Abstract
A number of viruses in the family Flaviviridae are the focus of efforts to develop effective antiviral therapies. Success has been achieved with inhibitors for the treatment of hepatitis C, and there is interest in clinical trials of drugs against dengue fever. Antiviral therapies have also been evaluated in patients with Japanese encephalitis and West Nile encephalitis. However, no treatment has been developed against the prototype flavivirus, yellow fever virus (YFV). Despite the availability of the live, attenuated 17D vaccine, thousands of cases of YF continue to occur each year in Africa and South America, with a significant mortality rate. In addition, a small number of vaccinees develop severe systemic infections with the 17D virus. This paper reviews current efforts to develop antiviral therapies, either directly targeting the virus or blocking detrimental host responses to infection.
I- Introduction
A number of viruses in the family Flaviviridae are the focus of efforts to develop effective antiviral therapies. Success has been achieved for the treatment of hepatitis C virus (HCV) infection, and there is interest in clinical trials of drugs against dengue fever. Antiviral therapies have also been evaluated in patients with Japanese encephalitis and West Nile encephalitis. However, no treatments have been developed against the prototype flavivirus, yellow fever (YF) virus.
As an arbovirus, YFV is spread through the bite of an infected mosquito. The virus is endemic to areas of tropical Africa and Latin America, and causes significant morbidity and mortality throughout its range. Despite the availability of the live, attenuated 17D vaccine, thousands of cases of YF continue to occur each year. Severe cases may have a fatality rate as high as 50%. An increase in YF observed over the last 10 years may be due to many factors including declining population immunity, deforestation, urbanization, population movements and climate change (WHO website).
Vaccination is the only countermeasure available for those inhabiting or visiting YF-endemic areas. The 17D vaccine protects against all YFV strains, eliciting a protective antibody response within 10 days in 95-100% of vaccinated individuals (Barrett and Teuwen, 2009; Kay et al., 2011; Monath et al., 2002). However, a small number of vaccinees develop severe systemic infections with the 17D virus. This paper reviews current efforts to develop antiviral therapies, either directly targeting the virus or blocking detrimental host responses to infection, and briefly discusses some of the limitations and hazards associated with YF vaccination.
II- The need for antiviral therapies
One common theme with all flaviviruses is that there are no approved antiviral therapies for the treatment of human disease. Despite the availability of licensed vaccines for the prevention of human infection with YFV, JEV, and TBEV (Heinz and Stiasny, 2012), there is still a high burden of disease associated with infection from these viruses. This is likely due to underutilization of vaccines in endemic areas.
A large industry has been built around the discovery of compounds against HCV. Direct-acting antiviral drugs that specifically target the viral proteins of HCV, especially the viral RNA-dependent RNA polymerase and the NS3/4A serine protease, continue to be a viable avenue of drug development. In 2011, the protease inhibitors telaprevir and boceprevir were approved for clinical use in the United States and Europe, effectively replacing a combination of pegylated interferon-α and ribavirin as the standard of care for the treatment of genotype 1 HCV (Doyle et al., 2012). About 50 agents are now undergoing clinical trials for the treatment of hepatitis C.
Dengue virus (DENV) causes significant morbidity and mortality in tropical regions of the world. There have been efforts to develop drugs for the treatment of dengue. Clinical trials have been conducted in Viet Nam with the antimalarial drug, chloroquine, although no clinical benefit was observed (Tricou et al., 2010). Balapiravir, a nucleoside analog originally developed by Roche for the treatment of hepatitis C, has also been tested for efficacy in DENV-infected patients, although there did not appear to be any benefit from treatment in reducing viremia or fever duration (Nguyet et al., 2012). Further clinical studies are likely to follow for the evaluation of other compounds for the treatment of DENV, and many programs have been developed for the discovery and preliminary characterization of drug activity in various model systems.
Some clinical trials for agents effective in the treatment of encephalitic flaviviruses, including WNV and JEV, have also been conducted. Safety trials for the treatment of WNV with a humanized antibody, MGAWN1, with specificity for the WNV envelope protein were conducted and it was shown to be well-tolerated (Beigel et al., 2010). However, poor enrollment of patients infected with WNV has hampered further clinical evaluation. A controlled trial of ribavirin treatment of JEV-infected individuals in India was performed over a period of 3 years, which attracted enrollment of 153 patients. There was no improvement of disease, however, associated with ribavirin treatment (Kumar et al., 2009).
This review will focus on the development of antiviral therapies for the treatment of YF, but ideally, an effective antiviral would have broad-spectrum activity against other members of the Flaviviridae family. It is unlikely that an effective antiviral would be any better utilized than the vaccine in the resource-poor regions of YF endemicity. For a therapeutic agent to have utility in the treatment of disease such as YF, it would ideally be inexpensive, stable, safe, would have efficacy when administered before and after virus infection, and would be broadly active against a range of viruses. The most plausible scenario of use would be in the wake of an outbreak where a safe preventative could be administered at the onset of any symptoms that could be associated with YFV infection. Another scenario would be for the treatment of travellers to endemic regions, where vaccination may pose more of a risk than infection. An effective antiviral could be useful in such instances where travellers contract YF and could be treated as soon as symptoms arose. A third scenario would be the development of countermeasures for the treatment of adverse events associated with vaccination. Of course, to fulfill any one of these requirements is a challenge unto itself, and it may be difficult to find such a compound.
III. Classification
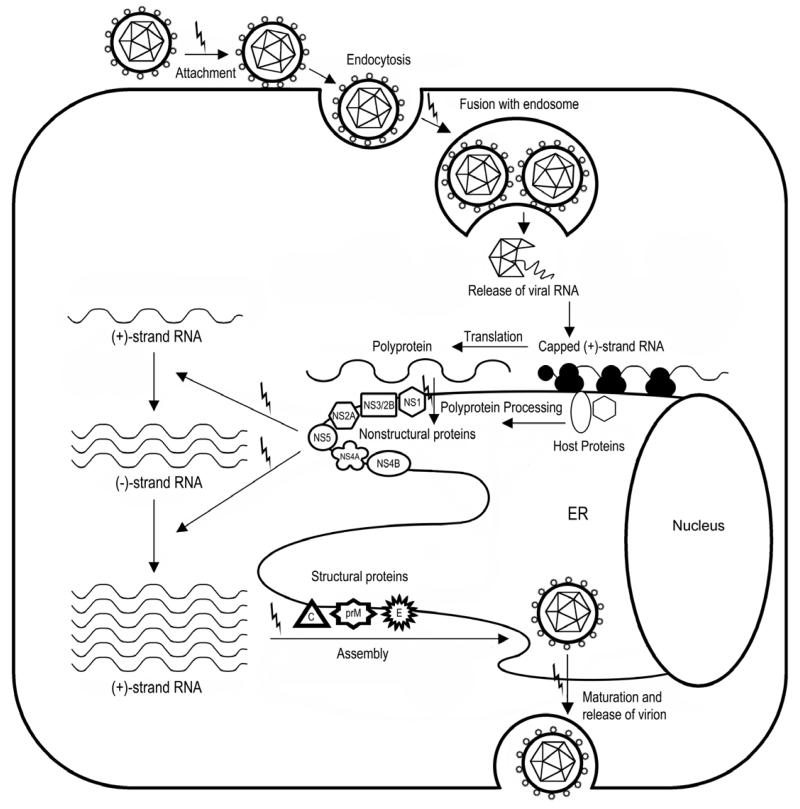
Yellow fever virus (YFV) is a member of the virus family Flaviviridae, which contains many other viruses of medical and veterinary concern, most notably including hepatitis C virus (HCV), Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), Tick-borne encephalitis virus (TBEV), and bovine viral diarrhea virus (BVDV). YFV and the other viruses listed above are further classified within 3 genera: flavivirus, hepacivirus, and pestivirus. These viruses share the characteristic of a single-stranded, positive-sense RNA genome that is approximately 10 KB in length and codes for a single long polyprotein (Figure 1). Host and viral proteases cleave the polyprotein to produce functional proteins that interact with host and viral components for production of progeny virions during viral replication. A diagram of the replication cycle of YFV and other flaviviruses is depicted in Figure 2.
Figure 1.
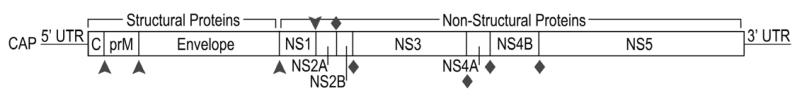
A graphical representation of the flavivirus genome. The structural capsid (C), premembrane (prM), and envelope proteins are situated at the 5′ end of the translated region. The nonstructural (NS) proteins 1, 2A, 2B, 3, 4A, 4B, and 5 are located at the 3′ end of the genome. The 3′ and 5′ terminal ends possess untranslated regions (UTR) that are important for replication. The 5′ end possesses a cap structure. Arrowheads represent host protease cleavage sites, while diamonds represent viral protease cleavage sites. Known functions of the various viral proteins are also included.
Figure 2.
Replication strategy of the flaviviruses, including YFV. The virus enters the cell by endocytosis and the viral RNA is released following fusion with an endosome. Viral RNA is translated by host ribosomes into a single polyprotein that is processed by host and virus-encoded proteases. The nonstructural proteins are involved in replication of the viral RNA. Viral RNA and structural proteins are packaged together into progeny virions, which bud from internal membranes before being released from the cell. “Lightning bolts” indicate potential targets for antiviral intervention; details of each target are contained in the text.
The flavivirus genus is separated into three major subdivisions: mosquito-borne viruses, tick-borne viruses, and flaviviruses with no known arthropod vector. The mosquito-borne group contains many well-known viruses that are important human pathogens. Yellow fever is the archetypal flavivirus with a transmission cycle that involves mosquito vectors. Within the mosquito-borne flaviviruses, the Yellow fever group encompasses YFV and related viruses such as Banzi virus (BANV), Edge Hill virus, Uganda S virus and Wesselsbron virus. The other viruses that are closely related to YFV and classified together in the YF group cause infrequent disease and are less frequently studied and therefore not as well characterized as YFV. In man, BANV infection results in a febrile illness (Smithburn et al., 1959; Williams and Woodall, 1964), although experimental infection of mice with BANV results in encephalitis (Bhatt et al., 1981). Antiviral studies with BANV have also been published (Julander et al., 2006; Smee et al., 1987), demonstrating the utility of such models in characterizing the activity of antiviral compounds against an encephalitic flaviviral disease in mice.
Other mosquito-borne virus groups within the flavivirus genus include the Dengue (DENV) and the Japanese encephalitis (JEV) viruses. Like the yellow fever group, human infection with the members of the dengue virus group may result in hemorrhagic manifestations and shock (Simmons et al., 2012). The viruses within the Japanese encephalitis virus group include, in addition to the nominate virus, West Nile virus (WNV), Murray Valley encephalitis virus and St. Louis encephalitis virus. Infection with this virus group is generally self-limiting, but may result in encephalitic disease (Turtle et al., 2012).
Tick-borne viruses within this genus contain viruses that cause encephalitic and hemorrhagic disease, and include Powassan virus, tick-borne encephalitis virus (TBEV), Langat virus, and Omsk hermorrhagic fever virus. Infection with these viruses may result in encephalitic or hemorrhagic disease manifestations, depending on the virus (Lasala and Holbrook, 2010). Little is known about the viruses with no known arthropod vector, and these viruses rarely if ever cause human disease.
One of the best-known viruses in the family Flaviviridae is HCV, which is classified within the genus Hepacivirus. Infection with this virus has resulted in around 180 million chronic infections worldwide, and continues to be one of the leading causes of hepatocellular carcinoma (El-Serag, 2012). Although there is some debate as to the taxonomy, some investigators classify the GB viruses, which are primate pathogens, within the hepacivirus genus, while others place these viruses in the proposed genus pegivirus (Stapleton et al., 2011), which would constitute a fourth genus within this family. These viruses and other members of the Flaviviridae family and may share common pathways and conserved sequences that may be useful in the development of broad-spectrum antiviral agents (Paula et al., 2009).
Within the genus Pestivirus is the veterinary pathogen bovine viral diarrhea virus (BVDV). This virus has been used historically as a surrogate for HCV, although with recent advances in model systems for HCV, this is no longer the case. Three other veterinary pathogens are also found in this genus.
IV. Limitations and adverse effects of the 17D vaccine
One limitation of the 17D vaccine was the rapid loss of titer after reconstitution, which has recently been countered by using new formulations to improve the stability of live, attenuated flavivirus vaccines (Wiggan et al., 2011). Changes of the sequence elements of a live vaccine during production is another area of potential danger for vaccinees, and efforts were recently made to more fully characterize the 17D vaccine throughout the manufacturing process. This study included 13 different preparations from many different manufacturers was undertaken to more fully characterize the molecular events that occurred during preparation at various institutions, which provides insight into potential important differences among the various preparations that might account for adverse events (Stock et al., 2011). Such advances are important in advancing the vaccine in regard to improved efficacy/use while mitigating adverse events.
Unfortunately, there are some drawbacks to the use of this live vaccine, including safety and inefficacy after onset of disease or during an epidemic. The safety risks of 17D vaccination have been recently reviewed (Barrett and Teuwen, 2009; Thomas et al., 2012), giving further understanding into potential risk factors for serious adverse events. Although occasional adverse events occur, it must be stressed that the 17D vaccine is exceedingly effective and safe, aside from some identified risk factors, where vaccination should be avoided. The virus is contraindicated in individuals who are immunocompromised, which is increasingly common. For example, individuals with inflammatory bowel disease (IBD) are prescribed different immunomodulators that may reduce the immune response. However, in a recent study of vaccination in individuals taking such medications, none showed adverse reaction to vaccination (Wilckens et al., 2011), although such factors should be seriously considered prior to administration of live attenuated vaccines. Another example is the compromised immunity in Africa where there is both a risk of YFV outbreaks as well as the underlying complication of a large population of individuals chronically infected with human immunodeficiency virus (HIV). Similar to the IBD study, no adverse events in 102 HIV-infected patients were observed in a recent investigation into YF vaccine complications, although significantly lower levels of protective neutralizing Ab titers (NT) were observed in these individuals as compared with non-HIV infected vaccinees (Veit et al., 2009). The reason for lower NT after vaccination of HIV-infected patients with 17D YFV was found to depend on the plasma levels of HIV at vaccination (Pacanowski et al., 2012).
The 17D vaccine virus was implicated in cases of neonatal meningoencephalitis after breast-feeding from recently vaccinated mothers (Kuhn et al., 2011; Traiber et al., 2011), further illustrating the potential for disease when contraindications are not heeded. The vaccine virus has been shown to persist up to 6 months after vaccination, with suggested implications in safety (Martinez et al., 2011). Another recent concern with vaccines in general was the newly discovered potential for risk of contamination with xenotropic murine leukemia virus-related virus (XMRV), which is associated with prostate cancer and chronic fatigue syndrome. YFV vaccine, as well as 7 other live attenuated vaccines, tested negative for XMRV and related viruses in a recent study (Switzer et al., 2011).
Serious adverse events are the most severe consequence of vaccination with the 17D vaccine, although such events are rare. One such adverse condition, YF vaccine associated viscerotropic disease (YEL-AVD), was reported in 0.06-3.2 per 100,000 vaccination events worldwide, depending on risk factors (Barrett and Teuwen, 2009). YEL-AVD was associated with a high case fatality rate around 60%. A cluster of 5 cases of YEL-AVD occurred after a 2007 vaccination campaign in Peru, although no uniform predictor of outcome could be identified (Whittembury et al., 2009). Analysis of a comprehensive data set from known cases of YEL-AVD revealed a higher incidence in men >60 years and in 19-34 year old women with no known immunodeficiency (Seligman, 2011). Recent studies revealed that intrathecal antibody production is produced during another adverse event, YF-vaccine associated syndrome involving neurotropic disease (YEL-AND), which confirms central nervous system infection by 17D vaccine virus after vaccination (Pires-Marczeski et al., 2011). These adverse vaccination events, although extremely rare, are an ongoing concern when administering live attenuated vaccines as the cause of such adverse events, and conversely how to prevent them, is largely unexplained.
To counteract the safety concerns associated with live attenuated vaccines, some inactivated vaccines have recently been developed and are currently being tested for efficacy in animal models and human subjects. One such vaccine, designated XRX-001, has shown suitable immunogenicity and protection in a hamster model of YFV infection and disease, protecting vaccinated animals from lethal infection (Monath et al., 2011; Monath et al., 2010). Antibodies (Ab) produced after vaccination and passively administered to naïve animals were protective after YFV challenge and corresponded to the concentration of neutralizing Ab present (Julander et al., 2011b). A neutralizing Ab titer of 1:10 was protective in this study, which is similar to that reported after 17D vaccination (Barrett and Teuwen, 2009). Human subjects challenged with XRX-001 developed a protective Ab response that was dose dependent (Monath et al., 2011). Another approach to improve safety concerns with live attenuated immunization is the development of a vaccinia delivery system utilizing intramuscular administration of non-replicating vaccinia-vectored precursor of membrane and envelope proteins, which protected BALB/c mice after intracranial challenge with YFV (Schafer et al., 2011). Further testing is needed to determine if this vaccination approach is useful against a more relevant course of disease. Overall, these efforts to improve the safety of YF vaccination appear to be making good progress towards this goal.
V. Tools for the discovery of new antivirals
To aid in the identification of antiviral agents with activity against YFV, various in vitro and in vivo systems have been developed. Initial screens, including blind screening of chemical libraries or initial tests of specifically designed compounds, are typically conducted in cell culture, but may also be tested in various in vitro systems to study the interaction of the compound with the specific target. The following sections contain only a brief account of recent advances in model systems that aid in the discovery of anti-YFV agents.
A. In vitro
Replicons containing the nonstructural genes of YFV and a reporter gene such as luciferase have been useful in high-throughput screening (HTS) applications to expedite the discovery of compounds with antiviral activity. A recently developed replicon HTS assay was recently developed and was used to screen a compound library, which resulted in the discovery of several compounds that were active against YFV in the high nM range (Patkar et al., 2009). Mutations generated in the presence of these compounds mapped to NS4B, suggesting a potential novel role of these compounds in the inhibition of this viral protein as a part of the replication complex.
B. In vivo
The hamster model of YF continues to provide a reliable small animal model for use in antiviral studies (Julander et al., 2011a; Julander et al., 2010; Julander et al., 2009; Julander et al., 2011b; Monath et al., 2010). It requires an adapted strain of either the Jimenez or the Asibi YFV strains. The pathogenesis is limited to viscerotropic disease, paralleling many aspects of human disease (Julander et al., 2007b; Sbrana et al., 2006; Tesh et al., 2001; Xiao et al., 2001). Recent studies in YFV-infected hamsters have identified key parameters of pathogenesis, including immunomodulation of cytokines in important tissues as well as immunopathogenic effects (Gowen et al., 2010; Li et al., 2008). A lack of reagents for use in hamster studies as well as the larger size, constitute the main impediments to their use in anti-YFV studies.
A mouse model of wild-type YFV infection that includes a relevant viscerotropic disease has recently been developed in A129 mice lacking the interferon alpha/beta receptor (Meier et al., 2009). Infection of this mouse strain with wild-type, but not attenuated, YFV strains results in a pantropic infection, including dissemination to several different tissues, elevated levels of relevant inflammatory cytokines, and hepatic pathology. Studies in AG129 mice, lacking both IFN alpha/beta and gama receptors, demonstrate the utility of these mice in YFV studies and provide a lethal model that has viscerotropic disease after infection with the 17D-204 vaccine strain (Thibodeaux et al., 2012). The use of this virus allows work to be conducted under BSL-2 conditions. Another benefit includes access to the plethora of reagents available for mouse studies. However, this model is in an immunocompromised mouse strain that is more expensive and less readily available than other rodent models such as the hamster, and also develops some neurological disease that constitutes a more difficult target for treatment.
Infection of nonhuman primates with YFV generally results in severe disease manifestations and represents a severe challenge model (Monath et al., 1981). Macaques are an important model for clinical development of various agents and will continue to be important to the discovery of improved vaccines or antiviral treatments for YFV (Maximova et al., 2008). Wild primates serve as sentinel species for sylvatic cycles of YFV and the observation of mortality of some monkey species may be an indication of current or impending YF outbreaks (Holzmann et al., 2010).
VI. Strategies for the development of YFV antivirals
The two broad areas of therapeutic intervention are 1) direct targeting of viral proteins or 2) targeting key host proteins involved in viral replication. For either of these istrategies, it is best to consider an agent that acts through conserved sequence/structural regions or shared host pathways that are common to a broad array of viruses.
Several important impediments to the development of antivirals for the treatment of YFV exist, including the high cost of development of drugs compounded with a limited number of YFV cases, the remote and often resource poor areas where outbreaks occur, and a limited treatment window for acute YF disease. For this reason, an effective therapy must be effective after the onset of clinical disease, has broad-spectrum activity against other agents of human disease, is relatively inexpensive, has good stability, low toxicity, and may be administered orally or through injection. Currently approved drugs may hold promise in the treatment of acute flaviviral disease, especially in regard to the number of compounds that have been shown to have efficacy in the treatment of HCV, as well as other drugs such as Ivermectin (discussed below), that could potentially be used in the treatment of neglected tropical diseases. A recent review discusses such approaches to the discovery of antivirals for the treatment of flavivirus infections (Botting and Kuhn, 2012), whereas this review will focus specifically on the treatment of YFV and advances made over the last several years.
Diagnosis of etiologic agents responsible for disease outbreaks in areas endemic for YFV has traditionally been slow and is considered a limiting factor in the implementation of timely intervention strategies. New technologies may mitigate this to some extent. During a recent outbreak of hemorrhagic fever in Uganda, Next generation sequencing (NGS) was used to identify YFV as the etiologic agent responsible (McMullan et al., 2012). The costs associated with this technology, however, are very high and may preclude the use of such assays locally. Sending samples across oceans for diagnosis does not lend to a speedy diagnosis for intervention, and until such capabilities are available in regions of endemicity, their use will likely not improve intervention response time to outbreaks.
As the development of compounds for acute viral diseases is generally not financially justifiable for pharmaceutical companies to pursue, aside from perhaps countermeasures for the treatment of dengue virus (DENV), services for discovery of treatment or prevention options for neglected tropical diseases are available from various funding agencies. One such avenue is the Antimicrobial Acquisition and Coordinating Facility program, which is a funded by the National Institute of Allergy and Infectious Diseases (NIAID) Division of Microbiology and Infectious Diseases (DMID) (Greenstone et al., 2008). The purpose of the AACF is to provide free and confidential evaluation of candidate antiviral agents in cell culture and animal models of viral disease of human concern. Potential antivirals are provided by a submitter and are subsequently tested against a diverse array of viral agents. Compounds found active in cell culture may be further evaluated in vivo. This facilitates a more rapid discovery of new antiviral drugs, including the initial steps of clinical evaluation to be conducted at little cost to the compound submitter.
A potential avenue for the development of antiviral compounds for the treatment of YFV is by testing compounds undergoing clinical trials for hepatitis C virus (HCV) for broad-spectrum activity against related viruses. Infection with HCV, a hepatotropic virus that is classified along with YFV in the virus family Flaviviridae, generally results in chronic liver disease, and afflicts around 180 million people throughout the world (Rosen, 2011). Many drugs have been developed or are under development for the treatment of HCV (Ilyas and Vierling, 2011; Vermehren and Sarrazin, 2011). Overlap between compounds that are effective against both HCV and YFV in vitro (Buckwold et al., 2007; Fogt et al., 2008; Lam et al., 2010; Yin et al., 2009) and in animal models (Julander et al., 2010; Julander et al., 2007b; Sbrana et al., 2004) has been reported. Harnessing the multi-billion dollar HCV market would allow the development of therapies for the treatment of YFV as a secondary indication agent. Also, the shorter course of treatment that would be required to treat patients with YFV and other related acute viral diseases may also serve to “rescue” HCV antivirals that fail clinical trials due to toxicity associated with long-term treatment regimens. Clinical development of HCV antivirals might potentially include preliminary studies against YFV in vitro and in animal models to support later investigation of utility in people infected with YFV.
VII. Targeting the virus
A- The YFV replication cycle
The flavivirus genome is comprised of a single-stranded, positive-sense RNA that codes for a single polyprotein, which is processed, initially by host proteases and later by viral proteases within the cytoplasm of the cell, into 3 structural and 7 nonstructural proteins (Figure 1).
The replication cycle of YFV occurs primarily in the cytoplasm of the cell (Figure 2). Various stages of the replication cycle serve as potential targets for antiviral development, many of which are discussed below. Please refer to Figure 2 for a graphic representation of the replication process.
B- Experimental drugs against specific viral proteins
Direct antivirals are likely key to a successful treatment regimen and much work has been done to identify antiviral compounds with efficacy against YFV. Further research into the mechanisms of flaviviral pathogenesis will continue to provide potential targets for antiviral development against various viral components. The following section discusses recent advances in the identification of various viral targets. Various high-resolution structures for YFV proteins, including the NS3 helicase, envelope domain III, the NS5 methyltransferase, have been elucidated, which are important in targeted drug design. These non-structural proteins of YFV are important targets for drug development. A recent review gives important insight into the use of protein sequence and crystal structures of flaviviral non-structural proteins in target-based approach to drug development (Bollati et al., 2010), and this aspect of rational drug design will not be discussed presently.
a. The envelope protein
The structural proteins of YFV, especially the envelope protein, may also be suitable targets for the development of antiviral compounds. Modeling studies have been employed for the identification of potential inhibitors of the envelope protein of YFV (Umamaheswari et al., 2011). A monoclonal antibody (mab) that recognizes an epitope of the fusion loop of the E protein has been shown to be potently neutralizing for not only YFV, but also for the related flaviviruses, DENV, WNV, JEV, and TBEV, including efficacy in animal models (Deng et al., 2011). This mab blocks a step subsequent to binding.
Compounds that bind to the envelope protein may also be useful inhibitors of viral entry. Several compounds within a series of derivatives of thiazoles targeting the envelope protein were shown to have potent activity against YFV in a cell-based assay (Mayhoub et al., 2011). One of these compounds, Compound 16 [5-methyl4-(dibromomethyl)-2-(4-chlorophenyl)thiazole-5-carbothioate], was relatively nontoxic (CC50= 369) and was active with an EC50 of 1.4 μM, giving a selective index (CC50/EC50) of 263. This activity warrants further studies in an animal model.
b. NS1
The NS1 protein has reported involvement in immune evasion strategies of various flaviviruses, including YFV. This non-structural protein will both form a complex with the compliment pathway proteins C1s and C4, which promotes cleavage of C4 to C4b (Avirutnan et al., 2010). In addition, NS1 will then directly associated with C4b, which results in inactivation through recruitment of C4BP (Avirutnan et al., 2011).
To overcome viral resistance, host cells have developed pathogen-specific responses, which is evident in the interferon-stimulated gene (ISG) production that has a different profile for different viruses (Schoggins et al., 2011). Interestingly, this study identified fewer (7) ISGs with activity against YFV as compared with those identified for other viruses, including other members of the family Flaviviridae WNV and HCV (10 and 9 ISGs, respectively). However, these active ISGs had a greater impact on replication, with 6 of 7 resulting in inhibition >50% as compared with 3 and 2 for WNV and HCV, respectively (Schoggins et al., 2011). It may be possible to stimulate certain ISGs, alone or in combination, to specifically inhibit YFV, but further research in this area is needed. In addition, several ISGs were found to enhance replication, which were similar for several unrelated viruses (Schoggins et al., 2011).
The recently discovered non-coding subgenomic flaviviral RNA (sfRNA) elements have been shown to be required for the pathogenesis of WNV, a related flavivirus (Funk et al., 2010). These sfRNAs were subsequently determined to be involved in immune evasion after it was observed that WNV strains deficient in producing sfRNA could only cause disease in mice deficient for certain interferon regulatory factors, lacking type 1 interferon (IFN) receptor, or under other conditions of IFN pathway inhibition (Schuessler et al., 2012). This suggests involvement of these non-coding RNAs in evasion of the type I IFN pathway. It is unknown if YFV has a similar method of immune evasion and future research in this area may elucidate a potential target for antiviral treatment.
The NS1 protein is also essential for viral RNA synthesis, and targeting NS1 would also potentially inhibit viral replication in addition to the interruption of immune evasion described above. Development of inhibitors of NS1 could therefor have potential utility in the treatment of YFV.
c. NS3
Position 349 within the helicase domain of the NS3 protein is involved in the assembly of virus progeny (Patkar and Kuhn, 2008). This represents another potential avenue for the development of antivirals and suggests that targeting specific regions of NS3, aside from the helicase function, may be useful targets for antiviral development. Further critical regions within this viral protein may be elucidated with the aid of computer modeling using the predicted 3D structure for the NS2b-NS3 protease complex (Kannappan and Narayanan, 2011).
Ivermectin, the anti-helminthic drug, was discovered through a targeted screen of a compound library for interaction with the flavivirus NS3 helicase. This was somewhat surprising, considering the original use of the compound to treat parasitic worm infections. This compound was potently active in cell culture against YFV, and displayed lesser activity against a broad range of flaviviruses (Mastrangelo et al., 2012). A compound like Ivermectin could hold great promise for the treatment of YFV, as it already has FDA approval, is relatively inexpensive, and is widely used throughout the world.
d. NS4B
Recent studies have identified an inhibitor of NS4B that is active against all four viral serotypes of DENV, but not against YFV (Xie et al., 2011). These results suggest that targeting this viral protein may also be useful in the development of antivirals for the treatment of YFV. As mentioned above, several inhibitors discovered during a replicon-based screening assay appear to inhibit NS4B (Patkar et al., 2009). These results also underscore the potential for the development of resistance mutations by the virus in NS4B, which should be taken into account if such inhibitors are advanced toward clinical use.
e. NS5
The importance of the YFV NS3 and NS5 proteins as targets for antiviral therapy has been strengthened by a recent study that utilized proteome mapping to identify 108 host proteins that interact with these two viral proteins (Le Breton et al., 2011). Le Breton and colleagues outline the cellular proteins and identify those that are known to be involved with other viral replication cycles, including HCV, herpesviruses, papillomaviruses and human immunodeficiency virus, providing potential targets for broad-spectrum antiviral therapy. Various cellular proteins involved in histone complex formation/chromatin remodeling process, which are important in the response of dendritic cells during pathogen infection, were identified, suggesting subversion or disruption of this process as an important mechanism of flaviviruses to establish replication in mononuclear cells (Le Breton et al., 2011).
Other potential targets revealed through this study are the cellular structural components, such as the microtubule network, which are utilized by the virus for use in trafficking and replication factory formation within the cell. Interaction of NS5 with the host protein U1A occurs at a site that is highly conserved across various flaviviruses, and may represent potential broad-spectrum inhibition of viral replication (Bronzoni et al., 2011). Knowledge of these various cellular components that are important in virus replication may result in novel targets for inhibition of YFV and also strengthens the case to develop broad-spectrum inhibitors of flavivirus NS3 and NS5.
Methyltransferase (MTase) capability of the NS5 protein is essential for the translation of YFV RNA (Bhattacharya et al., 2008). There are two basic functions of the flavivirus MTase: methylation at the N7 position of the blocking guanine bound to the viral RNA (Dong et al., 2008);(Ray et al., 2006), followed by methylation of the nucleoside-2′-O ribose (Egloff et al., 2002). The N7 methylation event is required, while inhibition of the 2′-O methylation only attenuates the virus (Liu et al., 2010). Direct inhibition of the viral NS5 MTase represents an important target of inhibition. The cellular protein casein kinase 1 was shown to interact with the methyltransferase domain of NS5 and targeting this enzyme resulted in decreased viral replication (Bhattacharya et al., 2009), supporting inhibition of host proteins as an additional method of inhibiting the virus. An in depth review of various known aspects of flaviviral MTase were recently reviewed, and include important insights into inhibition (Liu et al., 2010; Zhou et al., 2007).
The compound 2′-C-methylcytidine is an RNA polymerase inhibitor that was originally developed for and tested in clinical trials against HCV (Carroll and Olsen, 2006), although it ultimately failed due to toxicity associated with long-term treatment. In addition to its anti-HCV activity, it was also effective in cell culture as well as in vivo against YFV. In a study conducted using the hamster model of YF, efficacy was observed when treatment was initiated as late as 2 days after virus challenge (Julander et al., 2010). Various other derivatives of this compound have been synthesized, with various levels of activity against YFV in cell culture (Fogt et al., 2008; Pierra et al., 2006; Stuyver et al., 2006), and it is possible that a less toxic derivative with anti-YFV activity may be developed.
The pyrazinecarboxamide derivatives, T-705 (favipiravir) and T-1106, display broad-spectrum activity against many different RNA viruses, including YFV, likely through the inhibition of viral polymerase (Furuta et al., 2009). In the hamster YFV model, T-1106 is highly effective at doses as low as 32 mg/kg/d, and at a dose of 100 mg/kg was effective when treatment was initiated 4 days after virus challenge (Julander et al., 2007a). Although T-705 was slightly less effective in the hamster YFV model, this compound is currently undergoing clinical trials for use in the treatment of influenza virus (Hayden, 2009), and if clinically approved may represent an opportunity for off-label use in the treatment of patients infected by YFV. As reported on the Toyama Chemical Co website, T-1106 is currently undergoing non-clinical studies with HCV as the target agent. The clinical development of T-1106 for the treatment of HCV would also hold promise for future clinical use in the treatment of YFV.
The anti-dengue compound NIT008 was also shown to be active in cell culture against YFV (Yin et al., 2009). This compound displayed potent activity against DENV in a mouse model. Phosphorylation of this adenosine analog is required for inhibition of the viral polymerase as a chain terminator, and it is anticipated that similar activity will be observed in rodent models of YFV. The 2,3-dihydro-4H-pyridinon derivatives 4c cis and 6a were moderately effective in cell culture against YFV with selective index (SI) values of 5.6 and 10, respectively (Peduto et al., 2011). Compound 6a was also shown to inhibit HCV replicon in cell culture as well as the NS5B of HCV in vitro, suggesting inhibition of RNA dependent RNA polymerase as a possible mechanism of action.
f. Anti-YFV compounds with other or unknown mechanism
Several different mechanisms of action have been described for the broad-spectrum antiviral agent ribavirin. This compound has been shown to inhibit host inosine monophosphate dehydrogenase (IMPDH), which was shown in cell culture to be the primary mechanism of YFV replication inhibition (Leyssen et al., 2006). This compound has also been shown to interact directly with the viral RNA polymerase of influenza (Eriksson et al., 1977). Error catastrophe of flaviviruses has also been observed after passage of WNV in the presence of ribavirin (Day et al., 2005), although this mechanism does not likely play a major role in the antiviral activity of this compound against acute flavivirus infections. Ribavirin is highly effective in the hamster model (Julander et al., 2007b), but such efficacy has not been observed in primate models of disease (Monath, 2008). Ribavirin has been extensively used as a therapeutic for the treatment of HCV and exhibits potent efficacy when combined with interferon (Ilyas and Vierling, 2011).
Many compounds that have been shown to be active against YFV in cell culture have unknown mechanisms of action. The thiopurine, 6-methylmercaptopurine riboside (6MMPr) was shown to inhibit the replication of YFV, as well as other flaviviruses including West Nile virus (WNV), in cell culture (Lim et al., 2011). This compound appeared to exacerbate disease in a mouse model of WNV. It might be worthwhile, however, to investigate the efficacy of 6MMPr in the YFV hamster model to determine its effect in a non-neurological flavivirus model.
Direct targeting of viral RNA through pathways of small RNA interference has been shown to be effective in the inhibition of many viruses (Stein and Shi, 2008), although efficient delivery to key sites is one important hurdle to clinical development. Recent advances in delivery of such agents may help overcome this issue. Inhibitory RNA therapy has been shown to be effective in cell culture and in adult C57BL/6 mice infected intracerebrally with YFV, although treatment required intracerebral injection of plasmids coding for short hairpin RNAs directed against the NS1 and E viral proteins (Pacca et al., 2009).
VIII. Targeting host responses
A- Host responses to YFV infection
One of the key features of YF, as well as many other viral diseases, is the immunopathogenesis that occurs as a result of infection. Much of the pathology in the hamster model of YF occurs during the decline and even after clearance of much of the replicating virus (Julander et al., 2007b), suggesting the later stages of severe disease have a strong immunological component. This is an important consideration in the treatment of YF, as treatment with direct antivirals during later stages of severe disease will likely do nothing to quell the pathogenesis, as the virus is being cleared by the immune system.
A number of studies have been conducted to identify key components responsible for the immunopathogenesis in cell culture when comparing infection of cells with the vaccine strain to infection with a wild-type non-attenuated strain. One such study identified a reduction in cytokine induction in cells infected with Asibi wild-type YFV as compared with cells infected with 17D vaccine in a hepatocyte cell line, suggesting potential limiting of immune response as a mechanism of pathogenesis (Woodson and Holbrook, 2011). The opposite, however, was true for comparative infection of Asibi and 17D in Kupffer cells, where Asibi infection resulted in significant elevation of inflammatory cytokines (Woodson et al., 2011).
It is evident from these studies that virus infection of different cell or tissue types may stimulate the immune response differently, resulting in diverse effects on pathogenesis. This is similar to observations on cytokine induction in YFV-infected hamsters, in which tissue-specific differences in cytokine profiles were observed (Li et al., 2008). These studies emphasize the complexity of disease associated with YFV infection. Further research in this area could result in a more clear understanding of how potential immunomodulation may help alleviate disease associated with YFV infection.
B- Experimental drugs against specific host responses
It has been suggested that focus be placed on agents that may potentially reduce the immunopathology associated with systemic inflammatory response syndrome that is observed in fatal cases of YFV (Monath, 2008). The complexity and lack of understanding of many areas of the immune response to YFV infection makes this a difficult prospect. By characterizing the timing and action of key inflammatory intermediates, a mechanism to ameliorate the effects of an overactive response to virus infection may be discovered. Inhibitors of the immune system also have the potential for combination therapy together with a direct antiviral.
Stimulation of the immune system through treatment with a range of immunomodulators may ameliorate disease associated with YFV infection. Interferon (IFN) therapy, generally in combination with ribavirin, has been used to treat chronic hepatitis C. Such a combination may also be useful in the treatment of other acute flaviviral diseases, and it is likely that a short treatment course would be sufficient to ameliorate YF, with the added bonus of fewer administrations and lower toxicity. Unfortunately, the treatment window for therapy with interferon may be limited. Treatment with interferon alfacon-1 or the adenovirus-vectored interferon DEF201 was effective in a hamster model of disease and both agents were effective up to 2-3 days after virus challenge, which was prior to severe disease manifestations (Julander et al., 2011a; Julander et al., 2007b). This construct was also effective when administered 7 days before virus challenge, suggesting the potential for prophylactic use in the event of an outbreak. Treatment with DEF201 did not preclude the development of neutralizing antibodies (Julander, unpublished data). The compound CCG-4088 was recently discovered to possesses anti-YFV activity (Patkar et al., 2009). This compound has a similar structure to a class that inhibits cellular RNAse L, an antiviral protein that is involved in the innate immune response and cleaves single-stranded RNA.
In addition to modulation of the innate immune response, there is also potential for exploiting adaptive immune responses through utilization of therapeutic antibodies. A relatively low percentage of neutralizing antibody was found to be directed to sites that result in effective neutralization of flaviviruses (Throsby et al., 2006), suggesting a potential benefit for the administration of exogenous antibody with specificity for these sites. While this has important implications in the field of vaccine development, antibody therapy has also been shown to be effective in the treatment of YFV and other viruses in animal models (Julander et al., 2011b; Morrey et al., 2006; Morrey et al., 2007) as well as in afflicted patients (Shimoni et al., 2001). ‘Reverse vaccinology’, the elucidation and targeting of antigens that results in potent neutralization of virus, may be a useful tool in the development of therapeutic antibodies for the treatment of YFV and other acute viral diseases (Pierson and Diamond, 2008).
It is likely that patients suffering from YF do not present at the clinic until they experience severe disease manifestations, at which point intervention with IFN or other immunomodulators may be too late. Ideally, an effective immune activator would have broad-spectrum activity and would be very safe, so that administration of could be initiated at the earliest onset of disease symptoms, regardless of the causative agent. Other options for immunomodulatory treatment include the specific targeting of effector products stimulated by IFN. Recent work by Schoggins et al (Schoggins et al., 2011) has given additional insight into the antiviral effect of interferon, and may provide valuable information into improving treatment by selectively activating specific ISGs. Further research is needed to identify potential uses of immunomodulators in the treatment of YF and other acute viral diseases.
Interruption of virus replication through inhibition of host proteins that are important for virus replication are potentially very useful due to the fact that mutational escape is generally not an issue and many host-targeting therapeutics have been approved clinically. Several compounds with activity against YFV, including the heterocyclic compound 1 (Nair et al., 2009), have been shown to inhibit host inosine monophosphate dehydrogenase (IMPDH), suggesting the importance of this enzyme to viral replication.
Treatment of YFV in cell culture with the broad-spectrum kinase inhibitor SFV785 resulted in a 5-log reduction in virus titer at a concentration of 5 μM (Anwar et al., 2011). The mode of SFV785 action against the related flavivirus DENV was determined to occur through a disruption of viral assembly through a sequestration of the viral envelope protein away from the reticulate network to discrete vesicles. It will be interesting to see if such activity is also observed in vivo.
Various natural products demonstrate some antiviral activity against YFV. The venom component sPLA2s from the rattlesnake Crotalus durissus terrificus was shown to be highly active against YFV and DENV in cell culture, although this activity was primarily restricted to pretreatment/inactivation of the virus (Muller et al., 2012). The venom component crotoxin has been shown in mice to inhibit edema and cell migration at the site of carrageenan polysaccharide injection, suggesting anti-inflammatory activity (Nunes et al., 2010). Various essential oils have shown efficacy in cell culture assays against YFV (Meneses et al., 2009), although this action is likely due to inactivation of the virus and would not be suitable for clinical development.
IX. Conclusion
The prospect for the development of anti-YF countermeasures is a difficult one. However, the efforts of researchers to discover vaccines and antivirals for the prevention and treatment of YF have resulted in various candidates for further evaluation. It is likely that countermeasures for this age-old disease will be realized in the near future.
Highlights from “Experimental Therapy for Yellow Fever”.
Proposes methods for the development of countermeasures for the treatment of yellow fever
Includes discussion on the classification and replication of yellow fever virus
Discusses in vitro and in vivo model systems used for the discovery of antiviral agents
Identifies recently discovered viral pathways and their potential as targets for drug development
Discusses potential avenues of host response pathway manipulation in order to ameliorate yellow fever disease
Table 1A.
Antiviral compounds targeting specific YFV proteins that have shown activity in cell culture.
| Compound | Mechanism | Parameters | Treatment schedule |
Effecti ve dose |
Reference |
|---|---|---|---|---|---|
| mAb 2A10G6 |
Binds E | Neutralization | Pre- incubation for 1 h |
3.6 μg/ml |
(Deng et al., 2011) |
| Compound 16 |
Binds β-OG binding pocket of E |
Inhibition | Addition of compound to cells just after virus challenge |
1.4 μM | (Mayhoub et al., 2011) |
| Ivermectin | Targets NS3 helicase |
Inhibition of replication |
Addition of compound to cells just prior to virus challenge |
0.5 nM | (Mastrangelo et al., 2012) |
| 2′-C- methylcyti dine |
Targets NS5 polymerase |
Inhibition of replication |
Addition of compound to cells just prior to virus challenge |
0.7 μg/ml |
(Julander et al., 2010) |
| NITD008 | Polymerase inhibitor |
Virus titer reduction |
Addition of compound to cells just after virus challenge |
1-3 μM | (Yin et al., 2009) |
| 2,3- dihydro- 4H- pyridinon 4c cis |
Polymerase inhibitor |
Virus titer reduction |
Addition of compound to cells 1 h after virus challenge |
18 μM | (Peduto et al., 2011) |
| 2,3- dihydro- 4H- pyridinon 6a |
Polymerase inhibitor |
Virus titer reduction |
Addition of compound to cells 1 h after virus challenge |
10 μM | (Peduto et al., 2011) |
| Ribavirin | Inhibition of IMPDH |
Reduced GTP, Reduced virus RNA |
5 passages of YFV in the presence of ribavirin (5 d) |
120- 300 μg/ml |
(Leyssen et al., 2006) |
Table 1B.
Antiviral compounds targeting specific YFV proteins that have shown protective activity in rodent models.
| Compound | Model | Mechanism | Parameters | Treatment schedule |
Effective dose |
Reference |
|---|---|---|---|---|---|---|
| 2′-C- methyl- cytidine |
Hamster | Targets NS5 polymerase |
Survival/ protection from disease |
Twice daily for 7 days beginning −4 h to 2 days post- virus inoculation (dpi) |
120 mg/kg/d |
(Julander et al., 2010) |
| T-705 | Hamster | Likely targets polymerase |
Survival/ protection from disease |
Twice daily for 7 days beginning −4 h to 3 dpi |
200-400 mg/kg/d |
(Julander et al., 2009) |
| T-1106 | Hamster | Likely targets polymerase |
Survival/ protection from disease |
Twice daily for 7 days beginning −4 h to 4 dpi |
32-100 mg/kg/d |
(Julander et al., 2007a) |
| shRNA | Mouse | NS1, E | Mortality, disease |
Treated 12 h prior to virus challenge |
50 μg plasmid expressing shRNA |
(Pacca et al., 2009) |
| Ribavirin | Hamster | Inhibition of IMPDH |
Survival/ protection from disease |
Twice daily treatment for 7 days, administere d beginning −4 to 3 dpi |
50 mg/kg/d |
(Julander et al., 2007b) |
Table 2A.
Antiviral compounds targeting host responses that have shown activity in cell culture.
| Compound | Mechanism | Parameters | Treatment schedule |
Effective dose |
Reference |
|---|---|---|---|---|---|
| CCG-4088 | Inhibits RNAse L |
Replicon inhibition |
Compound administered as overlay just after challenge |
0.13-0.4 μM |
(Patkar et al., 2009) |
| SFV785 | Kinase inhibition |
Reduced virus titer |
Compound added 1 h after virus attachment |
5 μM | (Anwar et al., 2011) |
| sPLA2s | Inactivatio n (?) |
Reduced virus titer |
Added prophylacticall y or incubated with virus prior to virus challenge |
0.04-0.1 ng/ml |
(Muller et al., 2012) |
Table 2B.
Antiviral compounds targeting host responses that have shown activity in rodent models.
| Compound | Model | Mechanism | Parameters | Treatment schedule |
Effective dose |
Reference |
|---|---|---|---|---|---|---|
| Interferon alfacon-1 |
Hamster | IFN pathway | Survival, protection from disease |
Once daily treatment for 7 days beginning −4 h to 2 dpi |
5 μg/ animal |
(Julander et al., 2007b) |
| DEF201 | Hamster | IFN pathway | Survival, protection from disease |
Single treatment administer ed on −7 to 2 dpi |
5 × 107 pfu/ animal |
(Julander et al., 2011a) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anwar A, Hosoya T, Leong KM, Onogi H, Okuno Y, Hiramatsu T, Koyama H, Suzuki M, Hagiwara M, Garcia-Blanco MA. The kinase inhibitor SFV785 dislocates dengue virus envelope protein from the replication complex and blocks virus assembly. PloS one. 2011;6:e23246. doi: 10.1371/journal.pone.0023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, Diamond MS, Atkinson JP. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. The Journal of experimental medicine. 2010;207:793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P, Hauhart RE, Somnuke P, Blom AM, Diamond MS, Atkinson JP. Binding of flavivirus nonstructural protein NS1 to C4b binding protein modulates complement activation. Journal of immunology. 2011;187:424–433. doi: 10.4049/jimmunol.1100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Current opinion in immunology. 2009;21:308–313. doi: 10.1016/j.coi.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrobial agents and chemotherapy. 2010;54:2431–2436. doi: 10.1128/AAC.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt PN, Johnson EA, Smith AL, Jacoby RO. Genetic resistance to lethal flaviviral encephalitis. III. Replication of Banzi virus in vitro and in vivo in tissues of congeneic susceptible and resistant mice. Archives of virology. 1981;69:273–286. doi: 10.1007/BF01317342. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Ansari IH, Striker R. The flaviviral methyltransferase is a substrate of Casein Kinase 1. Virus research. 2009;141:101–104. doi: 10.1016/j.virusres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Hoover S, Falk SP, Weisblum B, Vestling M, Striker R. Phosphorylation of yellow fever virus NS5 alters methyltransferase activity. Virology. 2008;380:276–284. doi: 10.1016/j.virol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, Gould EA, Grard G, Grimes JM, Hilgenfeld R, Jansson AM, Malet H, Mancini EJ, Mastrangelo E, Mattevi A, Milani M, Moureau G, Neyts J, Owens RJ, Ren J, Selisko B, Speroni S, Steuber H, Stuart DI, Unge T, Bolognesi M. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antiviral Res. 2010;87:125–148. doi: 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting C, Kuhn RJ. Novel approaches to flavivirus drug discovery. Expert Opin Drug Discov. 2012;7:417–428. doi: 10.1517/17460441.2012.673579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzoni RV, Madrid MC, Duarte DV, Pellegrini VO, Pacca CC, Carmo AC, Zanelli CF, Valentini SR, Santacruz-Perez C, Barbosa JA, Lutz CS, Rahal P, Nogueira ML. The small nuclear ribonucleoprotein U1A interacts with NS5 from yellow fever virus. Archives of virology. 2011;156:931–938. doi: 10.1007/s00705-011-0927-x. [DOI] [PubMed] [Google Scholar]
- Buckwold VE, Wei J, Huang Z, Huang C, Nalca A, Wells J, Russell J, Collins B, Ptak R, Lang W, Scribner C, Blanchett D, Alessi T, Langecker P. Antiviral activity of CHO-SS cell-derived human omega interferon and other human interferons against HCV RNA replicons and related viruses. Antiviral Res. 2007;73:118–125. doi: 10.1016/j.antiviral.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Carroll SS, Olsen DB. Nucleoside analog inhibitors of hepatitis C virus replication. Infect Disord Drug Targets. 2006;6:17–29. doi: 10.2174/187152606776056698. [DOI] [PubMed] [Google Scholar]
- Day CW, Smee DF, Julander JG, Yamshchikov VF, Sidwell RW, Morrey JD. Error-prone replication of West Nile virus caused by ribavirin. Antiviral Res. 2005;67:38–45. doi: 10.1016/j.antiviral.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Deng YQ, Dai JX, Ji GH, Jiang T, Wang HJ, Yang HO, Tan WL, Liu R, Yu M, Ge BX, Zhu QY, Qin ED, Guo YJ, Qin CF. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein. PloS one. 2011;6:e16059. doi: 10.1371/journal.pone.0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhang B, Shi PY. Flavivirus methyltransferase: a novel antiviral target. Antiviral Res. 2008;80:1–10. doi: 10.1016/j.antiviral.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JS, Aspinall E, Liew D, Thompson AJ, Hellard ME. Current and emerging antiviral treatments for hepatitis C infection. Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B, Helgstrand E, Johansson NG, Larsson A, Misiorny A, Noren JO, Philipson L, Stenberg K, Stening G, Stridh S, Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrobial agents and chemotherapy. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogt J, Januszczyk P, Framski G, Onishi T, Izawa K, De Clercq E, Neyts J, Boryski J. Synthesis and antiviral activity of novel derivatives of 2′-beta-C-methylcytidine. Nucleic Acids Symp Ser (Oxf) 2008:605–606. doi: 10.1093/nass/nrn306. [DOI] [PubMed] [Google Scholar]
- Funk A, Truong K, Nagasaki T, Torres S, Floden N, Balmori Melian E, Edmonds J, Dong H, Shi PY, Khromykh AA. RNA structures required for production of subgenomic flavivirus RNA. Journal of virology. 2010;84:11407–11417. doi: 10.1128/JVI.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Julander JG, London NR, Wong MH, Larson D, Morrey JD, Li DY, Bray M. Assessing changes in vascular permeability in a hamster model of viral hemorrhagic fever. Virol J. 2010;7:240. doi: 10.1186/1743-422X-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstone H, Spinelli B, Tseng C, Peacock S, Taylor K, Laughlin C. NIAID resources for developing new therapies for severe viral infections. Antiviral Res. 2008;78:51–59. doi: 10.1016/j.antiviral.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(Suppl 1):S3–13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- Holzmann I, Agostini I, Areta JI, Ferreyra H, Beldomenico P, Di Bitetti MS. Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. Am J Primatol. 2010;72:475–480. doi: 10.1002/ajp.20796. [DOI] [PubMed] [Google Scholar]
- Ilyas JA, Vierling JM. An overview of emerging therapies for the treatment of chronic hepatitis C. Clinics in liver disease. 2011;15:515–536. doi: 10.1016/j.cld.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Julander JG, Ennis J, Turner J, Morrey JD. Treatment of yellow fever virus with an adenovirus-vectored interferon (DEF201) in a hamster model. Antimicrobial agents and chemotherapy. 2011a doi: 10.1128/AAC.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Furuta Y, Shafer K, Sidwell RW. Activity of T-1106 in a hamster model of yellow Fever virus infection. Antimicrob Agents Chemother. 2007a;51:1962–1966. doi: 10.1128/AAC.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Jha AK, Choi JA, Jung KH, Smee DF, Morrey JD, Chu CK. Efficacy of 2′-C-methylcytidine against yellow fever virus in cell culture and in a hamster model. Antiviral Res. 2010;86:261–267. doi: 10.1016/j.antiviral.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Judge JW, Olsen AL, Rosenberg B, Schafer K, Sidwell RW. Prophylactic treatment with recombinant Eimeria protein, alone or in combination with an agonist cocktail, protects mice from Banzi virus infection. Antiviral Res. 2006 doi: 10.1016/j.antiviral.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 2007b;73:140–146. doi: 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011b doi: 10.1016/j.vaccine.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannappan P, Narayanan S. Mutation and docking studies on NS2b-NS3 complex from yellow fever virus towards drug discovery. Bioinformation. 2011;6:303–306. doi: 10.6026/97320630006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A, Chen LH, Sisti M, Monath TP. Yellow fever vaccine seroconversion in travelers. The American journal of tropical medicine and hygiene. 2011;85:748–749. doi: 10.4269/ajtmh.2011.11-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Twele-Montecinos L, MacDonald J, Webster P, Law B. Case report: probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. Cmaj. 2011;183:E243–245. doi: 10.1503/cmaj.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Tripathi P, Baranwal M, Singh S, Tripathi S, Banerjee G. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:400–406. doi: 10.1086/596309. [DOI] [PubMed] [Google Scholar]
- Lam AM, Murakami E, Espiritu C, Steuer HM, Niu C, Keilman M, Bao H, Zennou V, Bourne N, Julander JG, Morrey JD, Smee DF, Frick DN, Heck JA, Wang P, Nagarathnam D, Ross BS, Sofia MJ, Otto MJ, Furman PA. PSI-7851, a pronucleotide of beta-D-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrobial agents and chemotherapy. 2010;54:3187–3196. doi: 10.1128/AAC.00399-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasala PR, Holbrook M. Tick-borne flaviviruses. Clinics in laboratory medicine. 2010;30:221–235. doi: 10.1016/j.cll.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Le Breton M, Meyniel-Schicklin L, Deloire A, Coutard B, Canard B, de Lamballerie X, Andre P, Rabourdin-Combe C, Lotteau V, Davoust N. Flavivirus NS3 and NS5 proteins interaction network: a high-throughput yeast two-hybrid screen. BMC Microbiol. 2011;11:234. doi: 10.1186/1471-2180-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssen P, De Clercq E, Neyts J. The anti-yellow fever virus activity of ribavirin is independent of error-prone replication. Mol Pharmacol. 2006;69:1461–1467. doi: 10.1124/mol.105.020057. [DOI] [PubMed] [Google Scholar]
- Li G, Duan T, Wu X, Tesh RB, Soong L, Xiao SY. Yellow Fever Virus Infection in Syrian Golden Hamsters: Relationship between Cytokine Expression and Pathologic Changes. Int J Clin Exp Pathol. 2008;1:169–179. [PMC free article] [PubMed] [Google Scholar]
- Lim PY, Keating JA, Hoover S, Striker R, Bernard KA. A thiopurine drug inhibits West Nile virus production in cell culture, but not in mice. PloS one. 2011;6:e26697. doi: 10.1371/journal.pone.0026697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Dong H, Chen H, Zhang J, Ling H, Li Z, Shi PY, Li H. Flavivirus RNA cap methyltransferase: structure, function, and inhibition. Front Biol. 2010;5:286–303. doi: 10.1007/s11515-010-0660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MJ, Vilella A, Pumarola T, Roldan M, Sequera VG, Vera I, Hayes EB. Persistence of yellow fever vaccine RNA in urine. Vaccine. 2011;29:3374–3376. doi: 10.1016/j.vaccine.2011.02.075. [DOI] [PubMed] [Google Scholar]
- Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, de Lamballerie X, Neyts J, Hanson AM, Frick DN, Bolognesi M, Milani M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. The Journal of antimicrobial chemotherapy. 2012 doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximova OA, Ward JM, Asher DM, Claire M, Finneyfrock BW, Speicher JM, Murphy BR, Pletnev AG. Comparative neuropathogenesis and neurovirulence of attenuated flaviviruses in nonhuman primates. Journal of virology. 2008;82:5255–5268. doi: 10.1128/JVI.00172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhoub AS, Khaliq M, Kuhn RJ, Cushman M. Design, synthesis, and biological evaluation of thiazoles targeting flavivirus envelope proteins. Journal of medicinal chemistry. 2011;54:1704–1714. doi: 10.1021/jm1013538. [DOI] [PubMed] [Google Scholar]
- McMullan LK, Frace M, Sammons SA, Shoemaker T, Balinandi S, Wamala JF, Lutwama JJ, Downing RG, Stroeher U, MacNeil A, Nichol ST. Using next generation sequencing to identify yellow fever virus in Uganda. Virology. 2012;422:1–5. doi: 10.1016/j.virol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses R, Ocazionez RE, Martinez JR, Stashenko EE. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann Clin Microbiol Antimicrob. 2009;8:8. doi: 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am J Trop Med Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. An inactivated cell-culture vaccine against yellow fever. The New England journal of medicine. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, Levesque P, Bowick GC, Morin M, Fowler E, Trent DW. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, Shope RE, Thomas N, Schrader R, Furby D, Bedford P. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. The American journal of tropical medicine and hygiene. 2002;66:533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang H, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J Infect Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining limits of humanized neutralizing monoclonal antibody treatment for West Nile virus neurological infection in a hamster model. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller VD, Russo RR, Oliveira Cintra AC, Sartim MA, De Melo Alves-Paiva R, Figueiredo LT, Sampaio SV, Aquino VH. Crotoxin and phospholipases A(2) from Crotalus durissus terrificus showed antiviral activity against dengue and yellow fever viruses. Toxicon. 2012;59:507–515. doi: 10.1016/j.toxicon.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Nair V, Chi G, Shu Q, Julander J, Smee DF. A heterocyclic molecule with significant activity against dengue virus. Bioorg Med Chem Lett. 2009;19:1425–1427. doi: 10.1016/j.bmcl.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyet NM, Chau TN, Lam PK, Kien DT, Huy HL, Farrar J, Quyen TH, Hien TT, Van Vinh Chau N, Merson L, Long HT, Hibberd ML, Aw PP, Wilm A, Nagarajan N, Dung NT, Mai PP, Truong NT, Javanbaht H, Klumpp K, Hammond J, Petric R, Wolbers M, Chinh NT, Simmons CP. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes FP, Zychar BC, Della-Casa MS, Sampaio SC, Goncalves LR, Cirillo MC. Crotoxin is responsible for the long-lasting anti-inflammatory effect of Crotalus durissus terrificus snake venom: involvement of formyl peptide receptors. Toxicon. 2010;55:1100–1106. doi: 10.1016/j.toxicon.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Pacanowski J, Lacombe K, Campa P, Dabrowska M, Poveda JD, Meynard JL, Poirot JL, Fonquernie L, Girard PM. Plasma HIV-RNA is the key determinant of long-term antibody persistence following Yellow fever immunization in a cohort of 364 HIV-infected patients. Journal of acquired immune deficiency syndromes. 2012 doi: 10.1097/QAI.0b013e318249de59. [DOI] [PubMed] [Google Scholar]
- Pacca CC, Severino AA, Mondini A, Rahal P, D’Avila S,G, Cordeiro JA, Nogueira MC, Bronzoni RV, Nogueira ML. RNA interference inhibits yellow fever virus replication in vitro and in vivo. Virus genes. 2009;38:224–231. doi: 10.1007/s11262-009-0328-3. [DOI] [PubMed] [Google Scholar]
- Patkar CG, Kuhn RJ. Yellow Fever virus NS3 plays an essential role in virus assembly independent of its known enzymatic functions. Journal of virology. 2008;82:3342–3352. doi: 10.1128/JVI.02447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar CG, Larsen M, Owston M, Smith JL, Kuhn RJ. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrobial agents and chemotherapy. 2009;53:4103–4114. doi: 10.1128/AAC.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula T, Pablo R, Eugenia V, Pablo B, Sabino P, Jose M, Antonio M, Dolores HM, Pablo L, Javier GS, Vincente S. New drug targets for hepatitis C and other Flaviviridae viruses. Infectious disorders drug targets. 2009;9:133–147. doi: 10.2174/187152609787847749. [DOI] [PubMed] [Google Scholar]
- Peduto A, Massa A, Di Mola A, de Caprariis P, La Colla P, Loddo R, Altamura S, Maga G, Filosa R. 2,3-Dihydro-1,2-Diphenyl-substituted 4H-Pyridinone derivatives as new anti Flaviviridae inhibitors. Chem Biol Drug Des. 2011;77:441–449. doi: 10.1111/j.1747-0285.2011.01102.x. [DOI] [PubMed] [Google Scholar]
- Pierra C, Amador A, Benzaria S, Cretton-Scott E, D’Amours M, Mao J, Mathieu S, Moussa A, Bridges EG, Standring DN, Sommadossi JP, Storer R, Gosselin G. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J Med Chem. 2006;49:6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Diamond MS. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev Mol Med. 2008;10:e12. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-Marczeski FC, Martinez VP, Nemirovsky C, Padula PJ. Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in Argentina. Journal of medical virology. 2011;83:2208–2212. doi: 10.1002/jmv.22236. [DOI] [PubMed] [Google Scholar]
- Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, Shi PY. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. Journal of virology. 2006;80:8362–8370. doi: 10.1128/JVI.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HR. Clinical practice. Chronic hepatitis C infection. The New England journal of medicine. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Guzman H, Ye M, Travassos da Rosa AP, Tesh RB. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am J Trop Med Hyg. 2004;71:306–312. [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am J Trop Med Hyg. 2006;74:1084–1089. [PubMed] [Google Scholar]
- Schafer B, Holzer GW, Joachimsthaler A, Coulibaly S, Schwendinger M, Crowe BA, Kreil TR, Barrett PN, Falkner FG. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PloS one. 2011;6:e24505. doi: 10.1371/journal.pone.0024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler A, Funk A, Lazear HM, Cooper DA, Torres S, Daffis S, Jha BK, Kumagai Y, Takeuchi O, Hertzog P, Silverman R, Akira S, Barton DJ, Diamond MS, Khromykh AA. West Nile virus non-coding subgenomic RNA contributes to viral evasion of type I interferon-mediated antiviral response. Journal of virology. 2012 doi: 10.1128/JVI.00207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman SJ. Yellow fever virus vaccine-associated deaths in young women. Emerging infectious diseases. 2011;17:1891–1893. doi: 10.3201/eid1710.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerging infectious diseases. 2001;7 doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Farrar JJ, Nguyen v, V., Wills B. Dengue. The New England journal of medicine. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- Smee DF, McKernan PA, Nord LD, Willis RC, Petrie CR, Riley TM, Revankar GR, Robins RK, Smith RA. Novel pyrazolo[3,4-d]pyrimidine nucleoside analog with broad-spectrum antiviral activity. Antimicrobial agents and chemotherapy. 1987;31:1535–1541. doi: 10.1128/aac.31.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC, Paterson HE, Heymann CS, Winter PA. An agent related to Uganda S virus from man and mosquitoes in South Africa. S Afr Med J. 1959;33:959–962. [PubMed] [Google Scholar]
- Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. The Journal of general virology. 2011;92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DA, Shi PY. Nucleic acid-based inhibition of flavivirus infections. Front Biosci. 2008;13:1385–1395. doi: 10.2741/2769. [DOI] [PubMed] [Google Scholar]
- Stock NK, Boschetti N, Herzog C, Appelhans MS, Niedrig M. The phylogeny of yellow fever virus 17D vaccines. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.12.057. [DOI] [PubMed] [Google Scholar]
- Stuyver LJ, McBrayer TR, Tharnish PM, Clark J, Hollecker L, Lostia S, Nachman T, Grier J, Bennett MA, Xie MY, Schinazi RF, Morrey JD, Julander JG, Furman PA, Otto MJ. Inhibition of hepatitis C replicon RNA synthesis by beta-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antivir Chem Chemother. 2006;17:79–87. doi: 10.1177/095632020601700203. [DOI] [PubMed] [Google Scholar]
- Switzer WM, Zheng H, Simmons G, Zhou Y, Tang S, Shankar A, Kapusinszky B, Delwart EL, Heneine W. No evidence of murine leukemia virus-related viruses in live attenuated human vaccines. PloS one. 2011;6:e29223. doi: 10.1371/journal.pone.0029223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Thibodeaux BA, Garbino NC, Liss NM, Piper J, Blair CD, Roehrig JT. A small animal peripheral challenge model of yellow fever using interferon-receptor deficient mice and the 17D-204 vaccine strain. Vaccine. 2012;30:3180–3187. doi: 10.1016/j.vaccine.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. The Safety of Yellow Fever Vaccine 17D or 17DD in Children, Pregnant Women, HIV+ Individuals, and Older Persons: Systematic Review. The American journal of tropical medicine and hygiene. 2012;86:359–372. doi: 10.4269/ajtmh.2012.11-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throsby M, Geuijen C, Goudsmit J, Bakker AQ, Korimbocus J, Kramer RA, Clijsters-van der Horst M, de Jong M, Jongeneelen M, Thijsse S, Smit R, Visser TJ, Bijl N, Marissen WE, Loeb M, Kelvin DJ, Preiser W, ter Meulen J, de Kruif J. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. Journal of virology. 2006;80:6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiber C, Coelho-Amaral P, Ritter VR, Winge A. Infant meningoencephalitis caused by yellow fever vaccine virus transmitted via breastmilk. J Pediatr (Rio J) 2011;87:269–272. doi: 10.2223/JPED.2067. [DOI] [PubMed] [Google Scholar]
- Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS neglected tropical diseases. 2010;4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle L, Griffiths MJ, Solomon T. Encephalitis caused by flaviviruses. Qjm. 2012;105:219–223. doi: 10.1093/qjmed/hcs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umamaheswari A, Kumar MM, Pradhan D, Marisetty H. Docking studies towards exploring antiviral compounds against envelope protein of yellow fever virus. Interdiscip Sci. 2011;3:64–77. doi: 10.1007/s12539-011-0064-y. [DOI] [PubMed] [Google Scholar]
- Veit O, Niedrig M, Chapuis-Taillard C, Cavassini M, Mossdorf E, Schmid P, Bae HG, Litzba N, Staub T, Hatz C, Furrer H. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:659–666. doi: 10.1086/597006. [DOI] [PubMed] [Google Scholar]
- Vermehren J, Sarrazin C. New hepatitis C therapies in clinical development. European journal of medical research. 2011;16:303–314. doi: 10.1186/2047-783X-16-7-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittembury A, Ramirez G, Hernandez H, Ropero AM, Waterman S, Ticona M, Brinton M, Uchuya J, Gershman M, Toledo W, Staples E, Campos C, Martinez M, Chang GJ, Cabezas C, Lanciotti R, Zaki S, Montgomery JM, Monath T, Hayes E. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. 2009;27:5974–5981. doi: 10.1016/j.vaccine.2009.07.082. [DOI] [PubMed] [Google Scholar]
- Wiggan O, Livengood JA, Silengo SJ, Kinney RM, Osorio JE, Huang CY, Stinchcomb DT. Novel formulations enhance the thermal stability of live-attenuated flavivirus vaccines. Vaccine. 2011;29:7456–7462. doi: 10.1016/j.vaccine.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens V, Kannengiesser K, Hoxhold K, Frenkel C, Kucharzik T, Maaser C. The immunization status of patients with IBD is alarmingly poor before the introduction of specific guidelines. Scand J Gastroenterol. 2011;46:855–861. doi: 10.3109/00365521.2011.574734. [DOI] [PubMed] [Google Scholar]
- Williams MC, Woodall JP. An Epidemic of an Illness Resembling Dengue in the Morogoro District of Tanganyika. East Afr Med J. 1964;41:271–275. [PubMed] [Google Scholar]
- Woodson SE, Freiberg AN, Holbrook MR. Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology. 2011;412:188–195. doi: 10.1016/j.virol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Woodson SE, Holbrook MR. Infection of hepatocytes with 17-D vaccine-strain yellow fever virus induces a strong pro-inflammatory host response. The Journal of general virology. 2011;92:2262–2271. doi: 10.1099/vir.0.031617-0. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- Xie X, Wang QY, Xu HY, Qing M, Kramer L, Yuan Z, Shi PY. Inhibition of dengue virus by targeting viral NS4B protein. Journal of virology. 2011;85:11183–11195. doi: 10.1128/JVI.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard KA, Shi PY, Li H. Structure and function of flavivirus NS5 methyltransferase. Journal of virology. 2007;81:3891–3903. doi: 10.1128/JVI.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]