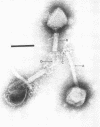
Abstract
Bacteriophage TSP-1 was isolated from soil in a search for phage which would form plaques on Bacillus subtilis W168 at 53 C. It forms clear plaques only at temperatures from 50 to 55 C. Approximately 95% of the free phage adsorb after 2 min at 53 C. The lytic cycle is between 55 and 60 min long with a burst size of about 55 particles per infected bacterium. The phage was shown to contain double-stranded deoxyribonucleic acid with a base composition of 44.7% guanine plus cytosine. This deoxyribonucleic acid does not contain a base analogue for thymine and has a molecular weight estimated at 56 × 106 daltons.
Full text
PDF

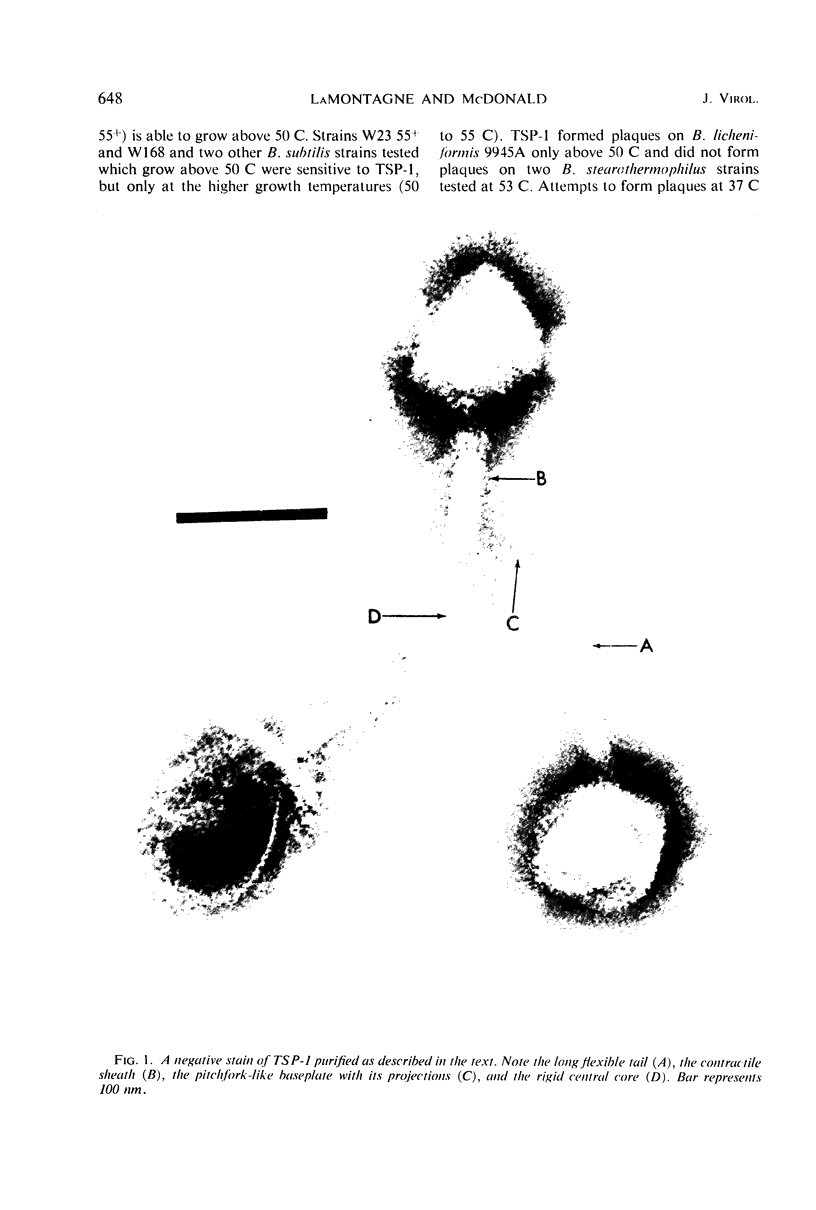
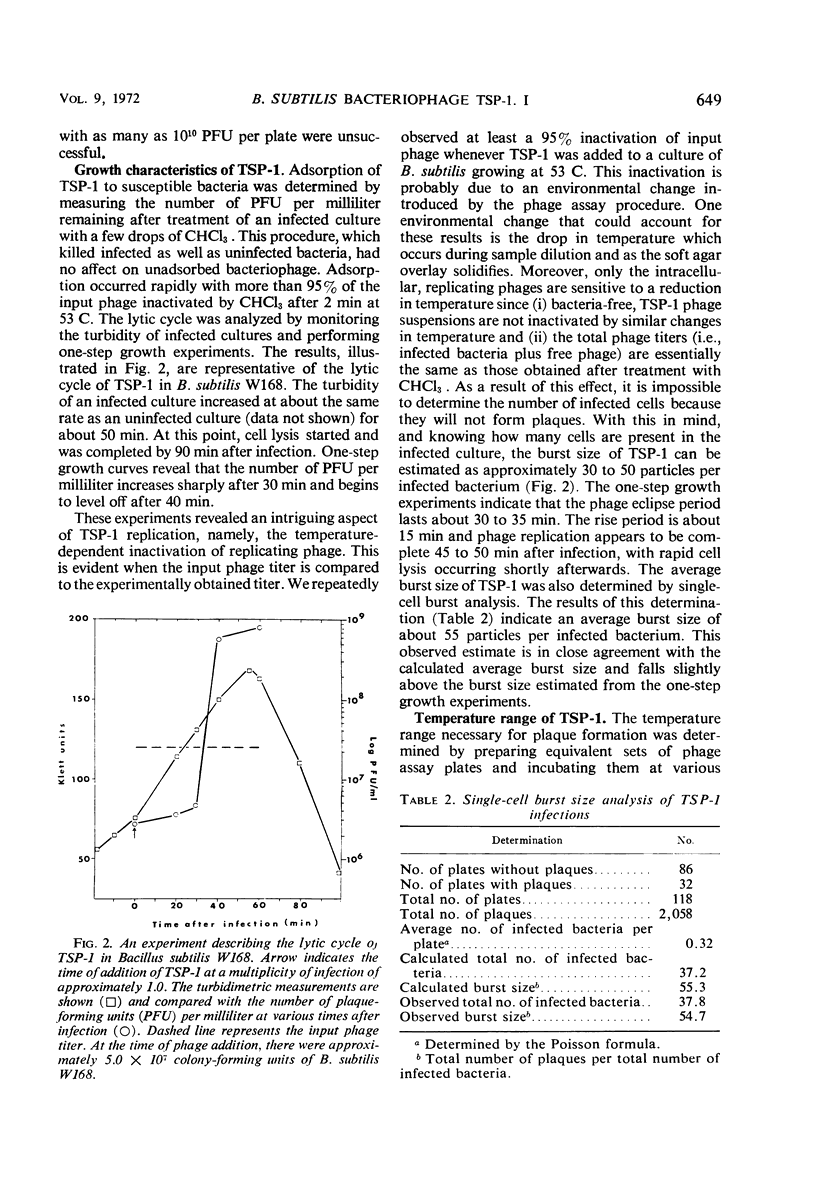


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON P. F. THE STRUCTURE OF BACTERIOPHAGE SP8. Virology. 1963 Oct;21:146–151. doi: 10.1016/0042-6822(63)90250-2. [DOI] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dul M. J., McDonald W. C. Morphological changes and antibiotic-induced thermal resistance in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 May;106(2):672–678. doi: 10.1128/jb.106.2.672-678.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISERLING F. A., BOYDELATOUR E. CAPSOMERES AND OTHER STRUCTURES OBSERVED ON SOME BACTERIOPHAGES. Pathol Microbiol (Basel) 1965;28:175–180. [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Hunter B. I., Yamagishi H., Takahashi I. Molecular weight of bacteriophage PBS 1 deoxyribonucleic acid. J Virol. 1967 Aug;1(4):841–842. doi: 10.1128/jvi.1.4.841-842.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- McDonald W. C., Matney T. S. GENETIC TRANSFER OF THE ABILITY TO GROW AT 55 C IN BACILLUS SUBTILIS. J Bacteriol. 1963 Jan;85(1):218–220. doi: 10.1128/jb.85.1.218-220.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBENSTEIN I., THOMAS C. A., Jr, HERSHEY A. D. The molecular weights of T2 bacteriophage DNA and its first and second breakage products. Proc Natl Acad Sci U S A. 1961 Aug;47:1113–1122. doi: 10.1073/pnas.47.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of a pyrimidine replacing thymine in bacteriophage DNA. Biochem Biophys Res Commun. 1964 Jun 1;16(2):106–110. doi: 10.1016/0006-291x(64)90344-4. [DOI] [PubMed] [Google Scholar]