Abstract
The liver is a central organ involved in inflammatory processes, including the elaboration of acute-phase proteins. Augmenter of liver regeneration (ALR) protein, expressed and secreted by hepatocytes, promotes liver regeneration and maintains viability of hepatocytes. ALR also stimulates secretion of inflammatory cytokines (tumor necrosis factor [TNF]-α and interleukin [IL]-6) and nitric oxide from Kupffer cells. We hypothesized that ALR may be involved in modulating inflammation induced by various stimuli. We found that hepatic ALR levels are elevated at 24 h, before or about the same time as an increase in the mRNA expression of TNF-α and IL-6, after portacaval shunt surgery in rats. Serum ALR also increased, but significantly only on d 4 when pathological changes in the liver become apparent. In rats, serum ALR was elevated after intraperitoneal administration of lipopolysaccharide alone and in a model of gram-negative sepsis. Serum ALR increased before alanine aminotransferase (ALT) in endotoxemia and in the same general time frame as TNF-α and IL-6 in the bacterial sepsis model. Furthermore, mathematical prediction of tissue damage correlated strongly with alterations in serum ALR in a mouse model of hemorrhagic shock. In vitro, monomethyl sulfonate, TNF-α, actinomycin D and lipopolysaccharide all caused increased release of ALR from rat hepatocytes, which preceded the loss of cell viability and/or inhibition of DNA synthesis. ALR may thus serve as a potential diagnostic marker of hepatocellular stress and/or acute inflammatory conditions.
INTRODUCTION
Augmenter of liver regeneration (ALR) is a novel protein, which was originally identified in the cytosolic fractions of the regenerating or hyper-plastic liver (1). It was postulated that on liver injury (for example, portacaval shunt [PCS] surgery) or during liver regeneration (for example, after partial hepatectomy), ALR is synthesized and released by nonparenchymal cells, which then exerts actions on hepatocytes (1). However, subsequent research showed that ALR is present in equivalent amounts in the hepatocytes of unmodified adult rat liver and is not synthesized by nonparenchymal cells (2). These findings led to the hypothesis that ALR has intracellular physiologic functions. Indeed, depletion of ALR from hepatocytes on introduction of ALR antisense-oligonucleotide was found to cause progressive apoptosis and necrosis of the cells, which was accompanied by depletion of cellular ATP stores (3). ALR has been shown to function as protein sulfhydryl oxidase (4) and cytochrome c reductase (5,6) and to cause Fe/S maturation of proteins (7,8).
Although these observations indicate that intracellular ALR has a critical physiological role in hepatocytes, its constitutive secretion by hepatocytes and increased serum levels shortly after partial hepatectomy (2) suggested that ALR might have extracellular effects as well. A G-protein–coupled receptor for ALR was identified on Kupffer cells, stimulation of which modestly increased the expression and release of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and nitric oxide (NO) (via induction of inducible NO synthase) (9). These mediators are known to play an important role in both acute inflammation (10,11) and hepatic regeneration (12,13). High expression of hepatic ALR in liver cirrhosis and hepatocellular carcinoma (14) suggests that it may also play a role in excessive proliferation or survival of neoplastic hepatocytes. In this regard, the human recombinant short 15-kDa ALR was observed to induce DNA synthesis in human hepatocytes to a similar or greater magnitude compared with the potent hepatocyte mitogens, hepatocyte growth factor (HGF) and transforming growth factor (TGF)-α (15–17). Interestingly, transfection of HepG2 cells (a human hepatoma cell line) with 15 kDa ALR was found to reduce cellular motility; this finding, along with increased expression of the 15-kDa ALR (determined via reducing Western blot analysis) in hepatocellular carcinoma tissue without angioinvasion (18) suggest that the short ALR may have antimetastatic potential. We note that this form of ALR is found at a very low level, if at all, in the rat liver (8). Although binding sites for the 15-kDa human recombinant ALR are reported on rat hepatocytes, the same could not be observed for the 22-kDa recombinant rat ALR (2), and this form does not stimulate DNA synthesis in rat hepatocytes (2,10) despite augmenting hepatocyte proliferation after partial hepatectomy in rats (10). Thus, it is apparent that ALR has both intracellular (hepatocytes) and extracellular (via receptors on nonparenchymal cells or hepatocytes) functions that may be critical in liver physiology and pathology.
On the basis of these observations, we hypothesized that circulating ALR levels might increase on hepatic injury of various types and that circulating ALR could be either a novel cytokine or an alarm signal (damage-associated molecular pattern [DAMP], in the setting of ongoing liver damage). We found that serum ALR levels increase in murine models of PCS, endotoxemia, sepsis and hemorrhagic shock, all before or in a similar time frame as the increase in inflammatory mediators or alanine aminotransferase (ALT). We also found that ALR secretion by cultured hepatocytes increases significantly on induction of various types of cellular stress or injury. These properties suggest that ALR is a novel inflammatory mediator or DAMP, given that DAMPs prototypically are produced in settings of injury, inflammation or stress and in turn promote further inflammation via induction of classic proinflammatory cytokines (19).
MATERIALS AND METHODS
Portacaval Shunt in Rats
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh in accordance with National Institutes of Health (NIH) guidelines. Under methoxyflurane anesthesia, laparotomy was performed in preweighed Lewis rats (200–250 g; Harlan Laboratories, Frederick, MD, USA), and hepatoesophageal plexus was ligated. Side-to-side anastomosis was created between the portal vein and inferior vena cava using a 10-0 Novafil suture as described previously (20). After the shunt was examined to ensure its patency, the portal vein was carefully separated from hepatic artery and ligated at the hepatic hilum to create total PCS. At the indicated times, blood was drawn and the liver was snap frozen in liquid nitrogen for various analyses.
Lipopolysaccharide Treatment of Rats
Lipopolysaccharide (LPS) (Escherichia coli lipopolysaccharide serotype 0111:B4; Sigma, St. Louis, MO, USA) (10 mg/kg) was administered intraperitoneally to male Sprague Dawley rats (200–250 g; Harlan Laboratories) to induce endotoxemia and liver injury (21–23). At the indicated times, blood was drawn and the liver was snap frozen in liquid nitrogen for various analyses.
Fibrin Clot plus E. coli–Induced Sepsis in Rats
Male Sprague Dawley rats (430–480 g; Harlan Laboratories) were anesthetized with sodium pentobarbital (40 mg/kg intraperitoneally). An E. coli (strain ATCC 25922; American Type Culture Collection, Manassas, VA, USA) inoculum in a fibrin clot (1.5 × 108 to 2 × 108 colony-forming unit [CFU]/clot) was introduced into the peritoneum via laparotomy essentially as described by Ahrenholz and Simmons (24). The rats were sacrificed at the indicated times, and blood and liver were acquired.
Experimental Trauma/Hemorrhage in Mice and Mathematical Model of Inflammation
Experimental trauma/hemorrhage and subsequent mechanistic mathematical modeling was carried out as described previously (25). In brief, C57BL/6 mice (25–40 g; Charles River Laboratories, Charles River, ME, USA) were anesthetized, and both femoral arteries were surgically prepared and cannulated. The mice were then subjected to withdrawal of blood with the mean arterial pressure maintained at 25 mmHg for 2.5 h with continuous monitoring of blood pressure. The mice were resuscitated over 10 min with their remaining shed blood plus two times the maximal shed blood amount in lactated Ringer solution via the arterial catheter. By using data from mice subjected to hemorrhagic shock as well as other mice subjected to surgical cannulation trauma or to bolus intraperitoneal injection of LPS (data not shown), we constructed a mathematical model of acute inflammation that consists of 15 ordinary differential equations that describe the time course of multiple inflammatory components. Included in the model equations is a systemic variable that represents global tissue dysfunction and damage, which induces further inflammation and thus can be considered a surrogate for DAMPs, as well as a surrogate for the overall health of the animal (25).
Assay of ALR and Inflammatory Cytokines
ALR in serum or the liver tissue was measured by enzyme-linked immunosorbent assay (ELISA) as described previously (2). Inflammatory cytokines were assayed by using species-specific ELISA kits (R&D Systems, Minneapolis, MN, USA). NO reaction products (NO2−/NO3−) were assayed by using the nitrate reductase method (Cayman Chemical, Ann Arbor, MI, USA).
Reverse Transcriptase–Polymerase Chain Reaction
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed to determine the messenger RNA (mRNA) expression of TNF-α and IL-6 as previously described (26,27). β-Actin mRNA expression was determined for comparison. The PCR primers specific for various complementary DNAs were as follows: TNF-α, CACGC TCTTC TGTCT ACTGA (forward) and GGACT CCGTG ATGTC TAAGT (reverse) (543 bp); IL-6, GAAAG TCAAC TCCAT CTGCC (forward) and CATAG CACAC TAGGT TTGCC (reverse) (681 bp); and β-actin, TTCTA CAATG AGCTG CGTGT G (forward) and TTCAT GGATG CCACA GGATT C (reverse) (561 bp). The PCR products were resolved in a 1.5% agarose gel and stained with 1× SYBR Green I (FMC Bioproducts, Rockland, ME, USA). The gels were scanned under blue fluorescence light by using a Phosphorimager™, and the band intensity was quantified by using ImageQuant® software (Molecular Dynamics, Sunnyvale, CA, USA).
Isolation and Culture of Hepatocytes
Hepatocytes, prepared by collagenase digestion of the liver and purified on a Percoll™ gradient were plated at a density of 0.0725 × 106 cells/cm2 in William’s medium E containing 2 mmol/L L-glutamine, 10−6 mol/L insulin, 10% fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin (26,28). The medium was renewed after a 3-h attachment period, and the cells were used after overnight incubation. Before beginning the experiment, cells in some wells were trypsinized and counted considering the intercell preparation attachment to normalize the data.
Determination of DNA Synthesis
Cells were incubated in serum-free medium containing 0.1% bovine serum albumin and 1 μCi/mL [3H]thymidine (3.18 terabequerel [TBq]/mmol; Amersham-Pharmacia, Piscataway, NJ, USA) for 4 h at 37°C and were then washed with ice-cold phosphate-buffered saline, treated with ice-cold 10% trichloroacetic acid (TCA) for 10 min, and washed once with TCA followed by 95% ethanol. Cells were then digested with 5% sodium dodecyl sulfate for determination of radioactivity.
Determination of Viability
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay (29). Lactate dehydrogenase (LDH) was measured by using a spectrophotometric assay kit (Stanbeo Laboratory, Boerne, TX, USA).
Statistical Analysis
For in vivo experiments, four to six animals were used for each time point. In vitro experiments were performed at least three times, each time in triplicate, by using cells from different animals. Results are expressed as means ± standard error of the mean (SEM). Statistical significance between groups was determined by the Student t test by using a Prism 5 Software package (GraphPad Software, GraphPad, San Diego, CA, USA) or by Kruskal-Wallis one-way analysis of variance on Ranks, followed by the Dunn method post hoc test. A p value <0.05 was considered statistically significant.
RESULTS
Hepatic and Serum ALR and Hepatic mRNA Expression of TNF-α and IL-6 in PCS
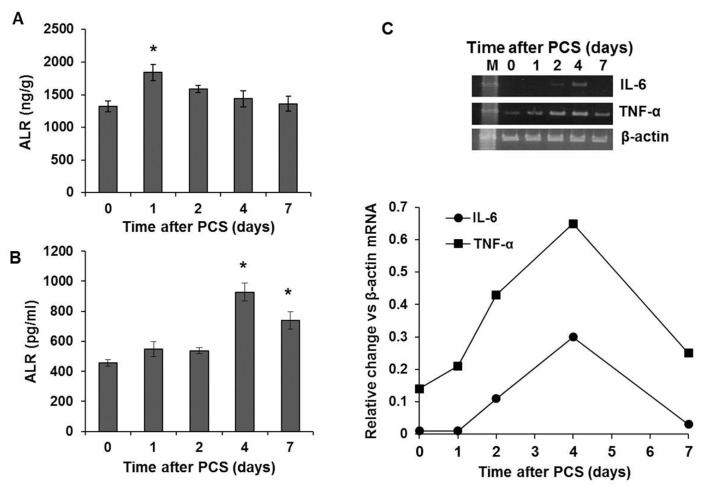
Hepatic ALR increased modestly but significantly at 24 h after PCS, but declined progressively to the basal value subsequently (Figure 1A). Interestingly, serum ALR increased significantly on d 4 and declined by d 7 after PCS, but was still significantly greater than the basal value (Figure 1B). We sought to correlate the expression of classic inflammatory mediators in the same animals and found that the mRNA expression both of IL-6 and TNF-α increased markedly on d 2 after PCS; the slight increase in TNF-α mRNA expression was also observed on d 1 after PCS (Figure 1C).
Figure 1.
Hepatic and serum ALR and mRNA expression of inflammatory cytokines after PCS. Lewis rats (five per time point) were subjected to PCS, and at indicated time points, hepatic (A) and serum (B) ALR protein levels were determined by ELISA. *p < 0.05 versus 0 time. (C) mRNA expression of IL-6 and TNF-α as determined by semiquantitative RT-PCR. Line graph shows expression of the respective cytokine mRNA versus that of β-actin (average of two independent determinations).
LPS Treatment Induces Release of ALR from the Liver
Shortly after LPS administration (10 mg/kg i.p.) in rats, hepatic ALR levels decreased (1 h) with significant reduction at 3 h (Figure 2A). ALR then rose to near-normal level after 6 h. The decrease in hepatic ALR corresponded to an increase in serum ALR, which was significant at 1 h, peaked at 3 h and remained elevated at 24 h. Serum ALT increased significantly at 3 h and remained elevated until 24 h after LPS administration (Figure 2C).
Figure 2.
Hepatic ALR release and injury after induction of endotoxemia. Sprague Dawley rats (four to five per time point) were administered LPS (10 mg/kg i.p.), and at indicated times, hepatic (A) and serum (B) ALR as well as serum ALT (C) were measured. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.001 versus control; ¶p < 0.025 versus 1-h LPS; ¶¶p < 0.01 versus 3-h LPS.
Serum ALR Increases on Induction of Sepsis
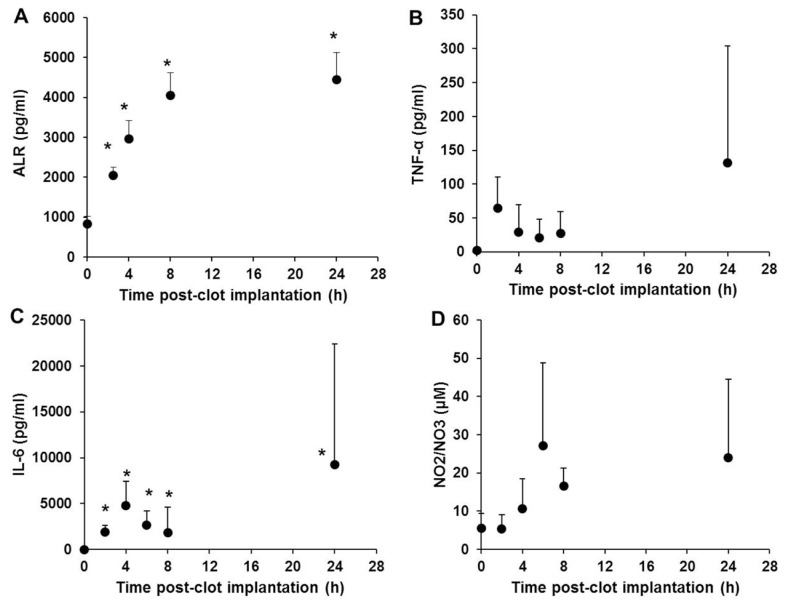
In a rat model of sepsis (implantation of E. coli–containing fibrin clot), serum ALR increased rapidly and significantly within 2 h, peaked at approximately 8 h and remained elevated at 24 h (Figure 3A). The levels of the inflammatory mediators TNF-α, IL-6 and NO (measured as NO2− + NO3−) also increased as early as 2–6 h (Figures 3B–D), with secondary (but variable) peaks observed at 24 h.
Figure 3.
ALR is elevated early and persistently in a gram-negative sepsis (peritoneal implantation of E. coli–impregnated fibrin clot). Sprague Dawley rats (five per time point) were subjected to surgical implantation of a fibrin clot containing E. coli. At the indicated time points, plasma ALR (A), TNF-α (B), IL-6 (C) and the NO reaction products NO2−/NO3− (D) were measured as described in Materials and Methods. *p < 0.05 versus 0 time.
Serum ALR Increases in a Mouse Model of Hemorrhagic Shock and Approximates Mathematically Predicted Damage/Dysfunction
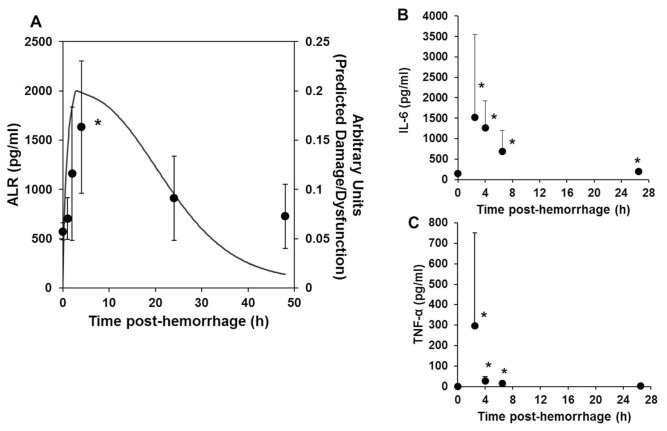
In mice, serum ALR increased within 1 h of induction of hemorrhagic shock, peaked at approximately 4 h and declined thereafter (Figure 4A). IL-6 and TNF-α peaked at ~2.5 h after hemorrhage and decreased rapidly thereafter (Figures 4B, C). We previously described a mechanistic mathematical model of acute inflammation in mice that incorporates a variable (“damage/dysfunction”) that roughly corresponds to the release of DAMPs, since this variable activates inflammatory cells to produce classic proinflammatory cytokines (25). The changes in ALR were found to correlate closely with the damage/dysfunction, as predicted by this mathematical model (Figure 4A).
Figure 4.
ALR is elevated in a mouse model of hemorrhagic shock, followed by resuscitation. C57BL/6 mice (three to four per time point) were subjected to hemorrhagic shock, followed by resuscitation for the indicated time points as described in Materials and Methods. The mice were euthanized by exsanguination under excess inhalation anesthesia and plasma obtained for measurement of ALR. (A) The line indicates predictions of “damage/dysfunction” induced by inflammatory stimuli after hemorrhagic shock, by using a mathematical model of acute inflammation (25). The time course of changes in serum ALR (shown in symbols) closely approximates that predicted for damage/dysfunction in the mathematical model. The damage/dysfunction variable in this model represents “alarm/danger” signals. (B, C) IL-6 and TNF-α in serum at the times indicated after hemorrhagic shock and resuscitation. *p < 0.05 versus 0 time control.
ALR Release by Hepatocytes in Response to Injury-Inducing Agents
Because serum ALR levels increase in all of the liver injury models described above and ALR is synthesized by hepatocytes (2) and biliary epithelial cells (14) in the liver, and hepatocytes have also been shown to secrete ALR constitutively (2), we examined the release of ALR from rat hepatocytes treated with methyl methanesulfonate (MMS), TNF-α, actinomycin D or LPS, the agents that may damage hepatocytes. We used LDH release, MTT assay and/or DNA synthesis ([3H]thymidine incorporation) assay to assess hepatocytes stress/injury. Hepatocytes challenged with the DNA-damaging agent MMS (30,31) secreted significantly greater amounts of ALR within 1 h compared with control hepatocytes (Figure 5A). Interestingly, significantly greater LDH release was observed at a later time (3 h) in MMS-challenged hepatocytes (Figure 5B). In the subsequent period, ALR and LDH release increased progressively in MMS-challenged hepatocytes. Hepatocyte viability reduced very strongly at 6 h (Figure 5C), after increased release of ALR and then LDH (Figures 5A, B). Over a 24-h time period, control hepatocytes secreted ALR at a steady rate without marked changes in LDH release or cell viability (Figure 5).
Figure 5.
ALR secretion increases before LDH release from hepatocytes challenged with DNA damaging agent MMS. Cultured hepatocytes were incubated in the presence of 500 μmol/L MMS for the indicated time points, at which point ALR (A) and LDH (B) release, and cell viability (C), were determined. Results shown are means of triplicate determinations ± SEM from a representative of three separate experiments. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.001 versus control.
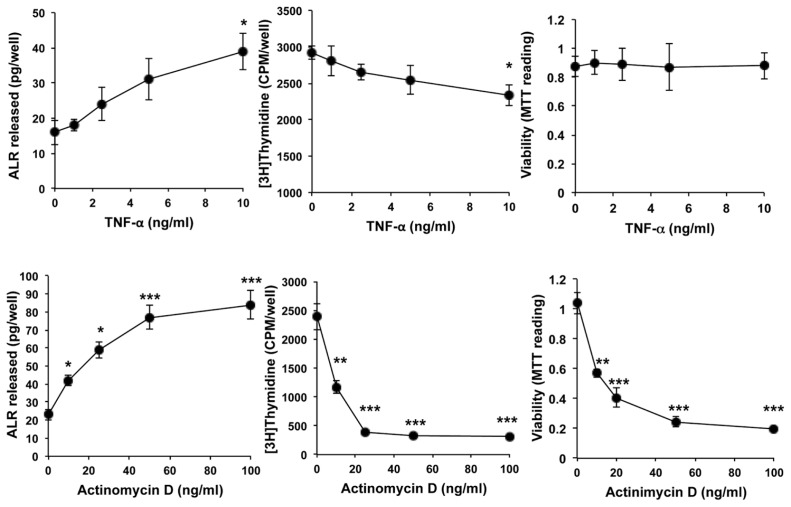
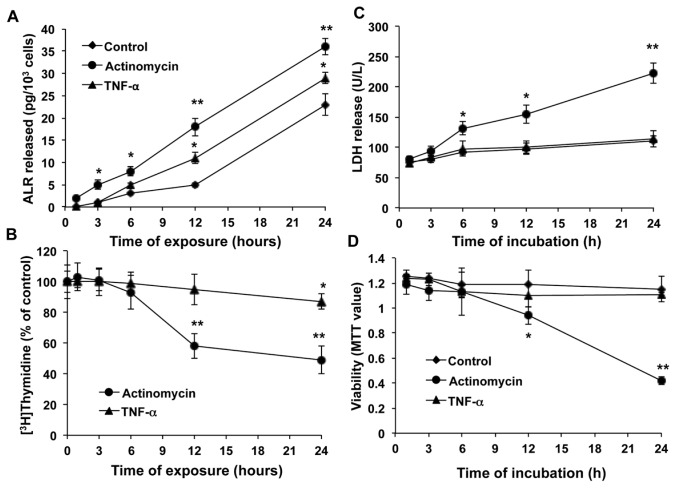
We next examined the effects of TNF-α and actinomycin D on the ALR release and DNA synthesis in rat hepatocytes. Both TNF-α and actinomycin D induced a concentration-dependent increase in ALR secretion from hepatocytes (Figure 6, left panels). However, the decrease in DNA synthesis by TNF-α was of much smaller magnitude than that induced by actinomycin D (Figure 6, middle panels). These results reflect no significant loss of viability of TNF-α–challenged hepatocytes, but loss of viability of actinomycin D–challenged hepatocytes paralleled the decrease in [3H]thymidine incorporation (Figure 6, right panels). Next, we used fixed concentrations of TNF-α (10 ng/mL) and actinomycin D (10 ng/mL) in a time-course experiment. A significantly greater increase in the secretion of ALR was observed after 12 h from hepatocytes challenged with TNF-α and after 3 h with actinomycin D (Figure 7A), earlier than the decrease in the DNA synthesis (24 and 12 h for TNF-α and actinomycin D, respectively) (Figure 7B), increase in LDH release by actinomycin D at 6 h (Figure 7C) or loss of viability with actinomycin D at 12 h (Figure 7D). We noted that TNF-α did not cause LDH release to levels greater than those observed in control-treated cells and also did not affect hepatocyte viability (Figures 7C, D).
Figure 6.
Concentration-dependent effects of TNF-α and actinomycin D on ALR secretion, DNA synthesis and viability of hepatocytes. Cultured hepatocytes were challenged with indicated concentrations of TNF-α or actinomycin D. The media were aspirated at 24 h for measurement of ALR release (left panels), and DNA synthesis (middle panels) and viability (right panels) of the cells were measured. Results shown are means of triplicate determinations ± SEM from a representative of three separate experiments. *p < 0.05 versus 0; **p < 0.01 versus 0; ***p < 0.001 versus 0.
Figure 7.
Time course of TNF-α– and actinomycin D–induced ALR secretion, DNA synthesis, LDH release and viability of hepatocytes. Hepatocytes were stimulated with 10 ng/mL TNF-α or actinomycin D for indicated time periods, after which the media were assessed for ALR concentration (A) and LDH release (C) and the cells for DNA synthesis (B) and viability (D). Results shown are means of triplicate determinations ± SEM from a representative of three separate experiments. *p < 0.05 versus 1 h (A, C, D) or 0 (B); **p < 0.01 versus 1 h (A, C, D) or 0 (B).
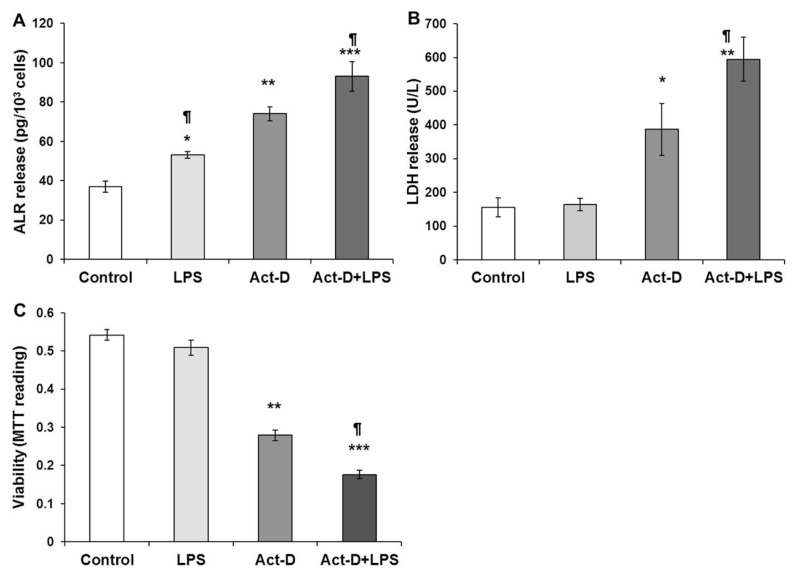
We have previously observed that LPS (up to 1 μg/mL) does not cause significant change in DNA synthesis in hepatocytes (26,28). However, LPS induces significant stress in hepatocytes that primes them to injury by other agents such as acetaminophen (32). Similar to TNF-α, challenging rat hepatocytes with 100 ng/mL LPS caused modestly increased secretion of ALR (Figure 8A) without any effect on LDH release (Figure 8B) or viability (Figure 8C). Actinomycin D (10 ng/mL) induced significantly greater release of ALR compared with LPS, and together, these agents caused greater release of ALR (Figure 8A). Furthermore, the magnitude of the actinomycin D– induced LDH release or loss of viability increased further in the presence of LPS (Figures 8B, C). Collectively, these data (Figures 5–8) suggest that increased ALR secretion by hepatocytes could be a stress alarm for their vulnerability to noxious stimuli.
Figure 8.
LPS augments actinomycin D–induced release of ALR additively and promotes loss of viability of hepatocytes. Cultured hepatocytes were challenged with 100 ng/mL LPS and/or 10 ng/mL actinomycin D (Act-D). The media were aspirated at 24 h for ALR (A) and LDH (B) measurements, and cell viability was determined via MTT assay (C). Results shown are means of triplicate determinations ± SEM from a representative of three separate experiments. *p < 0.05 versus control; **p < 0.01 versus control; ***p < 0.001 versus control; ¶p < 0.05 versus Act-D.
DISCUSSION
ALR is an enigmatic molecule that was originally found to augment partial hepatectomy–induced liver regeneration and to prevent PCS-induced hepatic pathology in animal models (1). Remarkable homology between ALR and ERV1 (33,34), a protein expressed by Saccharomyces cerevisiae that is essential for the survival of the yeast, and the abundance of ALR in normal quiescent hepatocytes (2) indicated that ALR may have physiological functions. Indeed, depletion of ALR from hepatocytes resulted in their apoptotic/necrotic death (3). Although ALR is secreted by hepatocytes constitutively, its rapid increase in serum after partial hepatectomy (2) suggested that increase in circulating ALR may be an indicator of hepatocyte stress/injury. In the present study, we used in vivo and in vitro models of liver or hepatocyte injury to examine whether increased ALR levels could be an alarm for ongoing hepatocyte stress/injury.
PCS that causes hepatocellular atrophy is accompanied by disruption of the rough endoplasmic reticulum and mitochondria due to diversion of the nutrient- and hormone-rich blood from the liver to systemic circulation (35,36). In rats, we found that PCS-induced liver atrophy and hepatocyte apoptosis is significant on d 4 after operation (20), which is consistent with the peak of pathological changes observed in dogs (35,36). Notably, serum ALR increased on d 4 after PCS. These pathological changes occur despite an immediate increase in hepatic levels of ALR as well as the mitogens HGF and TGF-α (20).
We sought to extend these findings and to demonstrate that the roles of ALR in inflammation are not restricted to a single species or a single form of acute inflammatory challenge, but rather that ALR production or release during inflammation is a general phenomenon. Hence, we used both mice and rats subjected to diverse inflammation paradigms. We also sought to leverage in silico modeling to better help define the role, if any, of ALR in acute inflammation. We found that the mRNA expression of inflammatory cytokines IL-6 and TNF-α, which are known to prime hepatic regeneration (12), were increased in approximately the same time frame as or after an increase in hepatic ALR. These results suggest that hepatic ALR may be induced along with Kupffer cell IL-6 and TNF-α after PCS (9) and that these mediators then function to limit injury and promote liver regeneration in conjunction with HGF and TGF-α. Indeed, there was no further decrease in the liver size or liver–to–body weight ratio up to 60 d after the initial atrophy up to 7 d after PCS (20). However, hepatocyte apoptosis peaked at 10 d and declined by 30 d but stabilized thereafter (20). Hepatic regeneration is a complex phenomenon involving induction of several proto-oncogenes, growth mediators and cytokines, including IL-6. Whereas we found that ALR stimulates IL-6 synthesis in Kupffer cells (9), stimulation of Foxa2 (HNF-3β)-transfected HepG2 hepatoma cells with IL-6 was reported to activate the ALR promoter (37), suggesting that cross-talk between IL-6 and ALR may have important implications in hepatic regeneration. Taken together, these findings suggest that the hepatic system attains equilibrium after PCS, establishing a balance between cell death and regeneration, with a plausible significant role of ALR in the latter.
Low-level hepatocyte apoptosis and modest increases in serum ALT levels occur in the established rat model of endotoxemia (38). Here, we found a rapid decrease in hepatic ALR with a concomitant increase in serum ALR on LPS administration. Interestingly, whereas hepatic ALR tended to normalize, serum ALR was still significantly elevated versus the basal level, even at 24 h. It is noteworthy that serum ALR increased before an increase in ALT, suggesting that increased ALR may be considered an indicator of ongoing hepatocyte stress/injury. In this regard, circulating ALR levels increase in patients with acute and fluminant hepatitis (39).
Induction of gram-negative bacterial sepsis by implantation of a fibrin clot–containing E. coli was the third model of liver injury in which we determined ALR levels at various times. In this experimental model, increases in serum ALR were observed early and remained elevated, even at 24 h. The levels of inflammatory cytokines and NO were elevated at approximately the same time (IL-6) or later (TNF-α and NO) than ALR, in a manner consistent with known differences between true gram-negative sepsis and endotoxemia (40). Our data suggest that serum ALR is an early and reliable indicator of inflammation and liver damage in the setting of sepsis, although these findings are yet to be validated in clinical studies.
Finally, we used a mouse model of hemorrhagic shock to examine whether ALR could be an indicator of the severity of organ damage in shock and also to see whether the dynamics of circulating ALR could match those of any of the variables included in a previously published mathematical model of trauma/hemorrhage-induced acute inflammation in mice (25,41,42). In our experimental sepsis model, as in the other experimental models we used, circulating ALR levels increased rapidly but returned to the basal value after peaking at approximately 4 h. We hypothesize that this result might be an effect of resuscitation that followed hemorrhagic shock. Application of our mathematical model of predicted organ damage after experimental trauma/hemorrhage (25,41,42) demonstrated a strong correlation of circulating ALR with the predicted dynamics of shock-induced organ damage, which we have previously suggested correlates with the release of DAMPs as well as acting as a proxy for the health status of the animal (25,41,42). This same mathematical model can account for quite different dynamics of classic inflammatory cytokines such as TNF-α, IL-6 and IL-10 (25,41,42); these cytokines are produced in the same general time frame as DAMPs such as high mobility group protein B1 (HMGB1) in the setting of trauma/hemorrhage (43,44).
These observations suggest that ALR may be a DAMP, a cytokine or a biomarker of inflammatory stress. Cytokines act via specific cytokine receptors that are generally coupled with serine/threonine or tyrosine kinases (45,46), whereas DAMPs often act via receptors that also sense pathogen-derived products (for example, toll-like receptors and nucleotide oligomerization domain [NOD]-like receptors) (19) that are often coupled with the same second-messenger signaling cascades as cytokine receptors. In this regard, a G-protein–coupled receptor for ALR was identified on Kupffer cells, stimulation of which modestly increased expression and release of TNF-α, IL-6 and NO (9). In addition, initial reports suggested that DAMPs were exclusively late mediators in settings such endotoxemia and sepsis (19,47,48), but, as mentioned above, more recent studies in trauma/hemorrhage have shown that DAMPs peak within ~2 h after injury, a time when classic cytokines are secreted as well (43,44). Thus, some ambiguity remains regarding the exact classification of ALR as a cytokine or a DAMP, and future studies may need to focus on the receptor for ALR to resolve this question.
Although ALR is expressed ubiquitously, its expression is much greater in the liver than in other organs except the testes (34,49). This observation suggests that the liver is the primary organ that contributes to the circulating ALR levels. It remains to be determined whether the liver is the only source or other organs contribute to increased serum ALR in the conditions studied herein. However, our in vitro experiments showed that LPS and TNF-α, which do not affect hepatocyte viability, induced greater release of ALR from hepatocytes than the unstimulated cells. These data suggest that even a minor stress can manifest into increased ALR release, which would indicate increased sensitivity of hepatocytes to injury by other stimuli. In this context, LPS treatment conditions hepatocytes to undergo necrosis when challenged in vivo with otherwise innocuous doses of acetaminophen (32). Indeed, in the present study, we found that actinomycin D–induced hepatocyte injury is strongly augmented in the presence of LPS, which by itself did not affect hepatocyte viability. Because both these agents induced modest release of ALR from cultured hepatocytes, their effect being additive when added together, it can be rationalized that hepatocytes under stress increase their ALR release, which is augmented on injury/damage. This proposal is supported by the observation that the DNA-damaging agent MMS-induced increase in ALR secretion preceded an increase in the LDH release from and loss of viability of cultured hepatocytes. This characteristic of ALR—the release under submaximal stress—is similar to the behavior of other DAMPs such as HMGB1 (19).
CONCLUSION
Our results have potential clinical implications, considering the need to identify a diagnostic biomarker that can help predict ongoing stress, which, if untreated in a timely manner, may develop into progressive and possibly irreversible pathology. The propensity of hepatocytes to release ALR when stressed or damaged provides a strong rationale to consider ALR as a novel diagnostic biomarker for hepatic stress and injury.
ACKNOWLEDGMENTS
This work was supported by VA Merit Review 1I1BX001174 and NIH grants R01-DK54411 and R21AA020846 (CR Gandhi). We thank Adam Kichler for technical assistance.
The content of this article does not represent the views of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
Y Vodovotz is cofounder and stakeholder in Immunetrics Inc.
REFERENCES
- 1.Francavilla A, et al. Augmenter of liver regeneration: its place in the universe of hepatic growth factors. Hepatology. 1994;20:747–57. [PubMed] [Google Scholar]
- 2.Gandhi CR, et al. A fresh look at augmenter of liver regeneration (ALR) in rats. Hepatology. 1999;29:1435–45. doi: 10.1002/hep.510290522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirunavukkarasu C, et al. Augmenter of liver regeneration: an important intracellular survival factor for hepatocytes. J Hepatol. 2008;48:578–88. doi: 10.1016/j.jhep.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisowsky T, Lee JE, Polimeno L, Francavilla A, Hofhaus G. Mammalian augmenter of liver regeneration protein is a sulfhydryl oxidase. Dig Liver Dis. 2001;33:173–80. doi: 10.1016/s1590-8658(01)80074-8. [DOI] [PubMed] [Google Scholar]
- 5.Farrell SR, Thorpe C. Augmenter of liver regeneration: a flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemistry. 2005;44:1532–41. doi: 10.1021/bi0479555. [DOI] [PubMed] [Google Scholar]
- 6.Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K. Erv1 mediates the Mia40- dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol. 2005;353:937–44. doi: 10.1016/j.jmb.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 7.Lange H, et al. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep. 2001;2:715–20. doi: 10.1093/embo-reports/kve161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klissenbauer M, Winters S, Heinlein UA, Lisowsky T. Accumulation of the mitochondrial form of the sulphydryl oxidase Erv1p/Alrp during the early stages of spermatogenesis. J Exp Biol. 2002;205:1979–86. doi: 10.1242/jeb.205.14.1979. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi CR, Murase N, Starzl TE. Cholera toxin-sensitive GTP-binding protein-coupled activation of augmenter of liver regeneration (ALR) receptor and its function in rat Kupffer cells. J Cell Physiol. 2010;222:365–73. doi: 10.1002/jcp.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peitzman AB, et al. Hemorrhagic shock. Curr Probl Surg. 1995;32:925–1002. doi: 10.1016/s0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- 11.Ring A, Stremmel W. The hepatic microvascular responses to sepsis. Semin Thromb Hemost. 2000;26:589–94. doi: 10.1055/s-2000-13215. [DOI] [PubMed] [Google Scholar]
- 12.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 13.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thasler WE, et al. Expression of augmenter of liver regeneration (ALR) in human liver cirrhosis and carcinoma. Histopathology. 2005;47:57–66. doi: 10.1111/j.1365-2559.2005.02172.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, et al. Identification and characterization of receptor for mammalian hepatopoietin that is homologous to yeast ERV1. J Biol Chem. 1999;274:11469–72. doi: 10.1074/jbc.274.17.11469. [DOI] [PubMed] [Google Scholar]
- 16.Dayoub R, et al. Regulation of polyamine synthesis in human hepatocytes by hepatotrophic factor augmenter of liver regeneration. Biochem Biophys Res Commun. 2006;345:181–7. doi: 10.1016/j.bbrc.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Thasler WE, et al. Repression of cytochrome P450 activity in human hepatocytes in vitro by a novel hepatotrophic factor, augmenter of liver regeneration. J Pharmacol Exp Ther. 2006;316:822–9. doi: 10.1124/jpet.105.094201. [DOI] [PubMed] [Google Scholar]
- 18.Dayoub R, et al. Liver regeneration associated protein (ALR) exhibits antimetastatic potential in hepatocellular carcinoma. Mol Med. 2011;17:221–8. doi: 10.2119/molmed.2010.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotze MT, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi CR, et al. Portacaval shunt causes apoptosis and liver atrophy despite increases in endogenous levels of major hepatic growth factors. J Hepatol. 2002;37:340–8. doi: 10.1016/s0168-8278(02)00165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geller DA, et al. Differential induction of nitric oxide synthase in hepatocytes during endotoxemia and the acute-phase response. Arch Surg. 1994;129:165–71. doi: 10.1001/archsurg.1994.01420260061008. [DOI] [PubMed] [Google Scholar]
- 22.Wan Y, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995;63:2435–42. doi: 10.1128/iai.63.7.2435-2442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, et al. Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia. Infect Immun. 1998;66:5089–98. doi: 10.1128/iai.66.11.5089-5098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahrenholz DH, Simmons RL. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery. 1980;88:41–7. [PubMed] [Google Scholar]
- 25.Chow CC, et al. The acute inflammatory response in diverse shock states. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 26.Uemura T, Gandhi CR. Inhibition of DNA synthesis in cultured hepatocytes by endotoxin-conditioned medium of activated stellate cells is transforming growth factor-β and nitric oxide-independent. Br J Pharmacol. 2001;133:1125–33. doi: 10.1038/sj.bjp.0704151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thirunavukkarasu C, Watkins S, Gandhi CR. Mechanisms of endotoxin-induced nitric oxide, interleukin-6 and tumor necrosis factor-a production in activated rat hepatic stellate cells: role of p38MAPK. Hepatology. 2006;44:389–98. doi: 10.1002/hep.21254. [DOI] [PubMed] [Google Scholar]
- 28.Thirunavukkarasu C, Uemura T, Wang LF, Watkins SC, Gandhi CR. Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol. 2005;204:654–65. doi: 10.1002/jcp.20366. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Lundin C, et al. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffatt P, Plaa GL, Denizeau F. Rat hepatocytes with elevated metallothionein expression are resistant to N-methyl-N′-nitro-N-nitroso-guanidine cytotoxicity. Toxicol Appl Pharmacol. 1996;136:200–7. doi: 10.1006/taap.1996.0025. [DOI] [PubMed] [Google Scholar]
- 32.Maddox JF, et al. Bacterial- and viral- induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J Toxicol Environ Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- 33.Lisowsky T. Dual function of a new nuclear gene for oxidative phosphorylation and vegetative growth in yeast. Mol Gen Genet. 1992;232:58–64. doi: 10.1007/BF00299137. [DOI] [PubMed] [Google Scholar]
- 34.Giorda R, et al. Analysis of the structure and expression of the augmenter of liver regeneration (ALR) gene. Mol Med. 1996;2:97–108. [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Porter KA, Putnam CW. Intra-portal insulin protects from the liver injury of portacaval shunt in dogs. Lancet. 1975;2:1241–6. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 36.Starzl TE, et al. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127–30. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dayoub R, et al. Foxa2 (HNF-3beta) regulates expression of hepatotrophic factor ALR in liver cells. Biochem Biophys Res Commun. 2010;395:465–70. doi: 10.1016/j.bbrc.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Huang C, Yang S, Geller DA, Stolz D, Gandhi CR. Interferon-β derived from endotoxin-stimulated hepatic stellate cells cause hepatocyte injury via JNK-mediated IRF-1 expression. FASEB J. 2011;25:A794.11. [Google Scholar]
- 39.Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112–9. doi: 10.1007/s005350050023. [DOI] [PubMed] [Google Scholar]
- 40.Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22–30. doi: 10.1046/j.1365-2168.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- 41.Vodovotz Y, et al. In silico models of acute inflammation in animals. Shock. 2006;26:235–44. doi: 10.1097/01.shk.0000225413.13866.fo. [DOI] [PubMed] [Google Scholar]
- 42.Vodovotz Y, An G. Systems Biology and Inflammation. In: Yan Q, Totowa NJ, editors. Systems Biology in Drug Discovery and Development: Methods and Protocols. Springer Science & Business Media; 2009. pp. 181–201. [Google Scholar]
- 43.Yang R, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–14. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen MJ, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathan C, Sporn M. Cytokines in context. J Cell Biol. 1991;113:981–6. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 48.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagiya M, et al. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci U S A. 1994;91:8142–6. doi: 10.1073/pnas.91.17.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]