublished data presented in oral presentations or in abstract form, see also Wallace and Porter 2011).
To date a small number of Type I, α7-nAChR-selective PAMs (defined as molecules that predominately affect the apparent peak current, agonist sensitivity, and Hill coefficient), and Type II PAMs (compounds that possess the aforementioned properties described for Type I PAMs, as well as the ability to modify the desensitization profile of agonist responses, see Bertrand and Gopalakrishnan, 2007) have been evaluated for effects on cognition. For example, in rats, the Type I (α7-nAChR-selective) PAM, NS1738, attenuated scopolamine-induced deficits in a water maze task and improved the performance of a social recognition task to a similar extent as nicotine (Timmermann et al., 2007). The Type I (α7-nAChR-selective) PAM known as compound 6 improved performance of a radial arm maze task in rats and normalized sensory-gating deficits in DBA/2 mice (Ng et al., 2007). Likewise, JNJ-1930942 (described as an α7-nAChR-selective, intermediate PAM since its profile is distinct from Type I and Type II PAMS) improved sensory-gating deficits in DBA/2 mice (Dinklo et al., 2011).
The allosteric nicotinic ligand evaluated in the current study, PNU-120596, is a urea analog and Type II (α7-nAChR-selective) PAM. The latter property was demonstrated in electrophysiological studies where PNU-120596 increased the maximal agonist (i.e., acetylcholine)-evoked α7-nAChR current and markedly slowed the decay of the current in the continued presence of agonist (i.e., it suppressed densensitization). Moreover, PNU-120596 also restored the agonist responses of desensitized receptors. Specifically, while continuous exposure to the nAChR agonist, nicotine, desensitized α7-nAChRs and eventually reduced the response to a non-detectable level, PNU-120596 applied during continued exposure to nicotine restored the current to a peak that was even larger than the initial peak evoked by the agonist alone (Hurst et al., 2005). In the same study, PNU-120596 improved the auditory gating deficit induced by amphetamine in rats. PNU-120596 has also been shown to reverse scopolamine-induced deficits in fear conditioning as well as NOR in rats, effects that were reversed by MLA demonstrating α7-nAChR selectivity (Wallace et al., 2007). In addition, subthreshold doses of PNU-120596 were shown to enhance the fear conditioning effects of a low (inactive) dose of nicotine whereas, combining active doses of PNU 120596 and nicotine retained the pro-cognitive effects (Wallace et al., 2007). More recently, PNU-120596 was demonstrated to attenuate the deficits induced by sub-chronic phencyclidine treatment in the extra-dimensional shift (EDS) phase of an attentional set-shifting task (McLean et al., 2011). The results of these studies, suggest that compounds like PNU-120596 might have a therapeutic potential in neuropsychiatric disorders like schizophrenia by improving deficits of information processing and cognitive flexibility.
It is important to note, that in several of the aforementioned behavioral studies, the α7-nAChR-selective PAM administered alone improved memory-related task function, whereas in our study, a combination with donepezil was required. The basis of this observation is unclear; however, there are several factors that could (at least theoretically) contribute to the difference. For example, in several of the cases where the PAM was effective when administered alone, the model system used was a pharmacologic impairment-reversal paradigm (e.g., scopolamine, phencyclidine, MK-801, amphetamine). Each of these compounds has been shown to increase acetylcholine release in the brain (see Bymaster et al., 1993; Hasegawa et al., 1996; Arnold et al., 2001 Del Arco et al., 12007). While speculative, it certainly seems possible that the elevated levels of acetylcholine associated with these compounds combined with allosteric modulation of the nAChR might contribute to observed pro-cognitive effect. In addition, it is also quite possible that specific behavioral tasks (radial arm maze, social recognition, or the anesthetized animal auditory evoked potential model) might engage cholinergic systems differently than the tasks we employed and result in higher basal states of acetylcholine allowing α-7 PAMs to activate the α-7 receptor.
In conclusion, the results of our studies combined with those described above suggest that α7-nAChR-selective PAMS like PNU-120596 might have therapeutic potential for the cognitive deficits associated with age-related illness such as AD as well as neuropsychiatric illnesses that are not necessarily associated with advanced aged (e.g. schizophrenia). The later subject (i.e., positive effects of the ACHEI-α7-nAChR PAM combination in young subjects) may be particularly interesting for future studies given our positive data in young rats in the NOR task, previous observations where donepezil-(alone) was associated with improvements in DMTS accuracy in young monkeys (Buccafusco and Terry, 2004), and the fact that we have not yet evaluated PNU-120596 in young monkeys. Our studies also appear to support the argument that PNU-120596 might work best as an adjunctive agent combined with an AChEI, whereas the other studies suggest that nicotinic PAMs might have efficacy when administered alone in some conditions. It is also important to note that while our studies support a role for PNU-120596 combined with an AChEI for age-related cognitive deficits, we did not evaluate the drug combination in AD animal models (e.g., AD transgenic mice) specifically, an interesting topic for future studies. Moreover, while we evaluated subthrehold doses of donepezil combined with PNU-120596, it would be interesting to determine if the efficacy of higher doses of donepezil could be further improved when combined with PNU-120596
Donepezil improved memory-related task performance in aged rats and monkeys
α7-nAChR PAM, PNU-120596 alone did not affect memory task performance
Combining a subthreshold dose of donepezil with PNU-120596 improved memory
Acknowledgments
This work was supported by the National Institute on Aging (AG032140) and Memory Pharmaceuticals, Inc.
Abbreviations
- AChE
acetylcholinesterase
- AD
Alzheimer’s Disease
- DMTS
Delayed Match to Sample
- MLA
methyllycaconitine
- NHP
non-human primate
- NOR
spontaneous novel object recognition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Nicol RM, Huston AE. The performance of amnesic subjects on tests of experimental amnesia in animals. Delayed matching-to-sample and concurrent learning. Neuropsychologia. 1988;26:265–72. doi: 10.1016/0028-3932(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Fadel J, Sarter M, Bruno JP. Amphetamine-stimulated cortical acetylcholine release: role of the basal forebrain. Brain Research. 2001;894:74–87. doi: 10.1016/s0006-8993(00)03328-x. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991 Mar-Apr;12(2):99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Bantrop RE. Non-human primates: essential partners in biomedical research. Immunological Reviews. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley Evans J, Iakovidou V, Tsolaki M. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2000:CD001191. doi: 10.1002/14651858.CD001191. [DOI] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada-Rovira M, Brodaty H, Cras P, Baloyannis S, Emre M, Zhang R, Bahra R 322 Study Group. Efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a global, multinational, clinical experience study. Drugs Aging. 2004;21(1):43–53. doi: 10.2165/00002512-200421010-00004. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989 Sep;48(1–2):29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004 Oct 5;101(40):14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991 May-Jun;12(3):233–8. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr Donepezil-induced improvement in delayed matching accuracy by young and old rhesus monkeys. Journal of Molecular Neuroscience. 2004;24:101–107. doi: 10.1385/JMN:24:1:085. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Schütz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schröder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res. 2000 Mar 29;76(2):385–8. doi: 10.1016/s0169-328x(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Möller HJ, Rogers SL, Friedhoff LT. The effects of donepezil in Alzheimer’s disease — Results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10(3):237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharmacology & Experimental Therapeutics. 1993;267:16–24. [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008 Mar 29;371(9618):1126–35. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention and The Merck Company Foundation. The State of Aging and Health in America. Whitehouse Station, NJ: The Merck Company Foundation; 2007. www.cdc.gov/aging/pdf/saha_2007.pdf. [Google Scholar]
- Del Arco A, Mora F, Mohammed AH, Fuxe K. Stimulation of D2 receptors in the prefrontal cortex reduces PCP-induced hyperactivity, acetylcholine release and dopamine metabolism in the nucleus accumbens. J Neural Transmission. 2007;114:185–193. doi: 10.1007/s00702-006-0533-3. [DOI] [PubMed] [Google Scholar]
- Dinklo T, Shaban H, Thuring JW, Lavreysen H, Stevens KE, Zheng L, Mackie C, Grantham C, Vandenberk I, Meulders G, Peeters L, Verachtert H, De Prins E, Lesage AS. Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the {alpha}7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 2011 Feb;336(2):560–74. doi: 10.1124/jpet.110.173245. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychol Med. 1996;26:975–89. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Crook T, Bartus RT. A visual recognition memory test for the assessment of cognitive function in aging and dementia. Exp Aging Res. 1987 Autumn;13(3):127–32. doi: 10.1080/03610738708259313. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995 Jul 1;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;8;1033(2):186–93. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Ann N Y Acad Sci. 2000;920(1):321–327. doi: 10.1111/j.1749-6632.2000.tb06942.x. [DOI] [PubMed] [Google Scholar]
- Goodman S, Check E. The great primate debate. Nature. 2002 Jun 13;417(6890):684–7. doi: 10.1038/417684a. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J Neurochem. 2000 Jan;74(1):237–43. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yamada K, Hasegawa T, Nabeshima T. Role of dopaminergic neuronal system in dizocilpine-induced acetylcholine release in the rat brain. J Neural Transmission. 1996;103:651–660. doi: 10.1007/BF01271225. [DOI] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- Harvey AL. The pharmacology of galanthamine and its analogues. Pharmacol Ther. 1995;68(1):113–28. doi: 10.1016/0163-7258(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Gearhart DA, Parikh V, Hohnadel EJ, Davis LW, Middlemore ML, Warsi SP, Waller JL, Terry AV., Jr Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. J Pharmacol Exp Ther. 2006 Feb;316(2):679–94. doi: 10.1124/jpet.105.093047. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E, Kessler J, Markowitsch HJ. Primate learning tasks reveal strong impairments in patients with presenile dementia of the Alzheimer’s type. Brain Cogn. 1987;6:429–49. doi: 10.1016/0278-2626(87)90138-2. [DOI] [PubMed] [Google Scholar]
- Jackson WJ, Reite ML, Buxton DF. The chimpanzee central nervous system: a comparative review, in Primates in Medicine, vol. 4. In: Reynolds HH, editor. Chimpanzee: Central Nervous System and Behavior; A Review. Karger; New York: 1969. pp. 1–51. [PubMed] [Google Scholar]
- King FA, Yarbrough CJ, Anderson DC, Gordon TP, Gould KG. Primates. Science. 1988;240:1475–82. doi: 10.1126/science.3287624. [DOI] [PubMed] [Google Scholar]
- Kinsella K, He W. An aging world US Census Bureau, International Population Reports, P95/09-1US. Government Printing Office; Washington, D.C: 2009. [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Effect of donepezil hydrochloride (E2020) on basal concentrations of extracellular acetylcholine in the hippocampus of rats. European J Pharmacology. 1999;380:101–107. doi: 10.1016/s0014-2999(99)00545-2. [DOI] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: A meta-analysis. CMAJ. 2003;169(6):557–564. [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+ dependent afterhyperpolarizations in rat hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol. 2000 Mar 30;393(1–3):165–70. doi: 10.1016/s0014-2999(00)00093-5. [DOI] [PubMed] [Google Scholar]
- Magness CL, Fellin PC, Thomas MJ, Korth MJ, Agy MB, Proll SC, Fitzgibbon M, Scherer CA, Miner DG, Katze MG, Iadonato SP. Analysis of the Macaca mulatta transcriptome and the sequence divergence between Macaca and human. Genome Biol. 2005;6:R60. doi: 10.1186/gb-2005-6-7-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995 Aug;109(4):579–93. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris N, Grayson B, Gendle D, Mackie C, Lesage A, Pemberton DJ, Neill J. PNU-120596, a positive allosteric modulator of α7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. 2012 Sep;26(9):1265–70. doi: 10.1177/0269881111431747. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993 Jan-Apr;18(1):33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Mintzer JE, Kershaw P. The efficacy of galantamine in the treatment of Alzheimer’s disease: comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. Int J Geriatr Psychiatry. 2003;18:292–297. doi: 10.1002/gps.826. [DOI] [PubMed] [Google Scholar]
- Myhrer T. The role of medial and lateral hippocampal perforant path lesions and object distinctiveness in rats’ reaction to novelty. Physiol Behav. 1988;42(4):371–7. doi: 10.1016/0031-9384(88)90279-x. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 2010 Jun;31(6):1020–31. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Winslow JT. Non-human primates: model animals for developmental psychopathology. Neuropsychopharmacology. 2009 Jan;34(1):90–105. doi: 10.1038/npp.2008.150. [DOI] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A. 2007 May 8;104(19):8059–64. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Bonner RT. Matching- and delayed matching- to-sample performance as measures of visual processing selective attention and memory in aging and alcoholic individuals. Neuropsychologia. 1985;23:639–51. doi: 10.1016/0028-3932(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Paule MG, Bushnell PJ, Maurissen JP, Wenger GR, Buccafusco JJ, Chelonis JJ, Elliott R. Symposium overview: the use of delayed matching-to-sample procedures in studies of short-term memory in animals and humans. Neurotoxicol Teratol. 1998 Sep-Oct;20(5):493–502. doi: 10.1016/s0892-0362(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Perryman KM, Fitten LJ. Delayed matching-to-sample performance during a double blind trial of tacrine (THA) and lecithin in patients with Alzheimer’s disease. Life Sci. 1993;53:479–86. doi: 10.1016/0024-3205(93)90699-4. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med. 1994;45:435–46. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- Purdy KS, McMullen PA, Freedman M. Changes to the object recognition system in patients with dementia of the Alzheimer’s type. Brain Cogn. 2002 Jul;49(2):213–6. [PubMed] [Google Scholar]
- Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney MJ. Facilitation of acetylcholine release and cognitive performance by an M2-muscarinic antagonist in aged memory-impaired rats. J Neuroscience. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000 Mar;3(3):238–44. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month, randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997 Aug;111(4):667–75. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Rice DC. Primate research: Relevance to human learning and development. Dev Pharmacol Ther. 1987;10:314–27. doi: 10.1159/000457762. [DOI] [PubMed] [Google Scholar]
- Riordan KC, Hoffman Snyder CR, Wellik KE, Caselli RJ, Wingerchuk DM, Demaerschalk BM. Effectiveness of adding memantine to an Alzheimer dementia treatment regimen which already includes stable donepezil therapy: a critically appraised topic. Neurologist. 2011 Mar;17(2):121–3. doi: 10.1097/NRL.0b013e31820aa383. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Aubert I, Kitaichi K, Richard J, Day J, Meaney MJ. Enhanced cognitive performance in aged memory-impaired rats treated with a selective muscarinic receptor antagonist. Annals of Psychiatry. 1999;7:319–330. [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007 Mar 21;27(12):3098–110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh DM. Learning skills of anthropoids. In: Rosenblum LA, editor. Primate Behavior: Developments in Field and Laboratory Research. Academic Press; New York, NY: 1973. pp. 1–70. [Google Scholar]
- Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer’s Disease: consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol. 2011 Feb 7;11:21. doi: 10.1186/1471-2377-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal A, Galloway PH, McKeith IG, Lloyd S, Cook JH, Ferrier IN, Edwardson JA. Matching-to-sample deficits in patients with senile dementias of the Alzheimer and Lewy body types. Arch Neuropsychol. 1992;49:1043–46. doi: 10.1001/archneur.1992.00530340059019. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lübbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003 Jun;305(3):1024–36. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Schiavetto A, Köhler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia. 2002;40(8):1428–42. doi: 10.1016/s0028-3932(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Nonhuman primate models of women’s health: introduction and overview. Am J Primatol. 2009 Sep;71(9):715–21. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Sibal LR, Samson KJ. Nonhuman primates: a critical role in current disease research. ILAR J. 2001;42(2):74–84. doi: 10.1093/ilar.42.2.74. [DOI] [PubMed] [Google Scholar]
- Smith D, Roberts J, Gage F, Tuszynski M. Age associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci USA. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–81. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992 Apr;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–3. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Summers JB, Prendergast MA, Hill WD, Buccafusco JJ. Localization of ubiquitin in the plaques of five aged primates by dual-label fluorescent immunhistochemistry. Alzheimer’s Res. 1997;3:11–21. [Google Scholar]
- Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, Green C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21(1):17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukumoto D, Ohba H, Sato K, Kakiuchi T. Effects of acute acetylcholinesterase inhibition on the cerebral cholinergic neuronal system and cognitive function: Functional imaging of the conscious monkey brain using animal PET in combination with microdialysis. Synapse. 2004;52(1):1–10. doi: 10.1002/syn.10310. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003 Sep;306(3):821–7. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Bartoszyk GD. Selective serotonin 5-HT2A receptor antagonist EMD 281014 improves delayed matching performance in young and aged rhesus monkeys. Psychopharmacology (Berl) 2005 Jun;179(4):725–32. doi: 10.1007/s00213-004-2114-1. [DOI] [PubMed] [Google Scholar]
- Terry AV, Callahan PM, Hall B, Webster SJ. Alzheimer’s Disease and Age-Related Memory Decline (Preclinical) in the Special Issue on Cognitive Enhancers and Neuropsychiatric Disorders. Pharmacology, Biochemistry and Behavior. 2011 Aug;99(2):190–210. doi: 10.1016/j.pbb.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Lanctôt KL, Herrmann N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin Drug Saf. 2004;3(5):425–440. doi: 10.1517/14740338.3.5.425. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, Ahring PK, Peters D, Holst D, Christensen JK, Malysz J, Briggs CA, Gopalakrishnan M, Olsen GM. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007 Oct;323(1):294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, Micheal TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neuroscience. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fischer 344 rats. J Neuroscience. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Struble RG, Wagster MV, Price DL, Cork LC. The neural basis of memory decline in aged monkeys. Neurobiol Aging. 1988 Sep-Dec;9(5–6):657–66. doi: 10.1016/s0197-4580(88)80130-1. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Rocha SM, Callahan PM, Misner D, Yeo H, Sahdeo S, Button D, Tehim A, Maag H, Santarelli L. Cognitive enhancing properties of positive allosteric modulators of the nicotinic α7 receptor. Biochemical Pharmacology. 2007;74:SMA-36. [Google Scholar]
- Wallace TL, Porter RHP. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition. Biochemical Pharmacology. 2011;82:891–903. doi: 10.1016/j.bcp.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol. 2011 Dec;80(6):1013–32. doi: 10.1124/mol.111.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P Donepezil Nordic Study Group. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001 Aug 14;57(3):489–95. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [accessed, July 2, 2012];The World is Fast Aging – 10 facts on ageing and the life course, April 2012. 2012 http://www.who.int/features/factfiles/ageing/en/index.html.
- Zeamer A, Decamp E, Clark K, Schneider JS. Attention, executive functioning and memory in normal aged rhesus monkeys. Behav Brain Res. 2011 May 16;219(1):23–30. doi: 10.1016/j.bbr.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]