Abstract
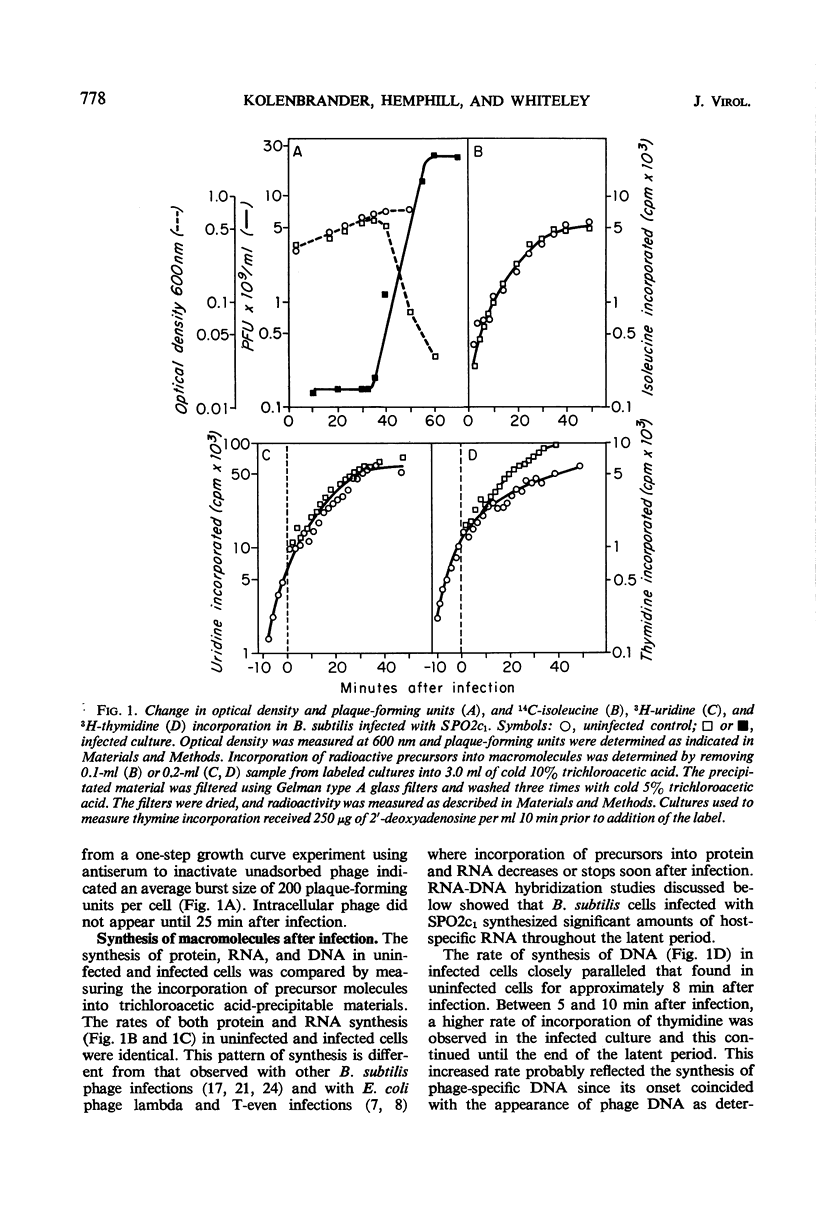
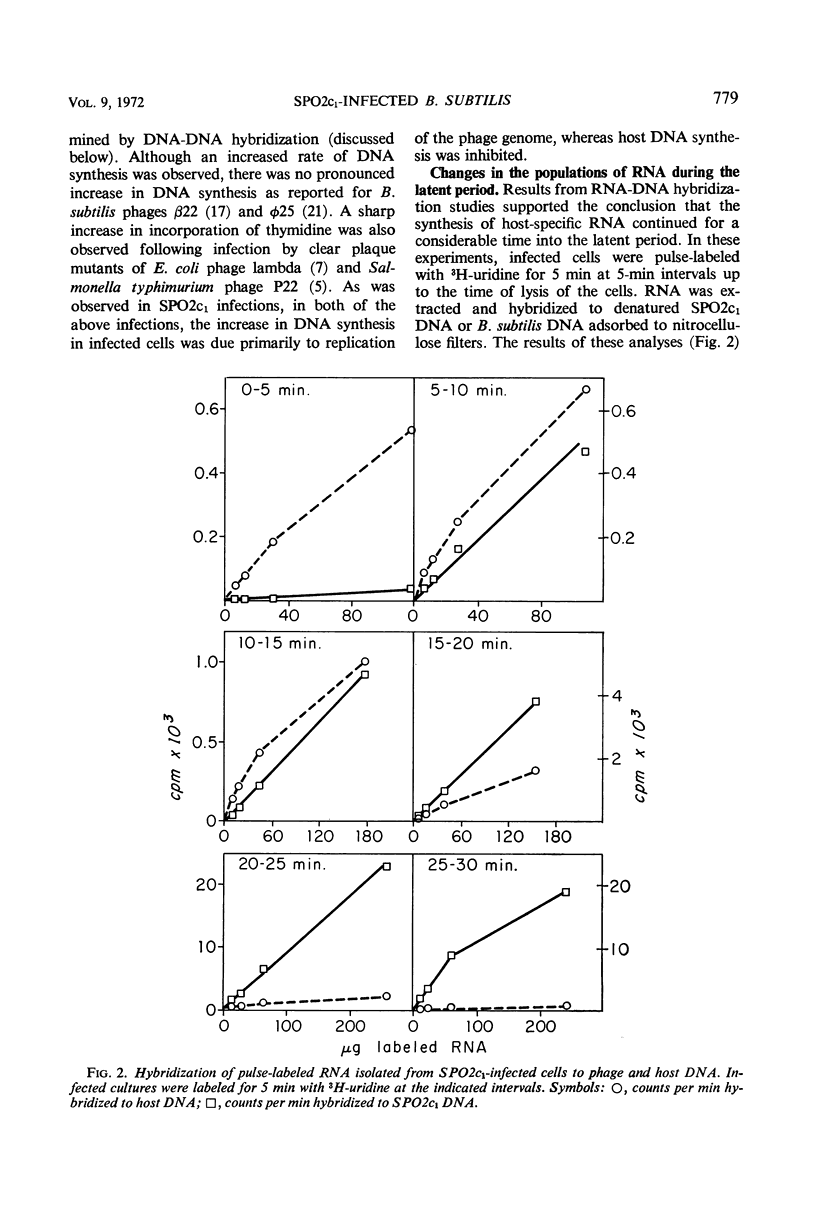
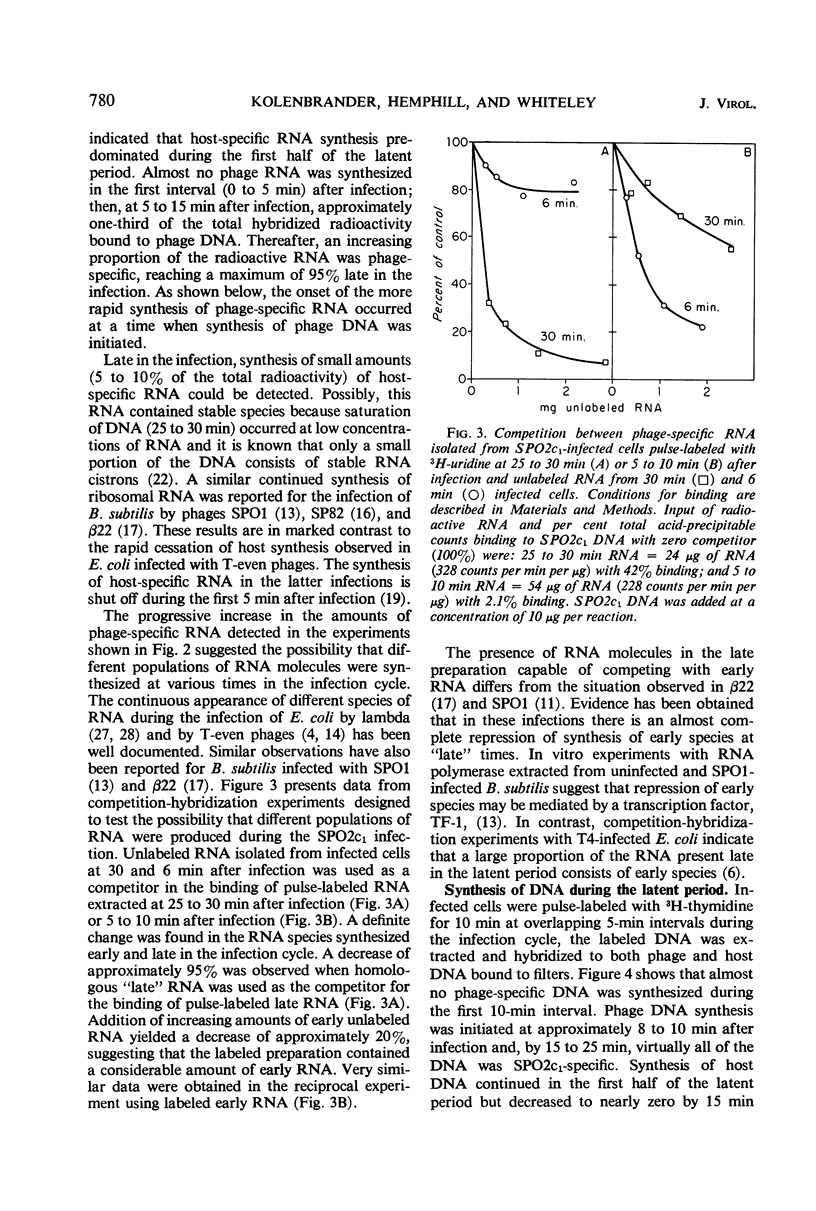
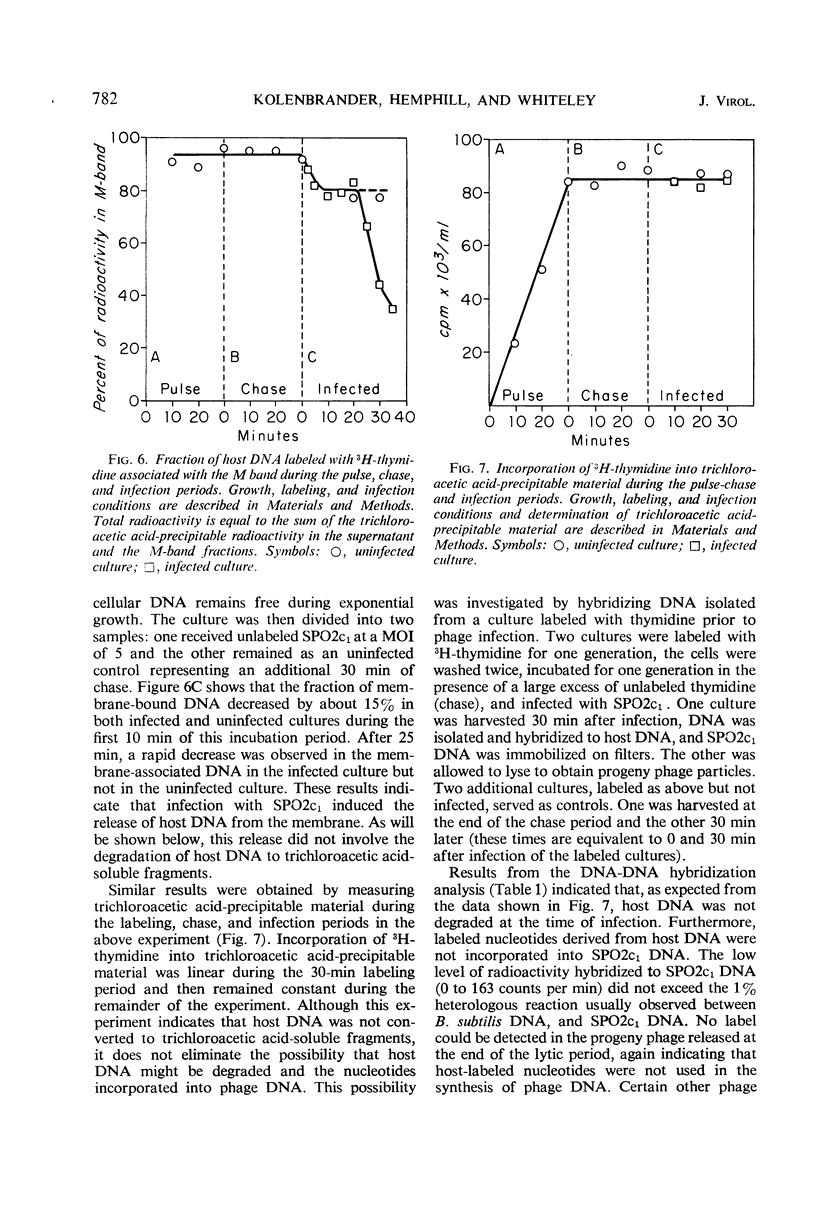
The synthesis of host macromolecules was shut off very slowly and incompletely by bacteriophage SPO2c1. No change in the rate of incorporation of radioactive precursors into protein and ribonucleic acid (RNA) could be detected after infection, and the rate of incorporation of thymidine was increased only slightly. The relative proportions of phage and host species of nucleic acids at various intervals in the latent period were determined by means of nucleic acid hybridization. Phage-specific RNA populations synthesized early were different from those synthesized late in the latent period. Host deoxyribonucleic acid (DNA) replication continued until 8 to 10 min after SPO2c1 infection and then decreased markedly as phage-specific DNA synthesis was initiated. Host DNA was not degraded to trichloroacetic acid-soluble fragments, and its nucleotides were not found in either newly synthesized intracellular phage DNA or in progeny phage particles. The average burst size of SPO2c1 was approximately 200 plaque-forming units per cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boice L. B. Evidence that Bacillus subtilis bacteriophage SP02 is temperate and heteroimmune to bacteriophage phi-105. J Virol. 1969 Jul;4(1):47–49. doi: 10.1128/jvi.4.1.47-49.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice L., Eiserling F. A., Romig W. R. Structure of bacillus subtilis phage SPO2 and its DNA: similarity of Bacillus subtilis phages SPO2, phi 1O5 and SPP1. Biochem Biophys Res Commun. 1969 Feb 21;34(4):398–403. doi: 10.1016/0006-291x(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Bruner R., Cape R. E. The expression of two classes of late genes of bacteriophage T4. J Mol Biol. 1970 Oct 14;53(1):69–89. doi: 10.1016/0022-2836(70)90046-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Genetic expression in bacteriophage lambda. 3. Inhibition of Escherichia coli nucleic acid and protein synthesis during lambda development. J Mol Biol. 1970 May 14;49(3):557–575. doi: 10.1016/0022-2836(70)90281-0. [DOI] [PubMed] [Google Scholar]
- Earhart C. F. The association of host and phage DNA with the membrane of Escherichia coli. Virology. 1970 Oct;42(2):420–436. [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., NYGAARD A. P., GREEN M. H. CONTROL OF T2-SPECIFIC RNA SYNTHESIS. J Mol Biol. 1964 Jul;9:143–153. doi: 10.1016/s0022-2836(64)80096-6. [DOI] [PubMed] [Google Scholar]
- Hallick L., Boyce R. P., Echols H. Membrane association by bacteriophage lambda-DNA: possible direct role of regulator gene N. Nature. 1969 Sep 20;223(5212):1239–1242. doi: 10.1038/2231239a0. [DOI] [PubMed] [Google Scholar]
- Hayward J. Inhibition of bacterial DNA and protein synthesis in Bacillus subtilis by phage SP82. Effect of changes of temperature on the inhibition. Virology. 1969 Aug;38(4):538–549. doi: 10.1016/0042-6822(69)90174-3. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in Bacillus subtilis infected with bacteriophage beta-22. J Virol. 1970 Oct;6(4):381–392. doi: 10.1128/jvi.6.4.381-392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. W., Eremenko-Volpe T., Greenwald L., Meadow W. L., Marmur J. Physical and genetic mapping of the SPO2 prophage on the chromosome of Bacillus subtilis 168. J Virol. 1969 Jun;3(6):627–628. doi: 10.1128/jvi.3.6.627-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Anderson D. L. Morphology and physiology of the intracellular development of Bacillus subtilis bacteriophage phi25. J Virol. 1970 Jul;6(1):114–124. doi: 10.1128/jvi.6.1.114-124.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Sueoka N. Location of genetic loci of ribosomal RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1965 Aug;54(2):483–491. doi: 10.1073/pnas.54.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Pène J. J. Host macromolecular synthesis in bacteriophage-infected Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):379–386. [PMC free article] [PubMed] [Google Scholar]
- Romig W. R. Infectivity of Bacillus subtilis bacteriophage deoxyribonucleic acids extracted from mature particles and from lysogenic hosts. Bacteriol Rev. 1968 Dec;32(4 Pt 1):349–357. [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H. Synthesis of DNA in phage-infected Bacillus subtilis. Virology. 1969 Aug;38(4):527–537. doi: 10.1016/0042-6822(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Skalka A., Butler B., Echols H. Genetic control of transcription during development of phage gamma. Proc Natl Acad Sci U S A. 1967 Aug;58(2):576–583. doi: 10.1073/pnas.58.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Yasunaka K., Tsukamoto H., Okubo S., Horiuchi T. Isolation and properties of suppressor-sensitive mutants of Bacillus subtilis bacteriophage SP02. J Virol. 1970 Jun;5(6):819–821. doi: 10.1128/jvi.5.6.819-821.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Brenner D. J. Lack of nucleotide sequence relationship among the temperate bacteriophage SPO2, Bacillus subtilis, and virulent bacteriophages beta 3 and beta 22. J Virol. 1970 Jan;5(1):39–44. doi: 10.1128/jvi.5.1.39-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Differential expression of bacteriophage genomes in vegetative and sporulating cells of Bacillus subtilis. J Virol. 1967 Oct;1(5):935–947. doi: 10.1128/jvi.1.5.935-947.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


