Abstract
Interleukin (IL)-15 is a ubiquitously expressed cytokine existing in both intracellular and secretory forms. Here we review the expression, regulation, and functions of IL15 and its receptors in the brain. IL15 receptors show robust upregulation after neuroinflammation, suggesting a major role of IL15 signaling in cerebral function. Involvement of the IL15 system in neuropsychiatric behavior is reflected by the effects of IL15, IL15Rα, and IL2Rγ deletions on neurobehavior and neurotransmitters, the effects of IL15 treatment on neuronal activity, and the potential role of IL15 in neuroplasticity/neurogenesis. The results show that IL15 modulates GABA and serotonin transmission. This may underlie deficits in mood (depressive-like behavior and decreased normal anxiety) and memory, as well as activity level, sleep, and thermoregulation. Although IL15 has only a low level of permeation across the blood-brain barrier, peripheral IL15 is able to activate multiple signaling pathways in neurons widely distributed in CNS regions. The effects of IL15 in “preventing” neuropsychiatric symptoms in normal mice implicate a potential therapeutic role of this polypeptide cytokine.
Keywords: cytokine, peptide, interleukin-15, depression, anxiety, memory, neurobehavior, neurotransmitter, neurogenesis, metabolic activity
1. Introduction
There is growing awareness of a role of cytokines in the structural and neurochemical basis of neuropsychiatric disorders. Cytokines are at the crossroads of inflammation and functional deficits in cognition and emotion. We review here the cerebral interleukin (IL)-15 system as a key player in the cytokine network that limits the consequences of neurochemical imbalance. IL15 receptors are robustly upregulated in mouse models of altered innate immunity and acquired immunity. Otherwise normal appearing IL15 receptor knockout mice show unusual cognitive deficits and mood disturbances. These exciting new findings prompted us to address the potential role of cerebral IL15 in mood and memory.
IL15 is a member of the 4 α-helix bundle family that was initially cloned in 1994 as a new T cell growth factor that competes with IL2 for receptor binding (Grabstein et al., 1994). In comparison with IL2 which has a relatively restricted expression in activated T cells, IL15 has a wider tissue and cellular distribution (Grabstein et al., 1994; Bamford et al., 1996; Kitaya et al., 2000). IL15 acts through a trimeric receptor complex comprising IL15Rα, IL2Rβ;, and IL2Rγ. The β and γ receptor subunits are shared by IL15 and IL2 and are essential in relaying signals for both cytokines (Waldmann et al., 1998; Waldmann and Tagaya, 1999). In many human and murine cell lines, IL15 shows high affinity binding, in contrast to IL2 that contains both high and low affinity binding sites (Giri et al., 1994). Like the multi-level control of IL15Rα and IL15 expression, the high affinity association between IL15Rα and IL15 suggests a highly efficient regulatory system.
A unique aspect of IL15 action is its juxtacrine and reverse signaling. With agonistic stimulation, membrane-bound IL15 phosphorylates mitogen-activated protein kinase (MAPK) and focal adhesion kinase (FAK) to increase cytokine secretion and cell adhesion and migration. IL15 can be trans-presented by its high-affinity receptor IL15Rα to adjacent cells that express IL2Rβ and IL2Rγ, without the necessity of IL15 binding to the heterotrimeric receptor complex Accepted Manuscript (Schluns et al., 2005; Bulfone-Paus et al., 2006). Although most findings were observed in lymphocytes, it is possible that CNS parenchymal cells show a similar phenomenon. If proven, trans-presentation of IL15 could represent a novel path for neuron-glial interactions.
This review focuses on the cerebral IL15 system in normal and pathophysiological conditions. We first describe the distribution and known functions of IL15 and its receptors, some of which are shared with IL2. We then summarize the effects of IL15, IL15Rα, and IL2Rγ deletion on the neurobehavior of these knockout mice, as well as the effects of IL15 treatment on neuronal activity, including neurogenesis and synaptic plasticity. IL15 and its receptors have complex regulation at multiple levels, and significantly influence cerebral activity and animal behavior. This is illustrated by findings from measurement of neurotransmitter profiles in the knockout mice and assays of regulatory changes of IL15 receptors during neuroinflammation and autoimmune challenge.
2. Distribution and cellular functions of IL15 and its receptors in CNS regions, cell types, and subcellular organelles
IL15 and its receptors are expressed throughout the brain by either glial cells or neurons, and show developmental changes, regional differences, and regulation by inflammatory challenges (Hanisch et al., 1997; Kurowska et al., 2002; Wu et al., 2010c). In human fetal brain, mRNA for IL15 and IL15Rα is higher in the hippocampus and cerebellum than in the cortex and thalamus (Kurowska et al., 2002). During inflammation with disruption of the BBB, both immune cells and CNS parenchymal cells become sources of IL15.
In peripheral cells, IL15 is induced by interferon-γ, lipopolysaccharide (LPS), mycobacteria, and Toxoplasma gondii in macrophages and monocytes (Doherty et al., 1996). In the CNS, basal expression of IL15 is mainly seen in astrocytes and some projection neurons. In patients with multiple sclerosis (MS), there is increased intrathecal production of IL15 (Lee et al., 1996; Satoh et al., 1998; Kivisakk et al., 1998;Beck, Jr. et al., 2005b). Within the CNS, IL15 affects nitric oxide production and growth of microglia (Waldmann and Tagaya, 1999). IL15 may play a protective role in host defense by increasing γδ-T cells, as shown by comparison studies in macrophages obtained from LPS-responsive C3H/HeN mice and LPS-hyporesponsive C3H/HeJ mice (Takano et al., 1998). IL15Rα might also confer neuroprotection, as IL15Rα knockout mice have five times more motor neuron death after facial nerve axotomy (Huang et al., 2007). IL15 induces sickness behavior; icv injection of IL15 dose-dependently increases non-rapid eye movement sleep and temperature, with the cost of an associated reduction of rapid eye movement sleep (Kubota et al., 2001).
IL15 can be proinflammatory to induce reactive gliosis. This is shown by reduction of the reactivity of both astrocytes and microglia by a blocking antibody against IL15 in the brain after LPS treatment (Gomez-Nicola et al., 2010). In cultured microglia, IL15 blockade reduces the activation of MAPK and nuclear factor (NF)-κB (Gomez-Nicola et al., 2008). However, IL15 is also anti-apoptotic and neurotrophic, and it suppresses nitric oxide production in neurons (Budagian et al., 2006). It is not yet clear whether its proinflammatory effects are beneficial for neuroregeneration, or whether the proinflammatory and anti-apoptotic effects represent two parallel processes in different courses of the associated disorders.
In contrast to the microglial changes related to innate immunity (LPS treatment), IL15-induced reactive astrocytes are implicated in MS. In demyelinating MS lesions, about 80-90% of astrocytes express IL15, whereas few astrocytes in normal control brain sections have detectable IL15; IL15 (+) microglia are more sparse and surrounded by infiltrating CD8+ T cells. Cultured astrocytes show increased surface IL15 levels after treatment with proinflammatory cytokines, and promote cytotoxicity of co-cultured CD8+ Tlymphocytes. By contrast, a blocking antibody against IL15 abrogates the functional enhancements of the CD8+ T cells (Saikali et al., 2010).
In sum, the intrathecal production of IL15 in the basal state is contributed to by both neurons and glia, and it provides neuroprotection with some regional specificity. The source of IL15 in the CNS includes both infiltrating and residential cells after inflammatory and autoimmune challenge; IL15 mainly serves as a proinflammatory cytokine. However, the detailed mechanisms of cell-cell interactions between IL15 (+) and IL15 receptor (+) cells are largely unexplored.
3. IL15 system at the blood-brain barrier (BBB): Crosstalk between tumor necrosis factor α (TNF) and IL15
The unique functions of IL15 at the BBB were initially identified from studies with TNF. TNF is a ubiquitous cytokine entering the CNS by upregulated transport across the BBB that may modulate neuroregeneration after spinal cord injury (Pan et al., 1999; Pan and Kastin, 2001), hippocampal trauma (Pan et al., 2003), and stroke (Pan et al., 2006). Microarray analysis of RBE4 cerebral microvessel endothelial cells at different time intervals after TNF treatment showed that IL15 and IL15Rα are among the most robustly upregulated genes. TNF increases the expression of IL15Rα mRNA and protein, and accelerates the post-translational processing of IL15. TNF also modulates the expression of IL2Rβ and IL2Rγ, the other components of the IL15 receptor trimeric complex. The effects of TNF on the IL15 system are greater than the increases of the other cytokines, chemokines and cell adhesion molecules measured. The results indicate that IL15 is a novel mediator of TNF signaling at the level of the BBB, serving to amplify and modulate TNF signaling (Pan et al., 2009).
Consistently, IL15 induces NF-κB signaling in primary brain microvessel endothelial cells and cerebral endothelial cell lines, similar to the effect of TNF. Though the JAK/STAT signaling pathway is known to mediate most of the effects of IL15 shown in other cellular models, NFκB seems to play a larger role in BBB endothelia. IL15 induces transactivation of an NFκB luciferase reporter, and activation and nuclear translocation of the p65 subunit of NFκB, an effect delayed and attenuated in cerebral endothelia from IL15Rα knockout mice and inhibited by the IκB kinase inhibitor Bay 11-7082. The combined effect can potentially increase BBB permeability by decreased expression of the tight junction protein claudin-2 and modulate endocytosis and intracellular trafficking of a subset of molecules that interact with caveolin-1 and vimentin (Stone et al., 2011).
An essential neuroprotective role of IL15 signaling is shown by increased motor neuron death in IL15Rα knockout mice after facial nerve axotomy (Huang et al., 2007), by increased deficits in IL15 knockout mice after induction of experimental autoimmune encephalomyelitis (EAE), and by the effect of IL15 treatment in ameliorating the symptoms of EAE (Wu et al., 2010c). Produced in the periphery as well as the CNS, IL15 has limited permeation across the BBB, but this passage can be modulated by LPS (Pan et al., 2008) and EAE (Hsuchou et al., 2009). Although endogenous IL15 in the circulation is low, inflammation induced by LPS increases the permeation of radioactively labeled IL15 across the BBB (Pan et al., 2008). The specificity of this regulatory response is further shown by a reduction (rather than increase) of IL15 permeation in mice with EAE, a model for the human disease MS (Hsuchou et al., 2009). The differential permeability changes in these two disease states might contribute to the worsening of neuropathology, since IL15 exacerbates microglial activation after LPS treatment (Gomez-Nicola et al., 2010) but benefits EAE recovery (Wu et al., 2010c). In contrast to LPS-induced increase of BBB permeability to IL15, the EAE mice show reduced BBB permeability to IL15.
Inflammation from a variety of sources increases blood levels of IL15. In Thai patients with scrub typhus infection, IL15 plasma concentrations increased from a mean of 13.4 pg/ml to 37.5 pg/ml (Chierakul et al., 2004). In Japanese patients with acute pancreatitis, serum IL15 increases 5-fold to 5.6 ± 0.5 pg/ml (Ueda et al., 2007). Patients with MS have significantly higher concentrations of IL15 in their serum (4.2 pg/ml) (Rentzos et al., 2006) than patients with a variety of other neurological diseases. Serum concentrations are also higher in patients with rheumatoid arthritis than with osteoarthritis or healthy controls (Cordero et al., 2001; Klimiuk et al., 2001; Petrovic-Rackov and Pejnovic, 2006), and in scleroderma (systemic sclerosis) (Wuttge et al., 2007). Another study showed increased IL15 blood concentrations in system lupus erythematosus, a related autoimmune disorder, but did not detect an increase in rheumatoid arthritis (Aringer et al., 2001). Even in patients with type I (autoimmune) diabetes mellitus, serum concentrations of IL15 are significantly higher than in control subjects (4.4 vs 2.9 pg/ml). Regardless of the absolute values, all these immune/inflammatory disorders were associated with increased blood concentrations of IL15, raising the question of how IL15 modulates CNS behavior. Even though blood concentrations, BBB permeation, and CNS effects are not linearly correlated, blood-borne IL15 is able to induce brain activation by direct actions on its receptors. This is shown by results from our laboratory that IL15 induces cFos activation in multiple brain regions 3 h after intravenous injection (He et al., 2010b) and activates several signaling pathways including phosphorylated ERK and STAT3. Moreover, blood-borne IL15 mainly activates neurons, and the effect is diminished in knockout mice lacking functional IL15Rα or IL2Rγ (Pan W et al., manuscript in review).
In cerebral microvessels composing the BBB, several IL15Rα isoforms are expressed, and the full-length isoform is the most abundant. TNF treatment of the isolated microvessels induced transcriptional upregulation of all the forms, most pronounced for the full-length isoform. This coincides with a summation of STAT3 activation in endothelia (Wu et al., 2010d).
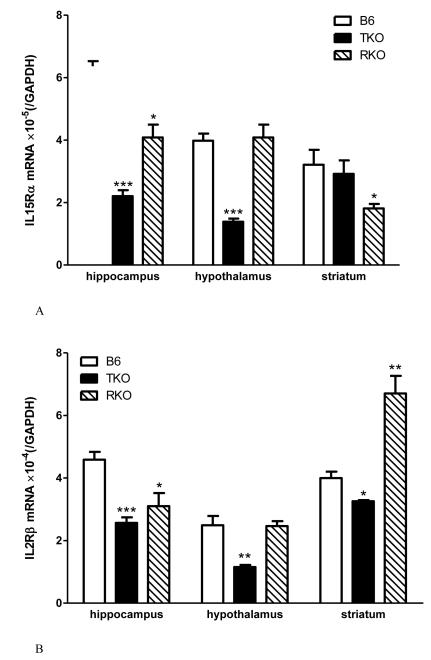
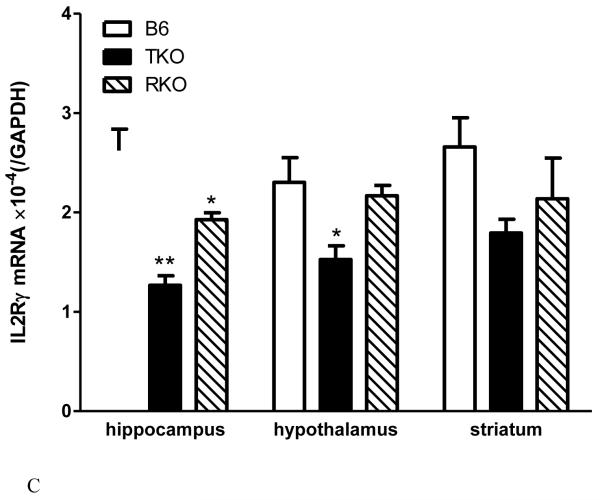
The interactions between TNF and the IL15 system at and beyond the BBB are further illustrated by changes of IL15 receptor expression in TNF knockout mice (TKO), TNF R1 and R2 dual receptor knockout mice (RKO), and the C57/B6J (B6) wildtype controls. IL15Rα mRNA is downregulated in the hippocampus and hypothalamus of the TKO mice and the striatum of the RKO mice (Fig.1A). IL2Rβ mRNA is decreased in all three regions in the TKO, in the hippocampus of RKO mice, but increased in the striatum of the RKO mice (Fig.1B). By contrast, IL2Rγ mRNA shows reduction in the hippocampus of both TKO and RKO mice, but in the hypothalamus only in the TKO mice (Fig.1C). The results seem to suggest that an absence of TNF leads to compensatory reduction of IL15 signaling by way of downregulation of IL15Rα and its co-receptors in the hippocampus and hypothalamus. However, an absence of TNF receptors results in a paradoxical increase of IL2Rβ in the striatum and less reduction of other receptor subtypes in the other regions. The differential regulation of receptor subtypes and regional variations might be pertinent to a role of cerebral TNF and IL15 in specific neurological diseases.
Fig.1.
The effects of TNF signaling on IL15 receptor expression in TNF knockout (TKO) and TNF dual receptor knockout (RKO) mice (n = 4 /group). The mRNA for IL15Rα (A), IL2Rβ (B), and IL2Rγ (C) showed differential regulation in three brain regions tested: hippocampus, hypothalamus, and striatum. *: p < 0.05; **: p < 0.01; ***: p < 0.005 in comparison with the B6 wildtype control in the same region.
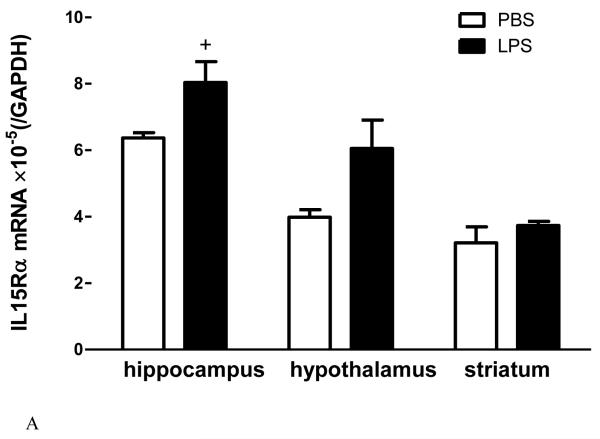
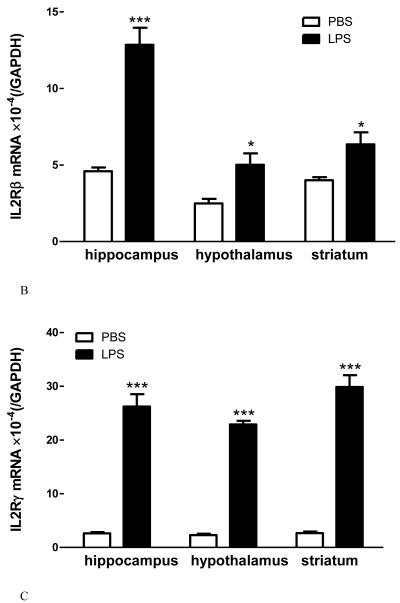
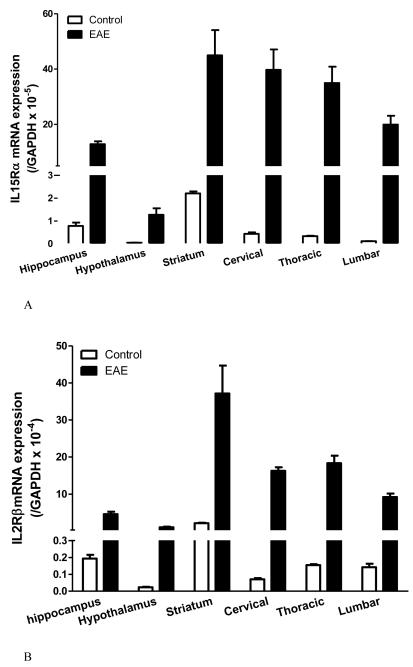
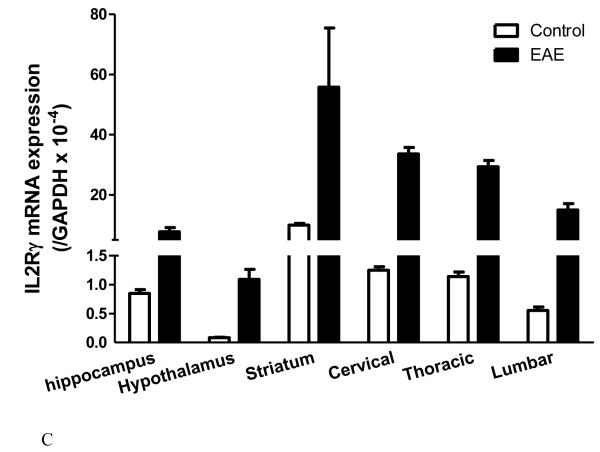
The degree of change for IL15 receptors differs in innate immune challenge and autoimmune disease. In adult male mice from a B6 strain background, LPS treatment (5 mg/kg ip) tended (p = 0.052) to increase IL15Rα mRNA in the hippocampus, significantly elevate IL2Rβ mRNA in all three regions tested (hippocampus, hypothalamus, and striatum), and remarkably elevate IL2Rγ mRNA in all these regions (Fig.2A-C). This occurred 48 h after LPS injection, when the sickness behavior was maximal. In female SJL/J mice of slightly younger age, there was a striking increase of the mRNA of all three receptor subtypes in all the regions at 12 d after EAE induction, immediately before the peak of neurological symptoms (Fig.3A-C). The effects were even more outstanding in the spinal cord, where inflammation, leukocyte infiltration, and demyelination were more severe than in the brain. There were different basal levels of gene expression in the LPS and EAE studies. This is probably related to the different experimental treatments, mouse strain, and gender. Overall, the high sensitivity of the IL15 receptors in response to pathological conditions indicates a prominent role of cerebral IL15 in immunomodulation.
Fig.2.
The effects of LPS treatment (5 ng/ml, 48 h) on IL15 receptor expression in three brain regions of B6 mice show differential regulation of mRNA for IL15Rα (A), IL2Rβ (B), and IL2Rγ (C). +: p = 0.052; *: p < 0.05; ***: p < 0.005 in comparison with the diluent-treated control in the same region (n = 4 /group).
Fig.3.
Changes of IL15Rα (A), IL2Rβ (B), and IL2Rγ (C) mRNA expression in brain and spinal cord regions in SJL/J female mice 12 days after induction of EAE by PLP139-151 peptide and RIBI adjuvant (n = 4 /group). In all regions, the increase in the EAE mice is significantly different from the adjuvant and naïve controls (p < 0.005).
Thus, IL15 production and signaling through IL15Rα and its co-receptors at the level of BBB endothelia are regulatory processes, subject to changes in response to TNF, LPS, and EAE. Together with TNF, peripheral sources of IL15 can induce NFκB signaling and alter BBB structure and functions. The regulatory changes of the BBB are an integral part of CNS inflammation and autoimmune disease, thereby providing a potential target for therapeutic intervention. It is yet to be determined whether IL15 and TNF interactions in neurons and glial cells have the same pattern.
4. IL15Rα signaling in mood and memory, and the underlying biochemical basis
IL15Rα knockout mice show a unique neurobehavioral phenotype with depressive-like behavior, reduced anxiety, and impaired memory. Contrary to the reduction of depressive-like behavior observed in several strains of other cytokine receptor knockout mice, IL15Rα knockout mice have increased immobility in the tail suspension and modified forced swimming tests, despite a lack of reduction in social interactions or an increase of sucrose intake. This is consistent with alterations of serotonin (5-HT) receptor and reuptake transporter expression in the hippocampus. The impairment of serotonin transmission is also supported by a therapeutic effect of the classic selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac) in reducing the immobility of the IL15Rα knockout mice in comparison with their pretreatment baseline. Wildtype mice respond to IL15 treatment with improvement of immobility induced by forced swimming, whereas the knockout mice fail to respond. The results suggest an antidepressive effect of IL15 signaling (Wu et al., 2011).
In ex-vivo studies, IL15 decreases synaptosomal uptake of 5-HT, and modulates the expression of 5-HT2C and the serotonin transporter SERT in cultured neurons in a dose- and time-dependent manner. Thus, the effect of IL15 on serotonin transmission may underlie the depressive-like behavior of IL15Rα knockout mice. While IL15 appears to be essential in maintaining neurochemical homeostasis with a mood-stabilizing effect, it paradoxically shows anxiogenic functions (Wu et al., 2010a). IL15Rα knockout mice have reduction of anxiety, more apparent than in the IL15 or IL2Rγ knockout mice that show somewhat similar changes. The IL15Rα knockout mice cross more inner grids in the open field, and spend more time being active in the open arm of the elevated plus maze. In addition to this hyperactivity, there is no reduction of locomotor activity on the rotorod apparatus in the IL15Rα knockout mice as compared with the wildtype controls. Overall, an intact IL15Rα system appears to help mice develop a normal sense of fear and anxiety while maintaining an adequate level of activity (Wu et al., 2010a).
The increased activity of the IL15Rα knockout mice was confirmed by telemetry and metabolic activity assays. During the entire circadian cycle, the knockout mice have a significantly higher acrophase in their locomotor activity and heat dissipation. During the light-phase, there is significantly greater food intake, oxygen consumption, and carbon dioxide production. The difference in the dark- and light-phases suggests that IL15Rα participates in circadian rhythm regulation. As a result of higher activity, the IL15Rα knockout mice have lower fat composition though the difference is only apparent with nuclear magnetic resonance measurement. Body temperature is 0.8 °C higher than the control in the light phase, consistent with higher oxygen consumption. Altogether, the results show an increase in adaptive thermogenesis in the IL15Rα knockout mice. Besides the metabolic chamber studies and circadian rhythm analyses, qPCR of hypothalamic homogenates show higher mRNA expression of orexin and transient receptor potential vanilloid 4 (TRPV4) cation channels. Concurrently, IL15 treatment of the wildtype mice induces c-Fos expression in the preoptic area of the hypothalamus, a main area for temperature regulation. Therefore, activation of hypothalamic neurons by IL15 contributes to thermoregulation and modifies the metabolic phenotype in mice (He et al., 2010b).
In contrast to the effects of IL15 in the hypothalamus with regulation of temperature, energy metabolism, circadian rhythms, and perhaps also partial regulation of locomotor activity, the effects of IL15 in the hippocampus are reflected by consolidation of learning and memory. IL15Rα knockout mice show less memory retention in the modified Stone 14-unit T maze test (He et al., 2010a). In the fear conditioning test, IL15Rα knockout mice show the same freezing response to tone conditioning suggesting intact emotional memory, but freezing to the preceding contextual fear conditioning is significantly reduced, indicating impaired contextual memory. Hippocampal activity is required for early consolidation of fear conditioning with a short trace interval (Corcoran et al., 2005; Burman and Gewirtz, 2007; Ji and Maren, 2007). Thus, the hippocampus is a major structural component mediating the memory deficits of the IL15Rα knockout mice.
γ-aminobutyric acid (GABA) is one of the major neurotransmitters involved in the early consolidation of fear memory (Myers and Davis, 2002; Heldt et al., 2004; Bergado-Acosta et al., 2008). The unusual combination of increased immobility in the depression tests and hyperactivity in the anxiety tests also suggests a malfunction of inhibitory circuitry in the brain. This points to a dysregulation of the GABA system. As predicted, GABA concentration is significantly reduced in hippocampal homogenates of the IL15Rα knockout mice. This contrasts with the lack of changes of monoamines (epinephrine, norepinephrine, and dopamine) measured simultaneously by mass spectrometry, suggesting some specificity of IL15 in maintaining GABA concentrations (He et al., 2010a). While concentrations of serotonin, 5-HIAA, and the 5-HT/5-HIAA ratio do not show a significant change, the IL15Rα knockout mice have decreased mRNA for 5-HT1A, increased mRNA for 5-HT2C, and region-specific changes of the serotonin transporter (SERT) immunoreactivity (Wu et al., 2011). Cellular assays of cultured neurons also support a direct effect of IL15 on GABA and serotonin systems.
Concurrent with an overall reduction of GABA concentration in hippocampal homogenates is a region specific change of the GABA synthesizing enzyme glutamic acid decarboxidase (GAD). There are increased GAD-67 immunopositive interneurons in the stratum oriens of the CA1 region of the hippocampus, accompanied by non-significant reduction of GAD-67 synapses in the CA3 region. Western blotting shows that GAD-65, rather than GAD-67, is increased in hippocampal homogenates. As the ultrastructure of the hippocampus remains intact without change in the number of inhibitory and excitatory synapses, the neurochemical imbalance appears to be mainly responsible for the disinhibitory behavior and reduced memory in these mice (He et al., 2010a). However, it is still debatable whether the changes of IL15 signaling are parallel to the changes of classical neurotransmitters in inducing behavioral changes, or whether the effects of IL15 have to be exerted by alterations of the classical neurotransmitters as the underlying mechanisms of altered neurobehavior.
Another prominent change in the IL15Rα knockout mice is the presence of mild reactive gliosis. In the hippocampus of these mice there is an increase of CD11b and cyclooxygenase (COX)-2, markers of reactive microgliosis, and an increase of glial fibrillary acidic protein (GFAP), a marker for reactive astrogliosis, cross-validated by western blotting, qPCR, and immunohistochemistry. Though treatment of the IL15Rα knockout mice with minocycline did not affect their open field performance, it is possible that neuroinflammation plays a direct role in the reduction of normal anxiety behavior in the IL15Rα knockout mice (Wu et al., 2010a).
IL15 treatment by intravenous injection also induces COX2 expression in mouse hypothalamus 3 h later. Double-labeling studies with cell phenotype markers show that the induction is mainly seen in neurons. Consistently, IL15 treatment increases COX-2 protein expression in cultured cells of the GT1-7 neuron line of hypothalamic origin in a time-dependent manner, an effect partially blocked by the ERK kinase inhibitor U0126 (Pan W et al., manuscript in review). As IL15 concentrations are higher in IL15Rα knockout mice than wildtype controls (Wu et al., 2010a), it is possible that IL15 acts through its co-receptors (IL2Rβ and IL2Rγ) to promote COX2 upregulation and reactive gliosis in the IL15Rα knockout mice discussed in the preceding paragraph.
In knockout mice lacking the IL-2/15Rβ receptor subunit, there is a significant reduction in acoustic startle reactivity. The mice are less fearful, spending less time in closed arms and more in open areas in the elevated plus-maze test. However, there is no significant difference in learning, memory, or locomotor activity when examined in either the plus-maze or the Morris water maze (Petitto et al., 2002).
In knockout mice lacking the IL2/15Rγ receptor subunit, there is an apparent depressive-like behavior. IL2Rγ knockout mice have reduced performance in the Porsolt forced swimming test and Nomura water wheel test. In contrast with that seen in the IL15Rα knockout mice with impaired contextual fear memory, male IL2Rγ knockout mice show increased freezing in the tone-paired foot shock fear conditioning test, suggesting a better emotional memory related to the amygdala. This contrasts with their lack of significant difference in the auditory conditioned stimulus-shock association that involves hippocampal dependent pathways (Wu et al., 2010b). Although depression is commonly associated with decreased memory, the dissociation of these two in the IL2Rγ knockout mice shows that IL2Rγ signaling makes differential contributions to mood and memory compared with IL15Rα. The anxiolytic effect of IL15Rα signaling might also be greater than that of IL2Rγ signaling, although there has been no side-by-side comparison of the IL15Rα KO mice and the IL2Rγ knockout mice (Petitto et al., 2002).
The influence of lack of IL15 signaling differs from that observed with other cytokines on classical neurotransmitters and associated behavioral changes. For example, TNF results in depressive-like and anxiogenic behavior in mice. TNF knockout mice show reduced immobility in a forced swimming test and reduced time spent in open areas in the open field and elevated plus maze tests, with an increase of serotonin levels in several brain regions, including the hippocampus (Yamada et al., 2000). However, TNF icv induces anxiogenic-like effects in the elevated plus maze in rats (Connor et al., 1998). TNF transgenic mice show altered exploratory activities in the hole-board and black/white box tests (Fiore et al., 1998). TNF blockade by etanercept treatment partially blunts Mycobacerium vacille Calmette-Guerin (BCG)-induced over-activation of indoleamine 2,3-dioxygenase (IDO), an enzyme that catalyzes pathways leading to neurotoxin production from tryptophan, and deviates tryptophan from serotonin synthesis (O’Connor et al., 2009). TNF increases SERT-mediated 5-HT uptake in cultured cells (Mossner et al., 1998), increases extracellular 5-HIAA in the nucleus raphe dorsalis in freely moving rats after ip or icv injection (Clement et al., 1997), and increases 5-HIAA in the hippocampus and prefrontal cortex in mice (Hayley et al., 1999). These effects are different from what we observed in the IL15 studies both in-vivo and in-vitro (Wu et al., 2011). TNF also facilitates exocytosis of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor and endocytosis of the GABA receptor (Stellwagen et al., 2005), whereas IL15 increases GABA uptake in cultured neurons (He et al., 2010a).
Besides depression, cytokines are associated with other neuropsychiatric disturbances. For instance, use of IFNα or IL2 as immunomodulatory therapy for autoimmune diseases and certain cancers has the side effects of anxiety, psychosis, suicidal ideation, and cognitive impairment in addition to depression (Myint et al., 2009). Elevated cytokine levels in cerebrospinal fluid are also seen in childhood-onset schizophrenia, obsessive-compulsive disorder, and attention deficit hyperactivity disorder (Mittleman et al., 1997). Furthermore, TNF G308A promoter polymorphism is associated with schizophrenia. This suggests linkage disequilibrium, and is consistent with clinical findings of increased levels of the TNF in schizophrenic patients (Morar et al., 2007). With a more apparent dissociative behavior, could IL15Rα knockout mice be a closer model for neuropsychiatric behavior?
It should be noted that IL15 ligand knockout mice do not show dramatic changes in neurobehavior, though the immune phenotype after EAE induction is more apparent than in the receptor knockout mice (Wu et al., 2010c). Many of the functions of IL15 in the periphery are known to overlap with IL2, though the distribution of IL2 and IL15 in the brain does not show significant overlap (unpublished observations). The IL2 knockout mice show impaired learning and memory in the water maze (Petitto et al., 1999). However, repeated systemic injection of IL2 in normal mice results in impairment of spatial working memory in the water maze (Lacosta et al., 1999). These seemingly contradictory results from cytokine knockout mice and cytokine treatment suggest compensatory changes after ligand deletion, and support the utility of cytokine receptor knockout mice for behavioral and biochemical assays.
The inverted U-shaped dose response relationship has long been reported for peptides (Kastin et al., 1987) and more recently for cytokines. For example, IL1β is known to affect the immune system and hippocampal dependent memory consolidation (Rachal et al., 2001; Yirmiya et al., 2002). Injection of IL1β into the dorsal hippocampus provides more direct evidence that it impairs the consolidation process in contextual fear conditioning for up to 24 h (Barrientos et al., 2002). There is a biphasic effect of IL1β icv on contextual memory, with a low dose (1 ng) improving and high dose (10 ng) decreasing it. Prenatal treatment with IL1 receptor antagonist (IL1ra) also results in impaired fear conditioning performance in mice (Goshen et al., 2007).
In addition, cytokines including TNF and IL1 are essential for normal brain development (Merrill, 1992). TNF knockout mice show poorer learning and retention in the novel object test, and deletion of both TNF receptors also results in partially reduced cognitive functioning (Baune et al., 2008). More interestingly, TNF knockout mice show age-dependent changes in performance in the Barnes maze, initially doing worse than wildtype controls at 3 months of age, but not at 6 and 12 months (McAfoose et al., 2009).
In summary, we show here that IL15Rα knockout mice have an array of neurobehavioral changes that may also underlie the metabolic phenotype. IL15Rα knockout is associated with reduction of GABA and dysregulation of serotonin turnover in the hippocampus and increased activation in the medial preoptic area of the hypothalamus consistent with impaired thermoregulation. IL15 also induces neurotransmitter changes in cultured neurons, but it is not certain whether the changes of neurotransmitters occur in parallel or mediate the altered neurobehavior. We propose that IL15Rα knockout mice show behavioral changes resembling a subset of neuropsychiatric disease, and this suggests a critical role of IL15 signaling in mood and cognitive stability.
5. IL15 and adult neurogenesis
Adult neurogenesis, mainly occurring in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus, is a critical part of recovery during neurodegenerative disorders, and it also is implicated in depression as well as memory (Zhao et al., 2008). Cytokines and chemokines are known to affect the adult neurogenesis process (Bauer, 2009).
There have been a few studies showing the effect of IL15 on neurogenesis. Concurrent with an increase of hippocampal IL15, male IL2 knockout mice show increased neurogenesis in both the infrapyramidal and suprapyramidal limbs of the granule cell layer of the dentate gyrus (Beck, Jr. et al., 2005b). However, the IL2 knockout mice also show selective loss of cholinergic neurons in the septohippocampal area, in association with a reduction of brain-derived neurotrophic factor (BDNF) but increased nerve growth factor concentrations (Beck Jr. et al., 2005a).
Neuronal precursor cells of the developing mouse olfactory epithelium express both IL-15 and IL15Rα; IL15Rα knockout mice have fewer mature olfactory neurons and proliferating cells (Umehara et al., 2009). Neuronal stem cells in SVZ also express IL15. IL15 knockout mice have reduced neurogenesis and accelerated differentiation of neural stem cells. This defect can be restored by intraventricular administration of IL15 (Gomez-Nicola et al., 2011). In vitro, IL15Rα is present in neurospheres derived from rat neural stem cells and in differentiating neurons, but not in astrocyte or oligodendrocyte progenitors. IL15 acts through STAT3 signaling and reduction of MAP-2 protein to reduce neurite outgrowth in differentiating neurons (Huang et al., 2009). The effects of IL15 in promoting neurogenesis contrast with an inhibitory effect of some proinflammatory cytokines. For example, IL1β has a suppressive effect on neurogenesis, and this is at least partly mediated by upregulation of kynurenine pathways (Eyre and Baune, 2012; Zunszain et al., 2012).
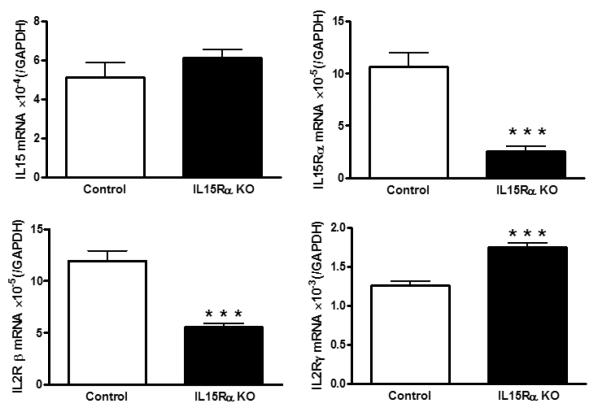
The pleiotropic function of IL15 signaling is reflected in compensatory changes of co-receptors when one receptor subunit is deleted embryonically. As shown in figure 4, IL15Rα deletion significantly reduces but does not completely abolish the level of IL15Rα mRNA, probably related to the presence of multiple splice variants. IL15 mRNA is unchanged; however, there is reduction of IL2Rβ and increase of IL2Rγ transcripts. The mechanisms underlying the differential regulation of receptor isoforms are not yet clear, but it appears that IL2Rγ overactivity might partially explain the behavioral phenotype of the IL15Rα knockout mice.
Fig.4.
In the hippocampus of the IL15Rα knockout (KO) mice, IL15 mRNA did not show a significant change. IL15Rα itself as well as the co-receptor IL2Rβ were reduced, whereas another co-receptor IL2Rγ was increased. ***: p < 0.001.
6. Summary and implications
In this review, we show that cerebral IL15 modulates neurotransmission and facilitates early consolidation of hippocampal memory. IL15 signaling also benefits mood stability; knockout mice show depressive-like behavior and reduced normal anxiety. In addition, IL15 contributes to thermoregulation in the hypothalamus and curtails hyperthermia. During neuroinflammatory challenge, such as occurs 4 – 48 h after LPS treatment, there is upregulation of cerebral IL15 receptors that help to limit metabolic consequences. Thus, cerebral IL15 links peripheral inflammatory and immune challenges to the maintenance of neurotransmission and hippocampal function. We hope that study of the cerebral IL15 system will provide better understanding of the cellular and biochemical mechanisms underlying behavioral changes commonly associated with neuroinflammation. These studies have direct implications to human situations of sickness behavior, neuropsychiatric disorders, and cognitive decline.
Highlights.
•Cerebral IL15 modulates neurotransmission and facilitates hippocampal memory
•IL15 signaling benefits mood stability, prevents depressive-like behavior and apathy
•IL15 contributes to thermoregulation in the hypothalamus and curtails hyperthermia
•Neuroinflammation upregulates cerebral IL15 receptors to limit metabolic consequences
Acknowledgement
The IL15 studies generated from our laboratory are supported by NIH funding (NINDS 62291 to WP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aringer M, Stummvoll GH, Steiner G, Koller M, Steiner CW, Hofler E, Hiesberger H, Smolen JS, Graninger WB. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:876–881. doi: 10.1093/rheumatology/40.8.876. [DOI] [PubMed] [Google Scholar]
- Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc Natl Acad Sci U S A. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF-and its receptors. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1056–1064. doi: 10.1002/ajmg.b.30712. [DOI] [PubMed] [Google Scholar]
- Beck RD, Jr., King MA, Ha GK, Cushman JD, Huang Z, Petitto JM. IL-2 deficiency results in altered septal and hippocampal cytoarchitecture: relation to development and neurotrophins. J Neuroimmunol. 2005a;160:146–153. doi: 10.1016/j.jneuroim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Beck RD, Jr., Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005b;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28:362–377. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval. Eur J Neurosci. 2007;25:2483–2490. doi: 10.1111/j.1460-9568.2007.05493.x. [DOI] [PubMed] [Google Scholar]
- Chierakul W, de FM, Suputtamongkol Y, Limpaiboon R, Dondorp A, White NJ, van der Poll T. Differential expression of interferon-gamma and interferon-gamma-inducing cytokines in Thai patients with scrub typhus or leptospirosis. Clin Immunol. 2004;113:140–144. doi: 10.1016/j.clim.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Clement HW, Buschmann J, Rex S, Grote C, Opper C, Gemsa D, Wesemann W. Effects of interferon-gamma, interleukin-1 beta, and tumor necrosis factor-alpha on the serotonin metabolism in the nucleus raphe dorsalis of the rat. J Neural Transm. 1997;104:981–991. doi: 10.1007/BF01273312. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1beta, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience. 1998;84:923–933. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero OJ, Salgado FJ, Mera-Varela A, Nogueira M. Serum interleukin-12, interleukin-15, soluble CD26, and adenosine deaminase in patients with rheumatoid arthritis. Rheumatol Int. 2001;21:69–74. doi: 10.1007/s002960100134. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- Eyre H, Baune BT. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-alpha. Physiol Behav. 1998;63:571–576. doi: 10.1016/s0031-9384(97)00514-3. [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Valle-Argos B, Nieto-Sampedro M. Blockade of IL-15 activity inhibits microglial activation through the NFkappaB, p38, and ERK1/2 pathways, reducing cytokine and chemokine release. Glia. 2010;58:264–276. doi: 10.1002/glia.20920. [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D, Valle-Argos B, Pallas-Bazarra N, Nieto-Sampedro M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol Biol Cell. 2011;22:1960–1970. doi: 10.1091/mbc.E11-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Valle-Argos B, Pita-Thomas DW, Nieto-Sampedro M. Interleukin 15 expression in the CNS: blockade of its activity prevents glial activation after an inflammatory injury. Glia. 2008;56:494–505. doi: 10.1002/glia.20628. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Lyons SA, Prinz M, Nolte C, Weber JR, Kettenmann H, Kirchhoff F. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. J Biol Chem. 1997;272:28853–28860. doi: 10.1074/jbc.272.46.28853. [DOI] [PubMed] [Google Scholar]
- Hayley S, Brebner K, Lacosta S, Merali Z, Anisman H. Sensitization to the effects of tumor necrosis factor-alpha: neuroendocrine, central monoamine, and behavioral variations. J Neurosci. 1999;19:5654–5665. doi: 10.1523/JNEUROSCI.19-13-05654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Hsuchou H, Wu X, Kastin AJ, Khan RS, Pistell PJ, Wang W-H, Feng J, Li Z, Guo X, Pan W. Interleukin-15 receptor is essential to facilitate GABA transmission and hippocampal dependent memory. J Neurosci. 2010a;30:4725–4734. doi: 10.1523/JNEUROSCI.6160-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wu X, Khan RS, Kastin AJ, Cornélissen GG, Hsuchou H, Robert B, Halberg F, Pan W. IL15 receptor deletion results in circadian changes of locomotor and metabolic activity. J Mol Neurosci. 2010b;41:315–321. doi: 10.1007/s12031-009-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Wu X, Kastin AJ. Cessation of blood-to-brain influx of interleukin-15 during development of EAE. J Cereb Blood Flow Metab. 2009;29:1568–1578. doi: 10.1038/jcbfm.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Cheng SN, Chueh SH, Tsai YL, Liou NH, Guo YW, Liao MH, Shen LH, Chen CC, Liu JC, Ma KH. Effects of interleukin-15 on neuronal differentiation of neural stem cells. Brain Res. 2009;1304:38–48. doi: 10.1016/j.brainres.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Huang Z, Ha GK, Petitto JM. IL-15 and IL-15R alpha gene deletion: effects on T lymphocyte trafficking and the microglial and neuronal responses to facial nerve axotomy. Neurosci Lett. 2007;417:160–164. doi: 10.1016/j.neulet.2007.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Galina ZH, Horvath A, Olson RD. Some principles in the peptide field. J Allergy Clin Immunol. 1987;79:6–11. doi: 10.1016/s0091-6749(87)80007-6. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2000;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Matusevicius D, He B, Soderstrom M, Fredrikson S, Link H. IL-15 mRNA expression is up-regulated in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis (MS) Clin Exp Immunol. 1998;111:193–197. doi: 10.1046/j.1365-2249.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik B, Skowronski J, Chwiecko J. Serum cytokines in different histological variants of rheumatoid arthritis. J Rheumatol. 2001;28:1211–1217. [PubMed] [Google Scholar]
- Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1004–R1012. doi: 10.1152/ajpregu.2001.281.3.R1004. [DOI] [PubMed] [Google Scholar]
- Kurowska M, Rudnicka W, Maslinska D, Maslinski W. Expression of IL-15 and IL-15 receptor isoforms in select structures of human fetal brain. Ann N Y Acad Sci. 2002;966:441–445. doi: 10.1111/j.1749-6632.2002.tb04245.x. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Influence of acute and repeated interleukin-2 administration on spatial learning, locomotor activity, exploratory behaviors, and anxiety. Behav Neurosci. 1999;113:1030–1041. doi: 10.1037//0735-7044.113.5.1030. [DOI] [PubMed] [Google Scholar]
- Lee YB, Satoh J, Walker DG, Kim SU. Interleukin-15 gene expression in human astrocytes and microglia in culture. NeuroReport. 1996;7:1062–1066. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Koerner H, Baune BT. The effects of TNF deficiency on age-related cognitive performance. Psychoneuroendocrinology. 2009;34:615–619. doi: 10.1016/j.psyneuen.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Merrill JE. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev Neurosci. 1992;14:1–10. doi: 10.1159/000111642. [DOI] [PubMed] [Google Scholar]
- Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159:2994–2999. [PubMed] [Google Scholar]
- Morar B, Schwab SG, Albus M, Maier W, Lerer B, Wildenauer DB. Evaluation of association of SNPs in the TNF alpha gene region with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:318–324. doi: 10.1002/ajmg.b.30451. [DOI] [PubMed] [Google Scholar]
- Mossner R, Heils A, Stober G, Okladnova O, Daniel S, Lesch KP. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem Int. 1998;33:251–254. doi: 10.1016/s0197-0186(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis. 2009;24:55–68. doi: 10.1007/s11011-008-9114-5. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNF alpha transport across the blood-brain barrier. Exp Neurol. 2006;198:222–233. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Yu C, Kastin AJ. Permeation of blood-borne IL15 across the blood-brain barrier and the effect of LPS. J Neurochem. 2008;106:313–319. doi: 10.1111/j.1471-4159.2008.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Upregulation of the transport system for TNFα at the blood-brain barrier. Arch Physiol Biochem. 2001;109:350–353. doi: 10.1076/apab.109.4.350.4238. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor α transport across the blood-brain barrier after acute compressive spinal cord injury. J Neurosci. 1999;19:3649–3655. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, McLay RN, Rigai T, Pick CG. Increased hippocampal uptake of TNFα and behavioral changes in mice. Exp Brain Res. 2003;149:195–199. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuhou H, Khan RS, Kastin AJ. Cerebral microvascular IL15 is a novel mediator of TNF action. J Neurochem. 2009;111:819–827. doi: 10.1111/j.1471-4159.2009.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto JM, Huang Z, Hartemink DA, Beck R., Jr IL-2/15 receptor-beta gene deletion alters neurobehavioral performance. Brain Res. 2002;929:218–225. doi: 10.1016/s0006-8993(01)03393-5. [DOI] [PubMed] [Google Scholar]
- Petitto JM, McNamara RK, Gendreau PL, Huang Z, Jackson AJ. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J Neurosci Res. 1999;56:441–446. doi: 10.1002/(SICI)1097-4547(19990515)56:4<441::AID-JNR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Petrovic-Rackov L, Pejnovic N. Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin Rheumatol. 2006;25:448–452. doi: 10.1007/s10067-005-0106-0. [DOI] [PubMed] [Google Scholar]
- Rachal PC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, Dimitrakopoulos A, Triantafyllou N, Vassilopoulos D. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. J Neurol Sci. 2006;241:25–29. doi: 10.1016/j.jns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kurohara K, Yukitake M, Kuroda Y. Interleukin-15, a T-cell growth factor, is expressed in human neural cell lines and tissues. J Neurol Sci. 1998;155:170–177. doi: 10.1016/s0022-510x(97)00310-9. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Stoklasek T, Lefrancois L. The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both? Int J Biochem Cell Biol. 2005;37:1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KP, Kastin AJ, Pan W. NFêB is an unexpected major mediator of interleukin-15 signaling in cerebral endothelia. 2011 doi: 10.1159/000331720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Nishimura H, Kimura Y, Mokuno Y, Washizu J, Itohara S, Nimura Y, Yoshikai Y. Protective roles of gamma delta T cells and interleukin-15 in Escherichia coli infection in mice. Infect Immun. 1998;66:3270–3278. doi: 10.1128/iai.66.7.3270-3278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Takase K, Matsumoto I, Fujita T, Ajiki T, Fujino Y, Kuroda Y. Serum interleukin-15 level is a useful predictor of the complications and mortality in severe acute pancreatitis. Surgery. 2007;142:319–326. doi: 10.1016/j.surg.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Umehara T, Udagawa J, Takamura K, Kimura M, Ishimitsu R, Kiyono H, Kawauchi H, Otani H. Role of interleukin-15 in the development of mouse olfactory nerve. Congenit Anom (Kyoto) 2009;49:253–257. doi: 10.1111/j.1741-4520.2009.00246.x. [DOI] [PubMed] [Google Scholar]
- Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Wu X, Hsuchou H, Kastin AJ, He Y, Khan RS, Stone KP, Cash MS, Pan W. Interleukin-15 affects serotonin system and exerts antidepressive effects through IL15Ralpha receptor. Psychoneuroendocrinol. 2011;36:266–278. doi: 10.1016/j.psyneuen.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, He Y, Hsuchou H, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun. 2010a;24:1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, Hsuchou H, Pan W. The effects of IL2Rgamma knockout on depression and contextual memory. Behav Brain Res. 2010b;213:319–322. doi: 10.1016/j.bbr.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, He Y, Hsuchou H, Kastin AJ. Cerebral interleukin-15 shows upregulation and beneficial effects in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010c;223:65–72. doi: 10.1016/j.jneuroim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, Stone KP, Zhang Y, Hsuchou H, Kastin AJ. Expression and signaling of novel IL15Rα splicing variants in cerebral endothelial cells of the blood-brain barrier. J Neurochem. 2010d;114:122–129. doi: 10.1111/j.1471-4159.2010.06729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttge DM, Wildt M, Geborek P, Wollheim FA, Scheja A, Akesson A. Serum IL-15 in patients with early systemic sclerosis: a potential novel marker of lung disease. Arthritis Res Ther. 2007;9:R85. doi: 10.1186/ar2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]