Abstract
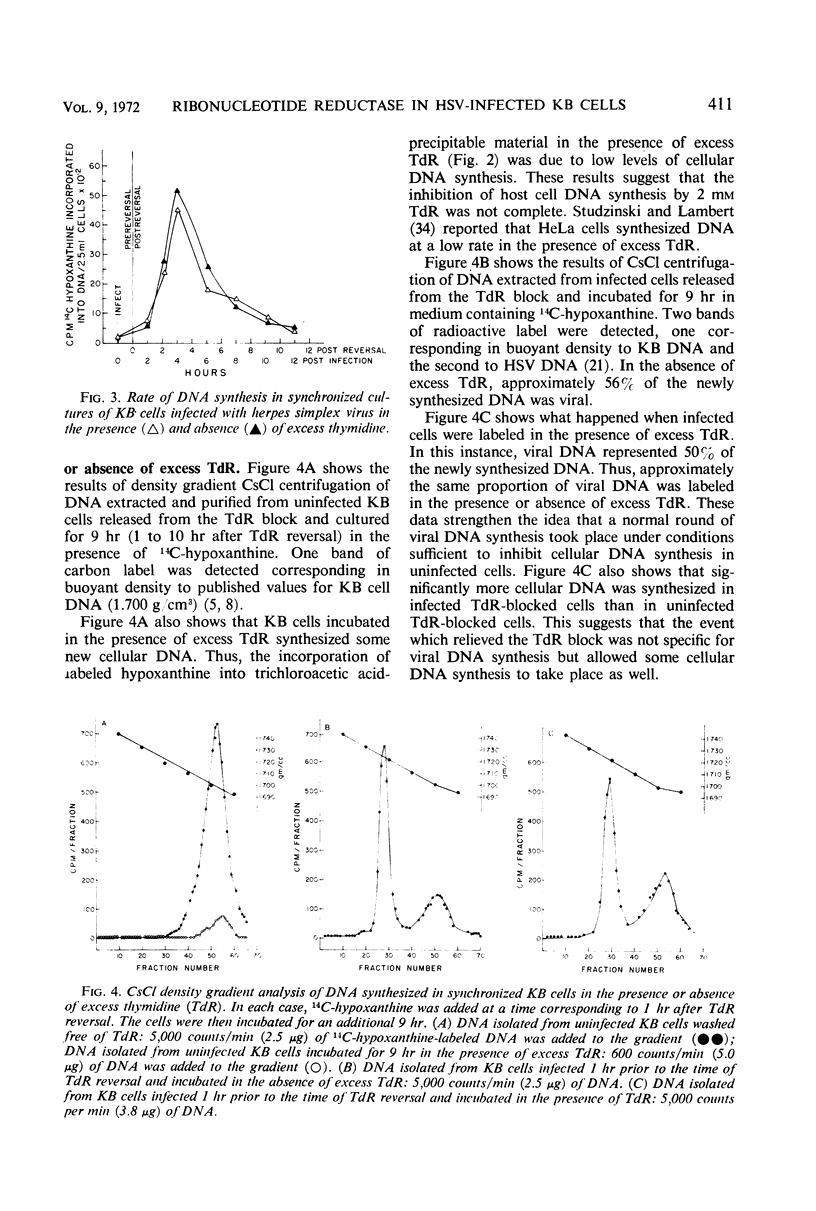
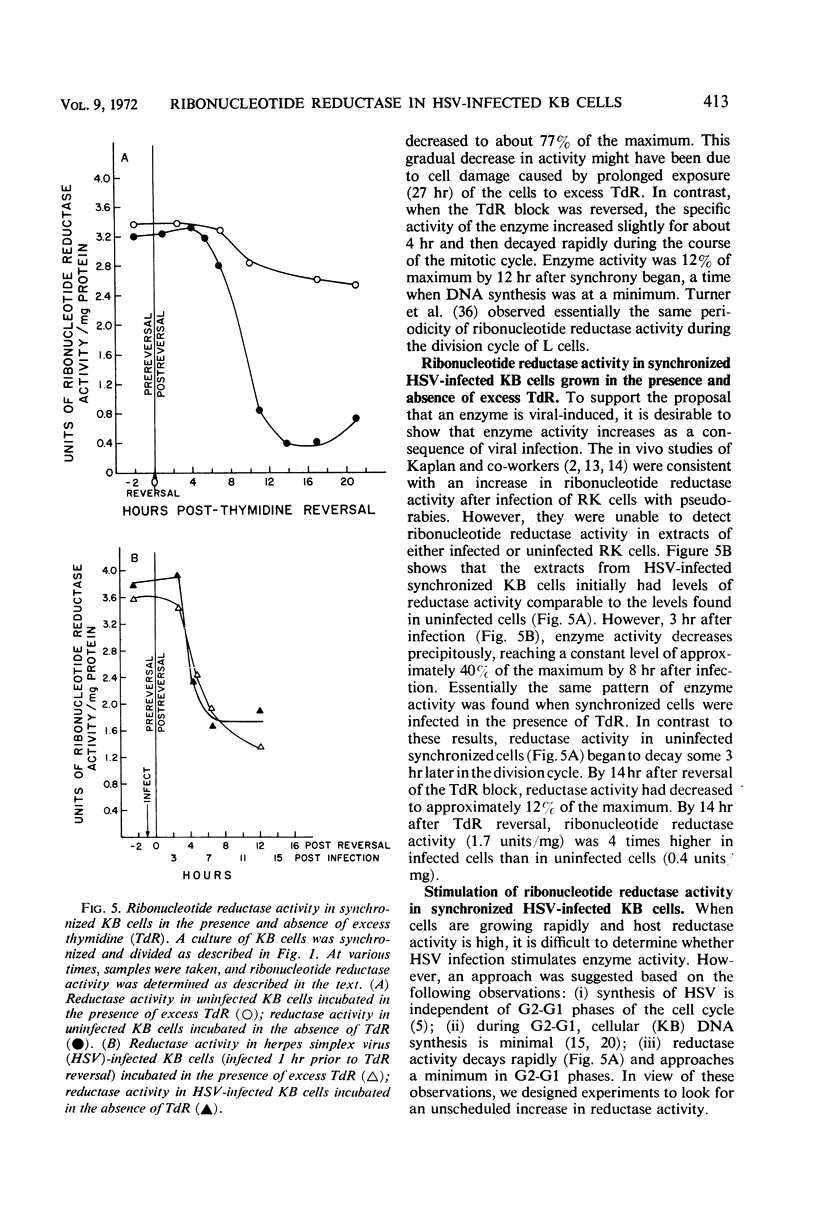
The replication of herpes simplex virus (HSV) is unimpeded in KB cells which have been blocked in their capacity to synthesize deoxyribonucleic acid (DNA) by high levels of thymidine (TdR). Studies showed that the presence of excess TdR did not prevent host or viral DNA replication in HSV-infected cells. In fact, more cellular DNA was synthesized in infected TdR-blocked cells than in uninfected TdR-blocked cells. This implies that the event which relieved the TdR block was not specific for viral DNA synthesis but allowed some cellular DNA synthesis to occur. These results suggested that HSV has a means to insure a pool of deoxycytidylate derivatives for DNA replication in the presence of excess TdR. We postulated that a viral-induced ribonucleotide reductase was present in the cell after infection which was not inhibited by thymidine triphosphate (TTP). Accordingly, comparable studies of the ribonucleotide reductase found in infected and uninfected KB cells were made. We established conditions that would permit the study of viral-induced enzymes in logarithmically growing KB cells. A twofold stimulation in reductase activity was observed by 3 hr after HSV-infection. Reductase activity in extracts taken from infected cells was less sensitive to inhibition by exogenous (TTP) than the enzyme activity present in uninfected cells. In fact, the enzyme extracted from infected cells functioned at 60% capacity even in the presence of 2 mm TTP. These results support the idea that a viral-induced ribonucleotide reductase is present after HSV infection of KB cells and that this enzyme is relatively insensitive to inhibition by exogenous TTP.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Bello L. J. Synthesis of DNA-like RNA in synchronized cultures of mammalian cells. Biochim Biophys Acta. 1968 Mar 18;157(1):8–15. doi: 10.1016/0005-2787(68)90258-x. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Brown M., Kaplan A. S. Effect of 1-beta-D-arabinofuranosylcytosine on DNA synthesis. II. In rabbit kidney cells infected with herpes viruses. Mol Pharmacol. 1968 Mar;4(2):139–146. [PubMed] [Google Scholar]
- Buchan A., Watson D. H., Dubbs D. R., Kit S. Serological study of a mutant of herpesvirus unable to stimulate thymidine kinase. J Virol. 1970 Jun;5(6):817–818. doi: 10.1128/jvi.5.6.817-818.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENTRY G. A., MORSE P. A., Jr, POTTER V. R. PYRIMIDINE METABOLISM IN TISSUE CULTURE CELLS DERIVED FROM RAT HEPATOMAS. 3. RELATIONSHIP OF THYMIDINE TO THE METABOLISM OF OTHER PYRIMIDINE NUCLEOSIDES IN SUSPENSION CULTURES DERIVED FROM THE NOVIKOFF HEPATOMA. Cancer Res. 1965 May;25:517–525. [PubMed] [Google Scholar]
- Groyon R. M., Kniazeff A. J. Vaccinia virus infection of synchronized pig kidney cells. J Virol. 1967 Dec;1(6):1255–1264. doi: 10.1128/jvi.1.6.1255-1264.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- KAPLAN A. S. STUDIES ON THE REPLICATING POOL OF VIRAL DNA IN CELLS INFECTED WITH PSEUDORABIES VIRUS. Virology. 1964 Sep;24:19–25. doi: 10.1016/0042-6822(64)90143-6. [DOI] [PubMed] [Google Scholar]
- KLENOW H. Further studies on the effect of deoxyadenosine on the accumulation of deoxyadenosine triphosphate and inhibition of deoxyribonucleic acid synthesis in Ehrlich ascites tumor cells in vitro. Biochim Biophys Acta. 1962 Dec 31;61:885–896. doi: 10.1016/0926-6550(62)90005-1. [DOI] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence W. C. Evidence for a relationship between equine abortion (herpes) virus deoxyribonucleic acid synthesis and the S phase of the KB cell mitotic cycle. J Virol. 1971 Jun;7(6):736–748. doi: 10.1128/jvi.7.6.736-748.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Nordenskjöld B. A., Reichard P., Skoog L. Thymidine phosphate pools and DNA synthesis after polyoma infection of mouse embryo cells. Cancer Res. 1969 Aug;29(8):1498–1506. [PubMed] [Google Scholar]
- MOORE E. C., HURLBERT R. B. Reduction of cytidine nucleotides to deoxycytidine nucleotides by mammalian enzymes. Biochim Biophys Acta. 1962 May 14;55:651–663. doi: 10.1016/0006-3002(62)90843-0. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Regulatory mechanisms in the synthesis of deoxyribonucleic acid in vitro. Biochim Biophys Acta. 1960 Jul 15;41:558–559. doi: 10.1016/0006-3002(60)90067-6. [DOI] [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Roizman B. The herpesviruses--a biochemical definition of the group. Curr Top Microbiol Immunol. 1969;49:3–79. [PubMed] [Google Scholar]
- Studzinski G. P., Lambert W. C. Thymidine as a synchronizing agent. I. Nucleic acid and protein formation in synchronous HeLa cultures treated with excess thymidine. J Cell Physiol. 1969 Apr;73(2):109–117. doi: 10.1002/jcp.1040730204. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Inhibition of Deoxyribonucleic Acid Synthesis in Synchronized Populations of L Cells Infected with Chlamydia psittaci. Infect Immun. 1971 Feb;3(2):363–364. doi: 10.1128/iai.3.2.363-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. K., Abrams R., Lieberman I. Levels of ribonucleotide reductase activity during the division cycle of the L cell. J Biol Chem. 1968 Jul 10;243(13):3725–3728. [PubMed] [Google Scholar]


