A 72-year-old man presented to the emergency department with shortness of breath. He had a history of left ventricular failure, hypertension, treated esophageal carcinoma and recurrent deep vein thromboses. His medications included warfarin, bumetanide, spironolactone, ramipril, bisoprolol, acetylsalicylic acid and simvastatin. Clinical examination, chest radiography and echocardiography confirmed worsening of congestive cardiac failure. The doses of the diuretics he was taking were adjusted and his symptoms improved.
Thyroid function tests done during the admission showed elevated serum free thyroxine at 54.0 (normal 12.0–22.0) pmol/L and total triiodothyronine at 3.3 (normal 1.3–2.6) nmol/L, and suppressed thyroid-stimulating hormone (TSH) at less than 0.02 (normal 0.27–4.20) mIU/L. He had never taken amiodarone and had not received iodinated contrast before his presentation. Clinical assessment showed no stigmata of Graves disease, but nodularity of the thyroid gland was noted. Ultrasonography of the thyroid confirmed enlargement of both thyroid lobes with multiple nodules bilaterally. Thyroid peroxidase antibody titre was 207 (normal < 35) IU/L, and TSH receptor antibodies were not measured.
A clinical diagnosis of toxic multinodular goitre was made. This was based on confirmation of multinodular goitre on ultrasonography, as well as the regional prevalence of thyroid disease, types of thyroid disease common in the patient’s age group, absence of pathognomonic features of Graves disease and poor specificity of thyroid peroxidase antibody testing for Graves disease in this population. The patient was given carbimazole and became euthyroid; 15 months later, he underwent definitive treatment with radioiodine. Carbimazole was stopped 2 weeks after radioiodine treatment. He subsequently remained clinically and biochemically euthyroid.
Three months after radioiodine therapy, at another centre, the patient underwent elective coronary angioplasty with 2 drug-eluting stents to the proximal left anterior descending artery, with no immediate complications. At the time of the angioplasty, he was clinically euthyroid. A total of 260 mL of iodinated contrast (iodixanol, iodine content 320 mg/mL) was infused intravenously during the procedure. A few days later, he reported shortness of breath and symptoms similar to his first presentation. On examination, his chest was clear, and an electrocardiogram showed no ischemic changes. An echocardiogram excluded pericardial effusion, and his left ventricular function, although moderately impaired, was no worse than previously. The doses of the diuretics he was taking were increased, but his symptoms did not improve.
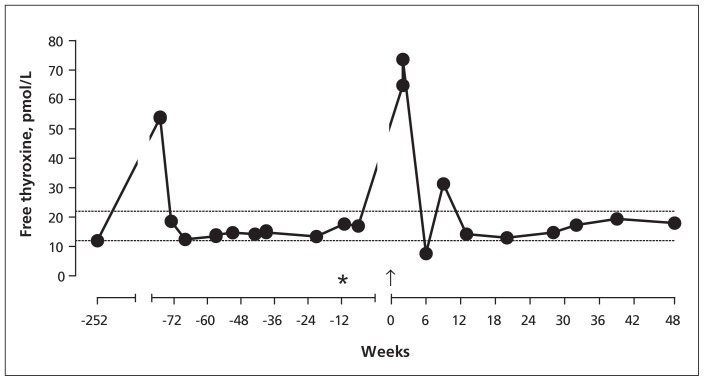
Eleven days after the angioplasty, thyroid function tests done in anticipation of a follow-up appointment in the endocrinology clinic showed a free thyroxine level of 73.6 pmol/L with a suppressed TSH of less than 0.02 mIU/L (Figure 1). Anti–thyroid peroxidase antibody titre was 754 IU/L, and TSH receptor antibody titre was not measured. On examination, the patient had no clinical features of Graves disease. Carbimazole was restarted and the patient’s symptoms improved rapidly. Results of blood tests swiftly returned to euthyroid status. Carbimazole was stopped 6 months later, and the patient remained well with normal thyroid indices.
Figure 1:
Serum concentrations of free thyroxine (6lled circles) in relation to coronary angioplasty. The patient had received radioiodine treatment (asterisk) 12 weeks preceding coronary angioplasty with iodinated contrast (week 0, arrow). Dotted horizontal lines mark the lower and upper limits of laboratory reference range for free thyroxine (12.0–22.0 pmol/L).
Discussion
Iodinated contrast media commonly used in various radiologic examinations and interventional procedures results in massive iodide exposure to the thyroid gland. Whereas people with normal thyroids usually experience no ill effects, those with pre-existing thyroid disease may experience thyrotoxicosis, which, if unrecognized, could lead to serious, potentially life-threatening consequences, including atrial arrhythmias, heart failure, pulmonary arterial hypertension, cerebrovascular and pulmonary embolism, and cardiomyopathy.1 Iodine administration to patients with underlying thyroid disease may lead to hypersecretion of thyroid hormones, a phenomenon known as the Jod–Basedow effect.2–4
Jod–Basedow hyperthyroidism usually develops over 2 to 12 weeks as the iodine is used as a substrate for new hormone formation. It typically occurs in people with nodular thyroid disease, although people predisposed to Graves disease, the elderly (among whom the prevalence of nodular thyroid disease is high) and people living in areas of iodine deficiency are also vulnerable. Exposure to a large iodide load such as occurs with iodinated contrast studies can also cause acute destructive thyroiditis in people without pre-existing thyroid disease.2
Our patient developed severe thyrotoxicosis of very rapid onset following exposure to iodinated contrast used in coronary angioplasty. The rapidity and severity of thyrotoxicosis; rise in antithyroid peroxidase antibody titre, reflecting increased thyroid antigen release into the circulation; and quick resolution of thyrotoxicosis suggest acute, destructive thyroiditis. Jod–Basedow hyperthyroidism in a patient with nodular thyroid disease remains a possibility, although thyroid function tests in this patient had been within the normal range in the period leading up to the angioplasty. However, Jod–Basedow hyperthyroidism develops slowly over weeks to months and persists for a longer period.2
Iodide load in radiologic procedures
Iodinated contrast media are used for various radiologic procedures, such as coronary angiography, computed tomography (Table 1),3,5 endoscopic retrograde cholangiopancreatography, hysterosalpingography, intravenous pyelography and digital subtraction angiography. In England, for example, as many as 40.12 million radiologic examinations were performed in 2011/12, of which 1.17 million involved interventional procedures.6 Whereas iodine-induced hyperthyroidism has been reported with as little as 300–500 μg of iodide, a typical dose of iodinated contrast medium contains about 13 500 μg of free iodide and 15–60 g of bound iodine that may be liberated as free iodide in the body.3 This represents an acute iodide load of 90 times to several hundred thousand times the recommended daily intake of 150 μg in adults,3 and can overwhelm physiologic thyroid hormone regulation in susceptible individuals. Thus, a dose–effect relation between different contrast studies and iodine-induced hyperthyroidism has not been described in the literature and is probably not clinically relevant.
Table 1:
Typical iodine load of common computed tomography studies involving use of iodinated contrast media for a 70-kg patient*3,5
| Examination | Typical dose of contrast material | Total iodine load, g |
|---|---|---|
| Brain parenchyma | 80 mL of 300 mgI/mL | 24.0 |
| Brain perfusion | 50 mL of 350 mgI/mL | 17.5 |
| Neck soft tissue | 100 mL of 300 mgI/mL | 30.0 |
| Neck and brain CTA† Pulmonary CTA† Aortic and coronary CTA† Liver and routine abdomen |
100 mL of 350 mgI/mL | 35.0 |
| Routine chest | 70 mL of 300–350 mgl/mL | 21.0–24.5 |
| Peripheral runoff CTA† | 125–140 mL of 350 mgI/mL | 43.8–49.0 |
| Pancreas (dual phase) Kidney | 100–120 mL of 350 mgI/mL | 35.0–42.0 |
Note: CTA = computed tomographic angiography, mgI/mL = mg of iodine per mL.
Recommended daily intake of iodine for adults is 150 μg.
16-detector computed tomography.
Prevention
Some researchers have investigated the role of prophylactic measures to reduce the risk of iodine-induced hyperthyroidism in patients undergoing interventional studies. In one prospective, randomized study of 1177 patients undergoing elective coronary angiography, 51 patients with euthyroid autonomy (i.e., suppressed TSH but normal thyroid hormone levels, negative thyrotropin-releasing hormone test and normal thyroidal radioisotope uptake study) identified as at increased risk of iodine-induced hyperthyroidism were randomly assigned to prophylactic treatment with perchlorate or thiamazole, or no treatment.7 Hyperthyroidism developed in 2 patients in the control group and 1 in each of the treatment groups. The authors concluded that short-term prophylactic thyrostatic therapy appeared to have a protective effect against iodine excess in patients with euthyroid autonomy. In another prospective study of 91 patients undergoing coronary angiography, thyroidal technetium uptake scintigraphy was used for stratification of the risk of iodine-induced hyperthyroidism.8 Fifty-six patients with a thyroidal technetium uptake of less than 1% proceeded directly to coronary angiography, and 19 patients with higher thyroidal technetium uptake were given prophylactic treatment with perchlorate alone or combined with thiamazole. Although there were no episodes of iodine-induced hyperthyroidism in the former group, hyperthyroidism developed in 2 patients in the group that received prophylactic drugs. The authors concluded that scintigraphy of the thyroid gland was suitable for risk stratification of iodine-induced hyperthyroidism in patients undergoing coronary angiography. However, thyroid scintigraphy is expensive and requires further logistical considerations, including the need for attendance at an imaging facility.
Furthermore, a large prospective study of 788 patients from an iodine-deficient area who were undergoing elective coronary angiography found only 2 instances of emergent hyperthyroidism, an incidence of less than 0.25%. These authors concluded that exposure to iodinated contrast media in unselected patients who were euthyroid, even from an iodine-deficient area, rarely led to hyperthyroidism.9
However, a recent large study involved a longitudinal nested case–control analysis of adult patients who were euthyroid at baseline and who were exposed to iodinated contrast media between January 1990 and June 2010; the study included 178 new hyperthyroid cases matched to 655 euthyroid control individuals and reported that exposure to iodinated contrast media was significantly associated with subsequent development of new hyperthyroidism (odds ratio 1.98, 95% confidence interval 1.08–3.60).3 This first large controlled study of the association between exposure to iodinated contrast media and new thyroid functional disease suggests that this association may occur more commonly than was previously recognized.3,4 In particular, a relatively large proportion of individuals who developed iodine-induced thyroid dysfunction in this study were not known to have underlying risk factors.
There may be a role for alternative contrast agents such as gadolinium in some radiologic procedures.
Existing guidelines
Guidelines on the subject are sparse. The Contrast Media Safety Committee of the European Society of Urogenital Radiology has concluded that routine monitoring of thyroid function before contrast medium injection in patients with a normal thyroid is not indicated.10 Prophylaxis was not recommended, although the committee conceded that prophylaxis may offer some protection in selected patients who are at high risk. However, high-risk patients (i.e., those with a history of Graves disease or nodular goitre, especially if they are elderly or live in areas of dietary iodine deficiency) who may be particularly unable to tolerate thyroid dysfunction, such as those with underlying unstable cardiovascular disease, should be carefully monitored after iodinated contrast studies.4,10 Patients who develop iodine-induced hyperthyroidism should be referred to endocrinologists, as the management of this condition can be challenging.
With the widespread use of radiologic investigations and interventional procedures involving iodine-containing contrast media, it is important for clinicians to be aware of the possibility of iodine-induced hyperthyroidism, particularly in patients who are at high risk.
Key points
Radiologic investigations and interventional procedures involving the use of iodine-containing contrast media may precipitate acute thyrotoxicosis in patients with a history of thyroid disease.
Patients with a history of Graves disease or nodular thyroid disease, especially if they are elderly or living in areas of dietary iodine de2ciency, are at higher risk of acute thyrotoxicosis.
Prophylaxis is not recommended; however, people at high risk who may be particularly unable to tolerate thyroid dysfunction, such as those with underlying unstable cardiovascular disease, should be carefully monitored after iodinated contrast studies.
Patients who develop iodine-induced hyperthyroidism should be referred to endocrinologists, because the management of this condition can be challenging.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Written consent from patients for publication of their story is a necessity and should accompany submissions. See information for authors at www.cmaj.ca.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Paul Dunne and Nisha Kaimal drafted the article, which John MacDonald and Akheel Syed revised. All authors approved the final version submitted for publication.
References
- 1.Biondi B. Mechanisms in endocrinology: heart failure and thyroid dysfunction. Eur J Endocrinol 2012;167:609–18 [DOI] [PubMed] [Google Scholar]
- 2.Calvi L, Daniels GH. Acute thyrotoxicosis secondary to destructive thyroiditis associated with cardiac catheterization contrast dye. Thyroid 2011;21:443–9 [DOI] [PubMed] [Google Scholar]
- 3.Rhee CM, Bhan I, Alexander EK, et al. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 2012;172:153–9 [DOI] [PubMed] [Google Scholar]
- 4.Pearce EN. Iodine-induced thyroid dysfunction: comment on “association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism”. Arch Intern Med 2012;172:159–61 [DOI] [PubMed] [Google Scholar]
- 5.Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61 [DOI] [PubMed] [Google Scholar]
- 6.UK Department of Health Imaging and radiodiagnostics — annual data. Leeds (UK): The Department; 2012. Available: http://transparency.dh.gov.uk/2012/07/10/annual-diagnostics-data/ (accessed 2012 Nov. 4). [Google Scholar]
- 7.Nolte, Muller R, Siggelkow H, et al. Prophylactic application of thyrostatic drugs during excessive iodine exposure in euthyroid patients with thyroid autonomy: a randomized study. Eur J Endocrinol 1996;134:337–41 [DOI] [PubMed] [Google Scholar]
- 8.Fricke E, Fricke H, Esdorn E, et al. Scintigraphy for risk stratification of iodine-induced thyrotoxicosis in patients receiving contrast agent for coronary angiography: a prospective study of patients with low thyrotropin. J Clin Endocrinol Metab 2004; 89:6092–6 [DOI] [PubMed] [Google Scholar]
- 9.Hintze G, Blombach O, Fink H, et al. Risk of iodine-induced thyrotoxicosis after coronary angiography: an investigation in 788 unselected subjects. Eur J Endocrinol 1999;140:264–7 [DOI] [PubMed] [Google Scholar]
- 10.van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004; 14:902–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.