Abstract
Little is known about initiation and completion among males who received the HPV vaccine on an off-label basis before 2009. This study utilized administrative claims data from a private insurance company to examine completion of the 3 dose HPV series among 514 males who initiated the vaccine between 2006 and May of 2009. Frequencies of HPV vaccination were examined and multivariate logistic regression estimated the odds of completing the entire series within 365 days of initiation. We found that only 21% of male initiators completed all 3 vaccine doses within 12 months and completion decreased over time. Series completion did not vary significantly by provider type. These findings suggest that difficulties may be encountered in fully vaccinating enough males to achieve adequate herd immunity in the future.
Keywords: HPV vaccines, human papillomavirus, papillomavirus vaccines, human papillomavirus vaccine L1, male HPV vaccine series completion
BACKGROUND
A small percentage of U.S. males elected to receive the HPV vaccine after the Food and Drug Administration (FDA) approved it for females in 2006 and before its approval for males in 2009. A nationally administered 2010 internet survey indicated that 2% of respondents’ adolescent sons had initiated HPV vaccination.[1] By 2011, however, 8.3% of 13–17 year old males in the US had initiated HPV vaccination, with 28% of those initiators having completed 3 doses.[2] Rates were lower among 19–26 year old males, with approximately 1% initiating the HPV vaccine by 2010.[3]
Almost no data are available, however, on the proportion of male initiators who completed all 3 doses of the HPV vaccine series prior to 2010. The purpose of this study was to examine the proportion of 9–26 year old insured males who completed the quadrivalent HPV vaccine series within 1 year among those who initiated the series between 2006 and 2009.
METHODS
Administrative insurance health record claims from one commercial insurance company that offers policies nationwide were used to identify all male enrollees who initiated the HPV vaccine between 2006 when it was approved for females and 2009, when the vaccine was approved for use in males by the FDA. Sociodemographic and socioeconomic data were not available in this dataset due to de-identification. There were 514 male cases that 1) had a Current Procedural Terminology (CPT) code (90649) indicating they had received a quadrivalent HPV vaccine 2) had been enrolled for 365 consecutive days from initiation of the HPV vaccine series, 3) were between 9 and 26 years old at first vaccination, and 4) initiated the vaccine series between January 1, 2006 and May 1, 2009. This study was exempted from full review by the institutional review board of the University of Texas Medical Branch, Galveston, TX.
Males with ≥3 claims for the HPV vaccine within 365 days of initiation were classified as completers. Completion was dichotomized with a 1 representing completers and 0 representing non-completing cases. Cases were categorized into four age groups: 9–12 years, 13–18 years, and 19–26 years. Healthcare providers that administered the first injection were categorized into: “obstetrician/gynecologist,” “pediatrician,” and “family care, internist, allied health, nurse practitioner.” The latter category included cases who received their first vaccine by family health practitioners, nurse practitioners, or in various emergency or convenience clinics.
Statistical Analyses
Frequencies of males that initiated the HPV vaccine was examined, and chi-square analysis was used to evaluate whether there were differences in completion status by age group and year of initiation. The proportion of males that received 1, 2, or completed the HPV vaccine series within 365 days was also graphed for each year of initiation. Logistic regression was used to estimate the odds of HPV vaccine series completion by age group and year of initiation. Data analyses for this study were completed using SAS ® 9.2 (SAS Institute Inc., Cary, NC, USA.)
RESULTS
The mean age of the 514 subjects in our study was 16.2 years (standard deviation = 4.1 years, median=15) at vaccine initiation. The proportion that completed the entire HPV vaccine series was 21% (Table 1). Of the 406 males who did not complete the HPV vaccine series, 10 received 3 vaccinations, but not within 365 days, so were considered non-completers. These males were not included in the “completers” category because their final vaccination occurred after the one year required enrollment period. The addition of these 10 to the completer’s category would have artificially inflated the proportion that completed.
Table 1.
Descriptive Characteristics of Males with 1 or More quadrivalent HPV Vaccinations (N=514)
| Total1 | Completed2 | Chi-Square | p-value | |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | |||
| Age Group | ||||
| 9–12 years at first vaccination | 91 (17.7) | 30 (33.0) | ||
| 13–18 years at first vaccination | 305 (59.3) | 60 (19.7) | ||
| 19–26 years at first vaccination | 118 (23.0) | 18 (15.2) | 10.5 | 0.005 |
| Provider Type | ||||
| Obstetrician/Gynecologist | 66 (12.8) | 14 (21.2) | ||
| Pediatrician | 278 (54.1) | 33 (19.4) | ||
| Family care, internist, allied health, nurse practitioner | 170 (33.1) | 34 (12.2) | 0.4 | 0.82 |
| Year at First Vaccination | ||||
| 2006 | 25 (4.9) | 9 (36.0) | ||
| 2007 | 265 (51.6) | 62 (23.4) | ||
| 2008 | 196 (38.1) | 35 (17.9) | ||
| 2009 | 28 (5.4) | 2 (7.1) | 8.7 | 0.03 |
Total number of males in sample for each category.
Completed human papillomavirus (HPV) vaccine series in <365 days.
Type of provider who initiated the HPV vaccine series.
Completion of the HPV vaccine series declined in later years of vaccine initiation (see figure). The proportion of males who received 2 vaccine doses as a proportion of those who initiated declined slightly after 2007, while the proportion of initiators who only received one HPV vaccine increased greatly among males who initiated between 2007 and 2009.
Figure 1.
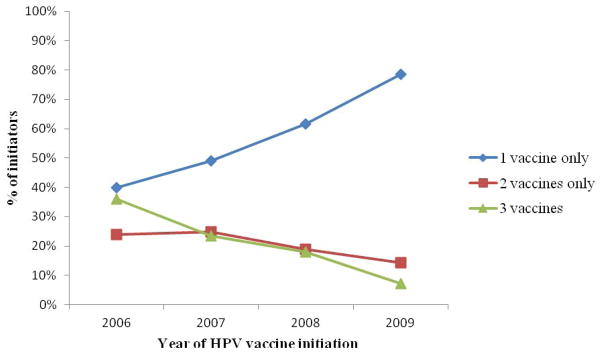
Figure Proportion of Males that Received 1, 2, or 3 Vaccines by Year of Initiation (N=514)
Older males were less likely to complete the series compared to 9–12 year olds (Table 2). The odds of completing did not vary significantly by provider type. Males who initiated the vaccine series in 2009 were more than 6 times less likely to complete all 3 injections compared to males who initiated in 2006. Males who initiated in 2008 were more than 2.5 times less likely to complete than 2006 initiators. Males who initiated the vaccine series in 2007, however, were as likely to complete the vaccine series as 2006 initiators.
Table 2.
Binary Logistic Regression Estimating Odds of Completing HPV 3 Vaccine Series in 365 Days (N=514)
| OR (95% CI)
|
|
|---|---|
| Age Group | |
| 9–12 years at first vaccination | Reference |
| 13–18 years at first vaccination | 0.52 (0.31–0.89) |
| 19–26 years at first vaccination | 0.34 (0.16–0.72) |
| Provider Type | |
| Obstetrician/Gynecologist | 1.39 (0.66–2.92) |
| Pediatrician | Reference |
| Family care, internist, allied health, nurse practitioner | 1.02 (0.62–1.69) |
| Year at First Vaccination | |
| 2006 | Reference |
| 2007 | 0.54 (0.24–1.30) |
| 2008 | 0.40 (0.16–1.00) |
| 2009 | 0.15 (0.03–0.79) |
Abbreviations: HPV=human papillomavirus, OR = odds ratio, 95% CI = 95% confidence interval, Reference=reference category
Among males who received only 2 vaccine doses, the mean time interval between the first and second injection was more than twice that of completers. Among males who received all three doses, but completed in more than 365 days, the interval between the first and second injection was greater than 2.5 times higher than that of completers. The mean time interval between the second and third dose of the vaccine was 288 days among males who received 3 injections outside of 365 days.
DISCUSSION
Consistent with Reiter et al.’s internet survey, which measured the proportion of adolescent males who initiated the HPV vaccine directly after FDA approval, we also found that a low proportion of males who initiated the series between 2006 and May of 2009 completed the quadrivalent HPV vaccine series within one year of initiation (21% in this study vs. 17% in Reiter et al.’s study).[1] The low proportion of completers in this study was somewhat surprising, since these patients, or their parents, must have been highly motivated to obtain the HPV vaccine due to the fact that they requested it before it was approved for males. It is possible that completion rates will improve among males now that it is both approved by the FDA and recommended for both genders by the Advisory Committee for Immunization Practices (ACIP).
Although our study focused on the off-label administration of the HPV vaccine, these results combined with evidence of low post-approval rates of vaccine initiation and completion indicate that it may be challenging to include males in the HPV vaccination effort.[1, 4, 5] Parents and physicians often are not aware of direct benefits of this vaccine for males, which is likely one reason for low levels of vaccination.[1, 6] Moreover, many physicians have indicated that they are reluctant to recommend this vaccine to males because they think it is a waste of time or that parents will not accept the vaccine for their male children.[6] Education about male HPV vaccination is needed because doctors who have offered the vaccine to parents of adolescent boys reported that a significant proportion did accept their recommendation to initiate the series.[6] In addition, strategies such as texting or calling to remind parents or older vaccine recipients about follow-up appointments could help increase completion of the vaccine series in this population. Permission to administer HPV vaccines in schools would also make it easier to administer all 3 of the vaccines within the recommended time limit.
Limitations of this study include lack of sociodemographic information about the enrollees and lack of information about motivation of physicians and enrollees. Further, the insurance company’s policy regarding the payment of claims for HPV vaccines for males was unclear until May of 2010 when the insurer issued a statement that it would not cover HPV vaccinations for males or adults ≥27 years old. Ambiguity of the insurance company’s policy before May of 2010 could have led to an underestimate of completion in our study. To address this, males who received their first vaccine after May, 2009 were excluded from the study, because data on vaccinations administered to males who elected to pay out-of-pocket after May 2010 would not have been available. We were also unable to verify with medical records that all participants in this study were male.[7]
In conclusion, only a small proportion of insured males completed the HPV vaccine series despite what must have been a strong motivation to initiate it before it was recommended or approved for use in this population. These findings, combined with low initial uptake of the vaccine series, [1–3, 5] show that it may be challenging to achieve a high rate of HPV vaccine series completion among males in the general population. However, broadening school administration of the vaccine, expanding the effort to remind patients of follow-up appointments through texts or calls, encouraging parents to vaccinate their sons, and educating physicians on the importance of recommending the HPV vaccine could help increase initiation and completion rates among males.
Highlights.
Male insurance records were used to estimate completion of the HPV vaccine series.
Association of age with completion of the HPV vaccine series was examined.
Association of initiation year with completion of the vaccine series was examined.
Acknowledgments
Federal support for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) as follows: Dr. Hirth is currently supported by ORWH (NICHD K12HD052023, PI: Berenson) and was supported at the time of initial submission by the NICHD through an institutional training grant (National Research Service Award T32HD055163, PI: Berenson); Dr. Berenson, under a mid-career investigator award in patient-oriented research (K24HD043659, PI: Berenson). This study also was supported by the Institute for Translational Sciences at the University of Texas Medical Branch, which is partially funded by a Clinical and Translational Science Award (UL1RR029876) from the National Center for Research Resources, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH.
Footnotes
There are no financial disclosures among the authors of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacqueline M. Hirth, Center for Interdisciplinary Research in Women’s Health, Obstetrics & Gynecology, University of Texas Medical Branch.
Alai Tan, Department of Preventive Medicine and Community Health, Senior Biostatistician, Sealy Center on Aging, University of Texas Medical Branch.
Gregg S. Wilkinson, Epidemiology and Biostatistics Group, University of Texas Medical Branch.
Abbey B. Berenson, Email: abberens@utmb.edu, Obstetrics & Gynecology, Director, Center for Interdisciplinary Research in Women’s Health. University of Texas Medical Branch, 301 University Blvd, Rte 0587, Galveston, TX 77573, Phone: 409-772-2417, Fax: 409-747-5129.
Bibliography
- 1.Reiter PL, McRee A-L, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29(34):5595–602. doi: 10.1016/j.vaccine.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years-United States, 2011. MMWR CDC Surveill Summ. 2012;61(34):671–7. [PubMed] [Google Scholar]
- 3.Williams WW, Lu P-J, Singleton JS, Bridges CB, Wortley P, Byrd KK, et al. Adult vaccination coverage - United States, 2010. MMWR CDC Surveill Summ. 2012;61(4):66–72. [Google Scholar]
- 4.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years-United States, 2009. MMWR CDC Surveill Summ. 2010;59(32):1018–23. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years-United States, 2010. MMWR CDC Surveill Summ. 2011;60(33):1117–23. [PubMed] [Google Scholar]
- 6.Perkins RB, Clark JA. Providers’ attitudes toward human papillomavirus vaccination in young men: challenges for implementation of 2011 recommendations. Am J Men’s Health. 2012 doi: 10.1177/1557988312438911. In press. [DOI] [PubMed] [Google Scholar]
- 7.Hirth JM, Tan A, Wilkinson G, Berenson A. Completion of the human papillomavirus vaccine series among insured females between 2006 and 2009. Cancer. 2012;118(22):5623–9. doi: 10.1002/cncr.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]


