Abstract
The mitogen-activated protein kinase p38α senses environmental stressors and orchestrates inflammatory and immunomodulatory reactions. However, the molecular mechanism how p38α controls immunomodulatory responses in myeloid cells remains elusive. We found that in monocytes and macrophages, p38α activated the mechanistic target of rapamycin (mTOR) pathway in vitro and in vivo. p38α signaling in myeloid immune cells promoted interleukin (IL)-10 but inhibited IL-12 expression via mTOR and blocked the differentiation of proinflammatory CD4+ T helper 1 cells. Cellular stress induced p38α-mediated mTOR activation that was independent of phosphoinositide 3-kinase (PI3K) but dependent on the kinase MK2 and on the inhibition of TSC1/TSC2 (tuberous sclerosis gene 1 and 2), a negative regulatory complex of mTOR signaling. Remarkably, p38α and PI3K concurrently modulated mTOR to balance IL-12 and IL-10 expression. Our data links p38α to mTOR signaling in myeloid immune cells that is decisive for tuning the immune response in dependence on the environmental milieu.
Introduction
Recognition of pathogen-associated molecular patterns (PAMPs) by innate immune receptors triggers inflammatory and immune responses involving several signaling molecules including the mitogen-activated protein kinases (MAPK) (1, 2). The MAPK p38α (also known as MAPK14) is one of four homologs of mammalian p38 and is essential in innate and adaptive immune signaling cascades (3). p38α is activated by diverse stimuli including Toll-like receptor (TLR) ligands, cytokines, and physicochemical stress signals such as ultraviolet (UV) irradiation, heat or osmotic shock, arsenite or anisomycin (4). p38α is ubiquitously expressed in most cell types and regulates diverse functions such as cell proliferation, differentiation, apoptosis, tissue repair, tumorigenesis, or inflammation (4, 5). For example, p38α was previously identified as activator of the proinflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, or cyclo-oxygenase 2 (6–8). Several kinases are activated by p38α such as mitogen- and stress-activated kinase (MSK) 1 and 2, MAPK-interacting serine/threonine-protein kinase (MNK) 1 and 2, or MAPK-activated protein kinase (MK) 2 and 3 that mediates TNF-α production (9, 10).
The finding that inhibiting p38 blocks lipopolysaccharide (LPS)-induced proinflammatory cytokine production (7) initiated the development of a wide range of p38 inhibitors for treatment of chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriasis or Crohn's disease (11). SB203580 is a competitive inhibitor of p38α and p38β by blocking ATP binding to the kinase, while BIRB796 is an allosteric inhibitor of p38α, p38β, and p38γ (12, 13). Remarkably, so far p38 inhibitors failed in clinical trials due to adverse and inflammatory side effects such as liver toxicity or skin rashes (11).
Recently, a more complex role of p38α has been reported (14–16). Expression of p38α in myeloid cells limits inflammation in an UV-induced irradiation model (14). These immunomodulatory effects of p38α may be mediated by the induction of the anti-inflammatory cytokine IL-10 (14, 15) and the inhibition of proinflammatory IL-12 (14, 16). However, the downstream pathway that controls coordinated IL-10 and IL-12 expression by p38α has remained elusive.
The classical insulin signaling pathway consisting of phosphoinositide 3-kinase (PI3K), Akt, and mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) has recently emerged as key regulator of innate immune cell homeostasis (17–20). Stimulation of innate immune cells by TLR ligands activates the mTOR pathway and it is in fact a major pathway activated in LPS-stimulated macrophages based on phosphoproteomics (21). The function of PI3K-Akt-mTOR is cell type-specific, but it has been shown that inhibition of PI3K by wortmannin or mTOR by rapamycin in myeloid cells, such as human monocytes, macrophages or myeloid dendritic cells, enhances IL-12 production but blocks the release of IL-10 in vitro and in vivo (22–28). Tuberous sclerosis 2 (TSC2) is a tumor suppressor that is phosphorylated and inactivated by the protein kinase Akt, which itself is activated by PI3K (29). TSC2 forms a heterodimeric complex with TSC1 and negatively regulates mTOR (29). Conversely, knockdown of TSC2 in human monocytes or macrophages enhances IL-10 but inhibits IL-12 production (24, 30). In line, genetic inactivation of mTORC1 reduces IL-10 production in intestinal CD11c+CD11b+ dendritic cells (31). On the other hand, TSC1-deficient macrophages show elevated production of TNF-α and IL-12p40 (32). Despite these observations, the precise regulatory units and upstream pathways controlling mTOR-dependent cytokine production are still unclear.
An outstanding question is how myeloid immune cells adapt and coordinate their immune response to an infectious trigger towards the status of the environmental milieu; e.g. how to avoid detrimental tissue-destructive CD4+ Th1 responses under conditions of tissue repair. Moreover, it is imperative to explore the molecular signaling pathways that regulate p38α-mediated immune responses for a deeper understanding of the effects of p38 inhibitors for human health and disease. Therefore, we tested whether p38α is connected to PI3K-TSC2-mTOR signaling to regulate innate inflammatory responses. We found that TLR ligands or environmental stress activate the TSC2-mTOR pathway via p38α and MK2 to regulate the balance of IL-12 and IL-10. Importantly, p38α acts in parallel to PI3K to control the IL-12/IL-10 equilibrium in response to the environmental milieu.
Material and Methods
Reagents
LPS (E. coli 0111:B4), wortmannin, anisomycin, and rapamycin were from Sigma. SAC (PANSORBIN) and SD169 were from Calbiochem. BIRB796 was a kind gift of Sir Philip Cohen or purchased from Axon Medchem. SB203580 was from Tocris Bioscience and IFN-γ from R&D. Heat-killed cells of Listeria monocytogenes (L.m.) were prepared by incubating the viable log-phase bacterial suspension at 70°C for 1 h. For UV exposure, cell culture plates were placed on a 20×20 UV-transilluminator (MWG Biotech) and activated with UV light for 10 sec, 30 sec or 1 min.
Cell culture
Human peripheral blood mononuclear cells (PBMC) and peripheral human myeloid DCs were isolated as described (24). Monocytes were isolated from PBMCs by MACS using CD14 Microbeads (Miltenyi Biotec). RPMI 1640 supplemented with 2 mM L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin (all from Gibco), and 10% FCS (Hyclone) was used as culture medium. Mouse embryonic fibroblasts (MEFs) were cultured in DMEM containing 4.5 g/L glucose, 2 mM L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% FCS. Tsc2+/+ p53−/− and Tsc2−/− p53−/− as well as Tsc1+/+ and Tsc1−/− MEFs were described previously (33, 34). p85α−/− p85β−/− MEFs were a kind gift of Lewis Cantley. p38αfl/fl and p38αΔM mice were described previously (14). Bone marrow-derived macrophages (BMDMs) from mice were isolated and grown as described (35) and were replated one day prior to stimulation in full medium containing 2% FCS. Mk2−/− immortalized murine macrophages stably reconstituted with MK2 or MK2K79R were described previously (36).
Measurement of cytokine production
Cells were pretreated for 90 min with the indicated concentrations of SB203580, BIRB796, rapamycin, or wortmannin and then stimulated with 100 ng/ml LPS (+30 ng/ml IFN-γ as indicated), 75 μg/ml SAC, or 107 L.m. in 48-well plates. Cell-free supernatants were collected after 22–24 h as indicated. Human and murine cytokines were determined by the Luminex bead system with beads from R&D Systems and Affymetrix and read on a Luminex 100 reader.
LPS injection
p38αΔM and p38αfl/fl mice were housed and maintained at the Massachusetts General Hospital and Harvard Medical School. Mice were injected intraperitoneally with 30 μg/mouse LPS. After 4 h, serum samples were taken and spleens were isolated. Cytokine levels in the sera were measured by Luminex. Homogenization of mouse tissue was performed by using the Precellys-ceramic kit 2.8 mm and the Precellys 24 tissue homogenizer (both from peQLab).
T cell differentiation
Monocytes were incubated with medium, 200 nM BIRB796, 2 μM SB203580 or 100 nM rapamycin for 90 min and stimulated with 100 ng/ml LPS for 24 h. The cells were then washed with PBS and incubated with allogeneic T lymphocytes at a ratio of 1:1 in 24-well plates in RPMI complete medium. After one week, IFN-γ production was determined in cell-free supernatants by Luminex. The primed cells were further activated for 5 h with 50 ng/ml phorbol-12-myristat-13-acetat (PMA) and 200 ng/ml ionomycin (both from Sigma) in the presence of 10 μg/ml brefeldin A (Sigma) for the last 3 h. Afterwards, cells were stained with FITC–labeled anti-IFN-γ, PE-labeled anti-IL-4, and APC-labeled anti-CD4 (all BD Bioscience) and analyzed by flow cytometry.
Analysis of signal transduction events
Monocytes, BMDMs, or 70% confluent MEFs starved overnight were treated and stimulated as indicated. Extract preparation and Western Blotting was done as described (24). Antibodies were p-p70S6K (Thr389), p70S6K, p-4E-BP1 (Thr37/46), p-p38 (Thr180/Tyr182), p-S6 (Ser 240/244), p-Akt (Ser473), p-MK2 (Thr334), GAPDH, S6-ribosomal protein, p38 MAPK, p38α MAPK, p38β MAPK, p38δ MAPK, Tuberin/TSC2 (all Cell Signaling Technology), p-Erk (Tyr204), IkBα and p38 (Santa Cruz Biotechnology).
Quantitative RT-PCR
RNA from human monocytes or murine immortalized macrophages was extracted in TRIzol (Invitrogen). cDNA was generated by Superscript II (Invitrogen). mRNA levels were determined by TaqMan Gene Expression Assays (Applied Biosystems) on a StepOnePlus™ Real-Time PCR System and normalized to ubiquitin.
Transfection of MEFs
Tsc2−/− MEFs in 6-well plates at 20–40% confluency were transfected in DMEM without antibiotics with 1μg pcDNA3-HA-TSC2 WT, pcDNA3-HA-TSC2 S1210A, or empty vector) with Lipofectamine 2000 Reagent (Invitrogen) for 36h and afterwards starved for 12 h in DMEM without antibiotics and FCS before stimulation.
Immunoflourescence microscopy
Cells were applied to 8-well Permanox™ chamber slides (Lab-Tek Chamber Slide System), fixed with 4% paraformaldehyde, quenched with 100mM glycine, permeabilized with methanol, blocked with 1% BSA, and stained with p-S6 antibody, p-MK2 antibody or isotype control overnight at 4° C. Cells were stained with AlexaFluor488-labeled goat anti-rabbit IgG (Invitrogen) followed by nuclear tracking using 0.1 μg/ml Hoechst-33342 (Invitrogen) and mounted in Vectashield® mounting medium.
Statistics
Results are expressed as means ± SEM. Student's t test was used to detect statistical significance.
Results
p38α modulates IL-12 and IL-10 production in mice and men
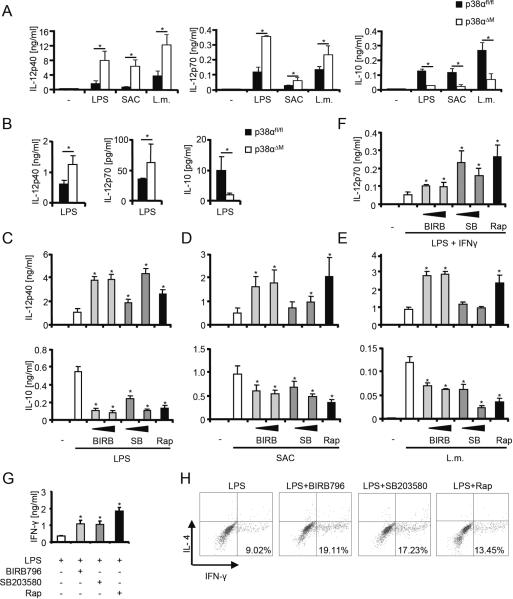
To evaluate the role of p38α in the myeloid immune system, we generated bone marrow-derived macrophages (BMDM) from mice with a deletion of p38α in cells expressing the lysozyme M gene (p38αΔM) and stimulated these cells with LPS, Staphylococcus aureus (SAC), or heat-killed Listeria monocytogenes (L.m.) (Fig. 1A). Deficiency of p38α enhanced IL-12p40 and IL-12p70 expression while the anti-inflammatory cytokine IL-10 was blocked compared to controls carrying homozygously the floxed p38α gene (p38αfl/fl) (Fig. 1A). Other cytokines such as IL-1β, IL-23, or TNF-α were not significantly altered (data not shown). Injection of LPS into p38αΔM mice similarly deviated the production of IL-12 and IL-10 in vivo (Fig. 1B). Next, we characterized the precise function of p38α, which is the most highly expressed p38 isoform in human monocytes. We found that inhibition of p38 with different concentrations of SB203580 or BIRB796 as well as with the mTOR inhibitor rapamycin strongly increased the production of IL-12p40 and IL-12p70 but blocked secretion of IL-10 after stimulation with LPS, SAC, or L.m. (Fig. 1,C–F). Notably, SB203580 did not augment IL-12p40 production in L.m.-stimulated monocytes. Enhanced IL-12p40 but reduced IL-10 expression was also observed in SB203580- or BIRB796-treated peripheral human myeloid dendritic cells stimulated with LPS, SAC or L.m. (data not shown).
FIGURE 1.
p38α modulates IL-12 and IL-10 in mice and men. (A) p38αfl/fl and p38αΔM bone marrow-derived macrophages (BMDM) were stimulated with medium (−), LPS, SAC or Listeria monocytogenes (L.m.) for 24 h. IL-12p40, IL-12p70, and IL-10 in the supernatants were determined by Luminex. Data are shown as means ± SEM for five mice. (B) Serum levels of IL-12p40, IL-12p70, and IL-10 were determined in p38αfl/fl and p38αΔM mice injected with LPS for 4 h (means ± SEM for 3 mice). (C–F) Human monocytes were treated with medium (−), BIRB796 (BIRB; 100 or 200 nM), SB203580 (SB; 200 nM or 2 μM) or rapamycin (Rap; 100 nM) and then stimulated with (C) LPS, (D) SAC, (E) L.m. or (F) LPS and IFN-γ for 22h. Cytokines in cell-free supernatants were measured by Luminex (means ± SEM of at least 3 donors). (G–H) Human monocytes were treated as indicated, washed and added to allogeneic T cells for one week. (G) IFN-γ of cell-free supernatants was determined by Luminex. Data represent means ± SEM for three independent experiments. (H) Primed T cells were activated for 5 h with PMA/Ionomycin in the presence of Brefeldin A. Intracellular cytokine staining for IL-4 and IFN-γ in CD-4 T cells is illustrated. One representative experiment out of three is shown. *, P < 0.05 compared with the respective controls.
The surface expression of the costimulatory molecule CD86, important for T cell activation and priming, was enhanced upon p38 blockade in human monocytes after stimulation with LPS, SAC or L.m. (data not shown). In line, we found that inhibition of p38 or mTOR in monocytes stimulated with LPS strongly enhanced the production of IFN-γ (Fig. 1G) and the differentiation of CD4+ Th1 cells (Fig. 1H) in an allogeneic T cell activation model. In summary, these data show that p38α differentially regulates the expression of IL-12 and IL-10 in human monocytes and DCs as well as in BMDM in vitro and in vivo in response to microbial insult.
p38 activation stimulates mTOR signaling
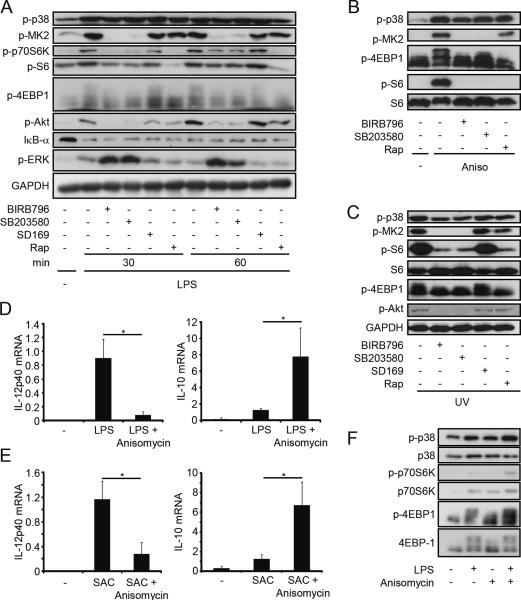
The concurrent regulation of IL-12/IL-10 by p38 and mTOR inhibitors indicated that these molecules might be connected. Therefore, we explored whether inhibition of p38 or mTOR might mutually influence the other kinase. Rapamycin did not modulate the phosphorylation of p38 or its downstream kinase MK2 after stimulation of human monocytes with LPS but blocked the phosphorylation of the mTOR substrates p70S6K and 4EBP1 (Fig. 2A). In contrast, inhibition of p38 with either SB203580 or BIRB796 blocked MK2 activation as expected, but also decreased the phosphorylation of 4EBP1 and p70S6K as well as S6 suggesting that p38 also activates mTOR signaling. Total levels of the investigated proteins were not altered by treatment with the inhibitors (data not shown). Interestingly, phosphorylation of Akt at Ser473 was blocked while activation of Erk was enhanced with BIRB796 and SB203580 (Fig. 2, A and C). However, inhibition of Erk did not inhibit mTOR and did not modulate the production of IL-12 or IL-10 (data not shown). Degradation of IκB-α was not influenced by either p38 or mTOR (Fig. 2A). Activation of p38 by the two environmental stress signals anisomycin or UV also activated mTOR signaling in human monocytes in a p38-dependend manner (Fig. 2, B and C). SD169, another reported inhibitor of p38α, was without effect in monocytes (Fig. 2, A and C). Next we tested whether hyperactivation of p38 with anisomycin in the presence of LPS or SAC could influence cytokine expression. Indeed, anisomycin reduced IL-12p40 mRNA levels but increased IL-10 mRNA levels in LPS- or SAC-stimulated macrophages (Fig. 2, D and E). Remarkably, hyperactivation of p38 with anisomycin further increased mTOR activity in LPS-activated monocytes (Fig. 2F). These results indicate that p38 activates the mTOR pathway and thereby regulates the expression of IL-12 and IL-10.
FIGURE 2.
p38 activation stimulates mTOR signaling. (A–C) Human monocytes were incubated with medium (−), BIRB796 (200 nM), SB203580 (2 μM), SD169 (200 nM) or rapamycin (Rap; 100 nM) and stimulated with (A) LPS (100 ng/ml), (B) anisomycin (50 ng/ml) or (C) UV (30 sec) for the indicated times. Cell lysates were analyzed by immunoblotting. Representatives of three independent experiments are shown. (D,E) Murine macrophages were stimulated with (D) LPS or (E) SAC in the presence or absence of anisomycin (50 ng/ml) for 1 h. IL-12p40 and IL-10 mRNA levels were measured by RT-PCR. Levels were normalized to ubiquitin and are shown relative to the (D) LPS- or (E) SAC-stimulated samples, which were set to 1 (means ± SEM; n=3). *, P < 0.05. (F) Monocytes were incubated with LPS (10 ng/ml) or anisomycin (10 ng/ml) for 30 min as indicated. Cell lysates were analyzed by immunoblotting.
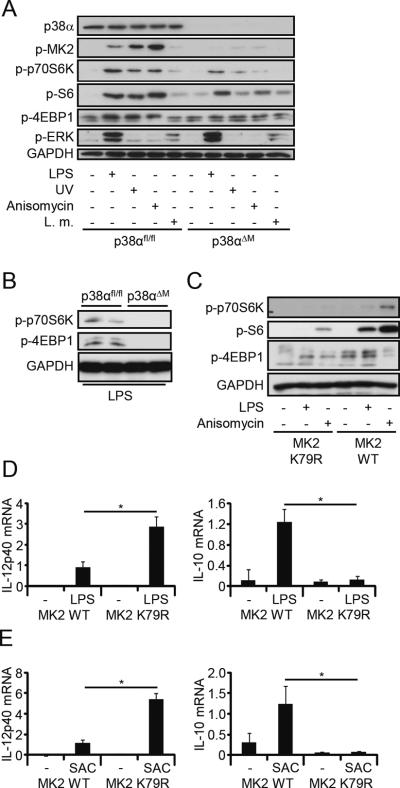
p38α deletion blocks mTOR activation in vitro and in vivo via MK2
To extend our results genetically, we examined the mTOR signaling pathway in p38αΔM BMDM. Deletion of p38α in macrophages abolished MK2 activation and also strongly diminished the phosphorylation of p70S6K, S6, and 4EBP1 after stimulation with LPS, UV, anisomycin, or L.m. (Fig. 3A and data not shown). Erk activation was only observed after LPS or L.m. treatment and not modified in p38αΔM BMDM (Fig. 3A). Moreover, activation of p70S6K and 4EBP1 was blocked in spleens from p38αΔM but not p38αfl/fl mice that were challenged with LPS (Fig. 3B) demonstrating that p38α activates mTOR signaling in vitro and in vivo. Next we analyzed whether MK2 may mediate the effect of p38α on mTOR signaling. Therefore, we used macrophages expressing a catalytic-dead mutant of MK2 (K79R) or its wild-type control. Strikingly, activation of mTOR after stimulation with LPS or anisomycin was severely compromised in the K79R mutant compared to wild-type MK2 (Fig. 3C). Moreover, the K79R macrophages showed absent IL-10 expression after LPS or SAC stimulation, while IL-12p40 was strongly increased (Fig. 3, D and E). These data indicate that the kinase activity of MK2 transmits the p38α signal to stimulate mTOR signaling and to regulate the production of IL-12 and IL-10.
FIGURE 3.
p38α activates mTOR via MK2. (A) p38αfl/fl and p38αΔM BMDM were stimulated with medium (−), LPS (100 ng/ml), anisomycin (100 ng/ml), UV (1 min) or L.m. (107 cells). Cell lysates were analyzed by immunoblotting. Representatives of three independent experiments are shown. (B) p38αfl/fl and p38αΔM mice were injected with LPS for 4 h. Isolated and homogenized spleens were analyzed by immunoblotting. Results for two mice per genotype are shown. Similar results were obtained in an independent experiment (data not shown). (C) MK2−/− macrophages reconstituted with either MK2K79R or wild-type (WT) MK2 were stimulated with medium, LPS (100 ng/ml) or anisomycin (100 ng/ml) for 1 h and whole-cell lysates were analyzed by immunoblotting. Representatives of three independent experiments are shown. (D,E) MK2 WT and MK2K79R macrophages were stimulated with medium (−), LPS (100 ng/ml) or SAC (75 μg/ml) for 2 h. mRNA levels of IL-12p40 and IL-10 were measured by RT-PCR. Levels were normalized to ubiquitin and are shown relative to the (D) LPS- or (E) SAC-treated MK2 WT samples, which were set to 1 (means ± SEM; n=3). *, P < 0.05.
p38 signals to mTOR via TSC1/TSC2 to regulate IL-12 and IL-10
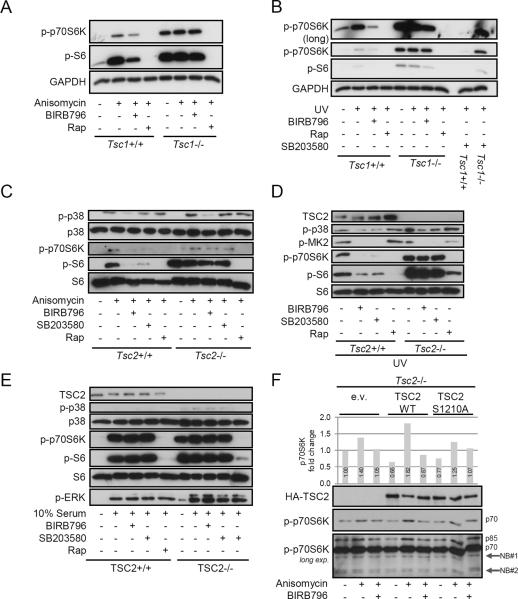
TSC1 and TSC2 act as dimer to inhibit activation of mTORC1 (29). To investigate whether p38-MK2 may mediate mTORC1 activation via TSC1/TSC2, we made use of murine embryonic fibroblasts (MEF) deficient in either TSC1 or TSC2. Treatment with anisomycin or UV stimulated p38 and mTOR signaling in Tsc1+/+ as well as in Tsc2+/+ cells and inhibition of p38 by either SB203580 or BIRB796 strongly reduced mTOR activation as shown by diminished phosphorylation of p70S6K or S6 (Fig. 4, A–D). Strikingly, inhibition of p38 did not block mTOR signaling in cells deficient of TSC1 or TSC2 (Fig. 4, A–D). Rapamycin, which acts downstream of TSC1/TSC2, still inhibited mTOR in these cells (Fig. 4, A–D). These results suggest that the TSC1/TSC2 complex is a critical signaling nodule that senses p38 activity to regulate mTOR activation. Interestingly, serum, a well known mTOR activator, did not stimulate p38 activation and inhibition of p38 did not influence mTOR activation induced by serum (Fig. 4E). Previously, it has been shown that MK2 phosphorylates serine 1210 (Ser1210) in TSC2 (37). However, it remained to be verified whether p38 activation regulates TSC2 activity via Ser1210 phosphorylation by MK2. To explore this possibility, we transfected Tsc2−/− cells with plasmids encoding either wild-type TSC2 or a S1210A TSC2 mutant and observed that wild-type but not mutant TSC2 restored the ability of anisomycin to activate p70S6K (Fig. 4F). Moreover, BIRB796 inhibited p70S6K activation upon p38 stimulation in cells transfected with wild-type but not mutant TSC2 (Fig. 4F). Overall, these results demonstrate that activation of p38 inhibits the TSC1/TSC2 complex allowing mTOR activation potentially via MK2-dependent phosphorylation of TSC2 at Ser1210.
FIGURE 4.
p38 signals to mTOR via TSC1/TSC2. (A, B) Tsc1+/+ and Tsc1−/− MEFs or (C–E) Tsc2+/+ and Tsc2−/− MEFs were starved overnight and treated with medium (−), BIRB796 (200 nM), SB203580 (2 μM) or rapamycin (Rap; 100 nM) for 90 min. Afterwards, MEFs were stimulated with (A, C) anisomycin (100 ng/ml), (B, D) UV (1 min) or (E) 10% serum for 60 min. Whole-cell lysates were analyzed by immunoblotting. (F) Tsc2−/− MEFs were transfected with empty vector (e.v.), HA-tagged wild-type TSC2 (WT), or HA-tagged TSC2 S1210A and then treated with medium, BIRB796, and anisomycin as indicated. Immunoblotting was performed with the indicated antibodies. Long exposure revealed the phosophorylation of the p85S6K (p85) isoform in addition the the p70S6K (p70) isoform. Nonspecific bands (NB). Densitometric analysis was performed on the intensity of p-p70S6K. One represantive of three different experiments is shown.
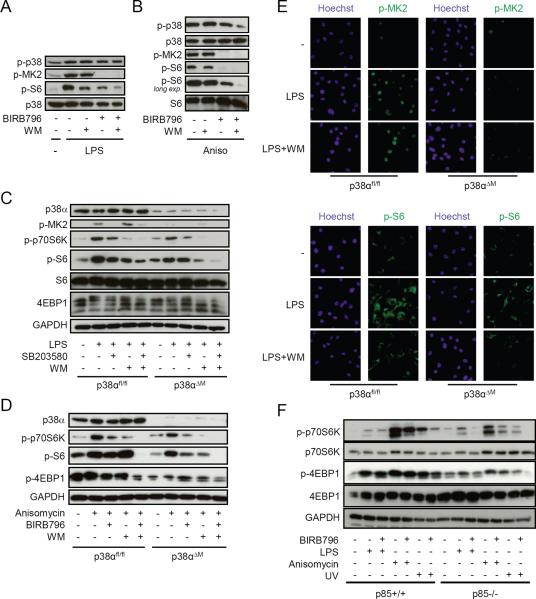
p38α and PI3K independently regulate mTOR
Classical activation of mTOR signaling by growth factors or TLR ligands is dependent on PI3K (17, 38). Our results so far established that p38α activates mTOR via MK2 involving a Ser1210-dependent regulation of TSC2 to modulate IL-12/IL-10 signaling. To further delineate the relative requirements of PI3K and p38α for stimulating mTOR, we inhibited p38 in the presence or absence of wortmannin, a specific covalent PI3K inhibitor. Treatment with either wortmannin or BIRB796 considerably diminished phosphorylation of S6 in human LPS- or anisomycin-activated monocytes (Fig. 5, A and B). The combination of both inhibitors led to a near complete abolishment of S6 phosphorylation (Fig. 5, A and B). Likewise, mTOR was blocked more efficiently in BMDM from p38αΔM than from p38αfl/fl mice after wortmannin treatment (Fig. 5, C and D). Immunofluorescence analysis confirmed that wortmannin inhibited the phosphorylation of S6 more completely in p38αΔM than in p38αfl/fl BMDM (Fig. 5E) suggesting that PI3K and p38 cooperatively stimulate mTOR signaling. Next, we analyzed MEFs deficient in p85α/p85β, the regulatory subunits of PI3K, to assess the importance of p38 versus PI3K for maximum activation of mTOR (Fig. 5F). Phosphorylation of p70S6K and 4E-BP1 induced by LPS, anisomycin or UV exposure was severely reduced but still detectable in p85−/− MEFs compared to their wild-type counterparts (Fig. 5F). However, concurrent inhibition of p38 by BIRB796 in p85−/− MEFs further inhibited activation of mTOR (Fig. 5F). Together, these results strongly suggest that full activation of mTOR in myeloid immune cells is dependent on p38 and PI3K.
FIGURE 5.
p38α and PI3K independently regulate mTOR. (A, B) Human monocytes were incubated with medium (−), BIRB796 (200 nM) and/or wortmannin (WM; 100 nM) for 90 min and stimulated with (A) LPS (100 ng/ml) or (B) anisomycin (100 ng/ml). Cell lysates were analyzed by immunoblotting. (C–E) p38αfl/fl and p38αΔM BMDM were treated with medium (−), BIRB796 (200 nM), SB203580 (2 μM) and/or wortmannin (WM; 100 nM) as indicated and stimulated with (C, E) LPS (100 ng/ml) or (D) anisomycin (100 ng/ml) for 1 h. (C, D) Cell lysates were analyzed by immunoblotting. (E) Phosphorylation of S6 and MK-2 was analyzed by immunofluorescence. One representative experiment of three is shown. (F) p85+/+ and p85−/− MEFs were treated with medium (−) or BIRB796 (200 nM) followed by stimulation with LPS (100 ng/ml), anisomycin (100 ng/ml) or UV (10 sec) for 60 min. Cell lysates were analyzed by immunoblotting.
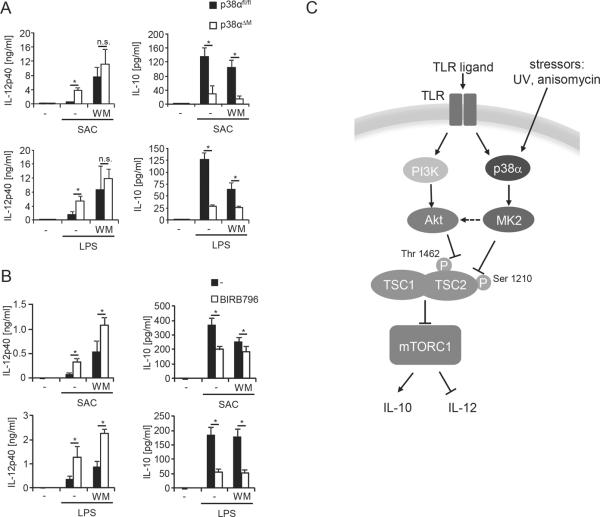
p38 and PI3K coordinately control expression of IL-12 and IL-10
Finally, we wanted to elucidate the relative contribution of p38α and PI3K for the production of IL-12 and IL-10. BMDM showed maximum production of IL-12p70 or IL-12p40 when PI3K was inhibited regardless of whether p38α was present or not (Fig. 6A and data not shown). This indicates that PI3K activation is dominant after LPS stimulation and that p38α modulates IL-12p40 within this context. In contrast, IL-10 production seems to more thoroughly depend on p38α as deletion of p38α already diminished IL-10 production of BMDM to a level that could not be further inhibited by PI3K inhibition (Fig. 6A). Similar results were obtained in human monocytes (Fig. 6B). In these cells, p38 was also dominant for IL-10 production, whereas PI3K and p38 both regulated Il-12p40 (Fig. 6B). In conclusion, these results suggest that PI3K and p38α coordinately modulate mTOR signaling to regulate the expression of IL-12 and IL-10 in myeloid immune cells.
FIGURE 6.
p38α and PI3K coordinately regulate the IL-12/IL-10 balance. (A) p38αfl/fl and p38αΔM BMDM were treated with wortmannin (WM; 100nM) and stimulated with medium (−), SAC (75 μg/ml), or LPS (100 ng/ml) for 24 h. Cytokine levels in cell-free supernatants were determined by Luminex (means ± SEM; n=5). *, P < 0.05; n.s., non significant. (B) Human monocytes were treated with wortmannin (WM; 100 nM) or BIRB796 (200 nM) as indicated and stimulated with LPS (100 ng/ml) for 24 h. IL-12p40 and IL-10 were determined in the supernatants by Luminex (means ± SEM; n=3). *, P < 0.05 (C) Model of mTOR-regulated production of IL-12/IL-10 via PI3K and p38 on the level of TSC1/TSC2.
Discussion
Monocytes, macrophages and DCs are emerging therapeutic targets in cardiovascular, malignant, and autoimmune disorders. Addressing gaps in knowledge about the function of these cells in response to environmental stimuli is required to understand the in vivo responses to therapies that target these cells. Stringent control of MAPK signaling is critical for balancing pro- versus anti-inflammatory signaling to enable efficient pathogen killing but also to limit detrimental tissue pathology. In that regard p38 is one of the most studied drug targets for anti-inflammatory therapy. This kinase directly or indirectly regulates many transcription factors and therefore participates in the gene induction of cytokines and other inflammatory molecules. p38 is also important in the post-transcriptional regulation of gene expression during inflammation (39). Animal studies have shown that p38 inhibitors are efficacious in several disease models, including inflammation, arthritis and other joint diseases, septic shock and myocardial injury (11). However, translation into the clinic has been difficult either due to lack of efficiency or the appearance of adverse effects including inflammation such as skin rashes.
p38α is activated in diverse cell types by a wide array of stress stimuli including genotoxic agents, PAMPs, proinflammatory cytokines, heat or osmotic shock, oxygen partial pressure or chemical insults (arsenite and anisomycin) (40). The simple view of an entirely proinflammatory kinase promoting the expression of TNF-α and IL-1β shifted to a more complex role in recent years demonstrating that p38α controls IL-12 and IL-10 expression (14–16). However, the downstream pathway that regulates these immunomodulatory cytokines remained unknown.
Here we have demonstrated that p38α utilizes the TSC2/mTOR signaling pathway to control the balance of IL-12 and IL-10. Our data suggest that TLR ligands or stress stimuli lead to an activation of p38α that in turn activates its downstream kinase MK2. The kinase activity of MK2 most likely phosphorylates Serine 1210 of TSC2 leading to inactivation of the TSC1/TSC2 complex and in turn activation of the mTOR pathway (Fig. 6C). Activation of mTOR then promotes IL-10 production, while reducing IL-12 expression. Our work indicates that p38α-mediated mTOR activation occurs in parallel to the well-known PI3K pathway that activates mTOR in response to TLR signals (17, 19). Hence, both pathways concurrently control mTOR activation to precisely allow the expression of pro- and anti-inflammatory cytokines in response to environmental stress. We are unaware of a report demonstrating that two stimuli additively regulate the activation of mTOR via the TSC complex. We suggest that p38α-mediated mTOR activation in addition to the PI3K pathway represents a tuning mechanism to regulate immunomodulatory cytokines to adapt the immune response to the environmental milieu. This is supported by the observation that hyperactivation of p38α by anisomycin can modulate IL-12 and IL-10 expression on top of a TLR signal (Fig. 2D). The p38/MK2 axis is required after excessive tissue damage to induce tissue repair (41, 42). In such situations p38α may promote mTOR activation in resident and recruited macrophages to reduce IL-12 and augment IL-10 production that limits the generation of a proinflammatory CD4+ Th1 response that would further exaggerate tissue damage (43).
A link from p38β to mTOR has been described in Drosophila that occurs via a TSC2-independent mechanism (44). In line, p38β was recently shown to phosphorylate the essential mTORC1 binding protein Raptor and to participate in arsenite-induced mTOR activation in fibroblasts (45). In contrast, in the same cell p38β can also inhibit mTOR upon energy starvation via phosphorylation and inactivation of Ras homologue enriched in brain (Rheb), a key component of the mTORC1 pathway (46). We now show that p38α via MK2 promotes mTOR activation dependent on the TSC1/TSC2 complex in myeloid immune cells. Indeed, MK2 was shown to phosphorylate TSC2 at Ser1210 in fibroblasts (37). In addition, MK2 was also described to phosphorylate Akt at Ser473 in neutrophils (47), in line with the inhibition of Akt Ser473 by the p38 inhibitors in our cells (Fig. 2A and Fig. 6). We have previously shown that mTOR regulates NF-κB and STAT3 signaling (24). Interestingly, also p38α and MK2 are required for STAT3 activation and IL-10 production (14, 36). These effects are likely to be indirectly mediated by p38 and mTOR and the precise downstream pathways how mTOR regulates IL-12 vs. IL-10 needs further investigation. In this study we focused on delineating the mechanism of p38-depdendent mTOR regulation in myeloid immune cells.
Rapamycin is currently evaluated as vaccine adjuvant, especially because of its ability to enhance memory CD8+ T cell responses. In addition, previous work also established that rapamycin exerts immunostimulatory effects via the innate immune system that may contribute to the adjuvant properties of rapamycin (23–25, 48, 49). However, its immunosuppressive activity will likely prevent the inclusion of rapamycin in widely-distributed vaccines. Our data suggest that inhibition of p38 might be similarly effective as adjuvant strategy where strong Th1 responses are desired and, moreover, it might avoid the potent immunosuppressive effects on the T cell compartment as p38 is regarded dispensable for T cell function (50). Indeed, in a mouse model of Leishmania major infection, vaccination with SB203580 was protective by inducing efficient Th1 immunity (16).
In summary, we have identified and characterized a pathway from p38α to mTOR via MK2 and TSC1/TSC2 in myeloid immune cells that tunes the immune response according to environmental input signals.
Acknowledgments
We thank Margarethe Merio for excellent technical assistance.
This work was supported by the Else-Kröner Fresensius Stiftung (to TW and MDS), by the Austrian Science Foundation FWF (SFB F28) and the Austrian Federal Ministry for Science and Research (GEN-AU III Austromouse) (to CL and MM), by the US National Institutes of Health grant AI074957 (to JMP) and by the Deutsche Forschungsgemeinschaft (to MG).
Footnotes
Disclosures The authors have no financial conflict of interest.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 3.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 4.Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–379. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature reviews. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 6.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 7.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 8.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehner MD, Schwoebel F, Kotlyarov A, Leist M, Gaestel M, Hartung T. Mitogen-activated protein kinase-activated protein kinase 2-deficient mice show increased susceptibility to Listeria monocytogenes infection. J Immunol. 2002;168:4667–4673. doi: 10.4049/jimmunol.168.9.4667. [DOI] [PubMed] [Google Scholar]
- 11.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69(Suppl 1):i77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002;9:268–272. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- 13.Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem. 2005;280:19472–19479. doi: 10.1074/jbc.M414221200. [DOI] [PubMed] [Google Scholar]
- 14.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Gerl RE, Schrader JW. Defining the involvement of p38alpha MAPK in the production of anti- and proinflammatory cytokines using an SB 203580-resistant form of the kinase. J Biol Chem. 2003;278:22237–22242. doi: 10.1074/jbc.M300847200. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Zhang X, Darrah PA, Mosser DM. The regulation of Th1 responses by the p38 MAPK. J Immunol. 2010;185:6205–6213. doi: 10.4049/jimmunol.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weintz G, Olsen JV, Fruhauf K, Niedzielska M, Amit I, Jantsch J, Mages J, Frech C, Dolken L, Mann M, Lang R. The phosphoproteome of toll-like receptor-activated macrophages. Mol Syst Biol. 2010;6:371. doi: 10.1038/msb.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 26.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ, Hengstschlager M, Muller M, Horl WH, Saemann MD. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 27.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, Wiltrout RH. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–4084. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, Liang TB. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–1372. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 31.Ohtani M, Hoshii T, Fujii H, Koyasu S, Hirao A, Matsuda S. Cutting Edge: mTORC1 in Intestinal CD11c+CD11b+ Dendritic Cells Regulates Intestinal Homeostasis by Promoting IL-10 Production. J Immunol. 2012;188:4736–4740. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 32.Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 35.Strobl B, Bubic I, Bruns U, Steinborn R, Lajko R, Kolbe T, Karaghiosoff M, Kalinke U, Jonjic S, Muller M. Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. J Immunol. 2005;175:4000–4008. doi: 10.4049/jimmunol.175.6.4000. [DOI] [PubMed] [Google Scholar]
- 36.Ehlting C, Ronkina N, Bohmer O, Albrecht U, Bode KA, Lang KS, Kotlyarov A, Radzioch D, Gaestel M, Haussinger D, Bode JG. Distinct functions of the mitogen-activated protein kinase-activated protein (MAPKAP) kinases MK2 and MK3: MK2 mediates lipopolysaccharide-induced signal transducers and activators of transcription 3 (STAT3) activation by preventing negative regulatory effects of MK3. J Biol Chem. 2011;286:24113–24124. doi: 10.1074/jbc.M111.235275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Inoki K, Vacratsis P, Guan KL. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J Biol Chem. 2003;278:13663–13671. doi: 10.1074/jbc.M300862200. [DOI] [PubMed] [Google Scholar]
- 38.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark A, Dean J, Tudor C, Saklatvala J. Post-transcriptional gene regulation by MAP kinases via AU-rich elements. Frontiers in bioscience: a journal and virtual library. 2009;14:847–871. doi: 10.2741/3282. [DOI] [PubMed] [Google Scholar]
- 40.Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thuraisingam T, Xu YZ, Eadie K, Heravi M, Guiot MC, Greemberg R, Gaestel M, Radzioch D. MAPKAPK-2 signaling is critical for cutaneous wound healing. The Journal of investigative dermatology. 2010;130:278–286. doi: 10.1038/jid.2009.209. [DOI] [PubMed] [Google Scholar]
- 42.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardi M, Caelles C, Serrano AL, Munoz-Canoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 44.Cully M, Genevet A, Warne P, Treins C, Liu T, Bastien J, Baum B, Tapon N, Leevers SJ, Downward J. A role for p38 stress-activated protein kinase in regulation of cell growth via TORC1. Mol Cell Biol. 2010;30:481–495. doi: 10.1128/MCB.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu XN, Wang XK, Wu SQ, Lu J, Zheng M, Wang YH, Zhou H, Zhang H, Han J. Phosphorylation of Raptor by p38beta participates in arsenite-induced mammalian target of rapamycin complex 1 (mTORC1) activation. J Biol Chem. 2011;286:31501–31511. doi: 10.1074/jbc.M111.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng M, Wang YH, Wu XN, Wu SQ, Lu BJ, Dong MQ, Zhang H, Sun P, Lin SC, Guan KL, Han J. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 48.Janes MR, Fruman DA. Immune regulation by rapamycin: moving beyond T cells. Sci Signal. 2009;2:pe25. doi: 10.1126/scisignal.267pe25. [DOI] [PubMed] [Google Scholar]
- 49.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr., Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 50.Kim JM, White JM, Shaw AS, Sleckman BP. MAPK p38 alpha is dispensable for lymphocyte development and proliferation. J Immunol. 2005;174:1239–1244. doi: 10.4049/jimmunol.174.3.1239. [DOI] [PubMed] [Google Scholar]