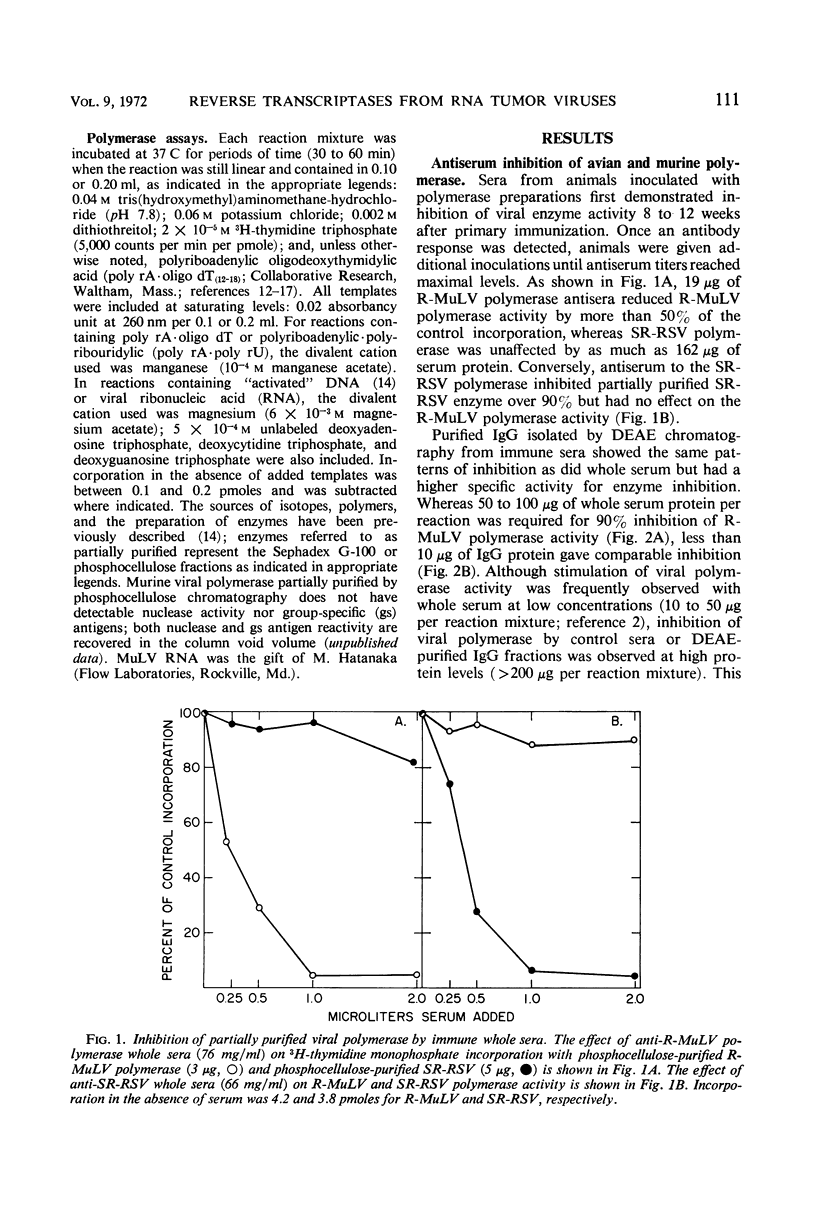
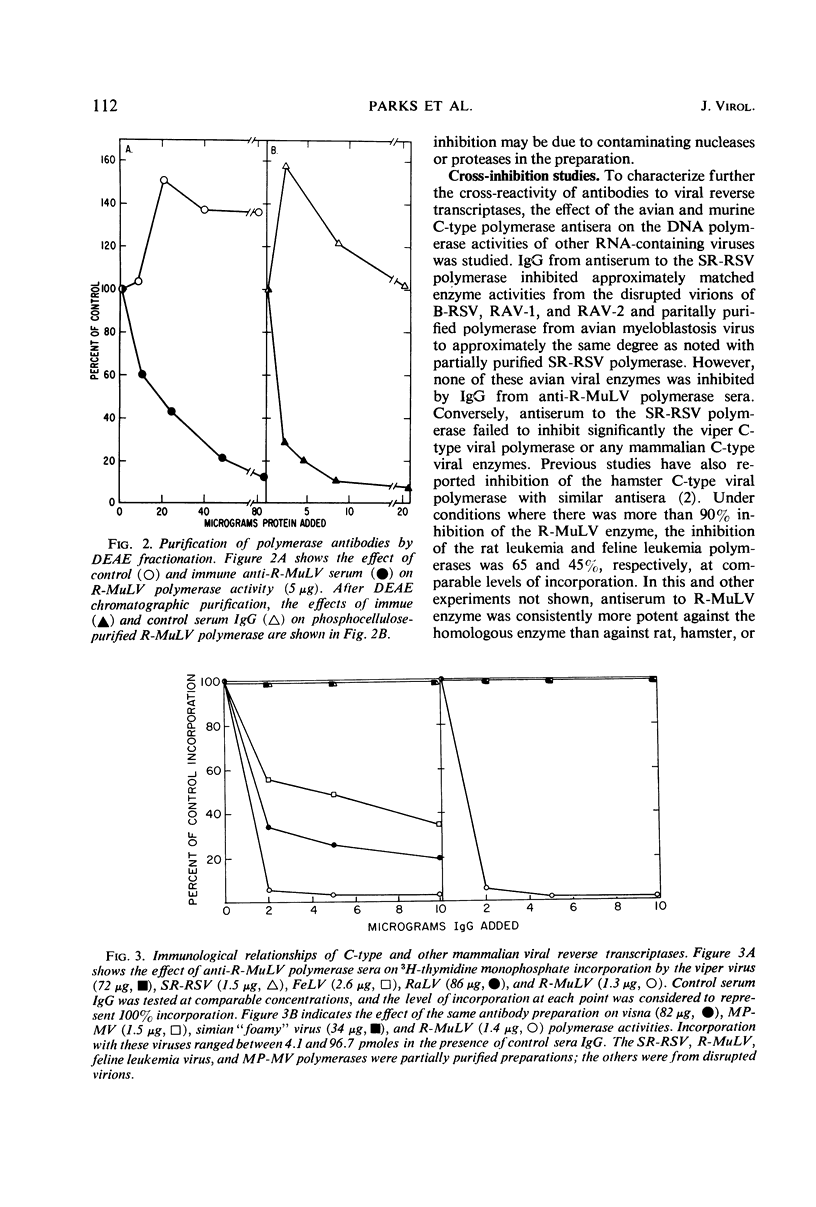
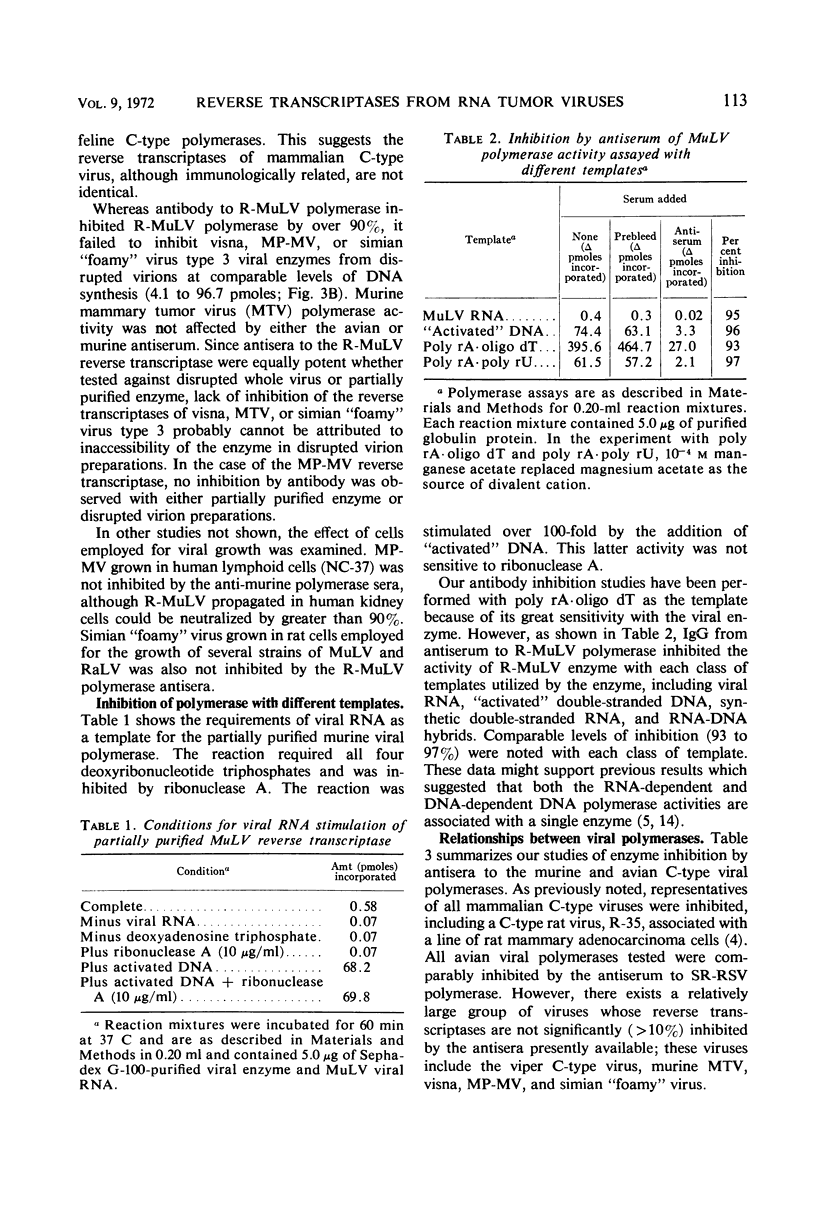
Abstract
Antiserum to partially purified reverse transcriptase from the Schmidt-Ruppin strain of Rous sarcoma virus has been prepared and characterized. Antibody to the avian polymerase inhibited the reverse transcriptase activity of avian C-type viruses but had no effect on the polymerase activity from C-type viruses of other classes. The known mammalian C-type viral polymerases were significantly inhibited only by the antiserum to murine C-type viral polymerases; reverse transcriptases from four other mammalian viruses were immunologically distinct from both avian and mammalian C-type viral polymerases. Partially purified murine leukemia viral DNA polymerase activity was comparably reduced by specific antibody regardless of the template used for enzyme detection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Isolation of a rat-tropic helper virus from M-MSV-O stocks. Virology. 1971 Apr;44(1):29–36. doi: 10.1016/0042-6822(71)90149-8. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Parks W. P., Scolnick E. M., Todaro G. J. Antibody to the RNA-dependent DNA polymerase of mammalian C-type RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 May;68(5):920–924. doi: 10.1073/pnas.68.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. W. Characterization of avian leucosis group-specific antigen from avian myeloblastosis virus. Biochim Biophys Acta. 1968 Feb 19;154(2):388–396. doi: 10.1016/0005-2795(68)90053-6. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Bogden A. E., Zelljadt I., Jensen E. M. Virus particles in a transplantable rat mammary tumor of spontaneous origin. Eur J Cancer. 1970 Aug;6(4):287–290. doi: 10.1016/0014-2964(70)90092-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S., Huebner R. J. Coexistence of intraspecies and interspecies specific antigenic determinants on the major structural polypeptide of mammalian C-type viruses. Nat New Biol. 1971 May 26;231(21):107–108. doi: 10.1038/newbio231107a0. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Old L. J. Isolation and some characteristics of a group-specific antigen of the murine leukemia viruses. Virology. 1969 Feb;37(2):189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. DNA polymerase activity associated with RNA tumor viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):143–147. doi: 10.1073/pnas.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. M., Zelljadt I., Chopra H. C., Mason M. M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970 Sep;30(9):2388–2393. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nowinski R. C., Edynak E., Sarkar N. H. Serological and structural properties of Mason-Pfizer monkey virus isolated from the mammary tumor of a Rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1608–1612. doi: 10.1073/pnas.68.7.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Boyse E. A., de Harven E., Geering G. Group-specific viral antigens in the milk and tissues of mice naturally infected with mammary tumor virus or Gross leukemia virus. Virology. 1968 Apr;34(4):617–629. doi: 10.1016/0042-6822(68)90083-4. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Hatanaka M., Gilden R. V., Huebner R. J. Specific inhibition of mammalian ribonucleic acid C-type virus deoxyribonucleic acid polymerases by rat antisera. J Virol. 1971 Nov;8(5):816–818. doi: 10.1128/jvi.8.5.816-818.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J., Scolnick E. M., Aaronson S. A. RNA dependent DNA polymerase in primate syncytium-forming (foamy) viruses. Nature. 1971 Jan 22;229(5282):258–260. doi: 10.1038/229258a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mattern C. F., Stone L. B., Coe J. E., Lavelle G. Antigenic and morphological similarities of progressive pneumonia virus, a recently isolated "slow virus" of sheep, to visna and maedi viruses. J Virol. 1971 Mar;7(3):301–308. doi: 10.1128/jvi.7.3.301-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigel R. F., Clark H. F. Electron microscopic observations on a "C"-type virus in cell cultures derived from a tumor-bearing viper. J Natl Cancer Inst. 1969 Nov;43(5):1097–1102. [PubMed] [Google Scholar]


