Abstract
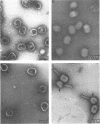
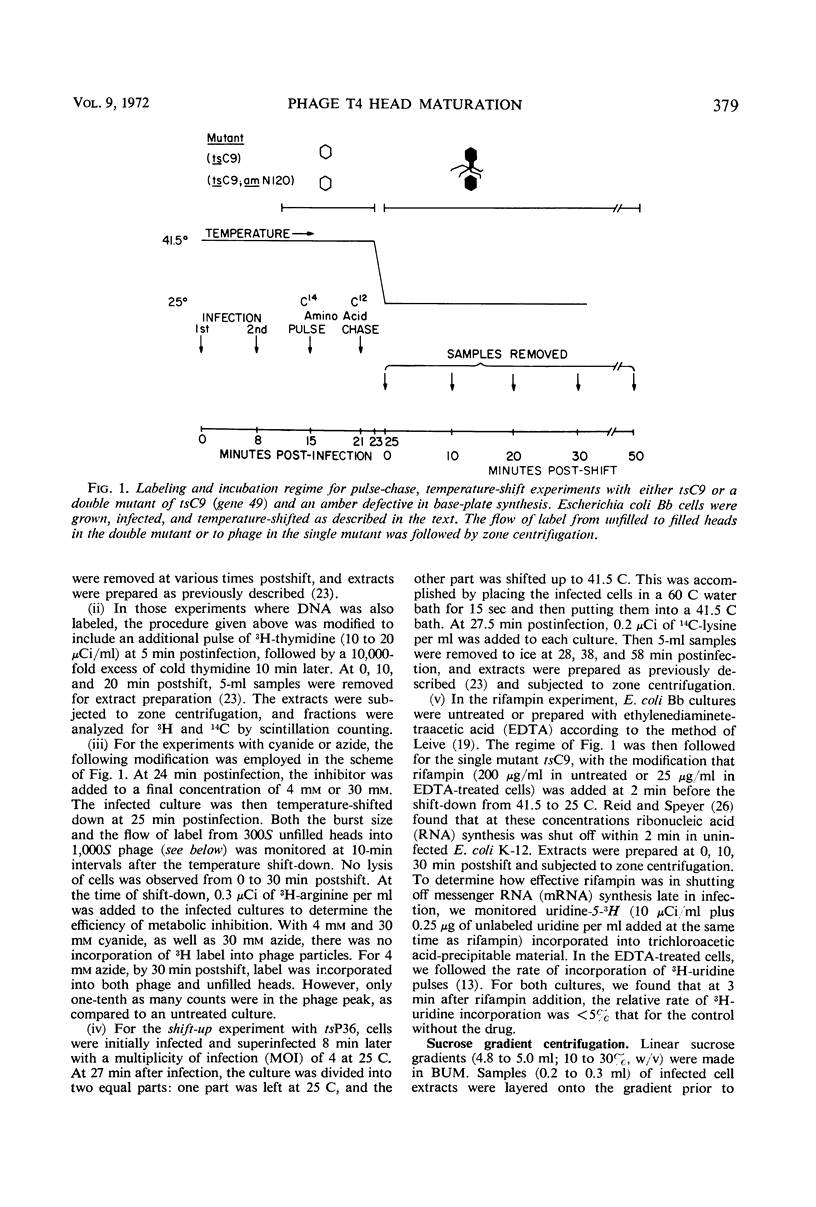
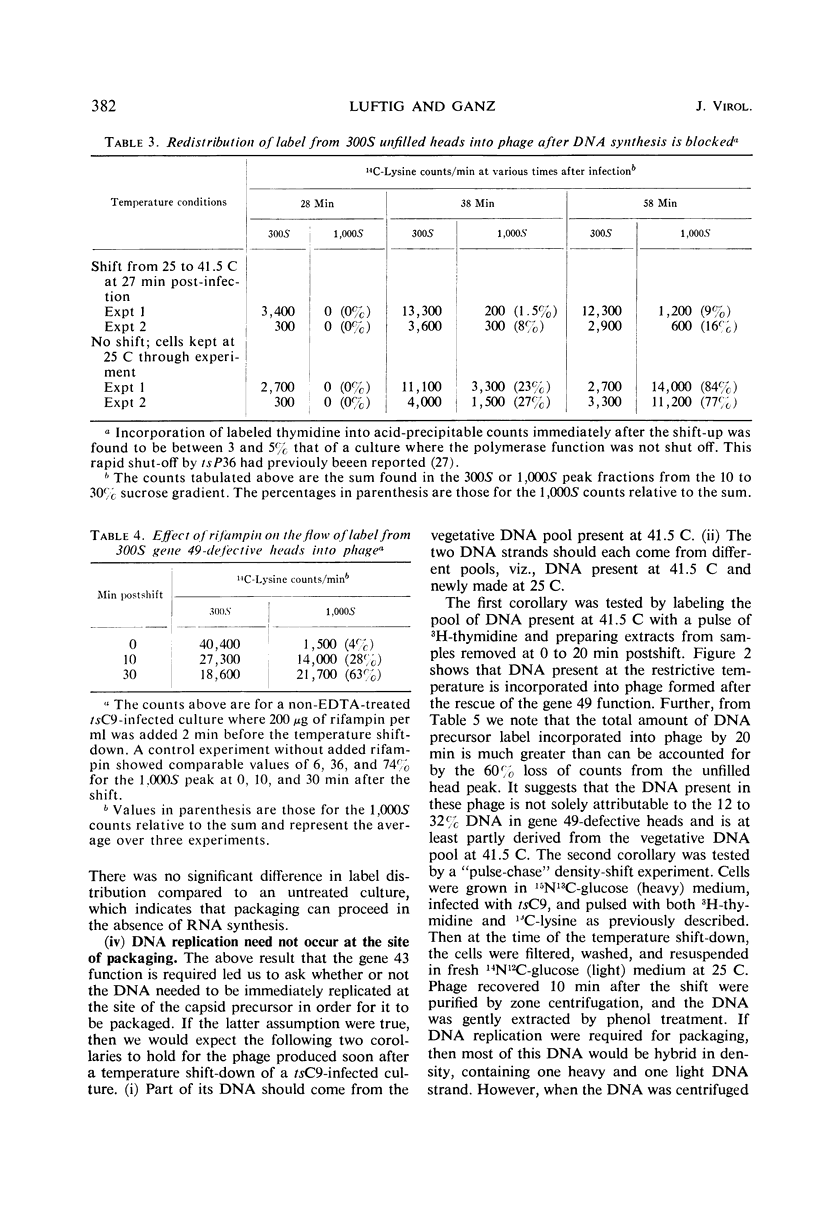
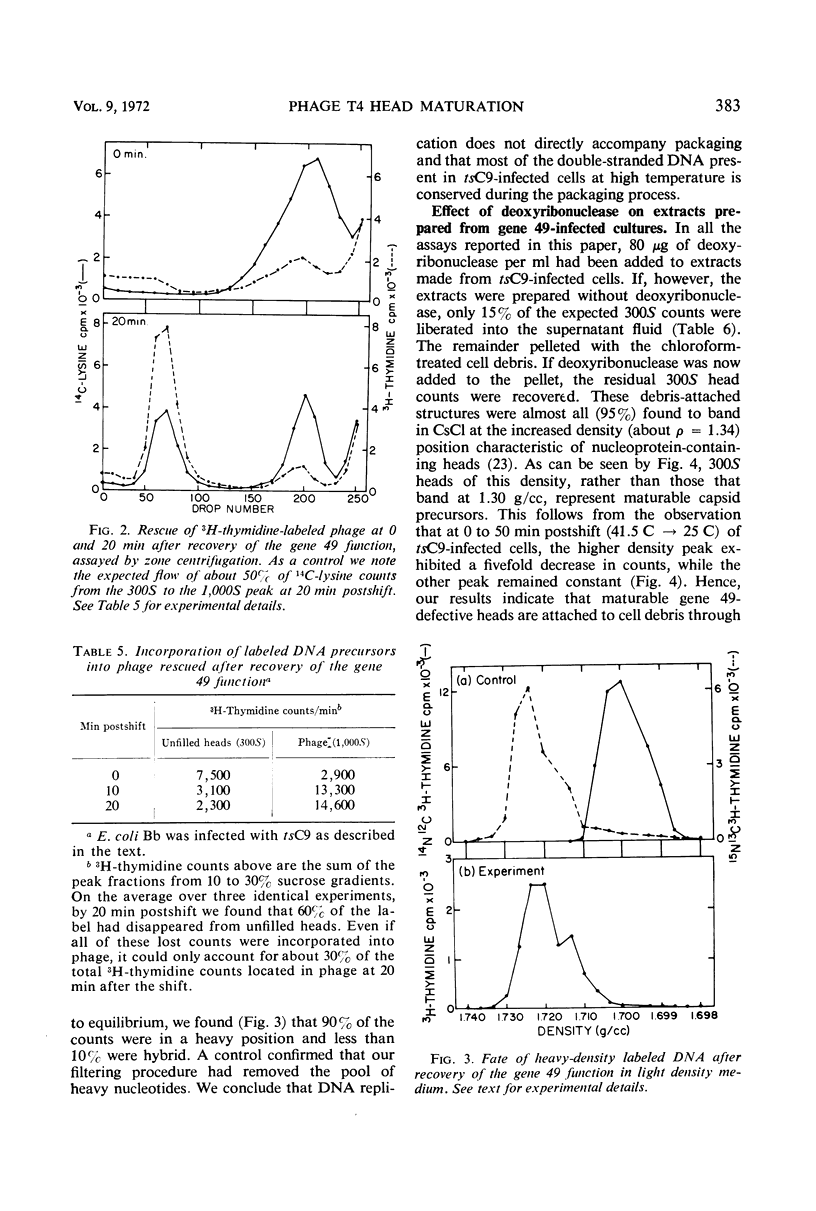
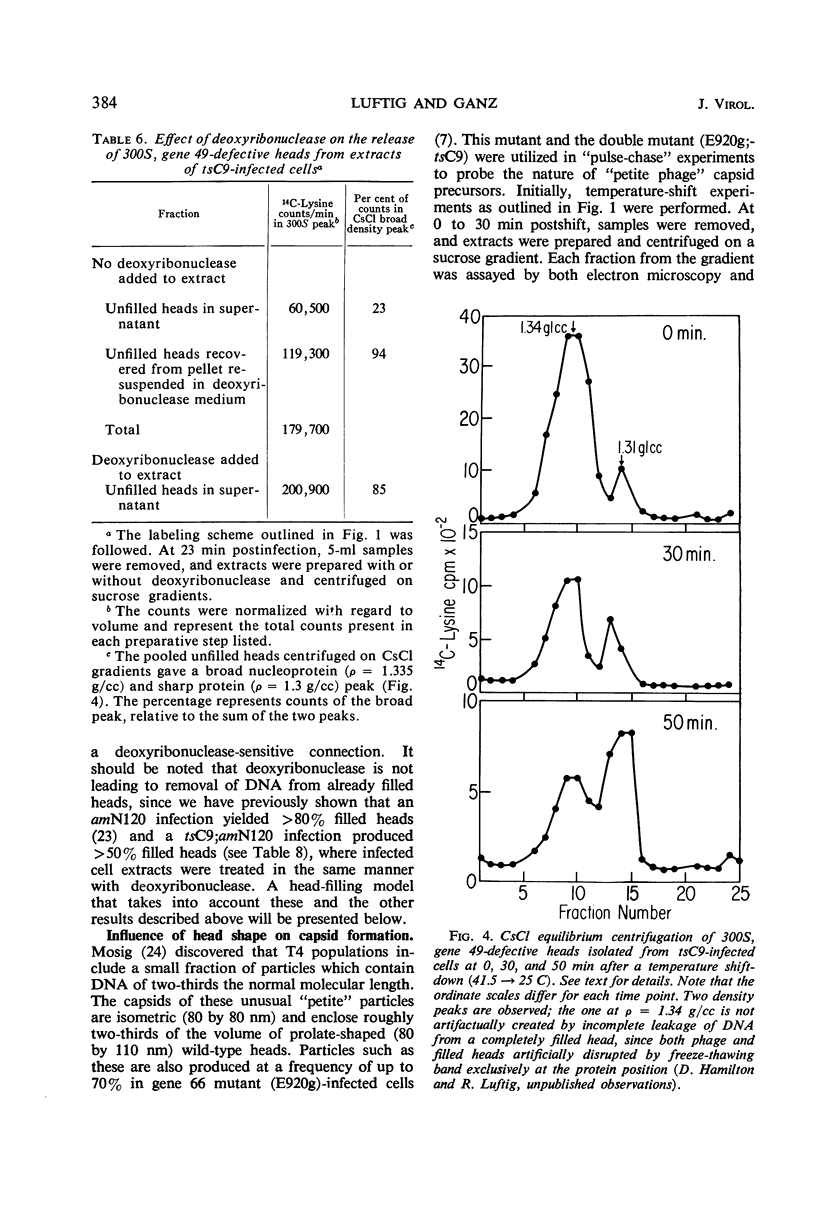
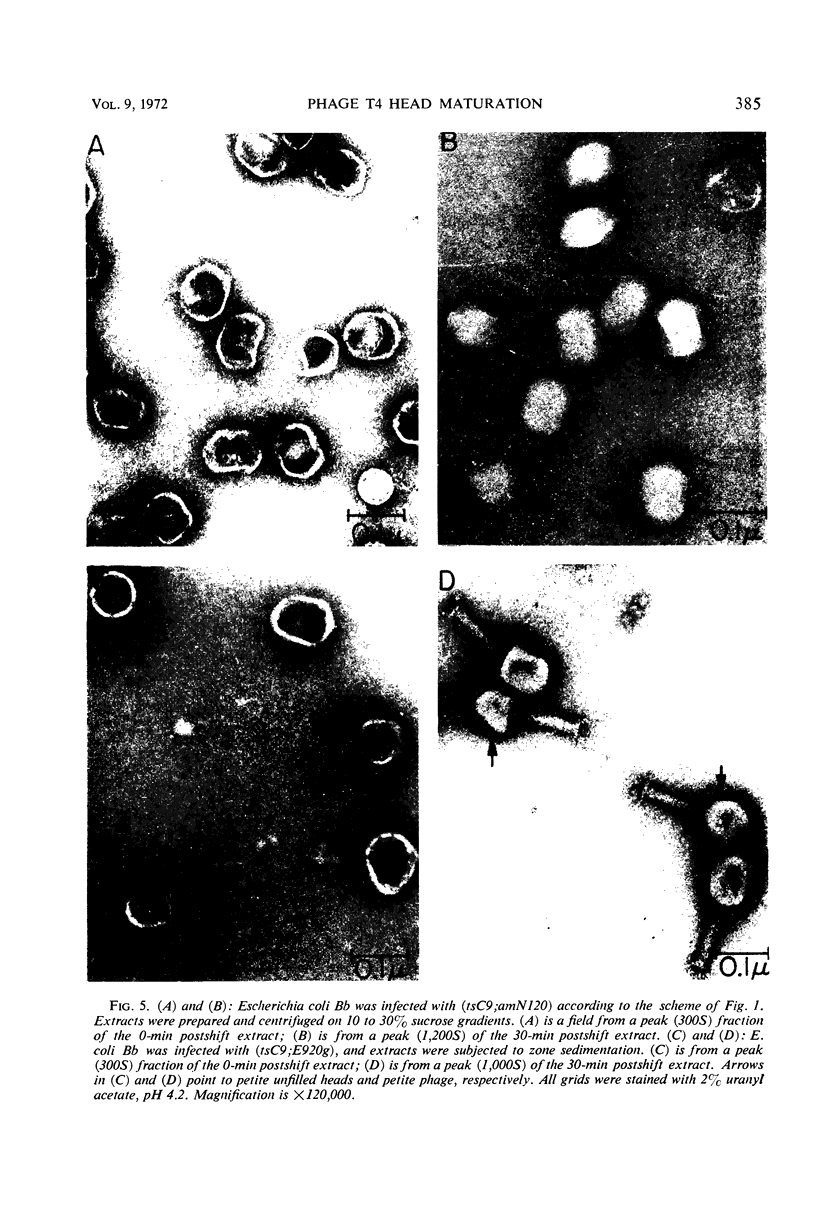
An investigation into the metabolic requirements for maturation of gene 49-defective heads indicated that adenosine triphosphate energy and continued deoxyribonucleic acid (DNA) but not ribonucleic acid synthesis were needed. The fate of DNA present at restrictive temperatures (41.5 C) in tsC9 (gene 49)-infected cells was also examined. After lysis of infected cells, the 12 to 32% deoxyribonuclease-resistant DNA associated with isolated gene 49-defective heads was found to be attached to a deoxyribonuclease-sensitive complex associated with the debris. Pulsechase experiments where 3H-thymidine was used to label the DNA at 41.5 C suggested that more DNA from this pool was present in phage recovered after rescue of the gene 49 function than could be accounted for by the deoxyribonuclease-resistant portion. Further, when these experiments were repeated with an additional density shift (15N13C-glucose to 14N12C-glucose), the DNA extracted from phage rescued at 10 min after the temperature shift-down was found to be 90% conserved. These results suggest a model whereby DNA packaging into capsid precursors is separated from DNA replication and the energy from DNA synthesis provides the driving force for packaging. Pulse-chase, temperature-shift experiments with E920g (gene 66) or E920g;tsC9 mutant-infected cells showed that gene (49, 66)-defective heads, which were isolated as small, isometric-shaped unfilled heads, were a precursor to “petite” phage. This suggests that the maturation process is independent of the size and shape of the head membrane. Similar experiments with the double mutant tsC9;amN120 indicate that gene 49-defective heads can also be filled in the absence of tails.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cascino A., Riva S., Geiduschek E. P. Host DNA synthesis after infection of Escherichia coli with mutants of bacteriophage T4. Virology. 1971 Nov;46(2):437–452. doi: 10.1016/0042-6822(71)90044-4. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. The sedimentation and conformational variance among T-even bacteriophages. Virology. 1969 Jan;37(1):94–108. doi: 10.1016/0042-6822(69)90310-9. [DOI] [PubMed] [Google Scholar]
- DOERMANN A. H. The intracellular growth of bacteriophages. I. Liberation of intracellular bacteriophage T4 by premature lysis with another phage or with cyanide. J Gen Physiol. 1952 Mar;35(4):645–656. doi: 10.1085/jgp.35.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Granboulan P., S echaud J., Kellenberger E. On the fragility of phage T4-related particles. Virology. 1971 Nov;46(2):407–425. doi: 10.1016/0042-6822(71)90042-0. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Josslin R. Physiological studies on the t gene defect in T4-infected Escherichia coli. Virology. 1971 Apr;44(1):101–107. doi: 10.1016/0042-6822(71)90157-7. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Nossal N. G. A T4 bacteriophage mutant which lacks deoxyribonucleic acid polymerase but retains the polymerase-associated nuclease. J Biol Chem. 1969 Jan 10;244(1):218–220. [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salivar W. O., Sinsheimer R. L. Intracellular location and number of replicating parental DNA molecules of bacteriophages lambda and phi-X174. J Mol Biol. 1969 Apr 14;41(1):39–65. doi: 10.1016/0022-2836(69)90124-7. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Kahana S. Conversion of T4 gene 46 mutant deoxyribonucleic acid into nonviable bacteriophage particles. J Virol. 1970 Sep;6(3):353–362. doi: 10.1128/jvi.6.3.353-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Luftig R. B., Wilson J. H., Eddleman H., Lyle H., Wood W. B. Assembly of bacteriophage T4 tail fibers. II. Isolation and characterization of tail fiber precursors. J Mol Biol. 1970 Nov 28;54(1):15–31. doi: 10.1016/0022-2836(70)90443-2. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]