Abstract
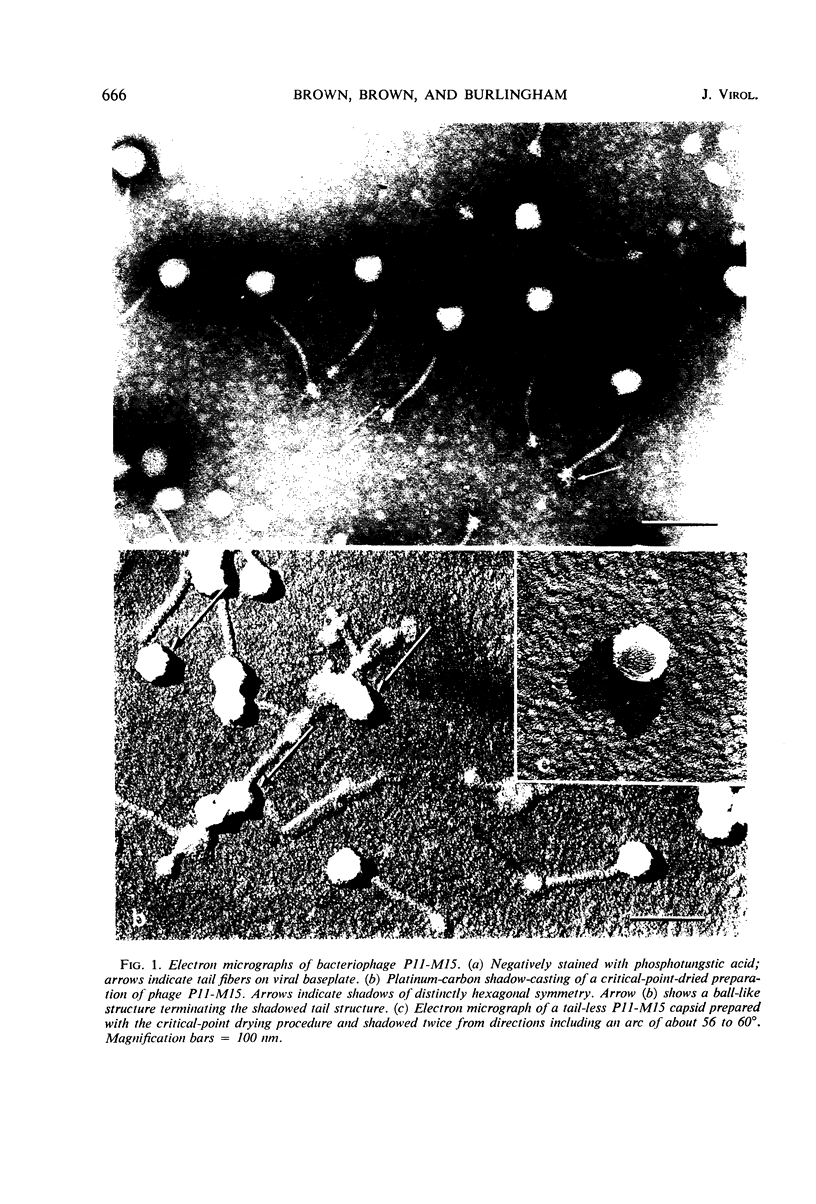
The Group B Staphylococcus phage P11-M15 is shown to be 51% protein and 49% deoxyribonucleic acid (DNA). The intact virion has a molecular weight of 66.7 × 106 daltons. The purified viral DNA has a molecular weight of 32.7 × 106 daltons. The intact virion is shown to be composed of a polyhedral head which is attached at one of its vertices to a flexible tail having helical symmetry. The tail structure is terminated by a complex baseplate which has sixfold symmetry. The virion contains a single molecule of double-stranded DNA which has no apparent single-strand nicks or single-stranded terminal redundancies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY D. E. The structure of some Staphylococcus and Pseudomonas phages. J Ultrastruct Res. 1963 Jun;8:552–565. doi: 10.1016/s0022-5320(63)80055-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Brown D. T., MacKenzie J. M., Bayer M. E. Mode of host cell penetration by bacteriophage phi X174. J Virol. 1971 Jun;7(6):836–846. doi: 10.1128/jvi.7.6.836-846.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. 6(p-Hydroxyphenylazo)-uracil: a reversible, selective inhibitor of the replication of deoxyribonucleic acid of staphylococcal bacteriophage P11-M15. J Virol. 1971 Nov;8(5):759–765. doi: 10.1128/jvi.8.5.759-765.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura R. K. Nucleotide analysis of deoxyribonucleic acid containing deoxybromouridylic acid. Anal Biochem. 1970 Jul;36(1):62–71. doi: 10.1016/0003-2697(70)90331-3. [DOI] [PubMed] [Google Scholar]
- Inman R. B. Some factors affecting electron microscopic length of deoxyribonucleic acid. J Mol Biol. 1967 Apr 28;25(2):209–216. doi: 10.1016/0022-2836(67)90138-6. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. II. Interaction of the protein with DNA in vitro. J Mol Biol. 1966 Nov 14;21(2):293–304. doi: 10.1016/0022-2836(66)90100-8. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM E. D., TYRONE S. SEROLOGY, DENSITY, AND MORPHOLOGY OF STAPHYLOCOCCAL PHAGES. J Bacteriol. 1964 Dec;88:1737–1742. doi: 10.1128/jb.88.6.1737-1742.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. C., SMITH K. M. The polyhedral form of the Tipula iridescent virus. Biochim Biophys Acta. 1958 Jun;28(3):464–469. doi: 10.1016/0006-3002(58)90507-9. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Blair J. E. Studies on polynucleotides. LXXI. Sedimentation and buoyant density studies of some DNA-like polymers with repeating nucleotide sequences. J Mol Biol. 1967 Jul 28;27(2):273–288. doi: 10.1016/0022-2836(67)90020-4. [DOI] [PubMed] [Google Scholar]