Abstract
Therapy resistance can be attributed to acquisition of anti-apoptotic mechanisms by the cancer cells. Therefore, developing approaches that trigger non-apoptotic cell death in cancer cells to compensate for apoptosis resistance will help to treat cancer effectively. Triple-negative breast cancers (TNBC) are among the most aggressive and therapy resistant to breast tumors. Here we report that manumycin A (Man A), an inhibitor of farnesyl protein transferase, reduces cancer cell viability through induction of non-apoptotic, non-autophagic cytoplasmic vacuolation death in TNBC cells. Man A persistently induced cytoplasmic vacuolation and cell death through the expression of microtubule-associated protein 1 light chain 3 (LC3) and p62 proteins along with endoplasmic reticulum (ER) stress markers, Bip and CHOP, and accumulation of ubiquitinated proteins. As inhibitors of apoptosis and autophagy failed to block cytoplasmic vacuolation and its associated protein expression or cell death, it appears that these processes are not involved in the death induced by Man A. Ability of thiol antioxidant, NAC in blocking Man A-induced vacuolation, death and its related protein expression suggests that sulfhydryl homeostasis may be the target of Man A. Surprisingly, normal human mammary epithelial cells failed to undergo cytoplasmic vacuolation and cell death, and grew normally in presence of Man A. In conjunction with its in vitro effects, Man A also reduced tumor burden in vivo in xenograft models that showed extensive cytoplasmic vacuoles and condensed nuclei with remarkable increase in the vacuolation-associated protein expression together with increase of p21, p27, PTEN and decrease of pAkt. Interestingly, Man A-mediated upregulation of p21, p27 and PTEN and downregulation of pAkt and tumor growth suppression were also mimicked by LC3 knockdown in MDA-MB-231 cells. Overall, these results suggest novel therapeutic actions by Man A through the induction of non-apoptotic and non-autophagic cytoplasmic vacuolation death by probably affecting ER stress, LC3 and p62 pathways in TNBC but not in normal mammary epithelial cells.
Keywords: cytoplasmic vacuolation, LC3, p62, manumycin A, cell death, ER stress, breast cancer
Cancer cells acquire anti-apoptotic mechanisms to facilitate their growth and expansion under stressful conditions, which often leads to their resistance to therapy. Triple-negative breast cancers (TNBC) are among the most aggressive and treatment-resistant breast tumors. Therefore, alternative approaches that trigger non-apoptotic cell death are sought to compensate for apoptosis resistance to eradicate cancers.
Cell death, induced by anticancer therapeutics in targeted cancer cells can be divided into two types: active or programmed cell death that requires active participation of cellular machinery and passive cell death or necrosis.1, 2 Apoptosis is the best-characterized form of programmed cell death with cells displaying cellular and nuclear condensation with membrane blebs, activation of caspases, cellular and nuclear fragmentation, and loss of asymmetry in phosphatidyl-serine distribution in the plasma membrane.3 Resistance to apoptosis is one of the hallmarks in the pathogenesis of cancer; together with proliferation, it leads to tumor formation and growth4, 5, 6 and ultimately drug resistance.7 TNBC, which are negative for expression of estrogen and progesterone receptors and HER2 receptor, are among the most aggressive forms of breast cancer that accounts for 15–25% of breast tumors. Most TNBCs fall into basal-like subgroup, which are defined by gene expression profiling8 and lack suitable therapeutic targets. TNBC often respond poorly to available chemotherapies and this resistance to chemotherapy occurs because of mutations or deletions of proapoptotic proteins and overexpression of anti-apoptotic molecules.9, 10, 11
It has been known that death processes independent of caspase proteolysis often exist in cells that are resistant to apoptosis, which are probably subjected to different controls than apoptosis. Several different forms of non-apoptotic cell death have been described on the basis of specific cellular or molecular criteria. These include autophagy-associated cell death,12, 13, 14 paraptosis,6, 15 oncosis,16, 17, 18 necroptosis,19, 20 entosis,21 and programmed necrosis.22, 23 In this connection, earlier we showed that cyclo-oxygenase-2 end product 15-deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) induced non-apoptotic and non-autophagic cytoplasmic vacuolation death in apoptosis-resistant cancer cells.24 This non-autophagic and non-apoptotic cell death is morphologically characterized by cytoplasmic vacuolation24 and at molecular level with increased expression of microtubule-associated protein 1 light chain 3 (LC3) and a polyubiquitin-binding protein sequestosome1 or p62.
Manumycin A (Man A) is a natural antibiotic produced by Streptomyces parvulus and was shown to competitively inhibit farnesyl protein transferase25 enzyme that is important in activating a variety of signaling proteins including Ras. Ras proteins are GTP-binding proteins that have important roles in signal transduction, proliferation, and malignant transformation,26 but are regulated by post-translational modifications like farnesylation, palmitylation and methylation and so on.27 Although Man A and other farnesyl protein transferase inhibitors exerted growth inhibitory activity in various cancer cells, the mechanisms by which they exhibited their antiproliferative effect was not directly considered through blocking of ras function.28, 29 As Man A also contained sulfhydryl (–SH)-reactive, α, β-unsaturated ketones in its structure, we explored whether antiproliferative actions of Man A are mediated through induction of cytoplasmic vacuolation death similar to 15d-PGJ2.24 Here, we characterized the potential tumoricidal function of Man A in the treatment of TNBC by examining its effects both in vitro cell cultures and in vivo xenograft models. For the first time, we show that Man A is capable of inducing a novel cytoplasmic vacuolation death pathway related to LC3 and p62 signaling axis involving endoplasmic reticulum (ER) stress and protein ubiquitination in therapy-resistant triple-negative breast cancer cells.
Results
Man A-induced non-apoptotic and non-autophagic cytoplasmic vacuolation death in triple-negative breast cancer cells
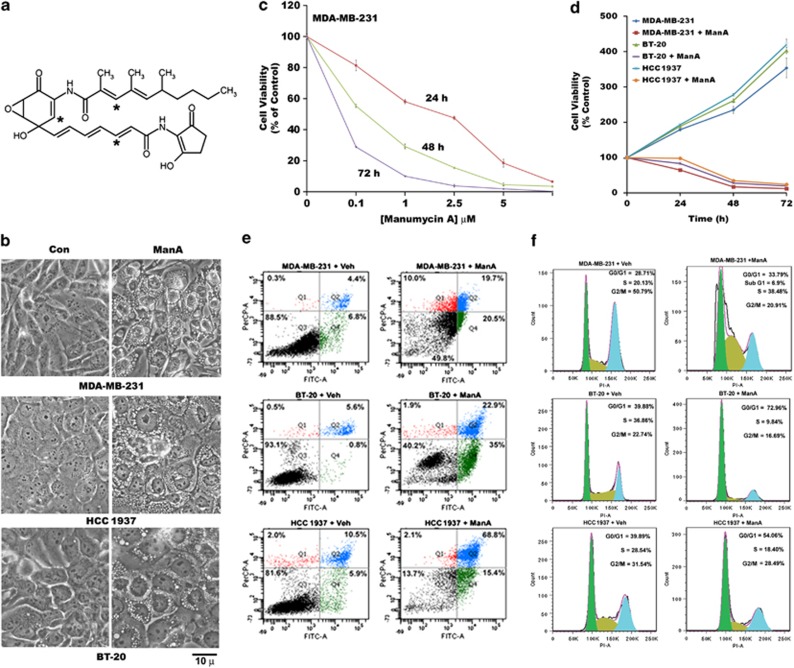
Our recent studies have shown that sulfhydryl-reactive prostaglandin, 15d-PGJ2 induces caspase-independent cytoplasmic vacuolation and cell death in different cancer cell types.24 Here we assessed the effect of Man A, a ras farnesylation inhibitor with three potential sulfhydryl-reactive α,−β, unsaturated carbonyl groups, (Figure 1a) on triple-negative breast cancer (TNBC) cells (MDA-MB-231, HCC1937, BT-20) in culture. Cytoplasmic vacuoles (Figure 1b) that started forming after 3 h of Man A treatment peaked at about 9 h in all TNBC cell lines. Initially, we used MDA-MB-231 cells to assess the effect of Man A on cell viability at different concentrations. A dose–response in MDA-MB-231 cells revealed that cytoplasmic vacuolation was seen at 1 μM concentration of Man A (see Supplementary Figure 1A), which resulted in considerable reduction in cell viability only after prolonged incubation (Figure 1c). At 5 μM concentration of Man A, cell viability, assessed by MTT assay, decreased by about 70% within 24 h of treatment (Figure 1c). Therefore, we chose to test Man A at 5 μM for all subsequent experiments. A time-course of cell viability after treatment with 5 μM of Man A in three different TNBC cell lines indicated >90% reduction of viability after 72 h in all cells (Figure 1d).
Figure 1.
Effect of mMan A on breast cancer cell viability. (a) Structure of Man A. * indicates reactive electrophilic carbons present in the molecule. (b) Phase-contrast images showing cytoplasmic vacuolation in MDA-MB-231, HCC1937 and BT-20 triple-negative breast cancer cells treated with 5 μM of Man A for 9 h. (c) Dose–response of Man A-induced cytotoxic effects in MDA-MB-231 breast cancer cells was determined by MTT assay after treatment at different times, as described in Materials and Methods section. Data represent average of three independent experiments with S.E. (d) Time course of cell viability of different TNBC cell lines after treatment with 5-μM Man A. Data represent average of three independent experiments with S.E. (e) Analysis of surface changes and cell permeability by FACS using Alexa Fluor 488 AnnexinV/Dead cell apoptosis kit in MDA-MB-231, BT-20, HCC1937 triple-negative breast cancer cells treated with vehicle (Veh) and Man A (5 μM) for 24 h. (f) Cell cycle analysis of MDA-MB-231, BT-20, HCC1937 triple-negative breast cancer cells after treating with vehicle (Veh) and Man A (5 μM) for 24 h by FACS after staining cells with propidium iodide
We further assessed cell death induced by Man A by annexin-V/PI staining and FACS analysis, which yielded comparable results to MTT-based assays with cell death in 24 h ranging between 50–86% among different cell lines (Figure 1e). To gain insights into the mechanism by which cell death occurred, we examined the effect of Man A on cell cycle distribution by FACS analysis in all three cell lines (Figure 1f). As shown in Figure 1f, the percentage of cells in each stage of the cell cycle: G0/G1, S and G2/M, are altered compared with the control after 24 h of treatment with Man A. Essentially, Man A induced cell cycle arrest before G2/M phase in all cell lines. In MDA-MB-231 cells, only a small percentage of cells appeared in sub G1 phase (<7%).
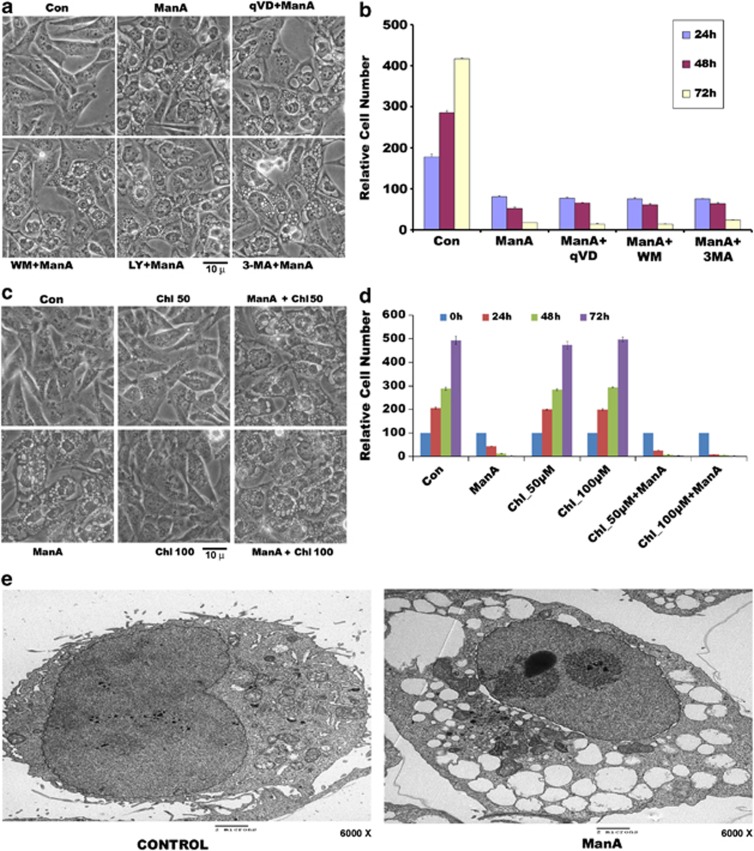
To find whether Man A-induced cytoplasmic vacuolation death was distinct from apoptosis and autophagy, we tested the effect of apoptosis and autophagy inhibitors on cytoplasmic vacuolation and cell death. As shown in Figure 2, inclusion of apoptosis inhibitor qVD or autophagy inhibitors wortmanin (WM), LY294002 or 3-methyl adenine (3-MA) along with Man A did not protect cells from either cytoplasmic vacuolation or cell death (Figures 2a and b). To further exclude autophagy in cytoplasmic vacuolation death, we tested the effect of chloroquine, another autophagy inhibitor on the Man A-induced vacuolation and death. As shown in Figure 2, autophagy inhibition by chloroquine has no effect on Man A-induced cytoplasmic vacuolation (Figure 2c), or cell death (Figure 2d). Treatment with chloroquine alone did not inhibit growth of TNBC (Figure 2d, Supplementary Figures 2A and B). Inhibition of lysosomes by chloroquine is consistent with modest increases in the levels of LC3 and p62 (Supplementary Figure 2C). In addition, we observed lack of caspase activation in cells dying by Man A-induced cytoplasmic vacuolation (Supplementary Figure.3). Man A failed to induce any cleavage of caspase-3 in MDA-MB-231 cells when compared with staurosporine-treated Hela cells (Supplementary Figure 3). Examination of cells treated with Man A by electron microscopy revealed that Man A-induced vacuoles were clear and lacked any visible cytoplasmic organelle components thus ruling out that these are autophagic vacuoles (Figure 2e). Together these results suggested that Man A induces non-apoptotic, non-autophagic cell death by cytoplasmic vacuolation in therapy-resistant triple-negative breast cancer cells.
Figure 2.
Man A-induced cytoplasmic vacuolation or cell death is not protected by autophagy or apoptosis inhibitors. (a) MDA-MB-231 cells were treated with either Man A (5 μM), or Man A (5 μM) with caspase inhibitor q-VD (20 μM) or Man A (5 μM) with wortmannin (WM, 1 μM)) or LY294002 (LY, 25 μM) or 3-methyl adenosine (3MA, 5 mℳ) autophagy inhibitors for 9 h and observed for cell vacuolation. (b) Viability of MDA-MB-231 cells was measured by MTT essay, as described in Materials and Methods section after various treatments for 24 h, 48 h, 72 h. (c) MDA-MB-231 cells were treated with Man A (5 μM) alone, or in the presence of chloroquine (50 μM and 100 μM) for 9 h and observed for cell vacuolation. (d) Viability of MDA-MB-231 cells treated as above for 24 h, 48 h, 72 h was measured by MTT assay. (e) Transmission electron micrograph of untreated (control) and treated (Man A, 5 μM) MDA-MB-231 cells at × 6000 magnification showing cytoplasmic vacuoles devoid of intracellular organelles
Man A induces ER stress and protein ubiquitination in breast cancer cells by disrupting sulfhydryl homeostasis
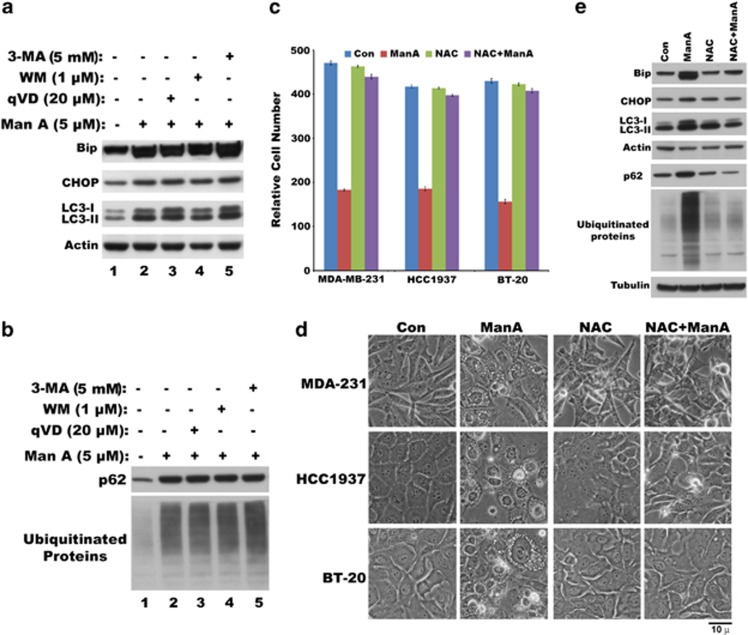
We previously showed that cytoplasmic vacuolation is due to ER stress resulting in marked dilation of ER cisternae.24 Consistent with our previous results, Man A-induced cytoplasmic vacuolation was also accompanied by increase in ER stress markers Bip, CHOP proteins (Figure 3a). In addition to ER stress markers, the cytosolic (LC3-I) and membrane bound forms (LC3-II) of an autophagy marker microtubule-associated protein 1 LC3 were also upregulated by Man A (Figure 3a). Immunofluorescence of LC3 in control cells revealed expression of LC3 in some punctated vesicular structures, whereas Man A-treated cells showed increased LC3 levels in both vesicles, as well as in the membrane boundaries of cytoplasmic vacuolations (Supplementary Figure 4). Again, inhibitors of both apoptosis (qVD) and autophagy (WM and 3-MA) failed to block the induction of ER stress markers and LC3 isoforms (Figure 3a), suggesting that cytoplasmic vacuolation is not related to either apoptotic pathway or autophagic pathway. In addition to ER stress markers and LC3, Man A also increased the levels of ubiquitinated proteins, as well as p62 protein (Figure 3b) that are not inhibited by either qVD, or WM or 3-MA, which indicated activation of unfolded protein response. As shown before,24 these ubiquitinated proteins are localized as aggregates in Triton-insoluble low-speed (1500 × g) and high-speed (100 000 × g) pellet fractions (not shown). However, unlike apoptosis and autophagy inhibitors, thiol antioxidant N-α-acetyl-L-cysteine (NAC) inhibited Man A-induced cytoplasmic vacuolation (Figure 3d), as well as cell death in MDA-MB-231, BT-20 and HCC1937 triple-negative breast cancer cells (Figure 3c). Notably, NAC prevented Man A-induced increase of ER stress markers, LC3, p62 and protein ubiquitination (Figure 3e). In addition to NAC, general inhibitors of transcription and translation, actinomycin D and cycloheximide respectively, also inhibited cytoplasmic vacuolation and associated protein changes (Supplementary Figure 5) indicating the requirement of new protein synthesis for cytoplasmic vacuolation death process. Overall, these results suggested that Man A, through its ability to disrupt the sulfhydryl homeostasis, essential for rapidly dividing cancer cells, induces cytoplasmic vacuolation death in TNBC.
Figure 3.
Failure of apoptosis and autophagy inhibitors and protection by NAC from Man A-induced cytoplasmic vacuolation death. (a) Man A-induced expression of ER stress markers (Bip, CHOP) and LC3 induction and processing in MDA-MB-231 cells in the absence or presence of inhibitors of autophagy (WM, 3-MA) and apoptosis (q-VD). (b) Effect of inhibitors of autophagy (WM, 3-MA) and apoptosis (q-VD) on Man A-induced expression of p62 and protein ubiquitination in MDA-MB-231 cells. (c) Effect of sulfhydryl-reactive ROS scavenger N-α-acetyl-L-cysteine (NAC) at 10 mM concentration on cell viability of Man A treated (24 h) TNBC cell lines (MDA-MB-231, HCC1937, BT-20). (d) Phase-contrast images of different triple-negative breast cancer cell lines treated with Man A in the absence or presence of NAC. (e) Immunoblots showing the effect of NAC (10 mℳ) on Man A (5 μℳ )-induced expression of ER stress markers (Bip and CHOP), LC3, p62 and ubiquitinated proteins
Man A failed to induce cytoplasmic vacuolation or cell death in normal human mammary epithelial cells
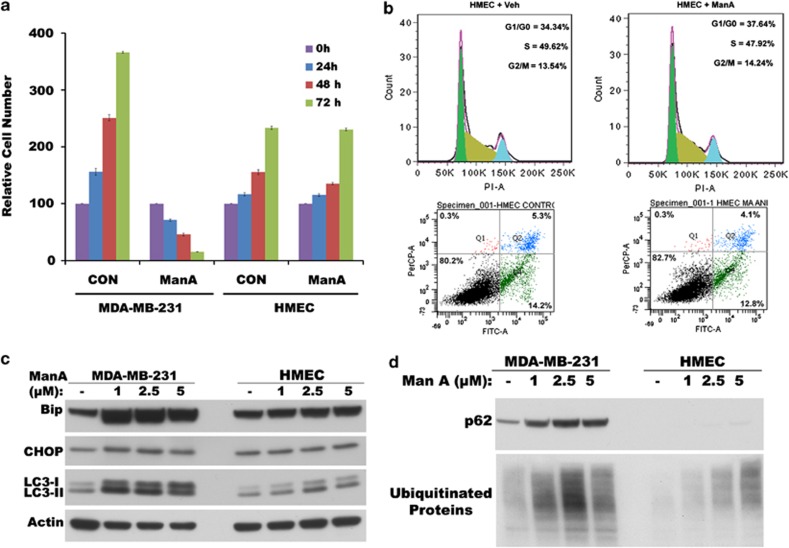
As one of the desired features of any potential cancer chemotherapeutic agent is its selective ability to induce cell death in malignant cancer cells without significant cytotoxic effect on normal cells, we examined the selectivity of Man A toward tumor cells. When the effect of Man A on normal human mammary epithelial cells (HMEC) immortalized by telomerase (hTERT) was tested, to our surprise, Man A failed to induce cytoplasmic vacuolation (Supplementary Figure 1B) or cell death (Figures 4a and b) in HMEC compared with MDA-MB-231 cells (Supplementary Figure 1A). Man A also failed to induce either cell cycle arrest or cell death in HMEC (Figure 4b, top panels). These results clearly indicated that Man A selectively targets transformed cancer cells over normal cells. As shown in Figure 4a, growth rate of HMEC are remarkably slower than rapidly dividing MDA-MB-231 cells with cell-doubling time of HMEC being almost double that of MDA-MB-231 cells. Notably, Man A failed to induce cytoplasmic vacuolation and cell death in HMEC at the same concentrations that induced vacuolation death in MDA-MB-231 cells (Figure 1 vs Figures 4a and b). Man A failed to significantly induce ER stress markers Bip, CHOP and LC3 proteins (Figure 4c) along with p62 and ubiquitinated proteins (Figure 4d) in HMEC-compared MDA-MB-231 cells. These results indicate that Man A exhibits selectivity toward rapidly dividing cancer cells to stimulate non-apoptotic and non-autophagic cytoplasmic vacuolation death through disruption of sulfhydryl homeostasis, which is unaffected in slow-dividing normal cells at the same concentrations of Man A.
Figure 4.
Normal HMEC are protected from Man A-induced cytotoxic effects. (a) Time course of cell viability measured by MTT assay in MDA-MB-231 cells and HMEC after treatment with 5 μM Man A. (b) Cell cycle analysis by FACS after propidium iodide staining (top panel) and analysis of surface changes and cell permeability by FACS using Alexa Fluor 488 AnnexinV/ dead cell apoptosis kit (bottom panel) in HMEC treated with vehicle (Veh) or Man A for 24 h. (c) Western blots of total cell lysates on the expression of Bip and CHOP, and expression and processing of LC3 in Man A-treated MDA-MB-231 cells and HMEC. (d) Western blots of total cell lysates for p62 expression and accumulation of ubiquitinated proteins in Man A-treated MDA-MB-231 cells and HMEC
Man A inhibited tumor growth in vivo of xenografts derived from MDA-MB-231 cells
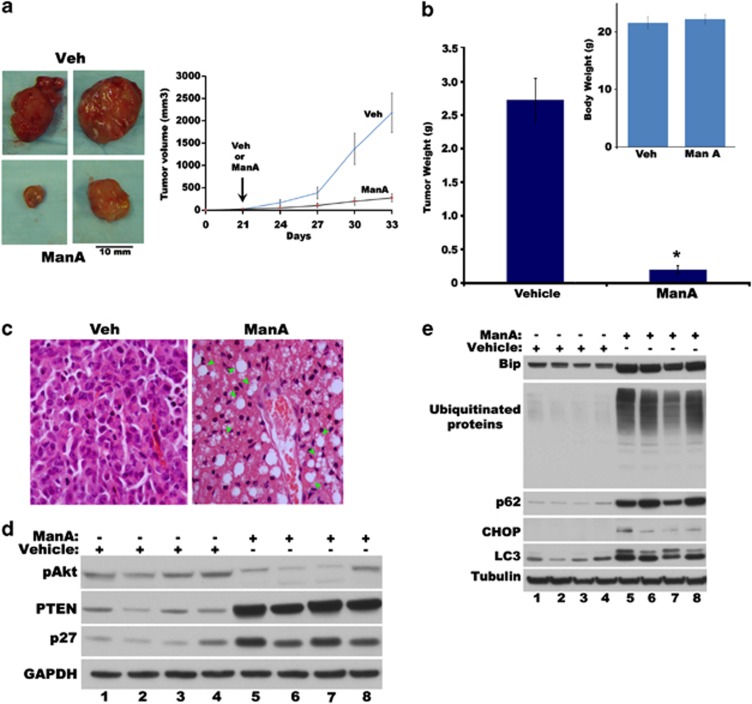
To study whether Man A might be effective in reducing breast tumor burden in vivo, we used a xenograft model in nude mice. One million MDA-MB-231 cells were inoculated subcutaneously and tumor growth was monitored. After 4 weeks of tumor growth, Man A was administered (5 mg/kg of body weight) by intraperitoneal injection. Tumors receiving Man A showed remarkable reduction of tumor volume and weight (Figures 5a and b) with extensive vacuolation in tumor (Figure 5c) without any significant difference in body weight (see Figure 5b inset). As Man A treatment showed extensive vacuoles in the growth-inhibited tumors (Figure 5c), we speculated the induction of ER stress, p62, LC3, and accumulation of ubiquitinated proteins. As expected, lysates from Man A-treated tumors showed remarkable increase in ER stress markers such as Bip, CHOP along with LC3 and p62 proteins as well as higher molecular weight ubiquitinated proteins (Figure 5e). Interestingly, Man A treatment also resulted in the elevation of growth-suppressive p21, p27 and PTEN proteins along with a reduction in the growth-promotive pAkt levels in the cultured cells (Figure 6c, lane 1 and 2), which became even more pronounced in the tumors (Figure 5d).
Figure 5.
Man A inhibits human breast tumor growth in nude mice. (a) Relative sizes of tumors (left panel) and relative progression of tumor volumes (right panel) in xenografts derived from MDA-MB-231 cells in animals treated with vehicle (Veh) and Man A (5 mg/kg body weight). (b) Relative weights of tumors isolated from mice treated with vehicle and Man A (*P<0.001). Inset, represents relative body weights of treated mice. (c) Hematoxylin and eosin (H&E)-stained sections of MDA-MB-231 xenografts treated with vehicle or Man A. Vacuoles are indicated by arrow heads. (d) Increased expression of PTEN, p27 and reduced activation of Akt (pAkt) in tumors treated with Man A. (e) Increased expression of Bip, CHOP, LC3, p62 and accumulation of ubiquitinated proteins in xenograft tumors treated with vehicle or Man A
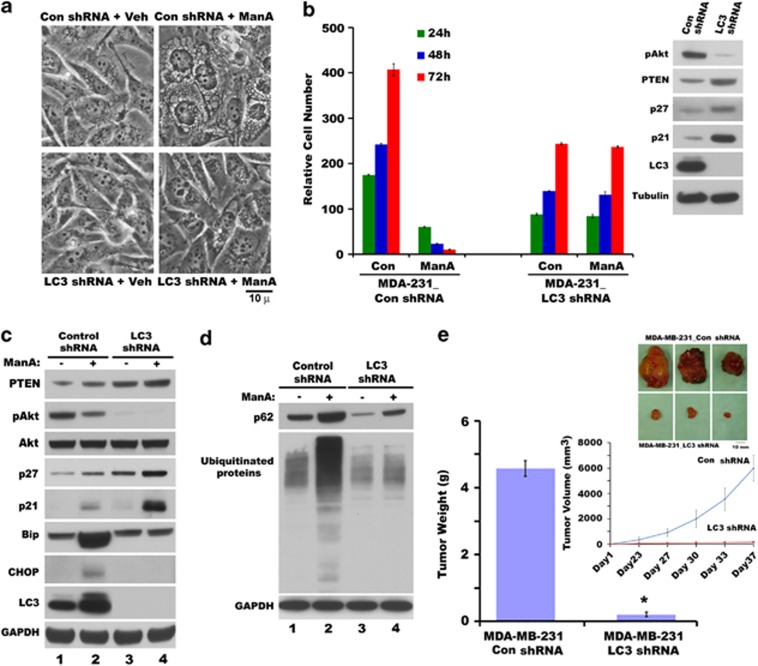
Figure 6.
LC3 deficiency reduces growth and protects from Man A-induced cytotoxicity of breast cancer cells. (a) Phase-contrast images of wild-type and LC3-deficient MDA-MB-231 cells treated with 5 μM Man A for 9 h. (b) LC3 deficiency in MDA-MB-231 cells decreased growth rate and protected from Man A-induced cytotoxicity. Inset represents pAkt, PTEN, p27, p21 and LC3 protein levels in MDA-MB-231 cells harboring control shRNA (Con shRNA) or LC3 shRNA expression vectors. (c) Western blot analysis of PTEN, pAkt, Akt, p27, p21, Bip, CHOP, LC3 and GAPDH in LC3-deficient MDA-MB-231 (LC3 shRNA) cells compared with control shRNA treated without or with Man A for 24 h. (d) Western blot analysis of p62, protein ubiquitination and GAPDH in LC3-deficient MDA-MB-231 (LC3 shRNA) cells compared with control shRNA cells treated without or with Man A for 24 h. (e) Reduced breast tumorigenic potential in vivo of LC3-knockdown cells (MDA-MB-231 LC3 shRNA) compared with control shRNA cells (MDA-MB-231 Con shRNA) as measured by tumor weight (*P<0.001). Inset shows relative sizes of tumors (top panel) and relative progression of tumor volumes (bottom panel) in xenografts derived from control shRNA (MDA-MB-231 Con shRNA) and LC3 shRNA (MDA-MB-231 LC3 shRNA)-expressing cells
LC3 deficiency prevented Man A-induced cell death and tumor growth in vivo
Inhibition of Man A mediated the induction of LC3 by reagents that prevented cytoplasmic vacuolation and cell death of breast cancer cells, and insignificant induction of LC3 in normal cells that are resistant to Man A-induced death suggested that LC3 might have an important role in cytoplasmic vacuolation death. To confirm the importance of LC3 in cytoplasmic vacuolation-mediated cell death, LC3 expression was inhibited by gene knockdown by stably expressing LC3 shRNA in MDA-MB-231 cells. LC3 deficiency not only blocked the Man A-induced cytoplasmic vacuolation (Figure 6a), but also prevented Man A-induced cell death in MDA-MB-231 cells by MTT assay (Figure 6b), as well as by annexin-V/PI staining and FACS analysis (data not shown). Knockdown of LC3 itself caused a structural change from an elongated messenchymal appearance to epithelial cobblestone morphology (Supplementary Figure 6A) with a remarkable reduction in colony-formation ability of MDA-MB-231 cells in soft agar in vitro assay (Supplementary Figure 6B). LC3 deficiency significantly inhibited MDA-MB-231 cells migration in transit well chamber as well as invasion in matrigel coated transit well chamber assay (Supplementary Figures 7 and 8). In addition, decreased LC3 expression caused cells to grow at a slower rate, which is further supported by high levels of PTEN, p27, p21 and low levels of pAkt (Figure 6b, inset). These later findings prompted us to analyze whether LC3 knockdown itself has any effect on in vivo tumor progression. Strikingly, LC3-deficient cells showed marked reduction in tumor volume and weight gain compared with control shRNA bearing MDA-MB-231 cells in nude mice xenograft model (Figure 6e). Most significantly, Man A failed to induce Bip, CHOP (Figure 6c) and p62, ubiquitinated proteins (Figure 6d), cytoplasmic vacuolation (Figure 6a) or cell death (Figure 6b) in LC3 knockdown cells. In addition, Man A did not inhibit the growth of LC3 knockdown cells (Figure 6b), albeit further increases in PTEN, p21 and p27 levels (Figure 6c, lanes 3 and 4), suggesting that Man A mainly acts on fast-growing cells but not on slow-growing LC3 knockdown cells, which formed severely diminished tumors compared with wild-type cells in vivo.
Discussion
Increased apoptosis resistance and recurrence of tumors are the major roadblocks to effective treatment of TNBC.9, 10, 11 Advanced cancer cells develop resistance to apoptosis through either inactivation or downregulation of pro-apoptotic effectors or upregulation of anti-apoptotic factors. Therefore, discovery of novel therapies is essential to treat advanced TNBC, which are resistant to current therapies. In this context, we explored the anti-cancer properties of Man A, a known farnesyl transferase inhibitor in treating apoptotic and conventional therapy-resistant TNBC. In this study, we identified that Man A-induced cytotoxicity in TNBC have common characterizations with 15d-PGJ2-induced cytoplasmic vacuolation death in various cancer cells,24 such as cytoplasmic vacuolation predominantly derived from ER stress, lack of caspase activation, and increased protein ubiquitination. Our current results showed that Man A-induced non-apoptotic and non-autophagic cell death in TNBC required both transcription and translation (Supplementary Figure 5), and was possibly mediated by LC3 (Figure 6).
Although Man A significantly reduced cell viability of three different TNBC cell lines, it had negligible effect on normal human mammary epithelial cells. Significantly, anti-cancer actions of Man A are independent of autophagic and apoptotic processes as the autophagy inhibitors LY, WM, 3-MA, chloroquinine and apoptosis inhibitor, Q-VD failed to block either cytoplasmic vacuolation or cell death (Figure 2). Nevertheless, our results clearly argue for Man A-mediated effects are specific to rapidly growing transformed cancer cells as it had no effect on normal human mammary epithelial cells, which still express endogenous LC3, Bip and CHOP. In fact, Man A showed very weak induction and activation of protein ubiquitination, ER stress markers (Bip and CHOP), LC3 and p62 in normal cells compared with breast cancer cells (Figure 4). Thus, in case of cancer cells, there appears to be a defect in their ability to eliminate Man A-induced misfolded proteins. These defects ultimately lead to the accumulation of misfolded protein aggregates and LC3-mediated cytoplasmic vacuolation death. Despite severe ER stress, cells avoided an apoptotic outcome as evident from the absence of activated caspase-3 (Supplementary Figure 3), but succumbed to novel non-apoptotic death signals that we earlier identified.24
Our results indicate that LC3, which has an important role in autophagy, may also have a central role in mediating Man A-induced non-apoptotic and non-autophagic cytoplasmic vacuolation death. Although decreased expression of LC3 in MDA-MB-231 cells by shRNA prevented cytotoxic effects of Man A (Figures 6a and b), it also blocked proliferation rates of knockdown cells (Figure 6b). This result was rather unexpected and was related to increased expression of growth inhibitory proteins PTEN, p21 and p27 and reduced formation of growth promoting pAkt in breast cancer cells (Figures 6b and c inset). In conjunction with low growth, LC3 deficient cells failed to form colonies (Supplementary Figure 6) and showed reduced migration, invasion properties in vitro (Supplementary Figures 7 and 8). Failure of chloroquine to inhibit cytoplasmic vacuolation death by Man A together with protection of LC3 knockdown from Man A-induced cytoplasmic vacuolation death through decreased growth point to the unresolved conundrum of paradoxical role of autophagy in cancer, where both stimulation and inhibition of autophagy had the same net effect on tumor growth.30 Nevertheless, LC3 knockdown inhibited the growth of MDA-MB-231 cells in xenograft tumors (Figure 6e), warranting further investigation into the role of LC3 in cancer cell progression and metastasis.
Another protein that can mediate Man A effects is p62, also referred as SQSTM1, a protein that was shown to be associated with polyubiquitinated proteins.31, 32 Generally, autophagy accelerates the degradation of p62 protein and that there is a good correlation between inhibition of autophagy and increased levels of p62.32, 33, 34 Increased abundance of p62 protein, following Man A treatment, in breast cancer cells but not HMEC (Figure 4) strongly suggests inhibition of autophagy by Man A in breast cancer cells. Thus autophagy may not be a direct player in Man A-induced cytoplasmic vacuolation and death. In fact, autophagy mediated cell death was mostly ruled out as possible mechanism of anticancer agent action by Dr. Kroemer's group after testing several hundreds of anticancer compounds.35 Although LC3 knockdown affected Man A actions by blocking p62, ER stress markers and protein ubiquitination induction (Figures 6c and d), the role of p62 in regulating Man A-induced cytoplasmic vacuolation death pathway is unclear and needs further investigation.
Increased accumulation of ubiquitinated proteins in Man A treated cells as Triton-insoluble aggregates also reflected possible inhibition of proteasomal activity required to remove unwanted misfolded proteins.36 Man A mediated accumulation of misfolded proteins also appears to be depended on the rate of cell division, since only rapidly dividing cancer cells appear to accumulate more ubiquitinated proteins than normal cells (Figure 4d) or LC3-deficient cancer cells (Figure 6d). In presence of Man A, cancer cells have increased rate of formation of misfolded proteins than their slow dividing counterparts, which ultimately affects misfolded protein load on ER. Overwhelming cells by drug induced misfolded proteins results in the failure of ER-associated degradation (ERAD), the cell protective ER quality control machinery, and ultimately cell death. This may be the main reason why cancer cells are much more susceptible to death inducing effects of proteasomal inhibitors than normal cells. We previously showed that cytoplasmic vacuolation induced by a sulfhydryl reactive 15d-PGJ2 was indeed due to ER dysfunction.24 Indeed, Man A may serve as a suitable anti-neoplastic agent because of its potential to cause protein misfolding, through covalent modification of sulfhydryl groups, while simultaneously inhibiting the proteasomal degradation machinery. As a result, only sulfhydryl anti-oxidants like NAC and NMPG (not shown) were able to protect cancer cells from Man A-induced cell death (Figure 3). Moreover,only newly synthesized proteins appears to be more sensitive than mature proteins for covalent modification by Man A because of the absolute requirement for new protein synthesis as Act D and CHX, drugs that block new protein synthesis, protected cancer cells from Man A-induced cytoplasmic vacuolation and death by reducing the overall load of misfolded proteins on the ER (Supplementary Figure 5). Therefore, only rapidly growing cancer cells, with extensive new protein synthesis, are much more susceptible than quiescent or slow dividing cells to Man A-induced cell death.
Notably, Man A showed great potential in the treatment of not only TNBC (present study) but also a wide variety of cancers including anaplastic thyroid cancer (ATC), pancreatic, hepatic and colon cancer.37, 38, 39, 40 Particularly, Man A was shown to exhibit insignificant toxic side effects in vivo.37, 41 Man A was shown to be effective in combination with paclitaxel in targeting anaplastic thyroid carcinoma cells in nude mice with no significant toxicity.38 Overall, our results showing Man A-induced cytoplasmic vacuolation with increased ER stress, LC3, p62 proteins as well as accumulation of ubiquitinated proteins and death are very significant and novel in reducing breast tumors derived from therapy-resistant triple-negative breast cancer cells. Thus, our studies provide a new paradigm to develop novel drugs for anticancer therapy to target treatment-resistant triple-negative breast tumors, which probably avoided apoptotic fate and tumor suppressive autophagy, through induction of novel cell death mechanisms that are not elicited in the normal cells.
Materials and Methods
Materials
Man A was obtained from Alexis and dissolved in tissue-culture grade DMSO and diluted to appropriate concentrations in tissue culture medium. DMSO concentration was kept below 0.1% (v/v) in all the experiments. LY294002, 3-MA, WM were purchased from Calbiochem. Chloroquine, Propidium iodide, STA, MTT, Noble agar was obtained from Sigma. qVD was purchased from MB Biomedicals. Alexa Fluor 488 AnnexinV/Dead cell apoptosis kit was obtained from Invitrogen.
Cell lines
Normal human mammary epithelial cells (HMEC) and triple-negative breast cancer cells, MDA-MB-231, BT-20, HCC1937 were grown in the medium recommended by ATCC as before.42 All cells were maintained at 37 °C in 5% CO2.
Cell morphology and cell proliferation assay
Cells were plated with suitable growth medium either in 35 mm tissue culture dishes or in 96 well plates. Phase contrast images of live cells were taken with an Olympus phase contrast microscope equipped with a digital camera. Cell viability was determined by MTT assay.29 Each data point represents average of at least three independent experiments in triplicates.
Annexin/Propidium iodide staining
MDA-MB-231, BT-20, HCC1937 and HMEC cells were treated with vehicle and Man A (5 μM) for 24 h and stained with Alexa Fluor 488 AnnexinV/ PI following kit manufacturer's instructions before FACS analysis at the Institutional core facility.
Cell cycle analysis
Cells were treated with vehicle and Man A (5 μM) for 24 h and used for cell cycle analysis by Propidium Iodide (PI) staining. In brief, harvested cells were washed with PBS and fixed in 1 ml cold 70% ethanol for 30 min on ice. Fixed cells were washed twice with PBS. To ensure that only DNA is stained, cell pellets were treated with 50 μl of RNase A solution (100 μg/ml stock solution) and 400 μl PI solution (50 μg/ml stock solution in PBS) and mixed well. Cells were incubated for 10 min at room temperature. All samples were analyzed by flow cytometry.
Electron microscopy
Electron microscopy was performed as described before.24 Briefly, vehicle and Man A treated cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacolylate buffer and were then incubated with osmium tetroxide (OsO4) at 4 °C for 1 h. The cells were then incubated overnight in 1% uranyl acetate at 4 °C after which they were further processed at the Electron Microscopy Core Facility in the Department of Pathology, UTHSCSA.
Western blot analysis
All proteins from different cell lysates were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) using 10% or 4–12% NuPAGE gels and were electroblotted onto PVDF membranes (0.2 mm) in Xcell II mini cell (Invitrogen, CA) using manufacturer's directions. Western blotting was performed on the above membranes using appropriate primary antibodies [LC3, Cleaved Caspase 3, PTEN, p27, pAkt (Cell Signaling); Bip, CHOP, p62 (Santa Cruz Biotechnology); Ubiquitin (Stressgen); Actin and p21 (Epitomics); Tubulin (Sigma); GAPDH (R&D Laboratories)] and peroxidase-conjugated suitable secondary antibodies (Jackson Immunoresearch). Chemiluminescent substrates from Pierce (Rockford, IL) were used to detect antigen-antibody complexes on the PVDF membrane.
Immunofluorescence of LC3
LC3 immunofluorescence was carried out using protocols described before.24 Briefly, vehicle and Man A treated cells on coverslips were fixed with 4% paraformaldehyde and permeabilized with 0.1% SDS before incubating with Primary (α-LC3, Cell Signaling) and secondary (AlexaFluor555 conjugated anti-rabbit, Invitrogen) antibodies. Coverslips were mounted on glass slides with ProLong antifade mounting medium containing DAPI (Invtrogen) to stain nuclei (blue).
Short hairpin RNA–mediated LC3 gene silencing
MDA-MB-231 cells stably expressing control shRNA and LC3 shRNA were generated using vectors described before.24
Soft agar Colony formation assay
Control shRNA and LC3 shRNA bearing MDA-MB-231 cells were used for soft-agar colony-formation assay. In brief, layer of 1 ml 0.5% agar in DMEM with 10% FCS was added in 60-mm tissue culture dish. Both control shRNA and LC3 shRNA containing MDA-MB-231 cells were suspended in 1 ml of 0.3% agar in mentioned media and poured over the bottom layer. The final concentration of cells in each culture was 0.4 × 105 cells/ml. Triplicate cultures were established for each experiment. After preparation of both bottom and top layers, the plates were examined under the inverted microscope to assure the presence of a good single-cell suspension. The plates were kept in 37 °C humidified incubator equipped with 5% CO2 for 21 days. The plates were stained with 0.005% crystal violet and colonies were counted using a dissecting microscope.
Migration and invasion assay
Migration and invasion asays were performed using MDA-MB-231-control shRNA and MDA-MB-231_LC3 shRNA as described before.42
In-vivo tumorigenesis
All animals were purchased from Harlan Laboratories and housed at the institutional facility. MDAMB-231 cells (1 × 106) were washed and suspended in sterile 1 × phosphate-buffered saline solution (PBS), mixed with 1 × Matrigel (BD Biosciences), and inoculated in 5–6-week-old female nude mice subcutaneously (s.c.) in the upper-right flank. When the tumors reached ∼10 mm in diameter, treatment was initiated by randomly dividing mice into two groups (five mice per group). Man A was dissolved in tissue culture grade 50% DMSO in sterile PBS. Final concentration of DMSO was kept at 0.05%. Mice were injected (i.p.) with vehicle (control group) or Man A (5 mg/kg/d) every alternate day for first week and every 2-day interval for following week for a total of 2-weeks period. Tumors were measured with a sliding caliper twice weekly and the volumes (mm3) were calculated according to the formula: V=A × B2 × 0.52, in which A is the largest superficial diameter and B is the smallest superficial diameter. The body weight, feeding behavior and motor activity of each animal were monitored as indicators of general health. At the end of the experiment, mice were sacrificed, tumors were excised and tumor weights and volumes were measured. Tumors were processed for hematoxylin–eosin staining and immunoblotting. Total lysates from tumor were used for immunoblotting. All experiments and procedures were done in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
To test the effect of LC3 knockdown on tumor growth, MDA-MB-231 cells (1 × 106) expressing control or LC3 shRNA were implanted s.c. in 5–6-week-old female nude mice (five animals per group). Tumor volumes were measured with a caliper twice weekly. The body weight, feeding behavior and motor activity of each animal were monitored as indicators of general health. After 6 weeks, mice were sacrificed and tumor weights were measured.
Statistical analysis
Data were expressed as the mean±S.D. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Tukey's test, using Graph Pad prism software. All values were considered statistically significant when P<0.05.
Acknowledgments
This research was supported by NIH grants DK54472 (P Saikumar), NIH DK37139 (MA Venkatachalam) and postdoctoral fellowship grant from Susan G Komen for the Cure (PK Singha). We also thank CTRC and Department of Pathology of UT Health Science Center at San Antonio, Texas for their support. We acknowledge Flow Cytometry and Optical Imaging Shared Resource Facilities at UTHSCSA for FACS and imaging.
Glossary
- manumycin A
Man A
- 15-deoxy-delta12,14-prostaglandin J2
15d-PGJ2
- triple-negative breast cancers
TNBC
- sequestosome1
SQSTM1
- farnesyl protein transferase
FPTase
- endoplasmic reticulum
ER
- human mammary epithelial cells
HMEC
- glucose-regulated protein 78
GRP78 or Bip
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Rufini
Supplementary Material
References
- Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, et al. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11:1066–1075. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA. Apoptosis: definition, mechanisms, and relevance to disease. Am J Med. 1999;107:489–506. doi: 10.1016/s0002-9343(99)00259-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li X, Wang L, Ding P, Zhang Y, Han W, et al. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci. 2004;117 (Pt 8:1525–1532. doi: 10.1242/jcs.00994. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Vaux DL. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene. 2003;22:7414–7430. doi: 10.1038/sj.onc.1206945. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others. Ann N Y Acad Sci. 2000;926:1–12. doi: 10.1111/j.1749-6632.2000.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–2419. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK, Chang SH, Phelps PC. The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther. 2003;2:863–872. [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Syntichaki P, Tavernarakis N. Death by necrosis. Uncontrollable catastrophe, or is there order behind the chaos. EMBO Rep. 2002;3:604–609. doi: 10.1093/embo-reports/kvf138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kar R, Singha PK, Venkatachalam MA, Saikumar P. A novel role for MAP1 LC3 in nonautophagic cytoplasmic vacuolation death of cancer cells. Oncogene. 2009;28:2556–2568. doi: 10.1038/onc.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Akasaka K, Akinaga S, Okabe M, Nakano H, Gomez R, et al. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci USA. 1993;90:2281–2285. doi: 10.1073/pnas.90.6.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Bio. 2012;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P, Gulbis B, Fokan D, Galand P. Effects of the farnesyltransferase inhibitor UCF-1C/manumycin on growth and p21-ras post-translational processing in NIH3T3 cells. Int J Cancer. 1998;76:601–608. doi: 10.1002/(sici)1097-0215(19980518)76:4<601::aid-ijc25>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK, et al. The autophagic paradox in cancer therapy. Oncogene. 2012;31:939–953. doi: 10.1038/onc.2011.295. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiheimo JP, Rantanen K, Heikkinen PT, Johansen T, Jaakkola PM. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28:334–344. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Kepp O, Michaud M, Martins I, Minoux H, Metivier D, et al. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30:4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- Canu N, Barbato C, Ciotti MT, Serafino A, Dus L, Calissano P. Proteasome involvement and accumulation of ubiquitinated proteins in cerebellar granule neurons undergoing apoptosis. J Neurosci. 2000;20:589–599. doi: 10.1523/JNEUROSCI.20-02-00589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Pan J, Martin C, Yeung SC. Angiogenesis inhibition in the in vivo antineoplastic effect of manumycin and paclitaxel against anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:1769–1777. doi: 10.1210/jcem.86.4.7374. [DOI] [PubMed] [Google Scholar]
- Yeung SC, Xu G, Pan J, Christgen M, Bamiagis A. Manumycin enhances the cytotoxic effect of paclitaxel on anaplastic thyroid carcinoma cells. Cancer Res. 2000;60:650–656. [PubMed] [Google Scholar]
- Di Paolo A, Danesi R, Nardini D, Bocci G, Innocenti F, Fogli S, et al. Manumycin inhibits ras signal transduction pathway and induces apoptosis in COLO320-DM human colon tumour cells. Br J Cancer. 2000;82:905–912. doi: 10.1054/bjoc.1999.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Kawata S, Tamura S, Matsuda Y, Inui Y, Yamasaki E, et al. Inhibition of cell growth of human hepatoma cell line (Hep G2) by a farnesyl protein transferase inhibitor: a preferential suppression of ras farnesylation. Int J Cancer. 1996;65:620–626. doi: 10.1002/(SICI)1097-0215(19960301)65:5<620::AID-IJC11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ito T, Kawata S, Tamura S, Igura T, Nagase T, Miyagawa JI, et al. Suppression of human pancreatic cancer growth in BALB/c nude mice by manumycin, a farnesyl:protein transferase inhibitor. Jpn J Cancer Res. 1996;87:113–116. doi: 10.1111/j.1349-7006.1996.tb03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha PK, Yeh IT, Venkatachalam MA, Saikumar P. Transforming growth factor-beta (TGF-beta)-inducible gene TMEPAI converts TGF-beta from a tumor suppressor to a tumor promoter in breast cancer. Cancer Res. 2010;70:6377–6383. doi: 10.1158/0008-5472.CAN-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.