Abstract
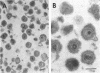
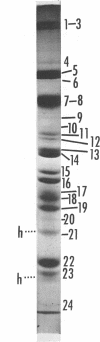
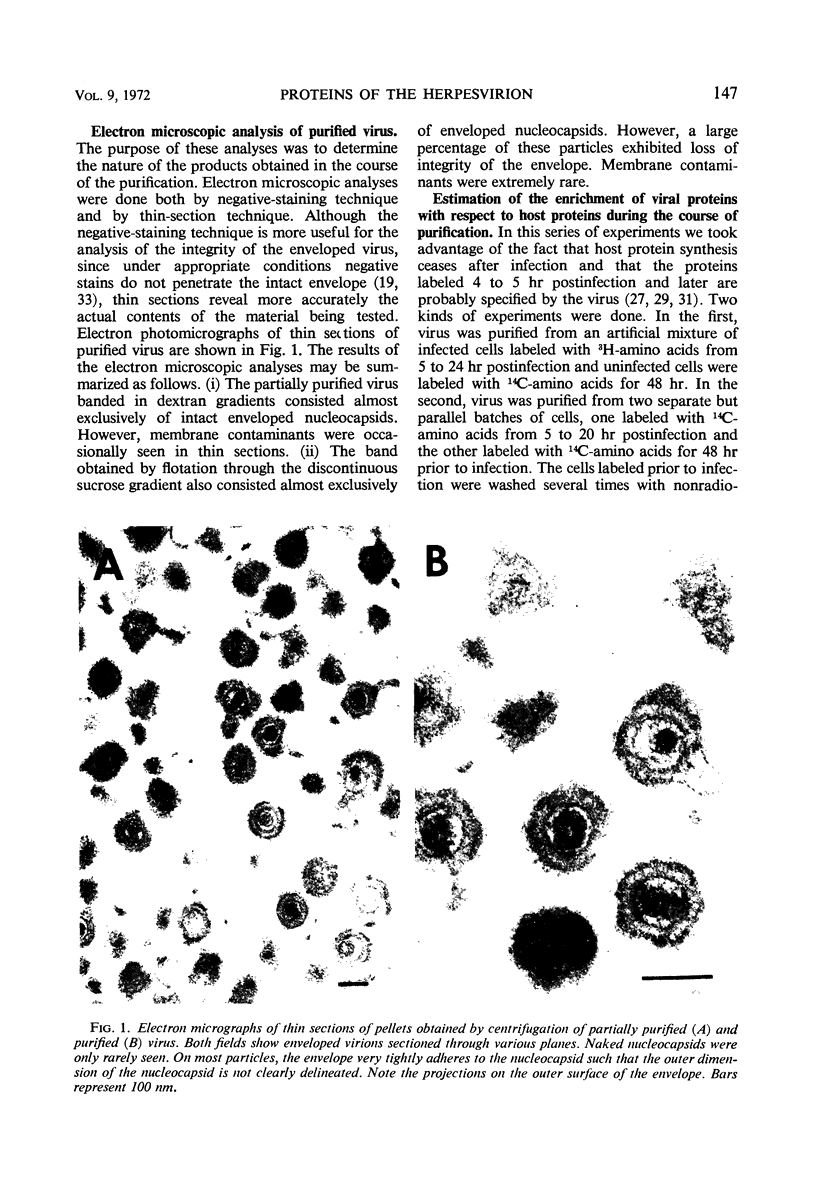
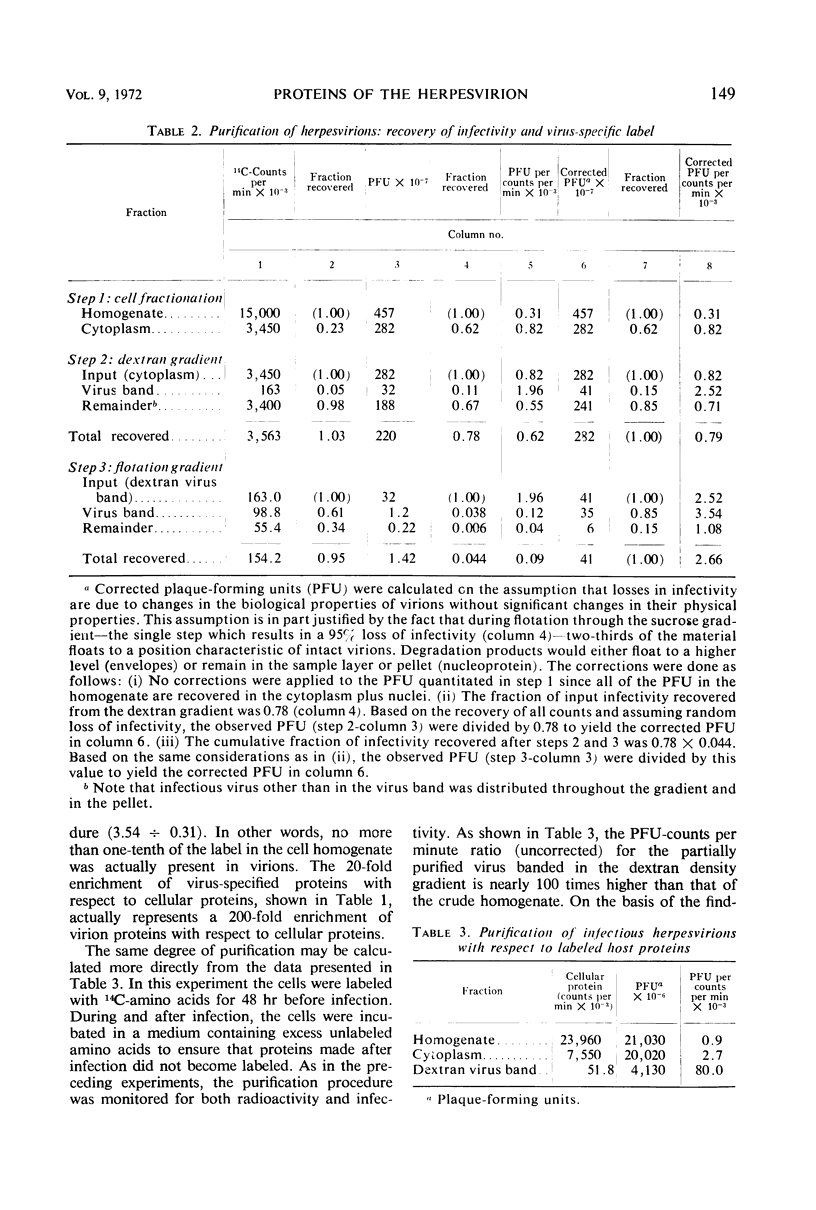
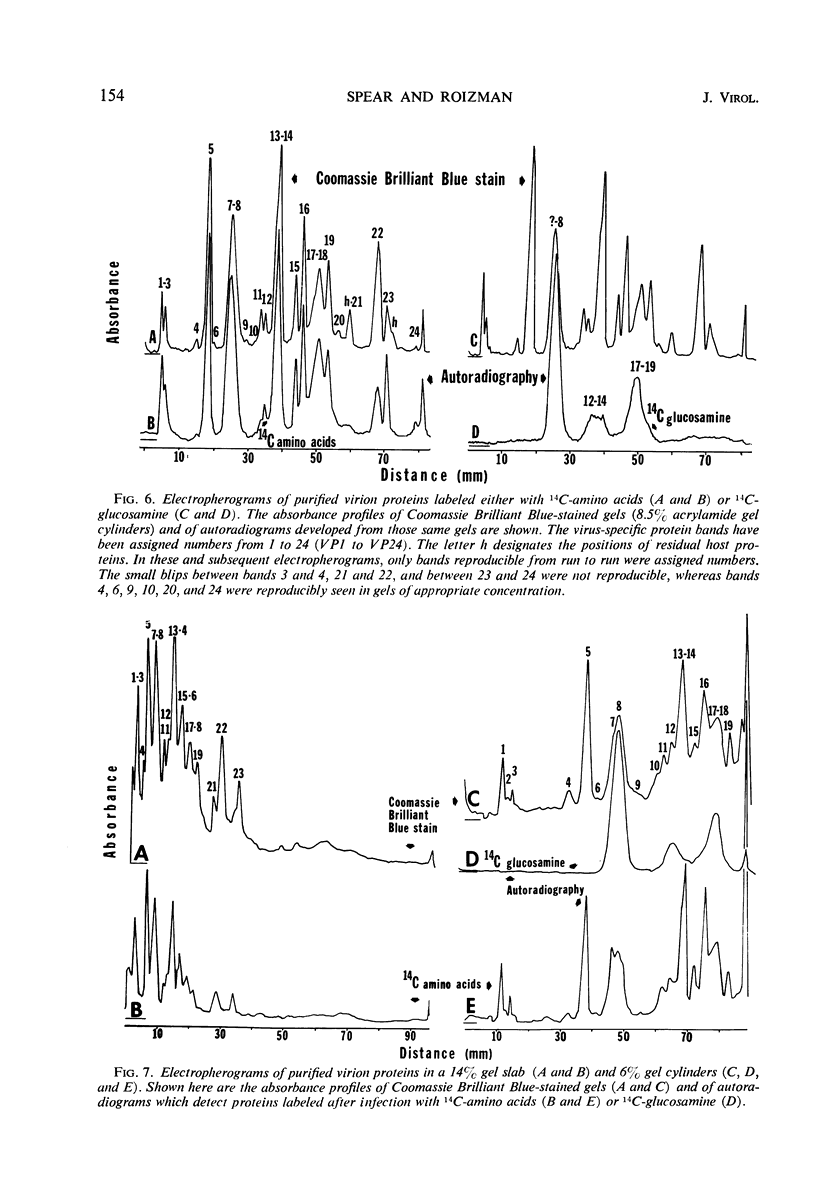
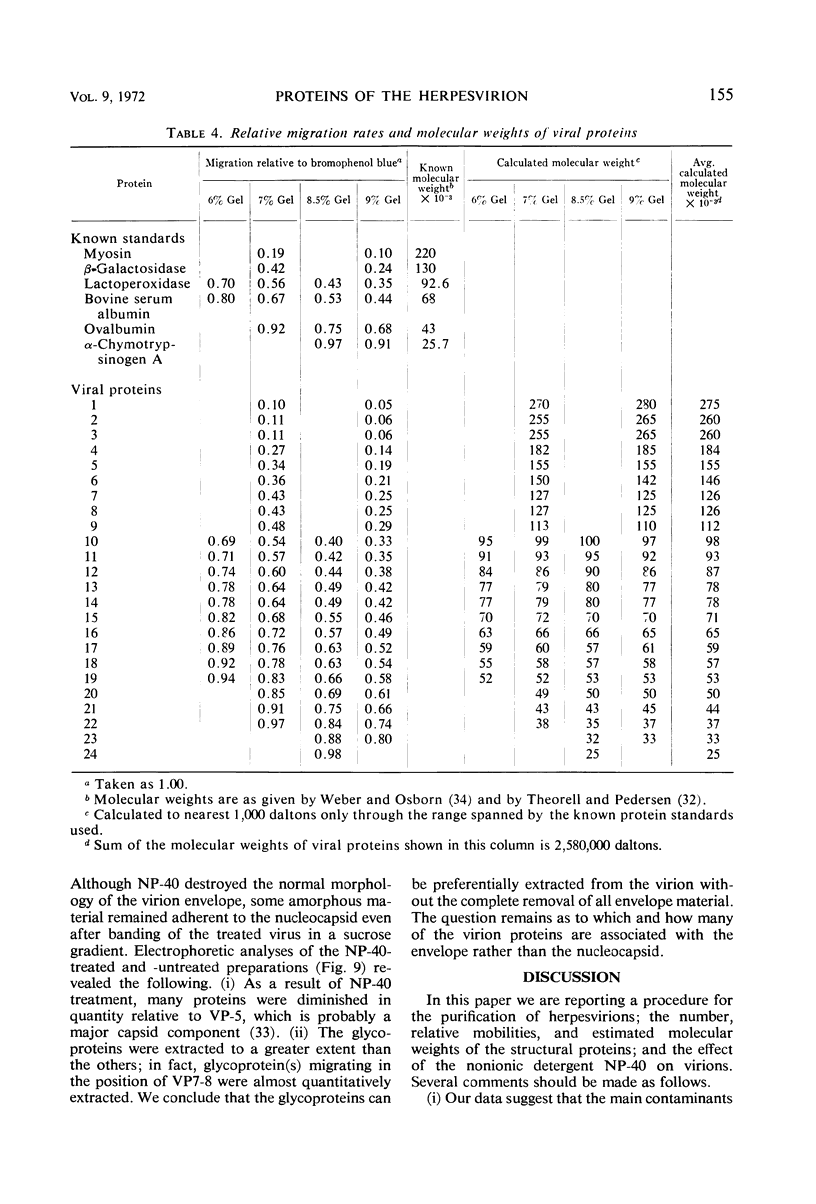
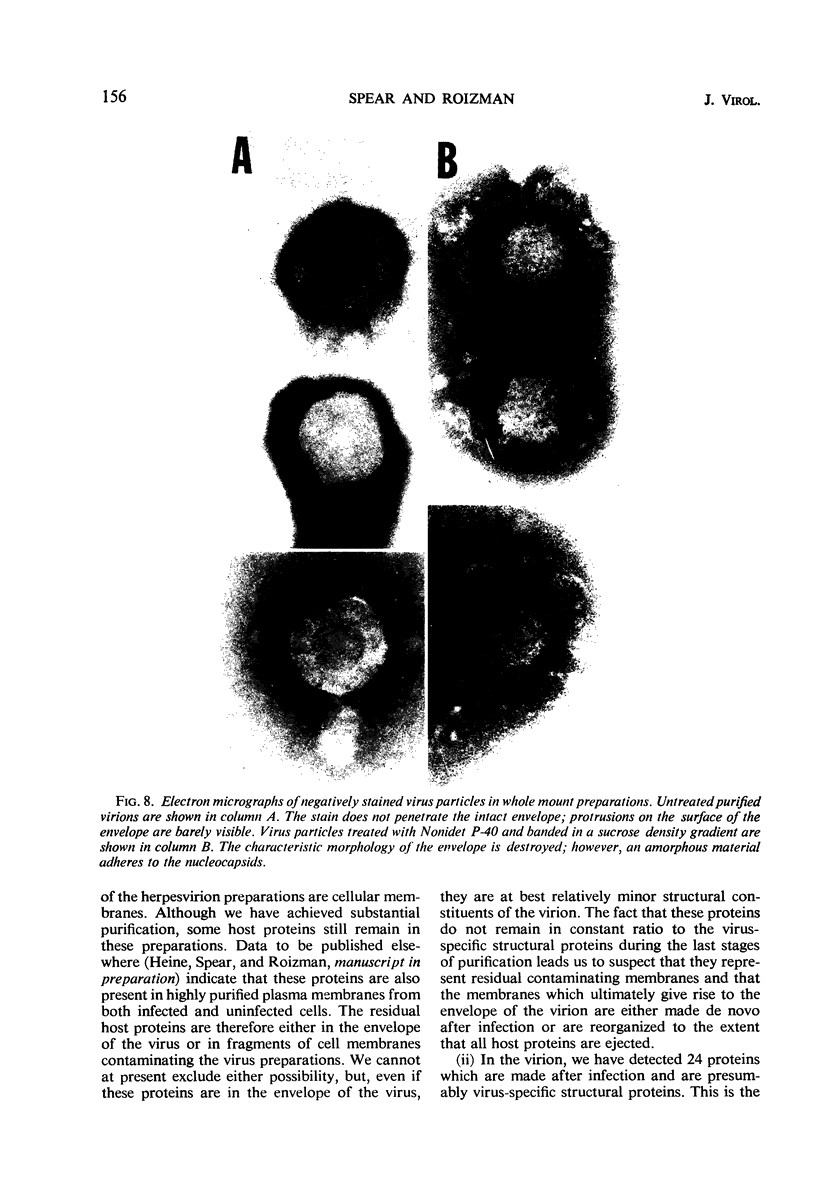
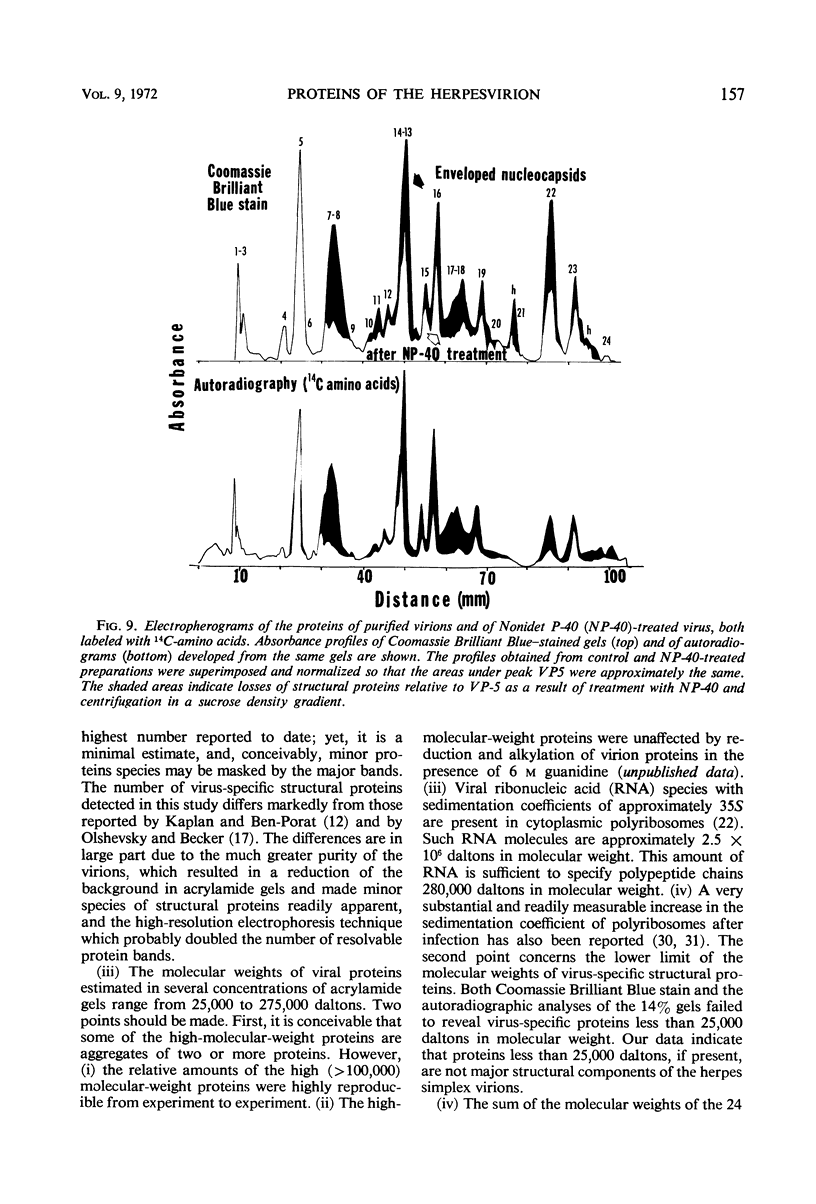
We are reporting a procedure for the purification of herpes simplex enveloped nucleocapsids (virions), an evaluation of the purification procedure and the results of analyses of the virion proteins by high-resolution acrylamide gel electrophoresis. The data may be summarized as follows. (i) The procedure for the purification of virions consists of careful extraction of cytoplasm to prevent nuclear breakage, separation of enveloped nucleocapsids from soluble proteins and membrane vesicles by rate zonal centrifugation of cytoplasmic extracts through dextran 10 gradients, treatment with urea to dissociate virus-debris aggregates, and, lastly, separation of virions from naked nucleocapsids and free membranes by isopycnic flotation in discontinuous sucrose gradients. (ii) Purity was evaluated in three ways, i.e., electron microscopic examination, analysis of purified virions produced in cells labeled with amino acids before infection, and analysis of purified virions from artificial mixtures of infected and labeled, uninfected cells. The extent of purification was 120-to 200-fold with respect to host proteins. Residual contaminants were identified as host and viral constituents of membrane vesicles. Residual host proteins are very likely contaminants and not structural components of the virion. (iii) Analyses by staining and autoradiography of structural proteins of purified virions in 6, 7, 8.5, 9, and 14% acrylamide gels revealed 24 bands of proteins and glycoproteins made and labeled after infection. Co-electrophoresis of viral proteins with six known standards ranging from 25,700 to 220,000 daltons in molecular weight in 6, 7, 8.5, and 9% acrylamide gels indicate that viral proteins range from 25,000 to 275,000 daltons. The sum of the molecular weights of viral proteins is 2,580,000 daltons. Assuming that messenger transcription is asymmetric and noncomplementary, this corresponds to 47% of the genetic information of the virus. (iv) The nonionic detergent NP-40 removes from purified virions some nonglycosylated proteins and a large fraction of the glycosylated proteins. It leaves behind traces of the envelope visible in the electron microscope as well as some glycoproteins thought to be in the envelope.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abodeely R. A., Lawson L. A., Randall C. C. Morphology and entry of enveloped and deenveloped equine abortion (herpes) virus. J Virol. 1970 Apr;5(4):513–523. doi: 10.1128/jvi.5.4.513-523.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abodeely R. A., Palmer E., Lawson L. A., Randall C. C. The proteins of enveloped and deenveloped equine abortion (herpes) virus and the separated envelope. Virology. 1971 Apr;44(1):146–152. doi: 10.1016/0042-6822(71)90161-9. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Herpes vimplex virus: genome size and redundancy studied by renaturation kinetics. J Virol. 1971 Oct;8(4):591–593. doi: 10.1128/jvi.8.4.591-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The effect of the temperature of incubation on the formation and release of herpes simplex virus in infected FL cells. Virology. 1959 Aug;8:508–524. doi: 10.1016/0042-6822(59)90052-2. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus, VI. Characterization of the proteins of the viral membrane. Proc Natl Acad Sci U S A. 1970 Jul;66(3):799–806. doi: 10.1073/pnas.66.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim R., Huang J. J., Davis G. A. Autoradiography with acrylamide gel slab electrophoresis. Anal Biochem. 1969 Apr 11;29(1):48–57. doi: 10.1016/0003-2697(69)90006-2. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Herpes simplex virus structural proteins. Virology. 1970 Apr;40(4):948–960. doi: 10.1016/0042-6822(70)90141-8. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- Robinson D. J., Watson D. H. Structural proteins of herpes simplex virus. J Gen Virol. 1971 Feb;10(2):163–171. doi: 10.1099/0022-1317-10-2-163. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Herpesvirus antigens on cell membranes detected by centrifugation of membrane-antibody complexes. Science. 1971 Jan 22;171(3968):298–300. doi: 10.1126/science.171.3968.298. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. The proteins specified by herpes simplex virus. I. Time of synthesis, transfer into nuclei, and properties of proteins made in productively infected cells. Virology. 1968 Dec;36(4):545–555. doi: 10.1016/0042-6822(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Straus J. H., Wallach D. F. A model for the behavior of vesicles in density gradients: implications for fractionation. Biochim Biophys Acta. 1970 Jun 2;203(3):385–393. doi: 10.1016/0005-2736(70)90179-3. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]