Abstract
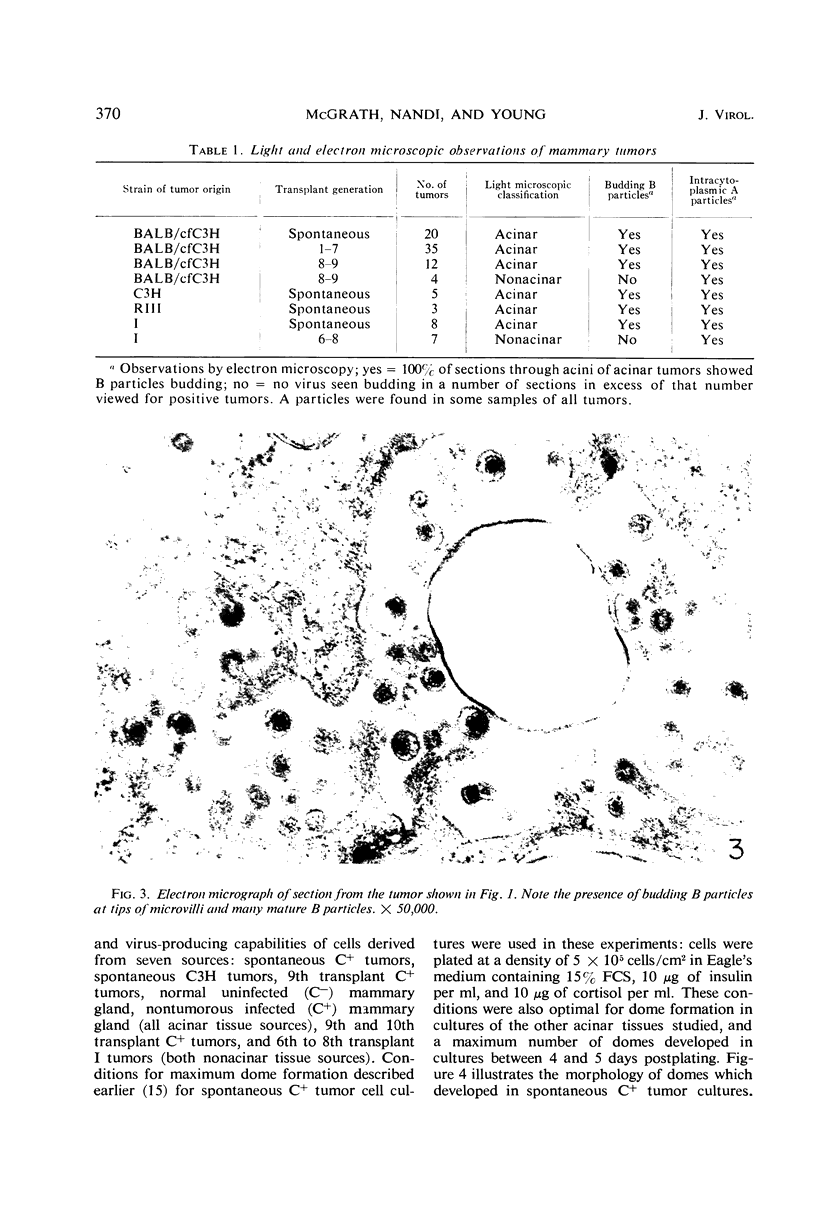
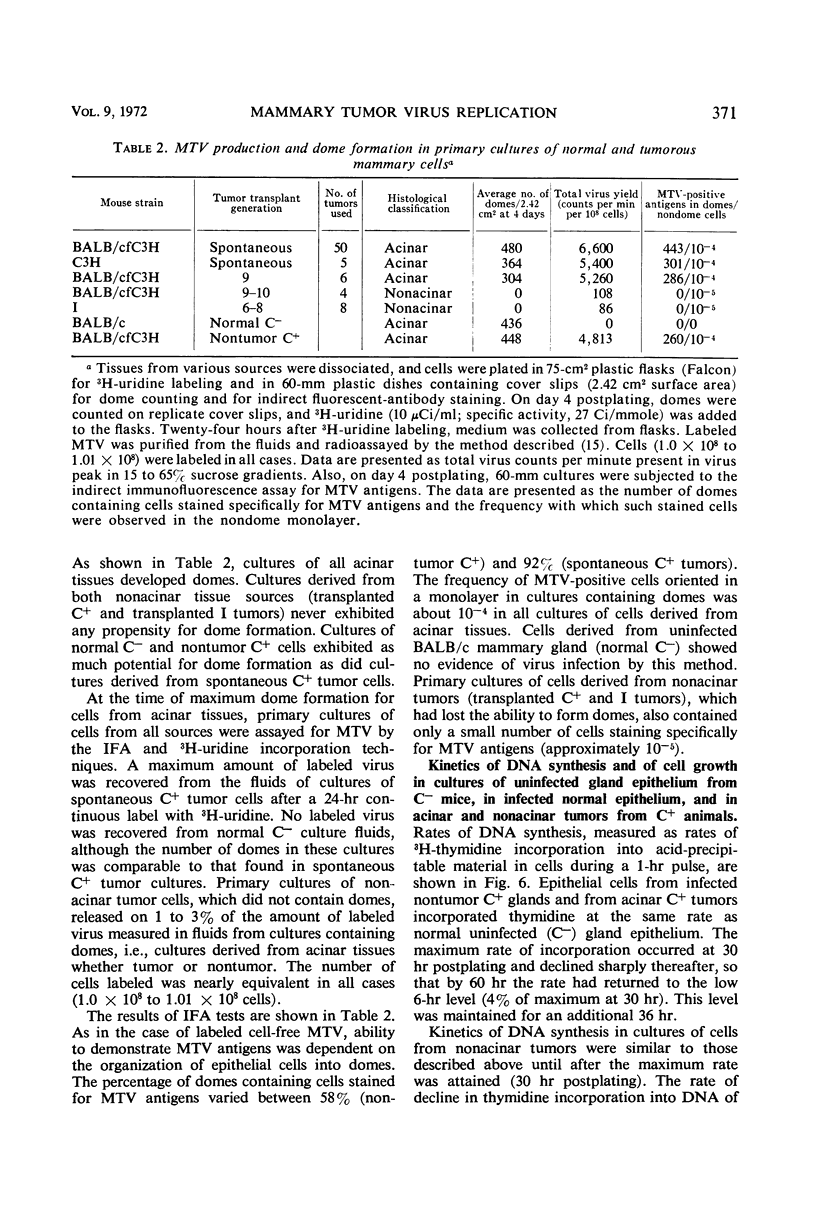
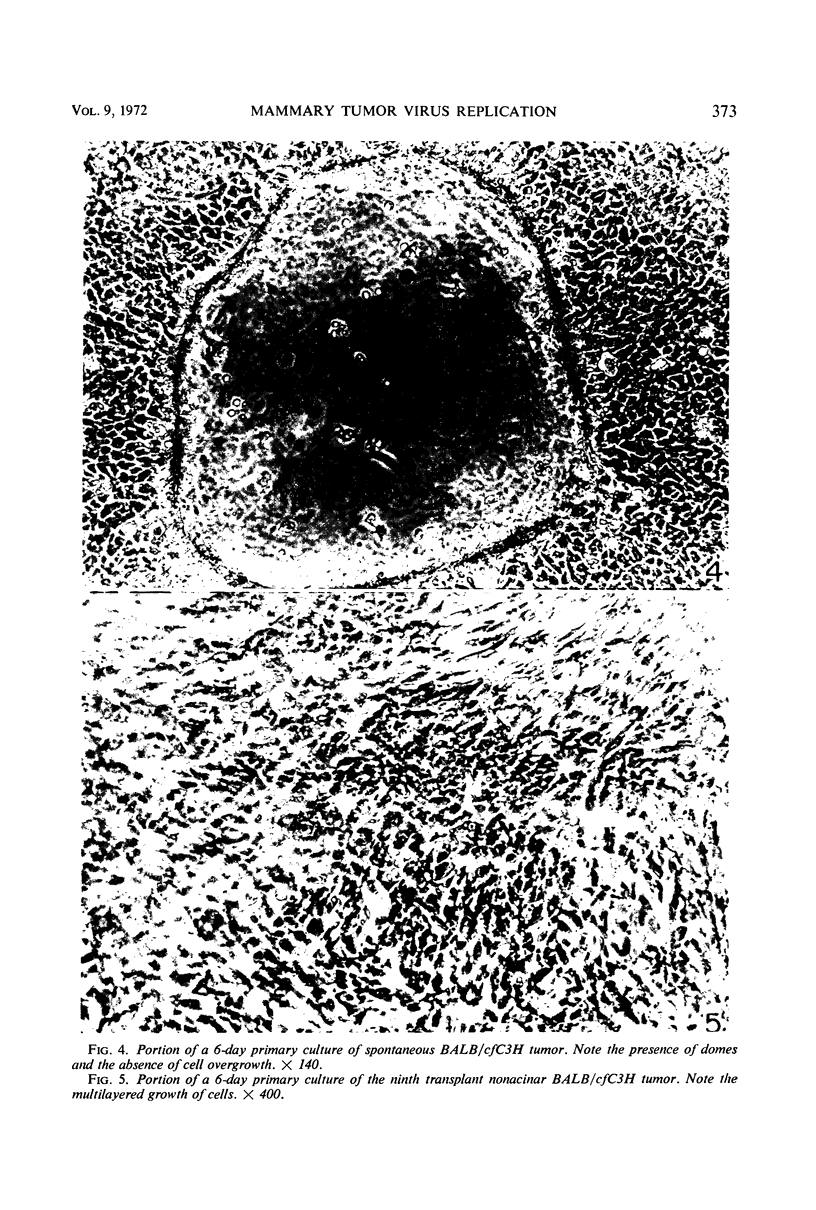
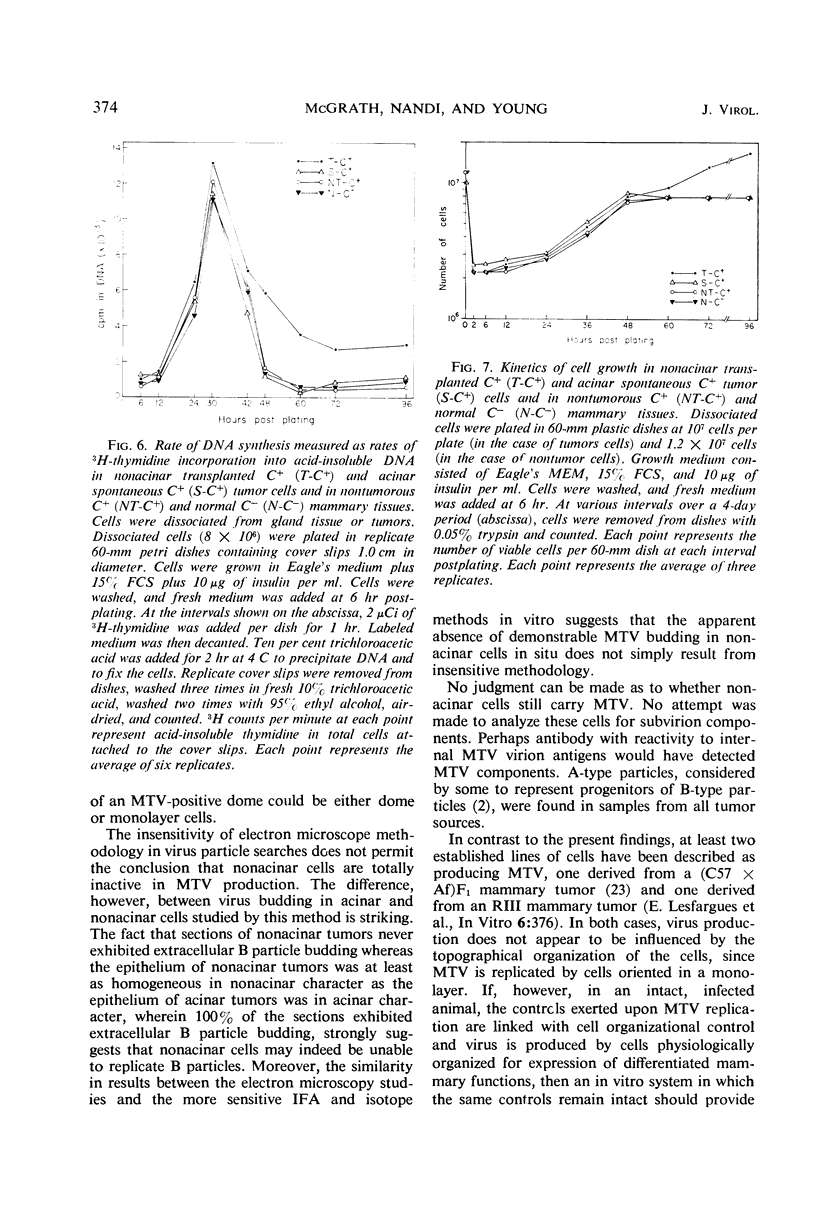
Mammary tumor virus (MTV) replication was confined primarily to cells organized as acini in intact mouse mammary glands. Primary mammary tumors maintained a high degree of acinar organization and cells therein continued to replicate MTV vegetatively. Nonacinar mammary cells, derived by serial transplantation of acinar tumor cells, no longer actively replicated MTV. This suggests that phenotypic differences exist among mammary epithelial cells in their ability to support virus replication, that a fundamental relationship exists between the organization of epithelium for secretion and active virus replication, and that this relationship is not altered as a primary consequence of neoplastic transformation. Mammary epithelial cells from pregnant, non-tumor-bearing, MTV-infected BALB/cfC3H mice or from acinar mammary tumors from a number of mouse strains were grown in primary monolayer cultures. Such cell cultures under the influence of insulin and cortisol exhibited the ability to organize into discrete three-dimensional structures called “domes.” MTV replication in such cultures took place primarily in cells within the organized domes. Cells cultured from nonacinar tumors did not exhibit any propensity to organize into domes, nor did they replicate MTV in primary culture. This suggests that the cell organizational requirement for MTV replication observed in vivo is conserved in primary culture. Dome formation is not an effect of virus replication, as cells from uninfected BALB/c animals organized into domes in culture without concomitant MTV replication. Growth-regulating signals, exerted between contiguous cells in cultures of non-MTV-infected mammary epithelium, were not modified by the occurrence of active virus replication nor as a direct consequence of neoplastic transformation. Cells derived from nontumor BALB/cfC3H glands and from spontaneous tumors exhibited cell growth kinetics, saturation densities, and deoxyribonucleic acid synthesis kinetics nearly identical to those of noninfected normal mammary epithelium in primary culture. Cell to cell growth regulatory signals were modified in cultures of nonalveolar tumor cells wherein evidence of overgrowth is documented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M. Contact-dependent behavior of normal cells and the possible significance of surface changes in virus-induced transformation. Cold Spring Harb Symp Quant Biol. 1962;27:427–431. doi: 10.1101/sqb.1962.027.001.040. [DOI] [PubMed] [Google Scholar]
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- DABROWSKA-PIASKOWSKA K. Observations on the histoformative capacities of tumor cells dissociated by digestion with trypsin. Exp Cell Res. 1959 Feb;16(2):315–323. doi: 10.1016/0014-4827(59)90259-9. [DOI] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FAULKIN L. J., Jr, DEOME K. B. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst. 1960 Apr;24:953–969. [PubMed] [Google Scholar]
- LASFARGUES E. Y. Cultivation and behavior in vitro of the normal mammary epithelium of the adult mouse. Anat Rec. 1957 Jan;127(1):117–129. doi: 10.1002/ar.1091270111. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson I. The characteristics of animal cells transformed in vitro. Adv Cancer Res. 1970;13:169–215. doi: 10.1016/s0065-230x(08)60166-9. [DOI] [PubMed] [Google Scholar]
- McGrath C. M., Blair P. B. Immunofluorescent localization of mammary tumor virus antigens in mammary tumor cells in culture. Cancer Res. 1970 Jul;30(7):1963–1968. [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- Nandi S., Handin M., Robinson A., Pitelka D. R., Webber L. E. Susceptibility of mammary tissues of "genetically resistant" strains of mice to mammary tumor virus. J Natl Cancer Inst. 1966 Apr;36(4):783–801. doi: 10.1093/jnci/36.4.783. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Briggs L. Observations on a cell line producing mammary tumor virus. J Natl Cancer Inst. 1968 Dec;41(6):1315–1327. [PubMed] [Google Scholar]