Abstract
The pathogenic hallmark of systemic lupus erythematosus (SLE or lupus) is the autoimmune response against self nuclear antigens, including dsDNA. The increased expression of the pro-inflammatory cytokine IL-1β has been found in the cutaneous lesion and peripheral blood mononuclear cells from lupus patients, suggesting a potential involvement of this cytokine in the pathogenesis of lupus. IL-1β is produced primarily by innate immune cells like monocytes and can promote Th17 cell response, which is increased in lupus. IL-1β production requires cleaving pro-IL-β into IL-1β by the caspase-1-associated multiprotein complex called inflammasomes. Here we show that self dsDNA induces IL-1β production from human monocytes dependently of serum or purified IgG containing anti-dsDNA antibodies by activating the NLRP3 inflammasome. Reactive oxygen species (ROS) and K+ efflux were involved in this activation. Knocking down the NLRP3 or inhibiting caspase-1, ROS and K+ efflux decreased IL-1β production. Supernatants from monocytes treated with a combination of self dsDNA and anti-dsDNA antibody-positive serum promoted IL-17 production from CD4+ T cells in an IL-1β dependent manner. These findings provide new insights in lupus pathogenesis by demonstrating that self dsDNA together with its autoantibodies induces IL-1β production from human monocytes by activating the NLRP3 inflammasome through inducing ROS synthesis and K+ efflux, leading to the increased Th17 cell response.
Introduction
The innate immune cells like monocytes, macrophages and dendritic cells (DCs) provide the first line of defense against microorganisms. These cells are armed with the germ line-encoded pattern recognition receptors (PRRs) which recognize pathogen-associated molecular patterns (PAMPs) commonly found in microorganisms (1, 2). Different classes of PRRs have been identified. These receptors include Toll-like receptors (TLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and absent in melanoma 2 (AIM2) (1–3). TLRs that exist on the cell surface or within the intracellular vesicular compartments, such as endosomes and lysosomes, recognize PAMPs present outside of cells or delivered into these compartments (1). RLRs, NLRs and AIM2, which are located in the cytosol, can detect PAMPs within the cytosol (1, 3).
Inflammasomes are multimeric protein complexes with the capacity to activate the caspase-1 that cleaves pro-IL-1β into IL-1β (2, 4). Different types of inflammasomes contain distinct PRRs responsible for the activation of the inflammasomes. For instance, the NLR family pyrin domain (PYD)-containing 3 (NLRP3) is associated with the NLRP3 inflammasome while AIM2 is found in the AIM2 inflammasome (2, 4). An array of molecules from host and environments as well as from microorganisms has been reported as inflammasome activators. AIM2 inflammasome is activated by cytosolic dsDNA from host and pathogens through its binding to C-terminal HIN domain of AIM2 (5, 6). Activators of the NLRP3 inflammasome are heterogeneous, ranging from self-originating uric acid, calcium pyrophosphate crystals, cholesterol crystals, ATP and glucose to environment-derived alum, silica and asbestos as well as molecules from pathogens (reviewed in (2, 4)). Although it is yet to be determined how molecules with such diverse structures could activate the NLRP3 inflammasome, reactive oxygen species (ROS) and K+ efflux appear to be important mediators for the activation of the NLRP3 inflammasome (7).
Systemic lupus erythematosus (SLE or lupus) is an autoimmune inflammatory disease of unknown etiology that affects multiple organs including the joint, skin, kidneys and hematologic system (8). The immunologic hallmark of lupus is autoantibodies against nuclear proteins and dsDNA. In particular, anti-dsDNA antibodies and circulating dsDNA/anti-dsDNA immune complexes are found in lupus patients (9, 10). A correlation of disease activity with titers of anti-dsDNA antibodies has been found in lupus patients (11, 12), suggesting a pathogenic role of these antibodies. In fact, the immune stimulatory property of dsDNA has been reported (10, 13–18). In the presence of anti-dsDNA antibodies, self dsDNA stimulated B cells and plasmacytoid DCs (pDCs) dependently of TLR9, leading to increased antibody and IFN-α production, respectively (10, 13, 14, 17). In addition, dsDNA from self and non-self could activate cytosolic AIM2 inflammasome in innate immune cells and keratinocytes when the cells were infected with virus or transfected with plasmid or host DNA in the presence of DOTAP (5, 6, 18–20). The production of IL-1β from the THP-1 cells and murine macrophages infected with adenovirus, a non-enveloped DNA virus, was dependent in part on the NLRP3 inflammasome, suggesting an activation of this inflammasome by DNA (21). Of interest, increased IL-1β gene or protein expression was found in the peripheral blood mononuclear cells (PBMCs) and skin lesions of lupus patients (22, 23). Similarly, ilb gene was detected in the nephritis tissues from lupus-prone mice (24–26). In addition, Th17 cell response, which is promoted by IL-1β, was increased in lupus patients(27–31). These observations raise the potential involvement of IL-1β and inflammasomes in the pathogenesis of lupus.
In the current study, we investigated whether and how self dsDNA, a molecular target of autoimmune responses in lupus, could induce IL-1β production from human monocytes, a major cellular source of IL-1β. Our results show that self dsDNA can induce IL-1β production from human monocytes in the presence of anti-dsDNA antibodies by activating the NLRP3 inflammasome. ROS and K+ efflux were responsible for this activation. Knocking down the NLRP3 or inhibiting ROS, K+ efflux, caspase-1 or TLR9 pathway decreased IL-1β production. In addition, supernatants from monocytes treated with dsDNA and anti-dsDNA antibody-positive serum promoted IL-17 production from CD4+ T cells in an IL-1β dependent manner. The results of our study indicate the potential role of IL-1β and NALP3 inflammasome in the pathogenesis of lupus by demonstrating that dsDNA together with its autoantibodies induces IL-1β production from human monocytes by activating the NLRP3 inflammasome through ROS synthesis and K+ efflux, leading to the increased Th17 response.
Materials and Methods
Human monocytes and sera
This work was approved by the institutional review committee of Yale University. Peripheral blood was obtained from the New York Blood Center or healthy adults after obtaining informed consent. Fresh monocytes were isolated from peripheral blood mononuclear cells (PBMCs) using a negative cell purification kit (Stem cell Technologies Inc, Canada). ANA-positive sera with or without anti-dsDNA antibodies were obtained from the L2 Diagnostic Laboratory. The presence of ANA and anti-dsDNA antibodies were determined by indirect immunofluorescence assay using Hep2 cells and Crithidia luciliae, respectively. Healthy human sera were obtained from peripheral blood of healthy adult donors.
Monocyte stimulation
Monocytes were resuspended in RPMI 1640 media supplemented with 10% FCS, penicillin and streptomycin at 5 × 105 cells/ml. Human genomic dsDNA was isolated from Jurkat and THP-1 cell lines using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). Monocytes were stimulated with or without human genomic dsDNA (5 μg/ml) in the presence or absence of serum (5% final concentration) or total IgG with or without anti-dsDNA antibodies. Total IgG was purified from sera using a NAb spin kit (Thermo scientific, Rockford, IL) according to the manufacturer’s instruction. To deplete anti-dsDNA antibodies from antibody-positive serum, serum was diluted with RPMI 1640 media (5% final concentration) and incubated overnight at 4°C in a sterile ELISA plate coated with dsDNA (20μg/ml). After the incubation, serum was collected and analyzed for anti-dsDNA antibodies by ELISA to determine levels of depletion. In some experiments, monocytes were additionally treated with the followings: ant-CD32 (FcγRII) antibodies (2.5 μg/ml, R&D systems, Minneapolis, MN) (10); NF-κB inhibitors (Bay11-7082 (5μM) and Cenostrol (5 μM), Invivogen, San Diego, CA); caspase-1 inhibitor (Ac-YVAD-CMK, 5μM, Enzo Life Sciences International Inc, PA); diphenyleneiodonium (DPI, 50 μM, Sigma-Aldrich); chloroquine (5μg/ml, Sigma-Aldrich), scavenger receptor blockers (tannic acid (20μM), dextran sulfate (50μg/ml), Sigma-Aldrich), and heparin (50U, Baxter, Deerfield, IL) (32–34); or inhibitory nucleic acid sequence for TLR9 (ODN, 5μM final concentration) (17). The TLR inhibitory sequence was pre-incubated for 15 min with DOTAP (Roche, Indianapolis, IN) at room temperature before adding to monocytes.
Purification and stimulation of IL-1 receptor I (IL-1RI)-positive memory CD4+ T cells
Human IL-1RI-positive memory (CD45RA−) CD4+ T cells were purified from PBMCs of healthy donors using a FACSAria as previously described (35). Purified cells were stimulated for 5 days with anti-CD3 and anti-CD28 antibody-coated beads in RPMI 1640 medium mixed at a 4:1 ratio with supernatants from stimulated monocytes in the presence or absence of IL-1R antagonist (100 ng/ml, R&D systems). IL-1R antagonist was re-added on day 2. Some IL-1RI+ memory CD4+ T cells were treated with culture medium alone or with anti-CD3 and anti-CD28 antibody-coated beads in the presence or absence of human recombinant IL-1β (20 ng/ml, R&D systems).
Measuring NF-κB activation
Monocytes were incubated for 4 hours with or without human genomic dsDNA in the presence or absence of healthy serum or anti-dsDNA antibody-positive serum (5% final concentration). This time point was determined based the results of a kinetic study (Supplemental Figure 2A). Following fixation and permeabilization, cells were stained with anti-phosphorylated NF-κB p65 antibodies (pS529) (BD bioscience, San Diego, CA). Cells were analyzed on a flow cytometer
Measuring caspase-1 activation and ROS synthesis
Monocytes were incubated for 7 hours (caspase-1) or for 3 to 4 hours (ROS) with or without healthy serum, anti-dsDNA antibody-positive serum (5% final concentration) or IgG purified from anti-dsDNA antibody-positive serum in the presence or absence of human genomic dsDNA. The active caspase-1 was detected by flow cytometry using FAM FLICA Caspase-1 assay kit (Immunochemistry Technologies Inc, Bloomington, MN) according to the manufacturer’s instruction. To measure ROS synthesis, stimulated cells were added with ROS detection reagent carboxy-H2DCFDA (C400) (Invitrogen) at the end of stimulation. Cells were then analyzed on a flow cytometer. The incubation time points were selected based the results of kinetic studies (Supplemental Figure 2B–C).
Knocking down the NLRP3 gene
Human monocytes were nucleofected with scrambed siRNA or NLRP3-specific siRNA (Invivogen) using the Amaxa Nucleofector System and a Human Monocyte Nucleofector Kit (Lonza, Walkersville, MD) according to the manufacturer’s instruction. Following 6 hours of resting, nucleofected cells were incubated for 18 hours with or without dsDNA and anti-dsDNA antibody-positive serum. Knock down of the NLRP3 gene was determined by Western blotting with anti-human NLRP3 antibody (Enzo life science) and qPCR with the following primers: forward primer 5′CCACAAGATCGTGAGAAAACCC3′ and reverse primer 5′ CGGTCCTATGTGCTCGTCA3′
ELISA and qPCR
IL-1β and IL-18 from monocytes and IL-17 from CD4+ T cells in culture supernatants were measured by ELISA (ebioscience, San Diego, CA). Pro-IL1B gene and pro-IL-1β protein in cell lysate were measured using real-time quantitative PCR and human pro-IL-1β ELISA kit (R&D systems), respectively. Briefly, total RNA was extracted from cells using RNeasy Plus Midi kit (QIAGEN) and cDNA was synthesized. Real-time quantitative PCR was done on an Mx3005P QPCR system (Stratagene, La Jolla, CA) using the 2XBrilliant SYBR green master mix (Stratagene). The primer sequences for the IL1B gene were: forward primer 5′CACGATGCACCTGTACGATCA3′ and reverse primer 5′GTTGCTCCATATCCTGTCCCT3′. The levels of gene expression were normalized to the expression of ACTINB (35). Also, the NLRP3 gene in monocytes was measured by qPCR using the primer sequences as described above. The comparative CT method (ΔΔCT) was used for quantification of gene expression.
Statistical analysis
Paired t-tests were performed for statistical analysis using Microsoft Excel. P values of less than 0.05 were considered statistically significant.
Results
Self dsDNA induces IL-1β production from human primary monocytes in the presence of anti-dsDNA antibodies
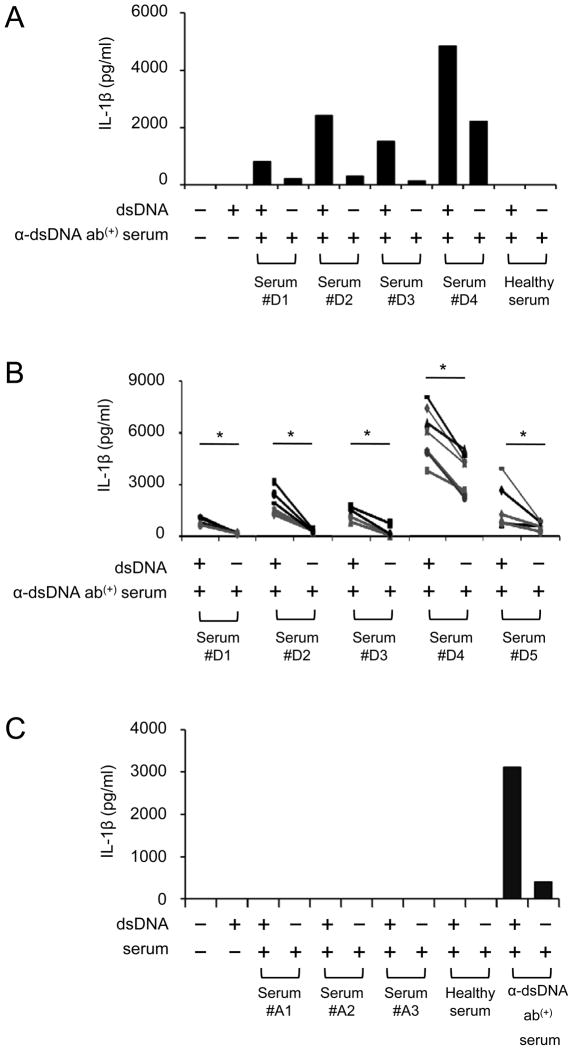
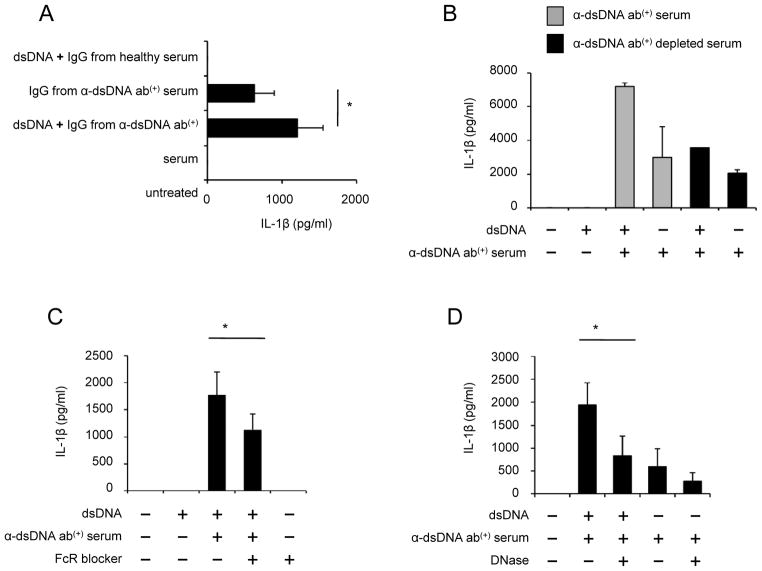
dsDNA from self and non-self could activate cytosolic AIM2 inflammasome in innate immune cells and keratinocytes when the cells were infected with virus or transfected with plasmid or host DNA in the presence of DOTAP (5, 6). We investigated whether human dsDNA alone could induce IL-1β production from human monocytes, which are a primary cellular source of this cytokine (36). dsDNA alone could not induce IL-1β production (Fig. 1). However, dsDNA induced IL-1β production from monocytes in the presence of serum containing anti-dsDNA antibodies (Fig. 1). IL-1β production was not detected from monocytes treated with dsDNA in the presence of healthy serum or serum positive for antinuclear antibodies (ANA) but negative for anti-dsDNA antibodies (Fig. 1C). Similarly, human monocytes produced IL-1β in response to dsDNA in the presence of IgG purified from anti-dsDNA antibody-positive serum (Fig. 2A). Next, we selectively depleted anti-dsDNA antibodies from the antibody-positiveserum by incubating the serum overnight in a plate coated with self dsDNA. Although we depleted these antibodies only partially (data not shown), it still decreased IL-1β production from monocytes treated with dsDNA (Fig. 2B). A previous study reported that immune complexes containing dsDNA bound the IgG receptor CD32 (FcgRII) on pDCs (10). Thus, we blocked CD32 on monocytes with anti-CD32 antibodies, which significantly reduced IL-1β production (Fig 2C). These data indicate that dsDNA induces IL-1β production from human monocytes in an anti-dsDNA antibody-dependent manner. Of interest, monocytes incubated with anti-dsDNA antibody-positive serum or IgG alone also produced IL-1β (Fig. 1A–Cfc and Fig 2A). However, the levels of IL-1β produced from such treated cells were lower than those produced from monocytes treated additionally with dsDNA. These findings suggest the presence of a complex of dsDNA and anti-dsDNA antibodies in anti-dsDNA-positive sera as previously reported (9, 10). Indeed, treating anti-dsDNA antibody-positive serum with DNase reduced IL-1β production from monocytes (Fig. 2D). Since anti-CD32 antibodies or DNase partially reduced IL-1β production from monocytes treated with dsDNA and anti-dsDNA antibody-positive serum, we considered scavenger receptor A as an alternative pathway for such IL-1β production. Previous studies reported the involvement of scavenger receptors in the uptake of nucleic acids and antigens into cells (32, 37). We found the expression of scavenger receptor A on human monocytes and decreased IL-1β production from monocytes treated with dsDNA and anti-dsDNA antibody-positive serum in the presence of known scavenger receptor A inhibitors including tannic acid, dextran sulfate and heparin (Supplemental Fig. 1A–B) (32–34).
Figure 1. Human monocytes produce IL-1β in response to self dsDNA in the presence of anti-dsDNA antibody-positive serum.
(A–C) Measuring IL-1β in cell culture supernatants of monocytes incubated for 18 hours in the following conditions by ELISA. (A–B) Monocytes purified from a single (A) or multiple healthy donors (B, symbols indicate individual donors) were incubated with human genomic dsDNA (5 μg/ml) in the presence or absence of healthy serum or anti-dsDNA antibody-positive serum (5% final concentration) from multiple donors (A, #D1–#D4; B, #D1–#D5). (C) ANA-positive sera without anti-dsDNA antibodies (donors, #A1–#A3) were added to monocytes from a single donor in the presence or absence of dsDNA. Representative data from 2 independent experiments (A and C). *P < 0.05.
Figure 2. Anti-dsDNA antibodies are required for IL-1β production from human monocytes treated with self dsDNA.
(A–D) Measuring IL-1β in cell culture supernatants of monocytes incubated for 18 hours in the following conditions by ELISA. (A) Monocytes from healthy donors were incubated with dsDNA in the presence or absence of total IgG purified from anti-dsDNA antibody-positive serum or healthy serum (n = 5). (B) Monocytes were incubated with dsDNA in the presence or absence of serum depleted or un-depleted of anti-dsDNA antibodies (2 independent experiments). (C) Monocytes were treated with anti-CD32 antibodies (FcR blocker, 2.5 μg/ml) and incubated with dsDNA and anti-dsDNA antibody-positive serum (n = 5). (D) Monocytes were incubated with anti-dsDNA antibody-positive serum or dsDNA and anti-dsDNA antibody-positive serum in the presence or absence of DNase (1 μg/ml) (n = 7). Bars and error bars indicate mean and SEM, respectively. *P < 0.05.
NF-κB and TLR9 are involved in producing IL-1β from human monocytes in response to a combination of self dsDNA and anti-dsDNA antibodies
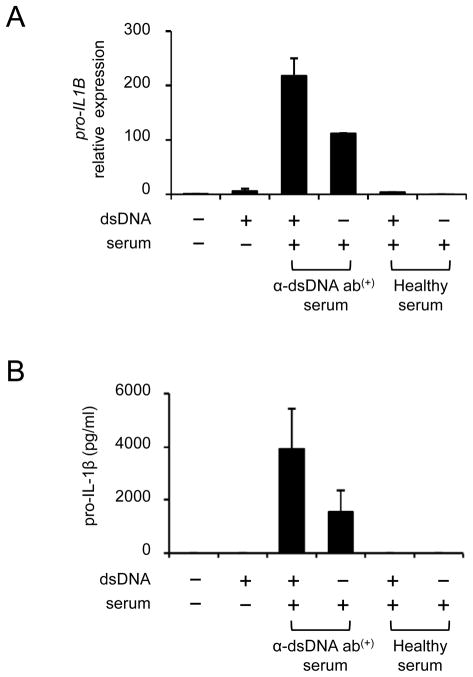
The production of IL-1β is tightly regulated at the transcriptional and post-translational levels through NF-κB and inflammasome activations, respectively (1). Triggering TLRs activates NF-κB, leading to increased synthesis of pro-IL-1β. Pro-IL-1β is then cleaved into the active form IL-1β by caspase-1 containing inflammasomes and secreted outside cells. Thus, we determined whether human monocytes stimulated with dsDNA and its antibodies had increased generation of pro-IL-1β gene and protein. Pro-IL1B transcripts were highly expressed in monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positiveserum although they were barely detected in monocytes treated with dsDNA alone or a combination of dsDNA and healthy serum (Fig. 3A). The same results were found when we measured pro-IL-1β protein in lysates from monocytes treated with dsDNA or a combination of dsDNA and anti-dsDNA antibody-positive serum (Fig. 3B).
Figure 3. Self dsDNA induces pro-IL-1β in human monocytes in the presence of anti-dsDNA antibody-positive serum.
(A–B) Monocytes from healthy donors were incubated for 6 (qPCR) or 10 (ELISA) hours with dsDNA (5 μg/ml) in the presence or absence of healthy or anti-dsDNA antibody-positive serum (5% final concentration). (A) Pro-IL1B gene expression was measured by qPCR. (B) Intracellular pro-IL-1β was measured using cell lysates by ELISA. Bars and error bars indicate mean and SEM, respectively (n = 2 and 3 independent experiments for A and B, respectively).
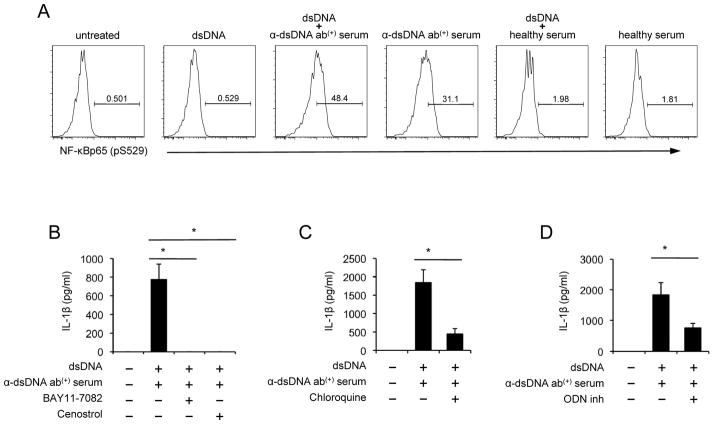
We next measured NF-κB activation in human monocytes treated with dsDNA in the presence or absence of anti-dsDNA antibody-positive serum or healthy serum. NF-κB was highly activated in monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum but not in monocytes incubated with dsDNA alone or with healthy serum in the presence or absence of dsDNA (Fig. 4A, Supplemental Fig. 2A). Although the activation of NF-κB was found in monocytes treated with anti-dsDNA antibody-positive serum alone, the levels of such activation were lower than those in monocytes treated additionally with dsDNA. Furthermore, IL-1β production from monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum or anti-dsDNA antibody-positive serum alone was blocked by NF-κB inhibitors (Fig. 4B, Supplemental Fig. 3). DNA is known to trigger TLR9 in the endosome, leading to NF-κB activation (1). Indeed, human monocytes expressed high levels of TLR9 (data not shown). The endosomal inhibitor chloroquine and inhibitory nucleic acid sequence for TLR9 significantly reduced IL-1β production from moncytes treated with dsDNA and anti-dsDNA antibody-positive serum (Fig. 4C and D, Supplemental Fig. 3). Our observations indicate the essential role of TLR9 and NF-κB in producing IL-1β by human monocytes in response to self dsDNA and anti-dsDNA antibodies.
Figure 4. The production of IL-1β from human monocytes by self dsDNA and anti-dsDNA antibody-positive serum is dependent on TLR9 and NF-κB activation.
(A–D) Monocytes were purified from healthy donors for the following experiments. (A) Monocytes were incubated for 4 hours with dsDNA in the presence or absence of healthy or anti-dsDNA antibody-positive serum. NF-κB activation (phosphorylation) was determined by flow cytometry. Numbers in histograms indicate the frequency (%) of cells stained for phosphorylated NF-κB (pS529). (B–D) Monocytes were incubated for 18 hours with dsDNA and anti-dsDNA antibody-positive serum in the presence or absence of the NF-κB inhibitors (B, 5 μM Bay11-7082 and 5 μM Cenostrol), chloroquine (C, 5 μg/ml) or inhibitory nucleic acid sequence for TLR9 (D, 5 μM ODN). IL-1β in cell culture supernatants were measured by ELISA. Representative data from 4 independent experiments (A). Bars and error bars indicate mean and SEM, respectively (n = 4, 7 and 8 for B, C and D, respectively). *P < 0.05.
The production of IL-1β by human monocytes in response to self dsDNA and anti-dsDNA antibodies requires caspase-1 and NLRP3
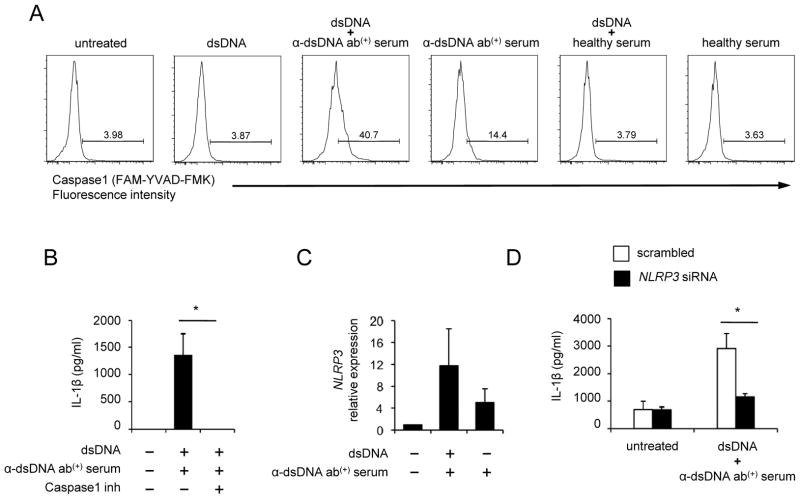
We investigated whether self dsDNA could activate caspase-1 in human monocytes in the presence or absence of anti-dsDNA antibody-positive serum. Increased caspase-1 activation was found in monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum but not in the cells treated with dsDNA alone or a combination of dsDNA and healthy serum (Fig. 5A, Supplemental Fig. 2B). Although increased caspase-1 activation was detected in monocytes treated with anti-dsDNA antibody-positive serum alone, the levels of such activation were lower than those in monocytes treated additionally with dsDNA. Similar findings were observed when monocytes were treated with IgG purified from anti-dsDNA antibody-positive serum (Supplemental Fig. 2D). Furthermore, caspase-1 inhibitor significantly suppressed IL-1β production from monocytes treated with dsDNA along with anti-dsDNA antibody-positive serum (Fig. 5B) or anti-dsDNA antibody-positive serum alone (Supplemental Fig. 3). Of interest, we noticed increased NLRP gene expression in monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum (Figure 5C), suggesting the role of the NLRP3 inflammasome in activating caspase-1. To determine whether this caspase-1 activation was dependent on the NLRP3 inflammasome, we knocked down NLRP3 expression in human primary monocytes (Supplemental Fig. 4). NLRP3 knockdown substantially reduced IL-1β production from monocytes stimulated with a combination of dsDNA and anti-dsDNA antibody-positive serum (Fig. 5D). These findings indicate that the NLRP3 inflammasome plays an important role in producing IL-1β from human monocytes in response to self dsDNA and anti-dsDNA antibodies.
Figure 5. Caspase-1 and NLRP3 are involved in the production of IL-1β from human monocytes in response to self dsDNA and anti-dsDNA-positive serum.
(A) Monocytes from a healthy donor were incubated for 7 hours with dsDNA in the presence or absence of healthy or anti-dsDNA antibody-positive serum. Active capspase-1 was measured using flow cytometry. Numbers in histograms indicate the frequency (%) of cells positive for active caspase-1. (B) IL-1β ELISA of culture supernatants from monocytes incubated for 18 hours with dsDNA and anti-dsDNA antibody-positive serum in the presence or absence of caspase-1 inhibitor (10 μM) (n = 4). (C) Measuring the NLRP3 gene expression in human monocytes incubated for 6 hours in the presence or absence of anti-dsDNA antibody-positive serum or a combination of dsDNA and anti-dsDNA antibody-positive serum by qPCR (n = 2). (D) IL-1β ELISA of culture supernatants from monocytes nucleofected with scrambled or NLRP3-specific siRNA followed by the incubation for 18 hours with dsDNA and anti-dsDNA antibody-positive serum (n = 6). Representative data from 4 independent experiments (A). Bars and error bars indicate mean and SEM, respectively. *P < 0.05.
ROS synthesis and potassium efflux are involved in producing IL-1β from human monocytes in response to self dsDNA and anti-dsDNA antibodies
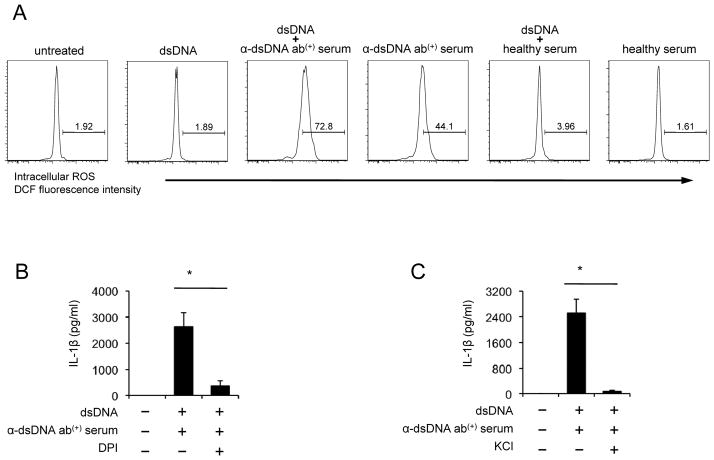
ROS has been suggested as a key mediator in the activation of the NLRP3 inflammasome (7). We measured intracellular ROS generation in human monocytes in response to self dsDNA and/or anti-dsDNA antibody-positive serum. The generation of ROS was increased in monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum compared to the cells treated with dsDNA or a combination of dsDNA and healthy serum (Fig. 6A, Supplemental Fig. 2C). However, monocytes treated with dsDNA or healthy serum with or without dsDNA could not increase ROS synthesis. Although ROS generation was detected in monocytes treated with anti-dsDNA antibody-positive serum alone, the levels of ROS generation were lower in these cells than those in monocytes treated additionally with dsDNA (Fig. 6A). We also measured ROS in monocytes treated with purified IgG from anti-dsDNA antibody-positiveserum in the presence of dsDNA. The increased generation of ROS was found in such treated monocytes (Supplemental Fig. 2E). Furthermore, blocking the generation of ROS with the NADPH oxidase inhibitor diphenylene lodonium (DPI) significantly decreased IL-1β production (Fig. 6B, Supplemental Fig. 3). In addition to ROS, potassium efflux has been suggested as an activator for the NLRP3 inflammasome (7). To study the role of potassium efflux in the IL-1β secretion, we suppressed potassium efflux by adding potassium to cell culture media. Indeed, increasing extracellular potassium significantly reduced IL-1β production from monocytes treated with the combination of dsDNA and anti-dsDNA antibody-positive serum (Fig. 6C). These results suggest the involvement of ROS and potassium efflux in inducing IL-1β production from human monocytes in response to dsDNA and anti-dsDNA antibodies by activating the NLRP3 inflammasome.
Figure 6. The production of IL-1β from human monocytes in response to self dsDNA and anti-dsDNA antibody-positive serum requires reactive oxygen species (ROS) synthesis and K+ efflux.
(A) Flow cytometric analysis of reactive oxygen species (ROS) in monocytes stimulated for 4 hours with dsDNA in the presence or absence of healthy or anti-dsDNA antibody-positive serum. (B–C) IL-1β ELISA of culture supernatants from monocytes incubated for 18 hours with dsDNA and anti-dsDNA antibody-positive serum in the presence or absence of the ROS inhibitor DPI (50 μM, B) or KCl (100 mM, C). Representative data from 3 independent experiments (A). Bars and error bars indicate mean and SEM, respectively (n = 5 and 7 for B and C, respectively). *P < 0.05.
IL-17 production from CD4+ T cells is promoted in the presence of culture supernatant from monocytes treated with dsDNA and its autoantibody-positive serum
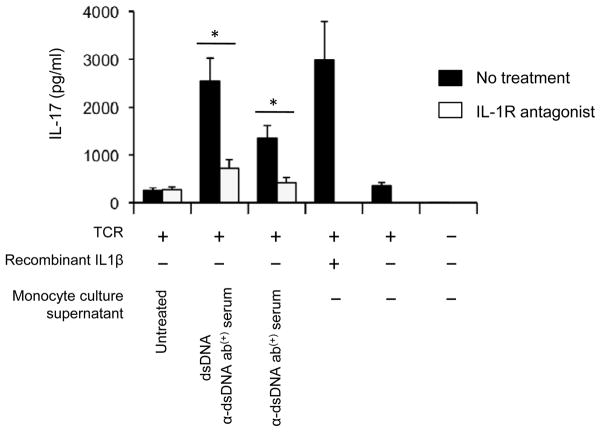
IL-1β promotes the differentiation of IL-17-producing Th17 cells (38, 39). Memory CD4+ T cells with the expression of IL-1RI potently produced IL-17, and IL-1β increased such cytokine production (35). A role for IL-17 in lupus pathogenesis has been suggested by recent studies showing enhanced antibody production from B cells by IL-17 (27) and increased Th17 cell response in lupus patients (27, 29, 30). However, the mechanism for the latter finding is unknown. Thus, we determined whether monocytes stimulated with a combination of dsDNA and anti-dsDNA antibody-positive serum could promote IL-17 production from IL-1R1+ memory CD4+ T cells through IL-1β production. Indeed, culture supernatant from such treated monocytes enhanced IL-17 production from IL-1R1+ memory CD4+ T cells, which was blocked by adding IL-1 receptor antagonist to the supernatant (Fig 7).
Figure 7. Cell culture supernatant from monocytes treated with a combination of self dsDNA and anti-dsDNA antibody-positive serum promotes IL-17 production from IL-1 receptor I (IL-1RI)-positive memory CD4+ T cells in an IL-1β-dependent manner.
ELISA of IL-17 production from sorted human IL-1RI+ memory CD4+ T cells that were stimulated for 5 days with anti-CD3 and -CD28 antibody-coated beads in 80% culture medium with 20% of supernatants from monocytes treated for 18 hours with self dsDNA in the presence or absence of anti-dsDNA antibody-positive serum. Some IL-1RI+ memory CD4+ T cells were incubated with culture medium alone or medium with anti-CD3 and anti-CD28 antibody-coated beads in the presence or absence of recombinant IL-1β (20 ng/ml). IL-1R antagonist (100 ng/ml) was added on days 0 and 2. Bars and error bars indicate mean and SEM, respectively (n = 5). The presence and absence of each treatment are indicated by + and −, respectively. *P < 0.05.
Discussion
Armed with the germ line-encoded PRRs that recognize PAMPs, innate immune cells such as monocytes provide the first line of host defense against microorganisms (1). In addition to PAMPs, some PRRs can be triggered by self molecules, called damage-associated molecular patters (DAMP), released in the setting of cell death (2). Inflammasomes are multimeric protein complexes with the capacity to activate caspase-1, which is required to mature and secrete the pro-inflammatory cytokine IL-1β by cleaving pro-IL-1β into IL-1β. Different types of inflammasomes contain distinct PRRs which are responsible for the activation of the inflammasomes by recognizing the ligands including nucleic acids (5, 6, 18, 21, 40–43). dsDNA is a molecular target of autoimmune responses in lupus that affects multiple organs including the skin and joints (8). In fact, anti-dsDNA antibodies and circulating dsDNA/anti-dsDNA immune complexes are frequently found in lupus patients (9, 10). Here we showed that self dsDNA could induce IL-1β production from human monocytes, a major cellular source of IL-1β, in the presence of anti-dsDNA antibodies by activating the TLR9 pathway and caspase-1-containing NLRP3 inflammasome. ROS and K+ efflux were likely responsible for the activation of the NLRP3 inflammasome in monocytes treated with self dsDNA and anti-dsDNA antibodies. Indeed, knocking down the NLRP3 or inhibiting ROS synthesis, K+ efflux, caspase-1 or TLR9 pathway decreased IL-1β production from such treated monocytes. Plus, Th17 cell response, which is known to be increased in lupus patients, was enhanced by supernatants from monocytes treated with dsDNA and anti-dsDNA antibody-positive serum in an IL-1β dependent manner. These findings elucidate a possible role of IL-1β and NLRP3 inflammasome in the pathogenesis of lupus as well as a mechanism involved in the production of such cytokine from monocytes in response to self dsDNA and its autoantibodies.
We showed that self dsDNA could stimulate human monocytes and induce IL-1β production in the presence of anti-dsDNA antibodies. The immune stimulatory property of dsDNA was reported in B cells, pDCs and keratinocytes (10, 13–17). In the presence of anti-dsDNA antibodies, self dsDNA stimulated B cells and pDCs dependently of TLR9, leading to increased antibody and IFN-α production, respectively (10, 13, 14, 17). In our study, treating dsDNA or anti-dsDNA antibody-positive serum with DNase suppressed IL-1β production from human monocytes, further supporting the immune stimulatory property of this molecule. Indeed, mice deficient of DNase I developed a lupus-like disease (44). Also, lupus patients with a nonsense mutation in one allele of DNase I had increased disease activity with reduced DNase I activity (45).
Previous studies showed the activation of AIM2 inflammasome by self and non-self dsDNA in innate immune cells and keratinocytes upon infection with virus or transfection with plasmid or host DNA (5, 6, 18–20). In addition, the NLRP3 inflammasome was involved in producing IL-1β from THP-1 cells and murine macrophages infected with adenovirus which is a non-enveloped DNA virus (21). DNA released from apoptotic hepatocytes induced pro-ilb gene expression in hepatocytes and liver sinusoidal endothelial cells from mice in a TLR9-dependent manner (46). In our study, knocking down NLRP3 or inhibiting TLR9 suppressed IL-1β production from monocytes in response to self dsDNA and anti-dsDNA antibody-positive serum. This clearly supports the role of the NLRP3 inflammasome and TLR9 in activating monocytes when self dsDNA are internalized into the cells dependently of its antibodies. Such an event is likely linked to the generation of ROS and K+ efflux, known NLRP3 inflammasome activators, as inhibiting ROS synthesis or K+ efflux decreased IL-1β production. In the generation of ROS, the endosome appears to be involved in that the endosomal inhibitor chloroquine reduced ROS synthesis by monocytes treated with dsDNA and anti-dsDNA antibody-positive serum (Supplemental Fig. 1C). Of interest, we noticed variability in the capacity to induce IL-1β production from dsDNA-treated monocytes among different anti-dsDNA antibody-positive sera (5% final concentration), ranging from below the level of detection to nanograms. Such variability could be related to the binding affinity of anti-dsDNA antibodies to dsDNA (11, 12). We found that self dsDNA alone could not activate NF-κB or induce IL-1β production from human monocytes unless anti-dsDNA antibodies were present. This finding could explain why inflammation may not occur in healthy people who do not have anti-dsDNA antibodies even though dsDNA could be released from dying cells. The NLRP3 inflammasome with active caspase-1 is also involved in cleaving pro-IL-18 to the active form IL-18 (2). Similarly to IL-1β, we detected IL-18 production from monocytes treated with a combination of dsDNA and anti-dsDNA antibody-positive serum or anti-dsDNA antibody-positive serum alone although the combination induced much higher levels of IL-18 production than the antibody-positive serum alone (Supplemental Fig. 2F).
IL-1β is a potent pro-inflammatory cytokine which can induce the expression of other pro-inflammatory molecules such as IL-6 and cycloxygenase-2 (47). Previous studies reported increased IL-1β gene or protein expression in PBMCs or skin lesions of lupus patients as well as in the kidneys of MRL-lpr and New Zealand Black/White mice with lupus-like disease (22–26). In an experimental mouse model of lupus induced by injecting anti-dsDNA monoclonal antibodies, nephritis was less severe in IL-1β-deficient mice compared to wild-type mice (48). In fact, decreased arthritis was noticed in patients with SLE who received recombinant IL-1 receptor antagonist anakinra (49). In a recent study, we also demonstrated IL-1β production from human monocytes in response to the autoantigen U1-small nuclear ribonucleoprotein (U1-snRNP) and anti-U1snRNP antibodies, which are found in lupus patients (50). These findings suggest an implication of IL-1β in the pathogenesis of lupus. Of interest, IL-1β is known to promote the development of T helper (Th) 17 cells which produce the pro-inflammatory cytokine IL-17. Patients with SLE had an increased frequency of Th17 cells in the peripheral blood that correlated with disease activity (28, 30). Plus, IL-17-producing T cells were found in the nephritis tissue from patients with SLE and lupus-prone mice (29, 51). Thus, it is conceivable that IL-1β produced from monocytes in response to a combination of dsDNA and anti-dsDNA antibodies could be a factor contributing to increased Th17 cell response in lupus. Indeed, in our study, IL-17 production was increased in human CD4+ T cells with the expression of IL-1 receptor I (35) by adding cell culture supernatant from monocytes-stimulated with dsDNA and anti-dsDNA antibody-positive serum. Such an increase was blocked by IL-1 receptor antagonist.
Taken together, we found that self dsDNA, a molecular target of autoimmune responses in lupus, could induce IL-1β production from human monocytes in the presence of anti-dsDNA antibodies. This phenomenon occurred by activating the TLR9 pathway and caspase-1-containing NLRP3 inflammasome that cleaves pro-IL-1β into mature IL-1β. The NLRP3 inflammasome activation by the self dsDNA and its autoantibodies was likely driven by ROS synthesis and K+ efflux. Indeed, the production of IL-1β from such treated monocytes was suppressed by knocking down the NLRP3 or inhibiting ROS synthesis, K+ efflux, caspase-1 or TLR9 pathway. Lastly, Th17 cell response was enhanced by supernatants from monocytes treated with dsDNA and anti-dsDNA antibody-positive serum in an IL-1β dependent manner. These findings provide new insights in lupus pathogenesis by linking the target autoantigen dsDNA to the NLRP3 inflammasome, IL-1β and IL-17 production. Our results also offer a scientific rationale for the therapeutic possibility of inhibiting this molecular pathway in human lupus.
Supplementary Material
Acknowledgments
We thank Ms. Laura Kramer and Yale Center for Clinical Investigation (UL1 RR024139) for assisting in the recruitment of human subjects. We are grateful to Dr. Mark Mamula for his critical review on the manuscript and Ms. Mary Lou Breitenstein for the identification of ANA-positive sera.
This work was supported in part by grants from the Department of Defense (W81XWH-10-1-0150) and the National Institute of Health (U19 AI082713, AI075157, AG028069, AT005241 and T32 AR007107). Insoo Kang is a participant of the World Class University Program of Republic of Korea.
Abbreviations used
- SLE
systemic lupus erythematosus
- ROS
reactive oxygen species
- PRRs
pattern recognition receptors
- PAMPs
pathogen-associated molecular patterns
- RLRs
retinoic acid-inducible gene (RIG)-I-like receptors
- NLRs
nucleotide-binding oligomerization domain (NOD)-like receptors
- AIM2
absent in melanoma 2
- NLRP3
NLR family pyrin domain (PYD)-containing 3
- pDCs
plasmacytoid DCs
- DPI
diphenyleneiodonium
Footnotes
Disclosures
The authors declare that they have no competing interests.
References
- 1.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis BK, Wen H, Ting JP. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 8.Kang I, Craft J. The Immunology of Systemic Lupus Erythematosus in the Autoimmune Diseases. In: Rose NR, MacKay IR, editors. The Autoimmune Diseases. 4. Elsevier; London: 2006. pp. 357–368. [Google Scholar]
- 9.Sano H, Takai O, Harata N, Yoshinaga K, Kodama-Kamada I, Sasaki T. Binding properties of human anti-DNA antibodies to cloned human DNA fragments. Scand J Immunol. 1989;30:51–63. doi: 10.1111/j.1365-3083.1989.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 10.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz LE, Gaipl US, Herrmann M. Predictive value of anti-dsDNA autoantibodies: importance of the assay. Autoimmun Rev. 2008;7:594–597. doi: 10.1016/j.autrev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 13.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of Autoreactive B Cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 14.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 20.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 22.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 23.Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, Nyberg F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–3645. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 24.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–3054. [PubMed] [Google Scholar]
- 25.Boswell JM, Yui MA, Endres S, Burt DW, Kelley VE. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988;141:118–124. [PubMed] [Google Scholar]
- 26.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470–3475. [PubMed] [Google Scholar]
- 27.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 29.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, Kang I. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanasescu C, Balanescu E, Balanescu P, Olteanu R, Badea C, Grancea C, Vagu C, Bleotu C, Ardeleanu C, Georgescu A. IL-17 in cutaneous lupus erythematosus. Eur J Intern Med. 2011;21:202–207. doi: 10.1016/j.ejim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Raycroft MT, Harvey BP, Bruck MJ, Mamula MJ. Inhibition of antigen trafficking through scavenger receptor A. J Biol Chem. 2012;287:5310–5316. doi: 10.1074/jbc.M111.316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thelen T, Hao Y, Medeiros AI, Curtis JL, Serezani CH, Kobzik L, Harris LH, Aronoff DM. The class A scavenger receptor, macrophage receptor with collagenous structure, is the major phagocytic receptor for Clostridium sordellii expressed by human decidual macrophages. J Immunol. 2010;185:4328–4335. doi: 10.4049/jimmunol.1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao Y, Karmali PP, Simberg D. Role of carbohydrate receptors in the macrophage uptake of dextran-coated iron oxide nanoparticles. Adv Exp Med Biol. 2012;733:115–123. doi: 10.1007/978-94-007-2555-3_11. [DOI] [PubMed] [Google Scholar]
- 35.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012 doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 40.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 44.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 45.Yasutomo K, Horiuchi T, Kagami S, Tsukamoto H, Hashimura C, Urushihara M, Kuroda Y. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28:313–314. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 46.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 48.Voronov E, Dayan M, Zinger H, Gayvoronsky L, Lin JP, Iwakura Y, Apte RN, Mozes E. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006;17:109–116. [PubMed] [Google Scholar]
- 49.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64:630–633. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, Kang I. U1-Small Nuclear Ribonucleoprotein Activates the NOD-like Receptor Family, Pyrin Domain-Containing 3 Inflammasome in Human Monocytes. J Immunol. 2012 doi: 10.4049/jimmunol.1103355. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, V, Kyttaris C, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.