Abstract
Asia is a populous and diverse region and potentially an important source of information on food allergy. This review aims to summarize the current literature on food allergy from this region, comparing it with western populations. A PubMed search using strategies "Food allergy AND Asia", "Food anaphylaxis AND Asia", and "Food allergy AND each Asian country" was made. Overall, 53 articles, published between 2005 and 2012, mainly written in English were reviewed. The overall prevalence of food allergy in Asia is somewhat comparable to the West. However, the types of food allergy differ in order of relevance. Shellfish is the most common food allergen from Asia, in part due to the abundance of seafood in this region. It is unique as symptoms vary widely from oral symptoms to anaphylaxis for the same individual. Data suggest that house dust mite tropomysin may be a primary sensitizer. In contrast, peanut prevalence in Asia is extremely low compared to the West for reasons not yet understood. Among young children and infants, egg and cow's milk allergy are the two most common food allergies, with prevalence data comparable to western populations. Differences also exist within Asia. Wheat allergy, though uncommon in most Asian countries, is the most common cause of anaphylaxis in Japan and Korea, and is increasing in Thailand. Current food allergy data from Asia highlights important differences between East and West, and within the Asian region. Further work is needed to provide insight on the environmental risk factors accounting for these differences.
Keywords: Food Allergy, Asia, West, Epidemiology, Prevalence, Shellfish
INTRODUCTION
Food allergy has been referred to as the second wave of the allergy epidemic, asthma being the first [1]. Peanut allergy is one of the main food allergies contributing significantly to this food allergy epidemic [2]. Its prevalence has doubled over the decade in the 1990s and 2000s in the United Kingdom [3, 4] and the United States of America [5, 6]. The reasons for this increase are not known. However, environmental lifestyle changes and modernization have been implicated. Complex environmental-gene interactions seem to have resulted in a loss of oral/gastrointestinal tolerance. Not only is there an apparent rise in IgE mediated food allergies, but immune disorders of the gut such as eosinophilic oesophagitis are also more frequently seen [7].
Large population based epidemiological surveys are relatively few in Asia. This region is, however, an important resource for research and should not be ignored. It is the most populous regions in the world with many diverse populations and vast genetic, cultural, racial, language and socio-economic differences [8]. The diets are also varied in the type of food, methods of cooking, and the age of weaning. Introduction of new foods in infants follow traditional rather than professional guidance. Amongst clinicians in Asia, the prevalence of food allergy is perceived to be low. With the recent research activity in food allergy, more evidence-based data is emerging from this region and this review attempts to provide a broad overview of the current knowledge of food allergy in Asia. To this end, published studies on food allergy in Asia over the last 8 years were examined and its patterns and trends analyzed. Comparisons were also made with studies from Europe, North America and Australia, as these are populations where the food allergy epidemic has been reported. This information may provide clues on modifiable lifestyle risk factors.
METHODS
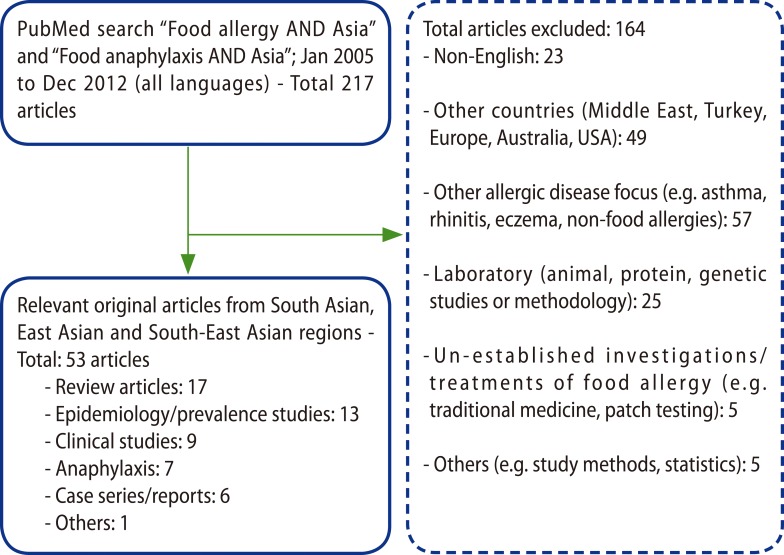
A PubMed search strategy was performed using the terms "Food allergy AND Asia" and "Food anaphylaxis AND Asia". To confirm that individual Asian countries were included, a search on "Food allergy AND several individual Asian countries - Brunei, Cambodia, China, East Timor, Hong Kong, India, Indonesia, Japan, Korea, Laos, Malaysia, Philippines, Singapore, Taiwan, Thailand, and Vietnam" was made. The initial search included all relevant articles in all languages, which resulted in 217 articles from January 2005 to December 2012 (inclusive). There were 5 countries - Brunei, Cambodia, East Timor, Laos and Myanmar - which yielded no results. There were also 6 countries - Bangladesh, Indonesia, Malaysia, Myanmar (Burma) and Vietnam, -which yielded no published epidemiological data. Articles from the Middle East and Turkey were excluded. Overall 53 original articles from South Asia, East Asia and South-East Asia were included in this review (Fig. 1).
Fig. 1.
Methodology: outlines of results of PubMed literature search.
The methodological concerns and food allergy prevalence in Asia
Self-reported food allergy surveys tend to overestimate the rates of food allergy. It is well documented that the prevalence of perceived food allergy exceeds that of true food allergy. Surveys that include skin prick testing and oral food challenges (OFC) (which remains the gold standard for the diagnosis of IgE-mediated food allergy) tend to give a better picture of the true prevalence [9]. However, despite OFC being the gold standard, the data may be compromised by many factors. These include open versus double blind placebo controlled food challenges, the choice of food used for the challenge (raw versus cooked), the challenge procedure itself (dose escalation, timing interval, outcome after 24 h or after a week), and the lack of universal criteria to define a positive challenge.
Population food allergy surveys in Asia also illustrate the importance of an accurate clinical history from well designed survey questionnaires and testing to provide a more precise evaluation of the food allergy in a community. In general, the prevalence of food allergy is higher in surveys that relied on questionnaires alone compared to those that incorporated allergy skin prick and oral food challenge testing (Table 1).
Table 1.
Population studies on food allergy prevalence in Asia
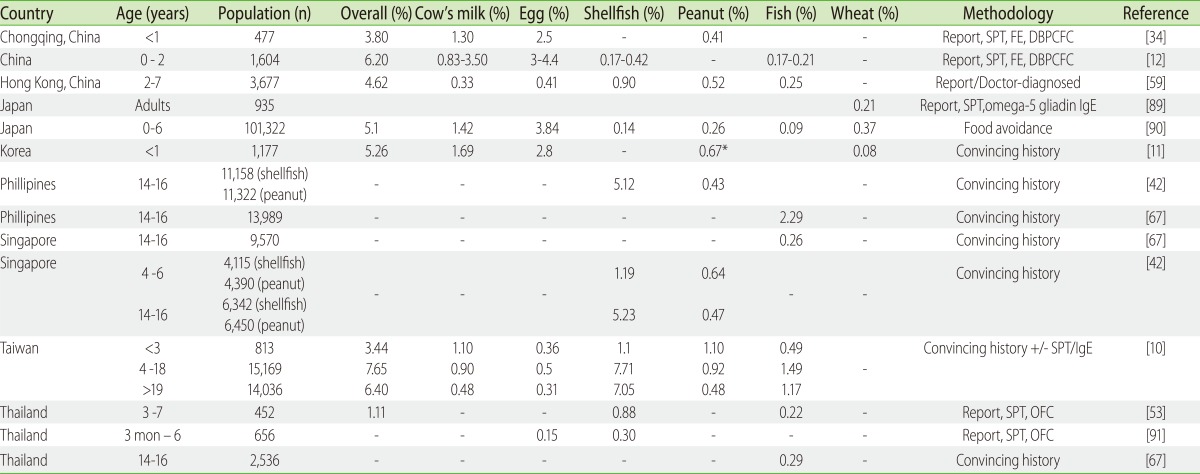
Report: self/parent-reported based on symptoms provided only; Convincing history: symptoms occurring in less than 2 h. SPT, skin prick test; FE, food elimination; DBPCFC, double-blinded placebo-controlled food challenge; OFC, open food challenge.
A self-reported random based expert screened questionnaire of Taiwanese children aged 4-18 years estimated the prevalence of food allergy as 7.02% [10]. In this questionnaire survey, the authors' criteria for food allergy required a convincing history of IgE allergy and included information on the age of the first attack, the clinical manifestations, and searched records for allergy tests, but OFC were not performed. Likewise a Korean study involving 1,176 infants with convincing history of reproducible food allergy symptoms via a telephone interview alone reported an overall prevalence of 5.2% [11]. The overall prevalence of self-reported or questionnaire-based food allergy in the paediatric age-group in Asia ranged from 3.4 to 11.1%. When more rigorous criteria were used in a study conducted in Northern Thailand on 452 children aged 4-7 years of age, where food challenges were included as part of the diagnostic criteria, a prevalence of 1.1% was reported. Another food challenge-proven study from three cities in China (published in Mandarin) demonstrated higher challenge-proven rates of 6.2% in a population of 1,604 children aged 0 to 2 years old [12]. The reason for the relatively higher prevalence of food allergy in this cohort is likely to be due to the inclusion of non-IgE food allergy, as well as conditions such as eczema.
Despite the notion that food allergy is less prevalent in Asia, these prevalence figures from Asia seem comparable with those reported from western populations (Table 2). In the European and US studies in the paediatric age-group (less than 16 years old) that are based on questionnaire surveys was reported to range from 3-35%, and those with added testing for allergen specific IgE and confirmatory food challenge tests from 1 to 10.8% [9].
Table 2.
Population studies on food allergy prevalence in Western countries
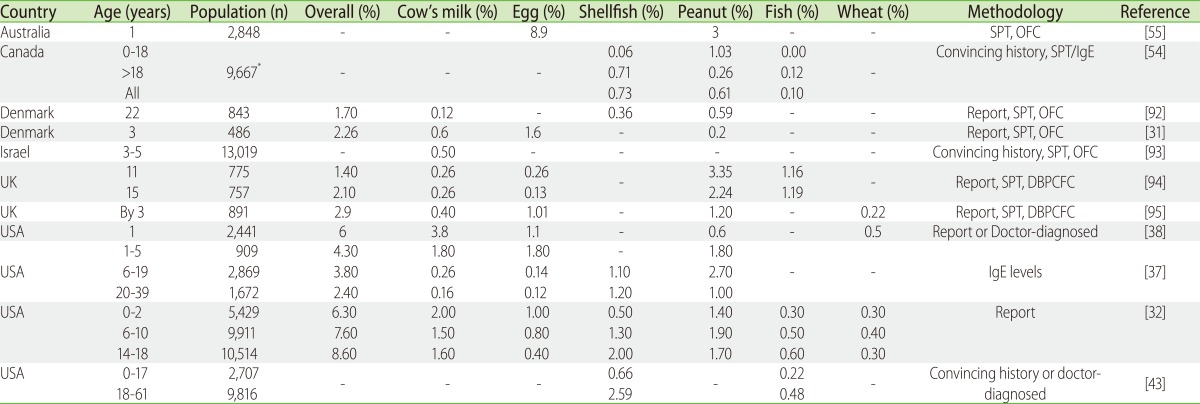
Report: self/parent-reported based on symptoms provided only; Convincing history: symptoms occurring in less than 2 h; SPT, skin prick test; DBPCFC, double-blinded placebo-controlled food challenge; OFC, open food challenge. *Individual population numbers for different age groups not provided.
There are only two Asian studies that have evaluated prevalence trends in food allergy. As with the global trend, a study of 0 to 2 year olds from Chongqing, China by Hu et al. [13] who used the exact same methodology 10 years apart showed that the prevalence of challenge-proven IgE-mediated food allergy has doubled from 3.5% in 1999 to 7.7% in 2009. In contrast, in a study of Korean school-children, based on food allergy symptoms alone, showed little change in prevalence over a 5 year period. The prevalence of 'ever having food allergy symptoms' for 6 to 12 year olds was 10.9% in 1995, and 8.9% in 2000, and for 12 to 15 year olds 11.3% and 12.6% respectively. It is therefore difficult to make firm conclusions on the trends in Asia, but they appear to be quite unlike those of western populations, where robust evidence shows doubling of peanut allergy prevalence over the last decade [5].
The most commonly reported food allergens in the European and North American populations are cow's milk, egg, peanuts, tree nuts, wheat, crustacean shellfish, fish and soy [14]. However, the true prevalence of fish and soy allergies are low in challenge-proven studies [9]. Egg and milk allergy are also the most prevalent food allergies in infants and young Asian children. Other than egg and cow's milk allergy (CMA), the pattern of food allergy in Asia appears quite different from other parts of the world and these will be discussed in the subsequent sections of the paper.
Food-induced anaphylaxis
There have been several hospital-based surveys evaluating anaphylaxis triggered by food. The epidemiology of food induced anaphylaxis is difficult to specify for a number of reasons. These include the lack of consensus on the definition of anaphylaxis, especially in those studies published prior to the recent consensus document by the Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium [15]. Published studies would suggest that anaphylaxis is increasing in the industrialised world specifically the United States, United Kingdom and Australia [16]. Although epidemiological studies in Asia are lacking the overall incidence data, appears to be low as suggested in a recent Singapore study [17].
The spectrum of foods responsible for causing anaphylaxis varies with age and is also country specific. In a recent cohort of Singaporean children (with non-local residents excluded) [17], the following food triggers were reported for each age group: egg and cow's milk in those less than 1 year old, peanut between 1 to 5 years old and shellfish after 10 years old. Peanut emerged as the most common trigger overall, whilst seafood and bird's nest continued to be important local triggers. This is of interest, since "nut" allergy and "nut induced anaphylaxis" has traditionally been unique to the West, and this may indicate a changing pattern of food anaphylaxis. In contrast, a similar Singapore study conducted 15 years ago did not identify any peanut or tree nut triggers for anaphylaxis and at that time bird's nest allergy was the most common trigger [18]. The authors postulated that migration and genetic predisposition are unlikely to account for this paradigm shift, and that possibly the change in exposure from boiled peanuts to processed peanut butter and previous peanut avoidance advice may in part account for this trend.
Likewise in a recent New York study [19] conducted in an urban paediatric emergency department that caters for 0-18 years of age, showed a similar pattern of food triggers of anaphylaxis. For children 6 years of age and below, cow's milk was the most common trigger and shellfish occurred mainly in children 7 through to 18 years of age. Also peanuts and tree nuts were reported more commonly as a trigger in the 0-6 years compared to the older age group but these findings were not statistically significant. Peanuts and tree nuts are a common trigger of anaphylaxis and an important culprit in fatal anaphylaxis in other Western populations including United Kingdom [20] and Australia [21].
A Taiwanese report by Hsin et al. [22] also focused on the younger paediatric age group where egg seemed to be the most common trigger followed closely by shellfish, and in a Japanese cohort where 50% of the population comprised less than 2 year olds, egg followed by cow's milk most commonly caused anaphylaxis [23]. This is similar to data from the US, where eggs are also commonly implicated in less than 5 year olds in the USA [24].
In the adult population, shellfish is the most frequently implicated food causing anaphylaxis as evidenced by presentation to emergency departments in adults and older children in most Asian countries such as Hong Kong (China), Singapore and Thailand [25-28]. Shellfish is also the leading cause of adult food-induced anaphylaxis in the US [24] and Australia [29]. In Korea, wheat and buckwheat are the main culprits of anaphylaxis [30].
Egg allergy
In most parts of Asia (China, Korea and some South East Asian countries), egg allergy predominates over cow's milk in children below the age of 5 (Table 1). This prevalence ranged from 3 to 4% in a Chinese food challenge-proven study [12]. These rates are higher than the western challenge-proven prevalence for egg allergy of 1 to 1.6% [31, 32]. With the exception of the Australian HealthNuts study [33] which reported one of the highest published raw egg challenge proven prevalence (9%), compared to the parent reported cumulative incidence of IgE-mediated CMA of only 2.7%. The authors suggested a possible explanation could be the use of raw versus cooked egg, the young age of the participants (11-16 months) and the high eczema rates in Australia. Furthermore, food challenges to raw eggs were undertaken based on a positive skin prick test rather than a history of clinical symptoms. Interestingly, children living in Australia with an East Asian parent have a higher risk of egg allergy compared to the other ethnic groups [33].
Cow's milk allergy
After egg allergy, CMA is the second most common food allergy in young Asian children. In China, the challenge-proven prevalence for large urban three cities (Chongqing, Zhuhai, and Hangzhou) ranged from 0.83-3.5% in 0 to 2 year olds. However, this study did not distinguish between IgE and non IgE-mediated CMA [12]. Their diagnostic criteria for challenge-proven allergy included symptoms reported up to 72 h post challenge [34]. The prevalence of IgE-mediated CMA from studies in Taiwan in less than 3 year olds [10] and from Korean infants [11] reported prevalence rates of IgE-mediated CMA as 1.1% and 1.7%, respectively. Though not challenge-proven, these two studies captured IgE-mediated food allergies via a history of convincing allergic symptoms occurring within minutes of exposure and/or sensitization through skin prick testing or IgE levels for the Taiwanese study, and within 2 h of exposure in the Korean study. In a clinical review of CMA cases in Thai children, non-IgE mediated CMA gastrointestinal symptoms were observed in 22% of cases, indicating that non-IgE mediated forms a significant proportion of CMA in at least some of our populations [35].
These prevalence rates are comparable to those reported in Western populations. In Europe, a meta-analysis by Rona et al. [9] reported the prevalence of IgE-mediated challenge-proven CMA to be as low as 0.4% by 3 years of age in Denmark, and when non-IgE mediated cases were included, it was 2.2% in children below 4 years in the Netherlands. In this latter study non-IgE mediated CMA comprised about half of the positive cases [36]. In the USA, the prevalence of 1.8% in 1 to 5 year olds was based on IgE sensitization levels that were above a threshold predictive of clinical allergy [37]. Another US study by Luccioli et al. [38] used doctor diagnosis or symptoms of angioedema and urticaria to define probable IgE-mediated food allergy, and reported CMA rates of 3.8% in infants. Despite the varied methodologies and the inclusion of non-IgE mediated CMA in some and not in others, the prevalence rates of CMA in young children in both Asia and the West ranged between <1% to <4%.
Shellfish allergy
Shellfish (crustaceans and molluscs) allergy in Asia is the most common food allergy in older children and adults [39, 40], and the leading causes of food-induced anaphylaxis in South-East Asia [22, 28, 41], Hong Kong [27], and Taiwan [22]. Population surveys show prevalence rates in teenagers in Philippines and Singapore of 5.12% and 5.23% respectively [42]. In contrast, a similar survey from the US showed a prevalence of shellfish allergy of 0.7% in 6-17 year olds [43]. Shellfish is also not a prominent cause of anaphylaxis and allergy in Korea [30] and Japan [44].
Due to the high prevalence of shellfish allergy in Asia, there is a special interest in the clinical manifestations of prawn/shrimp allergy in the South East Asian region. Two unique features in the clinical manifestations of prawn allergy have been described. Firstly, resembling the oral allergy syndrome, prawn allergy can result in mild oral symptoms such as itch and lip swelling [45]. Tropomyosin is a major allergen of prawn/shrimp and the invertebrate tropomyosin is considered a pan-allergen [46]. It is tempting to speculate that the mild oral allergies to shrimp/prawn observed in our South East Asian populations is due to cross reactivity with tropomyosin of dust mites which is so ubiquitous in our environment, and some case reports do support this notion [47-49]. This phenomenon may be akin to the oral allergy (inhalant-food) syndrome seen commonly in Europe, where individuals with pollen allergy react to cross reactive allergens in fruits and nuts. A multi-centre study in Italy [50] identified peach being the most frequently offending food in serious allergic reactions which was attributed primarily to sensitization to lipid transfer protein.
Secondly, prawn allergic reactions may fluctuate from severe anaphylaxis to mild reactions on separate occasions of exposure [51]. The reasons for this varied manifestations within the same patient is not clear. However, the prawn species [45], dose [51], the part of the prawn consumed (head versus body) [52] may be some of the reasons for these clinical observations.
Peanut and tree nut allergy
Although peanut and tree nut allergy constitutes an important part of the food allergy epidemic of the 1990's and 2000's in the western world, there is an impression amongst clinicians in Asia that these allergies are not as prevalent. Population prevalence studies of peanut and tree-nut confirms this clinical impression. It has been reported to be 0.67% in Korean infants [11], 0.47 to 0.64% in Singaporean children, 0.43% in Filipino children [42], 0.5 to 1.1% in Taiwan [10], and almost no cases in children in China [12] and Thailand [53]. These rates are at least half of those reported in the United Kingdom (1.2 = 3.3%) [3], Canada (0.26-1.0%) [54], USA (0.6-2.7%) and Australia (3%) [55]. In fact, these populations have experienced a doubling of prevalence rates over the 1990's to 2000's [5].
The reasons for this stark difference in peanut allergy compared to the western population are not known. It is tempting to speculate that early exposure to peanut which is cooked braised or boiled may be one of the reasons for the development of tolerance, as Asians including children are often exposed to peanut rice porridge. It has been shown that roasting of peanuts increases its allergenicity [56]. However, other environmental factors that modulate mucosal immunity of the gut and induce mucosal tolerance may also be implicated and requires further study. Environmental influences are suggested by studies on migrant populations (see section below on Risk factors for food allergy). In the Singapore survey, it was shown that irrespective of ethnicity, those born in Asia had lower risk of peanut and tree nut compared to those born in Western countries [57].
Wheat allergy
Within Asia, marked differences in wheat allergy prevalence are observed. Most notably, wheat allergy is prominent in Japanese school children [58] and the leading cause of food-anaphylaxis in both Japan and Korea [30, 44], and ranked above both shellfish and nuts. In Japanese adults, the prevalence of wheat allergy confirmed by skin prick test and serum ω-5 gliadin-specific IgE test is 0.21%. In contrast, the prevalence of wheat allergy prevalence is low in other parts of Asia. It was reported to be 0.08% less than one year olds in Korea, and in large population studies in Taiwan [10], Hong Kong [59], and China [12], the number wheat allergic children was uncommon. More recently, however, wheat allergy is increasingly reported from Thailand, with a report of 7 children described as having wheat anaphylaxis in Bangkok, Thailand in 2005 who demonstrated positive wheat skin prick test and IgE level results [60].
Although the reasons for this disparity in the prevalence of wheat allergy in the Asian region are not known, one possible postulation may be related to cooking methods and household exposure to wheat. Wheat flour in its form may be used more often in Japanese and Korean cooking, for example, dry flour is used in dishes such as tempura [61].
Wheat is the most common cause of food dependent exercise-induced anaphylaxis (FDEIA) in Japan [62] and Korea [30]. While shellfish was previously the most prominent cause of FDEIA [63], wheat is also an important trigger in Thailand [64], and in Singapore (personal communication Shek LPC, Singapore).
Buckwheat allergy
Common buckwheat (Fagopyrum esculentum) is not taxonomically related to other cereal grains (including wheat) and is termed a pseudocereal to highlight this. It is not even closely related to wild buckwheat (Polygonum convolvulus), which is grown in the US, which is from another genus level. However the similarity of the English terms makes this confusing, especially for those allergic to it. Common buckwheat is used to make buckwheat noodles in Japanese cooking and because of this frequent consumption, buckwheat allergy is the leading allergy in Japanese school children [58]. Inhalation or ingestion can often cause anaphylaxis as well. Currently in regions where buckwheat is not commonly consumed, allergy to buckwheat is rare [65]. However, there is an increasing number of cases seen in other Asian and western countries, especially as Japanese food popularises globally and common buckwheat is used as a substitute for wheat for gluten-free products [66].
Fish allergy
Despite the ubiquity of fish, fish allergy in Asia is relatively uncommon however there are some notable regional differences. In a recent population based study in late childhood, fish allergy was more prevalent in the Philippines (2.29%) compared to Singapore (0.26%) and Thailand (0.29%) [67]. Differences in prevalence were accounted for in part by food processing, dietary habits and other cultural practices. These figures were similar to a parent reported survey of adverse food reactions in Hong Kong where the estimated prevalence of fish allergy was 0.32% [59]. These figures are also similar to a US telephone survey, where the prevalence of 0.2% for fish allergy in children and 0.5% for adults [43].
The early weaning practices of Asian infants to fish (as early as 7 months) contrasts with Western populations where fish is introduced later [39]. This early introduction justifies the importance to better understand the allergen profiles of the commonly consumed tropical fish in this region. In Singapore Lim et al. [68] identified 12-kDa parvalbumin as the major allergen in threadfin, pomphret, indian anchovy and tengirri. This is important as parvalbumin is also the major allergen of cod (Gal d 1) and is a pan-allergen which shares various degrees of amino acid homologies, and therefore clinical cross reactivity amongst species is high. Children with tropical fish allergy had an early age of onset of clinical symptoms, male predominance and urticaria as the most common manifestation. In addition the majority developed symptoms on the first exposure to the particular fish suggesting alternate routes of sensitization.
Unique food allergens in Asia
There are food allergies that are unique to specific regions in Asia. In South East Asia, several unique allergies have been described. For example, bird's nest, a Chinese delicacy made from the saliva nests of cave swallows in South East Asia, is a common cause of anaphylaxis in Singapore [41]. In India, legumes, particularly chickpeas, are a major allergen due to high consumption in this populous region of vegetarians [69]. More recently, a cross sectional study from India established a high prevalence rate of eggplant allergy [70].
Specific to the tropics and subtropics, but not only confined to Asia [71], is the entity of ingested dust mite anaphylaxis occurring through the consumption of dust mite contaminated wheat flour has also been well described in several regions in Asia - Japan, Singapore and Taiwan [72-74]. It is common in regions where the warm and humid climate promotes growth of dust mites [72]. The use of wheat flour for cooking is also an important factor as dust mites contamination occurs in stored wheat flour and not in other forms of cereal [75].
From specific regions of Asia such as Japan [76] and Korea [77], where raw fish is traditionally consumed, allergy to Anisakis is common. Anisakis is a nematode which resides within raw fish. This allergy has also seen in other regions such as Spain where pickled anchovies are consumed. It is also expected to be increasingly reported elsewhere due to the rising popularity of raw fish consumption globally [78].
In addition, a new entity of allergy to galacto-oligosaccharide (GOS) containing formula has gained recent recognition in South East Asian countries such as Thailand, Vietnam and Singapore [79]. Despite GOS being approved for supplementation in infant formula in Europe since December 2001 [80], and conferred generally regarded as safe status by the US FDA in 2007 [81] - no such reports of GOS anaphylaxis have ever emerged from these western nations. This probably points to a environmental risk factor for the presence of GOS anaphylaxis in South East Asia, possibly similar to the postulation that carbohydrate sensitivity to alpha-galactose epitope in cetuximab and red meat allergic reactions in south eastern regions in the USA are a result of primary sensitization from bites of regionally distributed ticks [82].
Risk factors for food allergy
Food allergy, like other atopic disorders, is a complex trait where the interplay of genetic/epigenetic and environmental factors results in disease manifestation. There is a paucity of data emerging from Asia. However, studies on migration have contributed to the notion that environmental factors are paramount risk factors. The data shows that migrants appear to acquire the incident risk of their adopted country. Leung et al. [59] demonstrated that children born in mainland China compared to children born in and raised in Hong Kong had less parent reported adverse food reaction (4% versus 6.7%; p = 0.016). In the Singapore survey, it was shown that irrespective of ethnicity, those born in Asia had lower risk of peanut and tree nut compared to those born in Western countries [57]. Similarly data from outside Asia demonstrated that Jewish children in the United Kingdom have a prevalence of peanut allergy that is 10 fold higher than that of Jewish children in Israel [83]. The exact environmental factors contributing to these geographic differences are not fully understood. Factors that increase atopic susceptibility or reduce protection, such as early life microbial and parasitic exposure, the method of delivery (natural birth versus caesarean section), breast feeding rates; and factors relating to allergen exposure such as postpartum and weaning practices, the preparation and the processing of the food, and inhalant and cutaneous allergen exposure, are all likely candidates.
Genetics/epigenetics and ancestry are also important players in the development of food allergy. A study by Sicherer et al. [57] showed that monozygotic twins (64.3%) had significantly higher concordance rates for peanut allergy compared to dizygotic twins (6.8%). Similarly, in a Chinese twin study, food allergen sensitization as demonstrated by skin prick test showed that monozygotic twins were more likely to have concordant sensitization rates for peanut (odds ratio (OR) 3.7; 95% confidence interval (CI) = 1.4-10.0) and shellfish (OR 2.3; 95% CI = 1.0-5.2) than dizygotic twins [84]. Several genes have been implicated in genetic susceptibility to food allergy, and these include human leukocyte antigen, cytokine and immune genes: CD14, IL10, IL13, forkhead box P3 (FOXP3) [85]. Recently, mutations in loss of function variants in the filaggrin (FLG) gene have been identified as a risk factor (egg and peanut) allergy [86]. It is postulated that the impaired skin epithelial barrier resulting from these genetic defects facilitates epicutaneous sensitization through immune mechanisms, which in turn increases the susceptibility to food allergy. However these mutations are population specific as published by Chen et al. [87], where in a comparative study they identified 8 different FLG mutations account for 80% of the FLG mutations in Singapore while only 2 prevalent FLG null mutations dominated the European FLG spectrum. The relevance of the heterogeneity in FLG mutations in Asians is not well understood although in the HealthNuts study, infants with atopic eczema were much more likely to be of East Asian descent [88].
CONCLUSION
The prevalence of food allergy in Asia is increasing but still relatively lower, particularly for peanut and tree nut allergies, than the urbanized Western populations. These observed differences in prevalence are intriguing and deserve further study, since an affluent lifestyle is no longer an acceptable explanation as many urban regions of Asia are developed and affluent. The different pattern of food allergens also provides us with a unique opportunity to understand the environmental sources responsible for primary allergen sensitization. The marked genetic and environmental heterogeneity in Asia make future research challenging, and concerted efforts must be made to standardize definitions, procedures and food allergy outcomes to accurately identify risk and protective factors within the region.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr Motohiro Ebisawa, Department of Allergy, Clinical Research Center for Allergology and Rheumatology, Sagamihara National Hospital, Japan and Professor Pakit Vichyanond, Mahidol University, Siriraj Hospital, Bangkok, Thailand for the contribution of their data to this review.
References
- 1.Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22:155–160. doi: 10.1111/j.1399-3038.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120:491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, Higgins B, Dean T. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 4.Kotz D, Simpson CR, Sheikh A. Incidence, prevalence, and trends of general practitioner-recorded diagnosis of peanut allergy in England, 2001 to 2005. J Allergy Clin Immunol. 2011;127:623–630.e1. doi: 10.1016/j.jaci.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Kidambi T, Toto E, Ho N, Taft T, Hirano I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999-2009. World J Gastroenterol. 2012;18:4335–4341. doi: 10.3748/wjg.v18.i32.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shek LP, Lee BW. Food allergy in Asia. Curr Opin Allergy Clin Immunol. 2006;6:197–201. doi: 10.1097/01.all.0000225160.52650.17. [DOI] [PubMed] [Google Scholar]
- 9.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC, Huang IF, Chu CH, Lau BH, Wu L, Peng HJ, Tang RB. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42:1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol. 2011;22:715–719. doi: 10.1111/j.1399-3038.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Liao Y, Zhang HZ, Zhao H, Chen J, Li HQ. Prevalence of food allergy in children under 2 years of age in three cities in China. Zhonghua Er Ke Za Zhi. 2012;50:5–9. [PubMed] [Google Scholar]
- 13.Hu Y, Chen J, Li H. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr Int. 2010;52:820–824. doi: 10.1111/j.1442-200X.2010.03166.x. [DOI] [PubMed] [Google Scholar]
- 14.NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, Brown SG, Camargo CA, Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD, Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 16.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, Tan JH, Shih EC, Kidon M. Paediatric anaphylaxis in a Singaporean children cohort: changing food allergy triggers over time. Asia Pac Allergy. 2013;3:29–34. doi: 10.5415/apallergy.2013.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh DL, Lau YN, Chew FT, Shek LP, Lee BW. Pattern of food-induced anaphylaxis in children of an Asian community. Allergy. 1999;54:84–86. doi: 10.1034/j.1398-9995.1999.00925.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Chawla K, Järvinen KM, Nowak-Węgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129:162–168.e1-3. doi: 10.1016/j.jaci.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999-2006. J Allergy Clin Immunol. 2007;119:1018–1019. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–442. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 22.Hsin YC, Hsin YC, Huang JL, Yeh KW. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac J Allergy Immunol. 2011;29:307–312. [PubMed] [Google Scholar]
- 23.Akiyama H, Imai T, Ebisawa M. Japan food allergen labeling regulation--history and evaluation. Adv Food Nutr Res. 2011;62:139–171. doi: 10.1016/B978-0-12-385989-1.00004-1. [DOI] [PubMed] [Google Scholar]
- 24.Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121:166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Thong BY, Cheng YK, Leong KP, Tang CY, Chng HH. Anaphylaxis in adults referred to a clinical immunology/allergy centre in Singapore. Singapore Med J. 2005;46:529–534. [PubMed] [Google Scholar]
- 26.Techapornroong M, Akrawinthawong K, Cheungpasitporn W, Ruxrungtham K. Anaphylaxis: a ten years inpatient retrospective study. Asian Pac J Allergy Immunol. 2010;28:262–269. [PubMed] [Google Scholar]
- 27.Smit DV, Cameron PA, Rainer TH. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Lertnawapan R, Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011;60:283–289. doi: 10.2332/allergolint.10-OA-0256. [DOI] [PubMed] [Google Scholar]
- 29.Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–866. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 30.Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, Kwon HS, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008;100:31–36. doi: 10.1016/S1081-1206(10)60401-2. [DOI] [PubMed] [Google Scholar]
- 31.Osterballe M, Hansen TK, Mortz CG, Høst A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 32.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, Holl JL. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 33.Koplin JJ, Dharmage SC, Ponsonby AL, Tang ML, Lowe AJ, Gurrin LC, Osborne NJ, Martin PE, Robinson MN, Wake M, Hill DJ, Allen KJ. Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. 2012;67:1415–1422. doi: 10.1111/all.12015. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Hu Y, Allen KJ, Ho MH, Li H. The prevalence of food allergy in infants in Chongqing, China. Pediatr Allergy Immunol. 2011;22:356–360. doi: 10.1111/j.1399-3038.2011.01139.x. [DOI] [PubMed] [Google Scholar]
- 35.Ngamphaiboon J, Chatchatee P, Thongkaew T. Cow's milk allergy in Thai children. Asian Pac J Allergy Immunol. 2008;26:199–204. [PubMed] [Google Scholar]
- 36.Høst A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45:587–596. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806.e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luccioli S, Ross M, Labiner-Wolfe J, Fein SB. Maternally reported food allergies and other food-related health problems in infants: characteristics and associated factors. Pediatrics. 2008;122(Suppl 2):S105–S112. doi: 10.1542/peds.2008-1315n. [DOI] [PubMed] [Google Scholar]
- 39.Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–1061. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 40.Hill DJ, Hosking CS, Zhie CY, Leung R, Baratwidjaja K, Iikura Y, Iyngkaran N, Gonzalez-Andaya A, Wah LB, Hsieh KH. The frequency of food allergy in Australia and Asia. Environ Toxicol Pharmacol. 1997;4:101–110. doi: 10.1016/s1382-6689(97)10049-7. [DOI] [PubMed] [Google Scholar]
- 41.Thong BY, Cheng YK, Leong KP, Tang CY, Chng HH. Immediate food hypersensitivity among adults attending a clinical immunology/allergy centre in Singapore. Singapore Med J. 2007;48:236–240. [PubMed] [Google Scholar]
- 42.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Imamura T, Kanagawa Y, Ebisawa M. A survey of patients with self-reported severe food allergies in Japan. Pediatr Allergy Immunol. 2008;19:270–274. doi: 10.1111/j.1399-3038.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 45.Jirapongsananuruk O, Sripramong C, Pacharn P, Udompunturak S, Chinratanapisit S, Piboonpocanun S, Visitsunthorn N, Vichyanond P. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin Exp Allergy. 2008;38:1038–1047. doi: 10.1111/j.1365-2222.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 46.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 47.van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51:108–113. [PubMed] [Google Scholar]
- 48.Pajno GB, La Grutta S, Barberio G, Canonica GW, Passalacqua G. Harmful effect of immunotherapy in children with combined snail and mite allergy. J Allergy Clin Immunol. 2002;109:627–629. doi: 10.1067/mai.2002.122844. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 50.Asero R, Antonicelli L, Arena A, Bommarito L, Caruso B, Colombo G, Crivellaro M, De Carli M, Della Torre E, Della Torre F, Heffler E, Lodi Rizzini F, Longo R, Manzotti G, Marcotulli M, Melchiorre A, Minale P, Morandi P, Moreni B, Moschella A, Murzilli F, Nebiolo F, Poppa M, Randazzo S, Rossi G, Senna GE. Causes of food-induced anaphylaxis in Italian adults: a multi-centre study. Int Arch Allergy Immunol. 2009;150:271–277. doi: 10.1159/000222679. [DOI] [PubMed] [Google Scholar]
- 51.Gerez IF, Llanora G, Yap GC, Cheng YK, Thong BY, Tang CY, Van Bever HP, Shek LP, Curotto de Lafaille MA, Lee BW. Clinical characteristics of prawn allergy in an Asian population. J Allergy Clin Immunol. 2012;129:AB170. [Google Scholar]
- 52.Rosa S, Prates S, Piedade S, Marta CS, Pinto JR. Are there shrimp allergens exclusive from the cephalothorax? Allergy. 2007;62:85–87. doi: 10.1111/j.1398-9995.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 53.Lao-araya M, Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatr Int. 2012;54:238–243. doi: 10.1111/j.1442-200X.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- 54.Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, St Pierre Y, Godefroy SB, Elliott SJ, Clarke AE. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010;125:1327–1335. doi: 10.1016/j.jaci.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, Ponsonby AL, Wake M, Tang ML, Dharmage SC, Allen KJ. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676.e1-2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 56.Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000;106:763–768. doi: 10.1067/mai.2000.109620. [DOI] [PubMed] [Google Scholar]
- 57.Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106:53–56. doi: 10.1067/mai.2000.108105. [DOI] [PubMed] [Google Scholar]
- 58.Urisu A, Ebisawa M, Mukoyama T, Morikawa A, Kondo N. Japanese guideline for food allergy. Allergol Int. 2011;60:221–236. doi: 10.2332/allergolint.11-rai-0329. [DOI] [PubMed] [Google Scholar]
- 59.Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009;20:339–346. doi: 10.1111/j.1399-3038.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 60.Daengsuwan T, Palosuo K, Phankingthongkum S, Visitsunthorn N, Jirapongsananuruk O, Alenius H, Vichyanond P, Reunala T. IgE antibodies to omega-5 gliadin in children with wheat-induced anaphylaxis. Allergy. 2005;60:506–509. doi: 10.1111/j.1398-9995.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji S. Japanese cooking: a simple art. New York: Kondansha International; 2006. [Google Scholar]
- 62.Morita E, Kunie K, Matsuo H. Food-dependent exercise-induced anaphylaxis. J Dermatol Sci. 2007;47:109–117. doi: 10.1016/j.jdermsci.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Teo SL, Gerez IF, Ang EY, Shek LP. Food-dependent exercise-induced anaphylaxis - a review of 5 cases. Ann Acad Med Singapore. 2009;38:905–909. [PubMed] [Google Scholar]
- 64.Pacharn P, Jirapongsananuruk O, Daengsuwan T, Vichyanond P, Visitsunthorn N. Wheat-dependent, exercise-induced anaphylaxis in Thai children: a report of 5 cases. Asian Pac J Allergy Immunol. 2009;27:115–120. [PubMed] [Google Scholar]
- 65.Wang TC, Shyur SD, Wen DC, Kao YH, Huang LH. Buckwheat anaphylaxis: an unusual allergen in Taiwan. Asian Pac J Allergy Immunol. 2006;24:167–170. [PubMed] [Google Scholar]
- 66.Satoh R, Koyano S, Takagi K, Nakamura R, Teshima R, Sawada J. Immunological characterization and mutational analysis of the recombinant protein BWp16, a major allergen in buckwheat. Biol Pharm Bull. 2008;31:1079–1085. doi: 10.1248/bpb.31.1079. [DOI] [PubMed] [Google Scholar]
- 67.Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, Sangsupawanich P, Soh SE, Yap GC, Shek LP, Lee BW. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159:384–390. doi: 10.1159/000338940. [DOI] [PubMed] [Google Scholar]
- 68.Lim DL, Neo KH, Yi FC, Chua KY, Goh DL, Shek LP, Giam YC, Van Bever HP, Lee BW. Parvalbumin--the major tropical fish allergen. Pediatr Allergy Immunol. 2008;19:399–407. doi: 10.1111/j.1399-3038.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 69.Patil SP, Niphadkar PV, Bapat MM. Chickpea: a major food allergen in the Indian subcontinent and its clinical and immunochemical correlation. Ann Allergy Asthma Immunol. 2001;87:140–145. doi: 10.1016/S1081-1206(10)62209-0. [DOI] [PubMed] [Google Scholar]
- 70.Harish Babu BN, Mahesh PA, Venkatesh YP. A cross-sectional study on the prevalence of food allergy to eggplant (Solanum melongena L.) reveals female predominance. Clin Exp Allergy. 2008;38:1795–1802. doi: 10.1111/j.1365-2222.2008.03076.x. [DOI] [PubMed] [Google Scholar]
- 71.Sánchez-Borges M, Suárez Chacón R, Capriles-Hulett A, Caballero-Fonseca F, Fernández-Caldas E. Anaphylaxis from ingestion of mites: Pancake anaphylaxis. J Allergy Clin Immunol. 2013;131:31–35. doi: 10.1016/j.jaci.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 72.Tay SY, Tham E, Yeo CT, Yi FC, Chen JY, Cheong N, Chua KY, Lee BW. Anaphylaxis following the ingestion of flour contaminated by house dust mites--a report of two cases from Singapore. Asian Pac J Allergy Immunol. 2008;26:165–170. [PubMed] [Google Scholar]
- 73.Wen DC, Shyur SD, Ho CM, Chiang YC, Huang LH, Lin MT, Yang HC, Liang PH. Systemic anaphylaxis after the ingestion of pancake contaminated with the storage mite Blomia freemani. Ann Allergy Asthma Immunol. 2005;95:612–614. doi: 10.1016/S1081-1206(10)61027-7. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto T, Satoh A. The occurrence of mite-containing wheat flour. Pediatr Allergy Immunol. 2004;15:469–471. doi: 10.1111/j.1399-3038.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 75.Yi FC, Chen JY, Chee KK, Chua KY, Lee BW. Dust mite infestation of flour samples. Allergy. 2009;64:1788–1789. doi: 10.1111/j.1398-9995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 76.Hoshino C, Narita M. Anisakis simplex-induced anaphylaxis. J Infect Chemother. 2011;17:544–546. doi: 10.1007/s10156-011-0209-2. [DOI] [PubMed] [Google Scholar]
- 77.Choi SJ, Lee JC, Kim MJ, Hur GY, Shin SY, Park HS. The clinical characteristics of Anisakis allergy in Korea. Korean J Intern Med. 2009;24:160–163. doi: 10.3904/kjim.2009.24.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiang WC, Huang CH, Llanora GV, Gerez I, Goh SH, Shek LP, Nauta AJ, Van Doorn WA, Bindels J, Ulfman LH, Knipping K, Delsing DJ, Knol EF, Lee BW. Anaphylaxis to cow's milk formula containing short-chain galacto-oligosaccharide. J Allergy Clin Immunol. 2012;130:1361–1367. doi: 10.1016/j.jaci.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 80.The Commission of the European Communities. Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC. Official Journal of the European Union; 2006. [Google Scholar]
- 81.U.S. Food and Drug Administration. GRAS notice inventory, galacto-oligosaccharides. GRN No. 236. Silver Spring: U.S. Food and Drug Administration; 2007. [Google Scholar]
- 82.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293.e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, Fox AT, Turcanu V, Amir T, Zadik-Mnuhin G, Cohen A, Livne I, Lack G. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Zhang S, Tsai HJ, Hong X, Wang B, Fang Y, Liu X, Pongracic JA, Wang X. Genetic and environmental contributions to allergen sensitization in a Chinese twin study. Clin Exp Allergy. 2009;39:991–998. doi: 10.1111/j.1365-2222.2009.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong X, Wang X. Early life precursors, epigenetics, and the development of food allergy. Semin Immunopathol. 2012;34:655–669. doi: 10.1007/s00281-012-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–766. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 87.Chen H, Common JE, Haines RL, Balakrishnan A, Brown SJ, Goh CS, Cordell HJ, Sandilands A, Campbell LE, Kroboth K, Irvine AD, Goh DL, Tang MB, van Bever HP, Giam YC, McLean WH, Lane EB. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol. 2011;165:106–114. doi: 10.1111/j.1365-2133.2011.10331.x. [DOI] [PubMed] [Google Scholar]
- 88.Dharmage SC, Martin PE, Osborne NJ, Koplin JJ, Gurrin LC, Ponsonby A, Tang ML, Matheson MC, Lowe AJ, Dang T, Tan T, Anderson D, Thiele L, Sutherland M, Miles L, Wake M, Allen KJ. The epidemiology of food sensitization-associated eczema in infancy in HealthNuts, a population-based study. J Allergy Clin Immunol. 2011;127:AB35. [Google Scholar]
- 89.Morita E, Chinuki Y, Takahashi H, Nabika T, Yamasaki M, Shiwaku K. Prevalence of wheat allergy in Japanese adults. Allergol Int. 2012;61:101–105. doi: 10.2332/allergolint.11-OA-0345. [DOI] [PubMed] [Google Scholar]
- 90.Noda R. Prevalence of food allergy in nursery school (nationwide survey) Jpn J Food Allergy. 2010;10:5–9. [Google Scholar]
- 91.Santadusit S, Atthapaisalsarudee S, Vichyanond P. Prevalence of adverse food reactions and food allergy among Thai children. J Med Assoc Thai. 2005;88(Suppl 8):S27–S32. [PubMed] [Google Scholar]
- 92.Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev-Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20:686–692. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 93.Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, Leshno M. Early exposure to cow's milk protein is protective against IgE-mediated cow's milk protein allergy. J Allergy Clin Immunol. 2010;126:77–82.e1. doi: 10.1016/j.jaci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 94.Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol. 2005;116:884–892. doi: 10.1016/j.jaci.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 95.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, Arshad SH, Dean T. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]