Abstract
The tumor suppressor protein Pdcd4 is a nuclear/cytoplasmic shuttling protein that has been implicated in the development of several types of human cancer. In the nucleus, Pdcd4 affects the transcription of specific genes by modulating the activity of several transcription factors. We have identified the Daxx protein as a novel interaction partner of Pdcd4. Daxx is a scaffold protein with roles in diverse processes, including transcriptional regulation, DNA-damage signaling, apoptosis and chromatin remodeling. We show that the interaction of both proteins is mediated by the N-terminal domain of Pdcd4 and the central part of Daxx, and that binding to Pdcd4 stimulates the degradation of Daxx, presumably by disrupting the interaction of Daxx with the de-ubiquitinylating enzyme Hausp. Daxx has previously been shown to serve as a scaffold for protein kinase Hipk2 and tumor suppressor protein p53 and to stimulate the phosphorylation of p53 at serine 46 (Ser-46) in response to genotoxic stress. We show that Pdcd4 also disrupts the Daxx–Hipk2 interaction and inhibits the phosphorylation of p53. We also show that ultraviolet irradiation decreases the expression of Pdcd4. Taken together, our results support a model in which Pdcd4 serves to suppress the phosphorylation of p53 in the absence of DNA damage, while the suppressive effect of Pdcd4 is abrogated after DNA damage owing to the decrease of Pdcd4. Overall, our data demonstrate that Pdcd4 is a novel modulator of Daxx function and provide evidence for a role of Pdcd4 in restraining p53 activity in unstressed cells.
Keywords: Pdcd4, Daxx, protein–protein interaction, p53
Introduction
Pdcd4 (programmed cell death 4) is a tumor suppressor gene that was originally identified as a gene whose expression is increased during apoptosis.1 Subsequent work has shown that Pdcd4 is able to suppress tumor development in an in vitro mouse keratinocyte model of tumor promotion2 and in an in vivo mouse model of skin carcinogenesis.3 Decreased expression of Pdcd4 has been implicated in the development and progression of several types of cancer, including lung, colon, liver and breast cancer.4, 5, 6, 7, 8 Downregulation of Pdcd4 expression in tumor cells has been linked to increased expression of oncogenic micro-RNA miR-21, which targets the 3′-untranslated region of Pdcd4 mRNA.9, 10, 11 On the protein level, Pdcd4 is regulated by S6K-mediated phosphorylation, which triggers its ubiquitinylation via the E3 ubiquitin ligase complex SCF(βTRCP) and its subsequent degradation.12, 13 Downregulation of Pdcd4 appears to contribute to tumor development at least in two ways: a number of studies have shown that decreased Pdcd4 expression increases the mobility and invasiveness of tumor cells.8, 11, 14, 15 In addition, decreased Pdcd4 expression has been shown to deregulate the cellular response to DNA damage.16, 17
Pdcd4 encodes a highly conserved, predominantly nuclear phosphoprotein, which contains two so-called MA-3 domains, occupying the middle and C-terminal parts of the protein, and an N-terminal RNA-binding domain.18, 19 Pdcd4 is able to shuttle between the nucleus and the cytoplasm, and its subcellular localization is controlled by protein kinase Akt-mediated phosphorylation.20 Several studies have shown that Pdcd4 modulates the transcription of specific genes by affecting the activity of certain transcription factors, including c-Jun,21, 22 Sp115 and p53.16 An example is the upregulation of the p21 (Waf1/Cip1) gene after Pdcd4 knockdown, which is due to abrogation of Pdcd4-dependent inhibitory effects on the p300/CREB-binding protein-dependent acetylation of p53.16 In addition to its role in the nucleus, Pdcd4 acts as a translation suppressor. Pdcd4 interacts with the eukaryotic translation initiation factor eIF4A, a member of the DEAD-box family of ATP-dependent RNA helicases.23, 24 Binding of Pdcd4 to eIF4A is mediated by the MA-3 domains, whose structure and complex formation with eIF4A have been analyzed in detail.25, 26, 27, 28, 29, 30 Binding to Pdcd4 inhibits the RNA-helicase activity of eIF4A,23, 24 which is required to unwind secondary structures in the 5′-untranslated regions of certain mRNAs during translation initiation. Pdcd4 is therefore thought to suppress cap-dependent translation of mRNAs with 5′ structured untranslated regions.23, 24 Recently, proto-oncogene c-myb, p53 and procaspase-3 mRNAs were identified as natural translational targets of Pdcd4.31, 32, 33
The scaffold protein Daxx was initially identified as a protein that binds to the death domain of the CD95 death receptor.34 This interaction was thought to activate the JNK pathway and, ultimately, to lead to apoptosis.34, 35 However, the precise role of Daxx in apoptosis is controversial, because other work has shown that downregulation of Daxx by RNA interference also leads to increased levels of apoptosis,36 and disruption of the murine Daxx gene results in extensive apoptosis during embryonic development, indicating that Daxx also has antiapoptotic functions.37 Daxx is primarily a nuclear protein, which resides in the nucleoplasm or associates with the promyelocytic leukemia (PML) bodies, due to its ability to interact with sumoylated PML via a Sumo interaction motif.38, 39 Several splice variants of Daxx that differ at the C terminus and with regard to their ability to interact with PML have been described.40 Daxx is a well-established regulator of transcription. Daxx binds to the transcriptional coregulators, CREB-binding protein and histone deacetylase, to DNA methyltransferases41 as well as to numerous transcription factors, including members of the Pax and p53 families, C/EBPβ, ETS1, SMAD4 and glucocorticoid and androgen receptors.42, 43, 44, 45, 46, 47 In many cases, Daxx functions as transcriptional repressor, acting either through recruitment of histone deacetylase proteins48 or in a histone deacetylase-independent manner. An important function of Daxx is the regulation of p53-mediated apoptosis via cooperation with a Daxx/Axin/Hipk2/p53 complex49 and the DNA-damage-dependent dissociation of the Mdm2/Daxx/Hausp complex.50, 51 The interaction of Daxx with the de-ubiquitinylating enzyme Hausp has been shown to control the stability of Daxx52 and has also been implicated in the control of the subcellular distribution of the tumor suppressor protein PTEN.53 As shown recently, Daxx is also involved in chromatin remodeling. Daxx has been identified as a histone H3.3-specific histone chaperone that cooperates with ATRX in chromatin assembly at telomeres.54, 55, 56
In the work reported here, we show that Pdcd4 is a novel interaction partner of Daxx. Our work reveals that Pdcd4 modulates the stability and function of Daxx as a cofactor of Hipk2-dependent p53 phosphorylation, thereby providing a novel link between Pdcd4 and the DNA-damage response.
Results
Identification of Daxx as a novel interaction partner of tumor suppressor protein Pdcd4
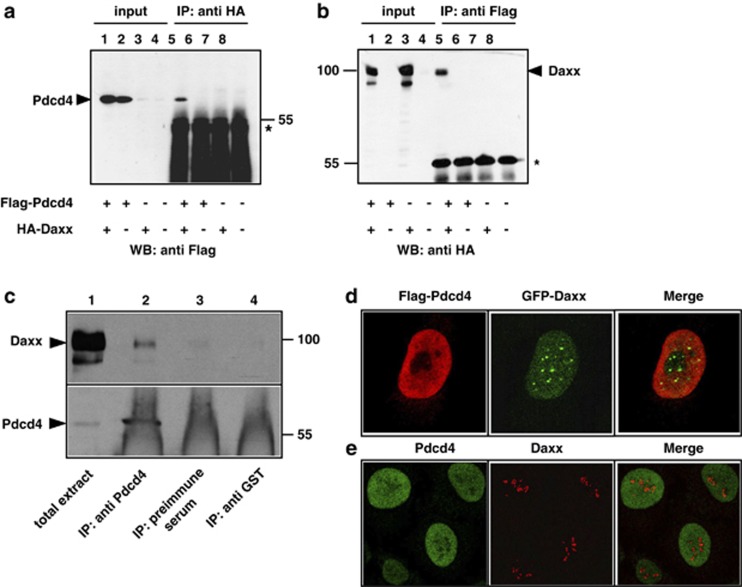
We have previously shown that Daxx interacts with the transcription factor C/EBPβ and inhibits its activity.47 During this work, we coincidentally found that Daxx also interacts with the tumor suppressor protein Pdcd4. Figure 1 illustrates co-immunoprecipitation experiments that demonstrate the interaction of Daxx and Pdcd4. When expression vectors for Flag-Pdcd4 and hemagglutinin (HA)-Daxx were cotransfected, Pdcd4 was co-immunoprecipitated with antibodies against the HA-tag of Daxx. Co-precipitation was not observed in the absence of HA-Daxx. This confirmed the specificity of the co-precipitation and excluded a crossreaction of Pdcd4 with the HA antibodies (Figure 1a). Conversely, HA-Daxx was co-precipitated with anti-Flag antibody in the presence of Flag-Pdcd4, but not in its absence (Figure 1b).
Figure 1.
Co-immunoprecipitation and subnuclear localization of Pdcd4 and Daxx. (a, b) QT6 cells were transfected with the indicated combinations of plasmids encoding Flag-Pdcd4 and HA-Daxx. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag or anti-HA antibodies, as indicated above the panels. Proteins were analyzed by SDS–PAGE and western blotting, using the antibodies indicated below the panels. Analyses of the crude protein extracts (input) demonstrate comparable expression levels of the proteins in the different samples. HA-Daxx and Flag-Pdcd4 are marked by arrowheads. The asterisks mark the immunoglobulin heavy chains of the HA and Flag antibodies. (c) Protein extracts of HeLa cells were immunoprecipitated with an antiserum against endogenous human Pdcd4 (lane 2). Controls were performed with preimmune serum from the same animal (lane 3) or with an antiserum against tubulin (lane 4). Total cell extract (lane 1) and precipitated proteins were analyzed by SDS–PAGE, followed by western blotting using an antiserum against Daxx (upper panel) or Pdcd4 (bottom panel). Daxx and Pdcd4 are marked by black arrowheads. The strong diffuse staining in lanes 2–4 at the bottom of the lower panel is due to the immunoglobulins from the antiserum used for immunoprecipitation. (d) HeLa cells were transfected with expression vectors for Flag-Pdcd4 and GFP-Daxx. After 24 h, cells were fixed and Flag-Pdcd4 was stained with anti-Flag and tetramethyl rhodamine iso-thiocyanate-conjugated secondary antibody (red). GFP-Daxx was detected using intrinsic GFP fluorescence (green). (e) Non-transfected HeLa cells were stained with antiserum against endogenous Pdcd4 (green) and endogenous Daxx (red).
Because the high concentrations of the expressed proteins in transfected cells might cause non-physiological interactions, we examined whether Daxx and Pdcd4 can also be co-precipitated from extracts of untransfected cells, that is, when both proteins are expressed at their endogenous levels. Protein extracts from untransfected HeLa cells were immunoprecipitated with anti-Pdcd4 antibodies. Figure 1c shows that Daxx was co-precipitated with Pdcd4-specific antiserum, while precipitation with the preimmune serum from the same animal or with an unrelated antibody failed to do so. These results confirmed that fractions of Daxx and Pdcd4 are also present in the same macromolecular complexes in unperturbed cells. As the co-precipitation experiments shown in Figure 1 were performed with crude cell extracts, it remains open whether both proteins are direct physical interaction partners or whether other proteins mediate their interaction.
Daxx is a nuclear protein, which is predominantly recruited in a sumoylation-dependent manner to the PML-oncogenic domains where it interacts with the PML protein.38, 39 In addition, a small fraction of Daxx has been shown to be present in the nucleoplasm where it exists in a dynamic equilibrium with the PML-oncogenic domain-associated Daxx.57 Because only small fractions of both proteins interact endogenously, we were interested to compare their subnuclear localization. To this end, we analyzed the localization of Daxx and Pdcd4 by immunofluorescence, using cells transfected with expression vectors for both proteins (Figure 1d) as well as untransfected cells expressing them at their endogenous levels (Figure 1e). These experiments showed that the bulk of both proteins do no show obvious colocalization. Pdcd4 is expressed throughout the nucleus, whereas Daxx shows a speckled pattern, presumably corresponding to the PML bodies. There is no obvious concentration of Pdcd4 in PML bodies, suggesting that they interact in the nucleoplasm. Because only a minor fraction of Daxx is present in the nucleoplasm,57 this might explain why only a small amount of Daxx is precipitated via Pdcd4 in the experiment shown in Figure 1c.
The interaction between Daxx and Pdcd4 is mediated by the N-terminal domain of Pdcd4 and the central domain of Daxx
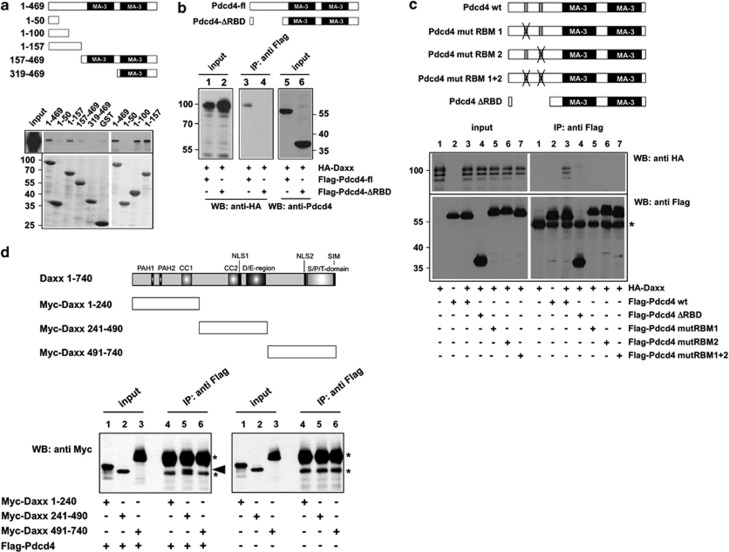
Pdcd4 contains two copies of the so-called MA-3 domain, which mediate the interactions between Pdcd4 and eIF4A, and are generally considered as protein–protein interaction domains. We were therefore interested to investigate whether Daxx also interacts with the MA-3 domains of Pdcd4. To identify the Daxx binding region of Pdcd4, we performed GST pull-down assays, using GST fusion proteins that contain different parts of Pdcd4. As shown in Figure 2a, bacterially expressed full-length GST-Pdcd4 as well as GST-Pdcd4 (1–157) were able to pull down HA-Daxx from extracts of cells expressing HA-tagged Daxx. HA-Daxx failed to bind to GST and to the part of Pdcd4 containing the MA-3 domains (amino acids 157–469). Thus, in contrast to the well-characterized interaction of Pdcd4 and eIF4A, Daxx binds to Pdcd4 via the N-terminal domain of Pdcd4. Binding experiments with additional GST-Pdcd4 fusion proteins encompassing different parts from the N terminus of Pdcd4 revealed that Daxx binds efficiently to a fusion protein containing amino acids 1–100 of Pdcd4 (Figure 2a). Taken together with the observation that GST-Pdcd4 (1–50) failed to interact with Daxx, this suggested that crucial residues for Daxx binding map to amino acids 50–100 of Pdcd4.
Figure 2.
Mapping of the Daxx and Pdcd4 binding sites. (a) GST-pull down experiments were performed with the indicated GST and GST-Pdcd4 fusion proteins, which were incubated with lysates of QT6 cells transfected with an expression vector for HA-Daxx. Bound proteins were analyzed by SDS–PAGE, followed by western blotting with antibodies against the HA-tag. Total protein extract of the transfected cells was used as control (input). Coomassie blue staining of GST proteins used in the pull-down experiments demonstrates comparable protein amounts (lower panels). (b, c) QT6 cells were transfected with the indicated combinations of plasmids encoding full-length Flag-Pdcd4, N terminally truncated or point-mutated versions of Flag-Pdcd4, and HA-Daxx. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting, using the indicated antibodies. Analyses of the crude protein extracts (input lanes) demonstrate comparable expression levels of the proteins in the different samples. The asterisk in panel c marks the immunoglobulin heavy chain of the Flag antibody. The Pdcd4 constructs used are shown schematically at the top of panels a–c. (d) QT6 cells were transfected with the indicated combinations of plasmids encoding full-length Flag-Pdcd4 and Myc-tagged Daxx proteins. Cells were lysed after 24 h and protein extracts were immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting with anti-Myc antibodies. Aliquots of the crude protein extracts (input lanes) were analyzed to demonstrate the expression levels and the sizes of the Daxx constructs. The Myc-Daxx (241–490) protein is marked by an arrowhead. Asterisks mark the immunoglobulin heavy chain of the Flag antibody and a nonspecific protein band present in all immunoprecipitates. The Daxx constructs used are shown schematically at the top.
To confirm the importance of the N-terminal domain of Pdcd4 for binding to Daxx, we coexpressed HA-Daxx with Pdcd4 mutants lacking the N-terminal domain or carrying specific amino-acid substitutions in the N-terminal domain. In these mutants, two clusters of basic amino acids in the N-terminal domain, which are involved in RNA binding by Pdcd4, have been replaced by alanine.19 As shown in Figures 2b and c, Daxx was not co-precipitated by these mutants, confirming that the integrity of the N-terminal domain of Pdcd4 is essential for the interaction with Daxx. Taken together, the data shown in Figures 2a–c indicate that Daxx does not interact with the MA-3 domains but rather with the N-terminal part of Pdcd4.
To map the binding site for Pdcd4 within Daxx, we performed in vivo co-immunoprecipitation experiments, using expression vectors for different Myc-tagged Daxx constructs. Coexpression of these constructs with Flag-Pdcd4 showed that Myc-Daxx (241–490) was co-precipitated via Pdcd4, whereas Myc-Daxx (1–240) failed to co-precipitate (Figure 2d). Myc-Daxx (491–740) comigrated with the immunoglobulin heavy chain of the antibody used for immunoprecipitation, making it impossible to determine whether or not Daxx (491–740) co-precipitates with Pdcd4. We therefore analyzed the samples shown in Figure 2d also by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) in the absence of reducing agent to shift the immunoglobulin heavy chain to a different position in the gel. This showed that Myc-Daxx (491–740) also failed to co-precipitate with Pdcd4 (Supplementary Figure 1). Taken together, these data indicated that the binding site for Pdcd4 resides between amino acids 241 and 490 of Daxx.
Attempts to demonstrate interaction of Pdcd4 and Daxx in pull-down experiments using bacterially expressed GST-Daxx proteins have been unsuccessful. It is therefore possible that another protein, a specific covalent modification of Daxx or a specific three-dimensional structure of the relevant part of Daxx that is missing in the bacterially expressed protein, is involved in the binding of Pdcd4.
Pdcd4 competes with Hausp for binding to Daxx and stimulates the turnover of Daxx
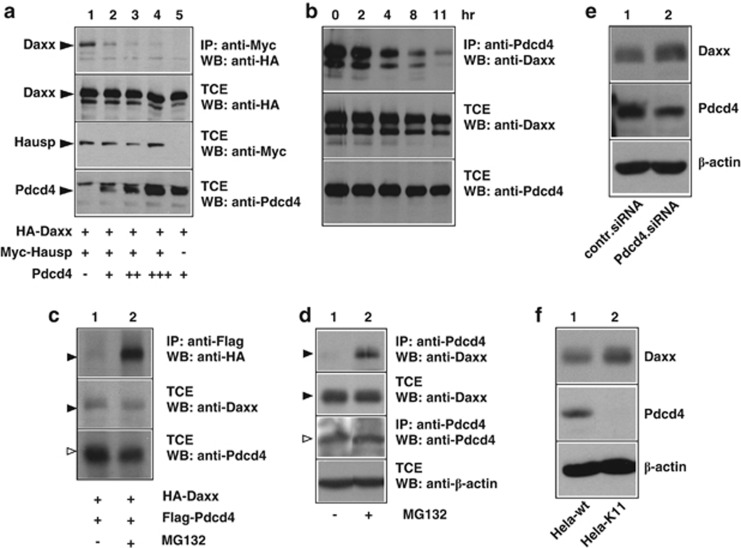
To address the functional consequences of the Daxx–Pdcd4 interaction, we decided to investigate the potential influence of Pdcd4 on the interaction of Daxx with known interaction partners. One of the proteins that we studied is the de-ubiquitinylating enzyme Hausp whose binding site in the amino-terminal half of Daxx overlaps with that of Pdcd4. Binding of Hausp has been shown to increase the stability of Daxx by reducing its ubiquitinylation.52 To address whether Hausp and Pdcd4 compete with each other for binding to Daxx, we co-transfected expression vectors for HA-Daxx and Myc-Hausp together with increasing amounts of a Flag-Pdcd4 expression vector and then analyzed the amount of Daxx interacting with Hausp. As shown in Figure 3a, a fraction of Daxx was co-precipitated via Hausp (lane 1), whereas no co-precipitation was observed in the absence of Hausp (lane 5). In the presence of increasing amounts of Pdcd4, the co-precipitation of Daxx was strongly diminished (lanes 2–4), indicating that Pdcd4 disrupts the Daxx–Hausp interaction. This observation suggested that Daxx might be prone to degradation when bound to Pdcd4. To find out if the interaction with Pdcd4 decreases the half-life of Daxx, we cotransfected cells with expression vectors for Daxx and Pdcd4, followed by treatment with cycloheximide to block new protein synthesis. The cells were then incubated for different times in the presence of cycloheximide before the total amounts of Daxx and Pdcd4, as well as the amounts of Daxx bound to Pdcd4 were analyzed. The result of this experiment is shown in Figure 3b. The amounts of Daxx and Pdcd4 present in the total cell extract decreased only slightly during an 11-h time period, consistent with a relatively slow turnover of Daxx. By contrast, the amount of Daxx that was co-precipitated via Pdcd4 decreased much faster. This suggested that the interaction with Pdcd4 shortens the half-life of Daxx, consistent with the displacement of the de-ubiquitinylating enzyme Hausp.
Figure 3.
Pdcd4 disrupts the interaction of Daxx and Hausp and decreases the half-life of Daxx. (a) QT6 cells were transfected with the indicated combinations of expression vectors for HA-Daxx, Myc-Hausp and Pdcd4, as indicated below the lanes. Cells were lysed after 24 h and protein extracts were either analyzed directly by western blotting (panels labeled TCE (total protein extract)) or were first immunoprecipitated with antibodies against the HA-tag before western blot analysis (top panel). (b) QT6 cells were transfected with expression vectors for HA-Daxx and Flag-Pdcd4. At 24 h after transfection, 50 μg/ml cycloheximide was added to the growth medium and the cells were harvested immediately or after growing them for additional times, as indicated at the top. Cell extracts were immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting with anti-HA antibodies (upper panel). Aliquots of the TCEs were analyzed with the indicated antibodies to demonstrate the Daxx and Pdcd4 expression levels (lower panels). (c) QT6 cells were transfected with expression vectors for HA-Daxx and Flag-Pdcd4. The cells were incubated with or without 10 nℳ MG132 for 4 h before they were lysed and immunoprecipitated with anti-Flag antibodies, followed by SDS–PAGE and western blotting with anti HA antibodies (upper panel). Aliquots of the TCEs were analyzed with the indicated antibodies to demonstrate the total expression levels of the proteins (lower panels). (d) HeLa cells were incubated with or without 10 nℳ MG132 for 4 h before they were lysed. Cell extracts were then immunoprecipitated with anti-Pdcd4 antibodies, followed by SDS–PAGE and western blotting with anti-Daxx antibodies (upper panel). Aliquots of the TCEs were analyzed with the indicated antibodies to demonstrate the expression levels of endogenous Daxx, Pdcd4 and β-actin (lower panels). To demonstrate the MG132-dependent increase of co-precipitated transfected or endogenous Daxx, the upper panels of (c) and (d) were exposed for a short time only. Daxx co-precipitated from cells not treated with MG132 is therefore only weakly visible. (e) MCF7 cells were transfected with control siRNA or Pdcd4-specific siRNA. The cells were analyzed after 2 days by western blotting for the expression of Daxx, Pdcd4 and β-actin. (f) HeLa wild-type cells or a clone of HeLa cells stably expressing Pdcd4-specific short hairpin RNA (HeLa-K11) were analyzed as described in (e).
We also used cells transfected with expression vectors for HA-Daxx and Flag-Pdcd4 to analyze the effect of the proteasome inhibitor MG132 on the total amount of Daxx and the amount of Daxx that was co-precipitated with Pdcd4. We reasoned that in case Pdcd4-bound Daxx had a shorter half-life than the bulk of Daxx inhibition of proteasomal degradation would exert a stronger effect on the amount of Pdcd4-bound Daxx than on the total amount of Daxx. Indeed, in cells transfected with expression vectors for Daxx and Pdcd4, treatment with MG132 significantly increased the amount of Daxx bound to Pdcd4 but not the total amount of Daxx (Figure 3c). A similar experiment was performed with untransfected HeLa cells to analyze the effect of MG132 on the amount of endogenous Daxx co-precipitated with endogenous Pdcd4 (Figure 3d). As in the experiment shown in Figure 3c, MG132 significantly increased the amount of Daxx bound to Pdcd4, while the total amount of Daxx was not affected. The results of these experiments are consistent with the notion that Pdcd4-bound Daxx is degraded faster than the bulk of Daxx.
An alternative interpretation of these results would be that the interaction of Pdcd4 and Daxx depends on the presence of an unknown protein with a short half-life. To address this possibility, we were interested to see if a reduction of the amount of Pdcd4 would affect the overall level of Daxx. We therefore performed knockdown experiments employing transient transfection of Pdcd4-specific small interfering RNA (siRNA) (Figure 3e) or stable expression of Pdcd4-specific short hairpin RNA (Figure 3f). In both cases, there was a slight increase of the amount of Daxx, supporting the notion that Pdcd4 decreases the half-life of at least a fraction of Daxx.
Pdcd4 disrupts the interaction of Daxx with protein kinase Hipk2 and inhibits Ser-46 phosphorylation of p53
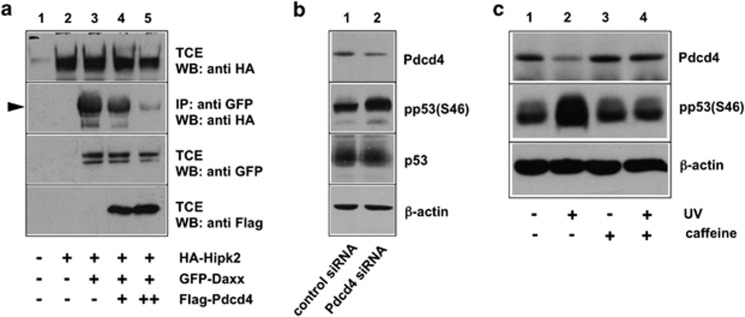
Daxx has been shown to act as a scaffold that stimulates the phosphorylation of p53 by the protein kinase Hipk2.49 Hipk2 interacts with the amino-terminal half of Daxx and phosphorylates the tumor suppressor protein p53 at Ser-46 in response to DNA damage.58, 59 We therefore wondered whether the interaction of Pdcd4 with Daxx would influence the phosphorylation of p53 at Ser-46. To see if Pdcd4 affects the binding of Hipk2 to Daxx, we performed a co-precipitation experiment, using cells transfected with expression vectors for HA-Hipk2 and green fluorescent protein (GFP)-Daxx together with increasing amounts of a Flag-Pdcd4 expression vector. We then analyzed the amount of Hipk2 that was co-precipitated with Daxx. Figure 4a shows that Hipk2 was efficiently co-precipitated via Daxx (lane 3), whereas no co-precipitation was observed in the absence of Daxx (lane 2), indicating that the co-precipitation was specific and that a significant amount of Hipk2 was associated with Daxx. The co-precipitation of Hipk2 was strongly diminished by increasing amounts of Pdcd4 (lanes 4 and 5), demonstrating that Pdcd4 interferes with the formation of the Daxx–Hipk2 complex.
Figure 4.
Pdcd4 inhibits Ser-46 phosphorylation of p53. (a) QT6 cells were transfected with the indicated combinations of expression vectors for HA-Hipk2, GFP-Daxx and Flag-Pdcd4, as indicated below the lanes. Cells were lysed after 24 h and TCEs were either analyzed directly by SDS–PAGE and western blotting with the indicated antibodies or were first immunoprecipitated with antibodies against GFP (second panel from top) before western blot analysis. Hipk2 co-precipitated via Daxx is marked by an arrowhead. (b) MCF7 cells were treated with Pdcd4-specific or control siRNA for 48 h. TCEs were subsequently analyzed with antibodies against Pdcd4, p53 and β-actin. In addition, p53 was first immunoprecipitated from the cell extracts and the immunoprecipitates were then analyzed by western blotting with phospho-p53- (Ser-46) specific antibodies. (c) HEK293 cells were UV irradiated (50 J/cm2) in the presence or absence of caffeine (concentration 6 mℳ). Unirradiated cells served as control. At 4 h after irradiation, TCEs were analyzed by western blotting with antibodies against Pdcd4, phospho-p53(Ser-46) and β-actin.
The data shown in Figure 4a are consistent with the idea that Pdcd4 disrupts the Daxx–Hipk2 interaction and, as a consequence, suppresses the phosphorylation of p53 at the Ser-46. To investigate whether the manipulation of the Pdcd4 expression level affects the phosphorylation of p53 also in cells not overexpressing Pdcd4, Daxx or Hipk2, we performed a Pdcd4 knockdown experiment and analyzed the level of the phosphorylation of p53. If Pdcd4 suppresses the phosphorylation, we expected the Ser-46 phosphorylation of p53 to increase after knock down of Pdcd4. To address this issue, we used an antiserum whose specificity for phosphorylated Ser-46 of p53 was confirmed by its ability to detect p53 in etoposide-treated but not in -untreated cells (Supplementary Figure 2). Figure 4b shows that Pdcd4 knockdown indeed increased the phosphorylation of p53 at Ser-46. This experiment, therefore, supports a model in which Pdcd4, by binding to Daxx and disrupting the Daxx–Hipk2 interaction, suppresses the phosphorylation of p53.
Figure 4c shows that the expression of Pdcd4 was decreased after ultraviolet irradiation concurrent with an increase of the p53 Ser-46 phosphorylation, confirming recent work.32 Ultraviolet irradiation activates Hipk2 by a signaling pathway involving the checkpoint kinases ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3-related) and the ubiquitin ligase Siah-1.59 We were interested to see if the blocking of this pathway by the ATM/ATR inhibitor caffeine would also have an effect on the ultraviolet-induced degradation of Pdcd4. Figure 4c shows that the decrease of Pdcd4 expression as well as the increase of p53 phosphorylation was abrogated in the presence of caffeine, indicating that ATM/ATR signaling is responsible for the activation of Hipk2 and the downregulation of its inhibitor Pdcd4.
Discussion
We have identified Daxx as a novel protein that is present in a complex with the tumor suppressor protein Pdcd4. Interactions of Daxx and Pdcd4 were demonstrated by in vitro pull-down experiments as well as by co-immunoprecipitation in vivo, utilizing cells that had been transfected with expression vectors for both proteins. Importantly, Pdcd4 and Daxx were also co-precipitated from extracts of untransfected cells expressing both proteins at their endogenous levels. This demonstrates that Daxx is a bona fide interaction partner of Pdcd4. However, this does not imply that the presence of Daxx and Pdcd4 in the same complex is due to their direct physical interaction, as we cannot exclude that other proteins are involved in the interaction.
Mapping experiments showed that the interaction of both proteins is mediated by the central part of Daxx (amino acids 241–492) and the N-terminal domain of Pdcd4 (amino acids 1–150). This part of Pdcd4 contains a large number of hydrophilic and charged amino acids and is predicted to have an intrinsically disordered structure. Such disordered regions are often found in proteins involved in cell signaling and transcriptional regulation and are able to fold into defined structures when they interact with other macromolecules. Intrinsically disordered regions are also interesting because they may fold into alternative structures, which allows them to interact with different partners and to function as nodal points in regulatory networks.60, 61 Analysis of the bacterially expressed N-terminal domain of Pdcd4 by circular dichroism and nuclear magnetic resonance spectroscopy has failed to reveal any stable secondary or tertiary structure in this part of Pdcd4 (LW and MDC, unpublished data), consistent with the idea that this region is intrinsically disordered. In contrast to the C-terminal two-thirds of the protein, which contain two MA-3 domains that form stable structures and allow tight interaction with eIF4A,25, 26, 27, 28, 29, 30 the function of the N-terminal domain of Pdcd4 is less well understood at present. Our recent work has demonstrated that the N-terminal domain of Pdcd4 functions as an RNA-binding domain and is required for stable association of Pdcd4 with translational initiation complexes in vivo.18, 19, 31 The binding of Daxx to the N-terminal domain of Pdcd4 highlights the potential of this domain to interact with protein as well as with RNA interaction partners. Whether there is cross-talk between the Daxx- and RNA-binding activities of Pdcd4 remains to be addressed in future work.
Daxx is sumoylated at several sites and contains two Sumo interaction motifs; hence, protein–protein interactions of Daxx are often mediated by sumoylation of one of the interacting proteins.39, 45, 46, 62 However, the binding of Pdcd4 and Daxx appears to be Sumo/Sumo interaction motif-independent. A fraction of Daxx in the nucleus is present in the PML oncogenic domains, due to binding of Daxx to sumoylated PML.38, 39 We have not observed a recruitment of Pdcd4 to PML-oncogenic domains, suggesting that Pdcd4 interacts with the nucleoplasmic fraction of Daxx.
To begin to address the functional relevance of the interaction of Daxx and Pdcd4, we have asked if Pdcd4 affects the interaction of Daxx with other proteins of known function whose binding regions within Daxx overlap with that of Pdcd4. Our data show that the binding of Pdcd4 to Daxx disrupts the interaction of Daxx and Hausp, suggesting that Pdcd4 interferes with the de-ubiquitinylation of Daxx by Hausp, leading to increased turnover of Pdcd4-bound Daxx. Furthermore, we have shown that Pdcd4 interferes with the binding of Hipk2 to Daxx and thereby diminishes the Hipk2-dependent phosphorylation of p53 at Ser-46. Phosphorylation of Ser-46 of p53 by Hipk2 is induced by DNA damage and stimulates the activation of proapoptotic genes by p53.63, 64 We have shown previously that the expression of Pdcd4 itself is decreased after induction of DNA damage.32 Based on our data, we propose a model in which Pdcd4 serves to suppress the activity of p53 in the absence of DNA damage, while the suppressive effect of Pdcd4 is abrogated after DNA damage due to the decrease of Pdcd4. Thus, one role of Pdcd4 appears to be to contribute to the maintenance of a low level of p53 phosphorylation at Ser-46 that is crucial for the homeostasis of unstressed cells. Interestingly, previous work has already demonstrated that Pdcd4 counteracts p53 in unstressed cells on several levels. We have shown that Pdcd4 inhibits the activity of p53 by interfering with the CREB-binding protein-dependent acetylation of p53.16 More recently, we have found that Pdcd4 suppresses the translation of p53 mRNA.32 Thus, Pdcd4 affects p53 by several mechanisms, resulting in the suppression of the activity and the synthesis of p53. The multiplicity of these inhibitory mechanisms underlines the importance of the role of Pdcd4 as a guardian of p53 in unstressed cells. Furthermore, the finding that Pdcd4 counteracts p53 on several levels also raises the intriguing possibility that Pdcd4 might also exert pro-oncogenic functions.
P53 has been implicated in numerous aspects of cellular physiology beyond its role in the response to acute genotoxic stress. There is evidence for a role of p53 in the regulation of the cellular energy metabolism and antioxidant function, autophagy, invasion and motility, angiogenesis, differentiation, necrosis and inflammation.65, 66, 67, 68, 69 By affecting p53 expression and activity, Pdcd4 is likely to exert pleiotropic effects on these biological processes and thereby influence the cellular homeostasis. Similarly, the interaction with Pdcd4 might affect other functions of Daxx in addition to its role as a scaffold for p53 phosphorylation. The identification of Daxx as a novel interaction partner of Pdcd4, therefore, opens new perspectives for future studies on the function of Pdcd4.
Materials and methods
Expression vectors
Eukaryotic expression vectors for Flag-tagged full-length human Pdcd4, the Pdcd4 mutants Flag-Pdcd4ΔRBD, Flag-Pdcd4mut1, Flag-Pdcd4mut2 and Flag-Pdcd4mut1+2 and the bacterial expression vectors for GST-Pdcd4 fusion proteins have been described.18, 19 Plasmids encoding HA- and GFP-tagged full-length Daxx and GFP-p53 have been described before.47 The expression vector for Myc-Hausp70 was obtained from M Maurice. Expression vectors for Myc-tagged Daxx constructs Myc-Daxx (1–240), Myc-Daxx (241–490) and Myc-Daxx (491–740) were gifts from T Matsuda.71 The expression vector for HA-Hipk2 was obtained from T Hofmann.72
Cell culture, transient transfections and siRNA experiments
Cells were grown in Iscove's modified Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Transfection of QT6 cells was carried out by calcium-phosphate co-precipitation, as described previously.73 RNA interference was performed as described.32
Immunoprecipitation and western blot analysis
For immunoprecipitation, cells were lysed in ELB buffer (50 mℳ Tris/HCl (pH 7.5); 120 mℳ NaCl; 20 mℳ NaF, 1 mℳ benzamidine; 1 mℳ EDTA; 6 mℳ EGTA; 15 mℳ sodium pyrophosphate; 1 mℳ phenylmethylsulfonyl fluoride; 0.5% NP-40). After incubation on ice for 30 min, lysates were centrifuged at 14 000 g for 30 min and the supernatant was used as total protein extract. Immunoprecipitations were carried out using aliquots of the total protein extract supplemented with the appropriate antibodies. After 1 h of incubation at 4 °C, protein-A Sepharose beads (GE Healthcare, München, Germany) were added and incubated further for 12 h at 4 °C under constant agitation. Immune complexes were then collected by centrifugation, washed three times with lysis buffer and finally subjected to SDS–PAGE. Immunostaining of proteins transferred to nitrocellulose membranes was performed with the following antibodies: anti-Flag (M2; Sigma-Aldrich, München, Germany), anti-HA (HA.11; Hiss Diagnostics, Freiburg, Germany), anti-Myc (9E11); anti-Daxx (Novocastra Laboratories, Newcastle upon Tyne, UK; NCL-Daxx), anti-Pdcd4,16 anti-p53 (Sigma-Aldrich), anti-phospho-p53 (Ser-46) (Cell Signaling Technonogies, Frankfurt, Germany) and anti-β-actin (Sigma-Aldrich). Commercial antibodies were usually used at a 1:1000 dilution for western blotting.
GST pull-down assay
GST fusion protein expression was induced in cultures of transformed Escherichia coli BL21-pLysS bacteria by adding isopropyl-𝒟-thiogalactopyranoside to a final concentration of 0.5 mℳ. After additional 3 h of growth at 37 °C, the bacteria were harvested by centrifugation for 10 min at 5000 g. Bacterial pellets were resuspended in GST lysis buffer (50 mℳ Tris–HCl (pH 8.0); 150 mℳ NaCl; 1% Triton X-100; 1 mℳ dithiothreitol; 0.1 mℳ phenylmethylsulfonyl fluoride) and lysed by three freeze–thaw cycles and sonication. An extract of soluble protein was prepared by ultracentrifugation for 1 h at 100000 g. Extracts containing 5–10 μg of GST fusion protein were then mixed with 30 μl of glutathione-Sepharose (GE Healthcare) and incubated at 4 °C for 1 h. The sepharose beads were then washed three times with ELB buffer and used for GST pull-down assays as follows: QT6 cells transfected with the appropriate expression vectors were lysed in ELB buffer and aliquots of the lysate were then incubated under constant agitation for 1 h at 4 °C with bacterially expressed GST fusion protein coupled to glutathione-Sepharose. Subsequently, beads were washed three times with ELB buffer. Bound proteins were eluted from the beads by boiling in SDS sample buffer and analyzed by SDS–PAGE, followed by staining with Coomassie Brilliant Blue or western blotting using appropriate antibodies.
Fluorescence microscopy
HeLa cells were seeded on coverslips and analyzed without transfection or were transfected with the desired plasmids. 24 h later, cells were washed with phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde in PBS for 10 min at room temperature. The cells were then permeabilized with PBST (PBS containing 0.1% Triton X-100) and incubated with blocking buffer (PBST containing 5% bovine serum albumin). Primary antibodies against the Flag-tag (Sigma), Daxx (Novocastra Laboratories) or Pdcd4 (Biomol, Hamburg, Germany) were diluted in blocking buffer and incubated for 1 h at room temperature. Subsequently, cells were washed five times with PBST, followed by incubation with tetramethyl rhodamine iso-thiocyanate-coupled goat-anti-mouse (Sigma-Aldrich), Alexa Fluor 546 Goat Anti-Mouse (Invitrogen, Darmstadt, Germany, A-11030) or Alexa Fluor 488 Goat Anti-Rabbit (Invitrogen A-11034) secondary antibody in blocking buffer for 1 h at room temperature in the dark. Finally, cells were washed five times with PBS, mounted in Aqua Poly/Mount (Polysciences, Eppelheim, Germany) and analyzed by confocal laser scanning microscopy.
Acknowledgments
We thank T Hofmann, M Maurice, T Matsuda and M Pagano for plasmids. This work was supported by grants from the Deutsche Krebshilfe, the Wilhelm-Sander-Stiftung and the Wellcome Trust. NK was supported by a fellowship from the Graduate School of Chemistry (GSC-MS) at the University of Münster.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166:297–301. doi: 10.1016/0378-1119(95)00607-9. [DOI] [PubMed] [Google Scholar]
- Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci USA. 1999;96:14037–14042. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol. 2003;200:640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene. 2004;23:8135–8145. doi: 10.1038/sj.onc.1207983. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, et al. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun Z, Yang H-S. Downregulation of tumour suppressor Pdcd4 promotes invasion and activates both β-catenin/TCF and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27:1527–1535. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- Dorello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–1260. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–4562. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- Bitomsky N, Wethkamp N, Marikkannu R, Klempnauer K-H. siRNA-mediated knock-down of Pdcd4 expression causes up-regulation of p21(Waf1/Cip1) expression. Oncogene. 2008;27:4820–4829. doi: 10.1038/onc.2008.115. [DOI] [PubMed] [Google Scholar]
- Singh P, Marikkannu R, Bitomsky N, Klempnauer K-H. Disruption of the Pdcd4 tumor suppressor gene in chicken DT40 cells reveals its role in the DNA-damage response. Oncogene. 2009;28:3758–3764. doi: 10.1038/onc.2009.239. [DOI] [PubMed] [Google Scholar]
- Böhm M, Sawicka K, Siebrasse JP, Brehmer-Fastnacht A, Peters R, Klempnauer K-H. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene. 2003;22:4905–4910. doi: 10.1038/sj.onc.1206710. [DOI] [PubMed] [Google Scholar]
- Wedeken L, Ohnheiser J, Hirschi B, Wethkamp N, Klempnauer K-H. Association of tumor suppressor protein Pdcd4 with ribosomes is mediated by protein–protein and protein–RNA interactions. Genes Cancer. 2010;1:293–301. doi: 10.1177/1947601910364227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005;65:11282–11286. doi: 10.1158/0008-5472.CAN-05-3469. [DOI] [PubMed] [Google Scholar]
- Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003;22:3712–3720. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- Bitomsky N, Böhm M, Klempnauer K-H. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene. 2004;23:7484–7493. doi: 10.1038/sj.onc.1208064. [DOI] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Cho MH, Zacowicz H, Hegamyer G, Sonenberg N, Colburn N. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRonde-LeBlanc N, Santhanam AN, Baker AR, Wlodawer A, Colburn NH. Structural basis for inhibition of translation by the tumor suppressor Pdcd4. Mol Cell Biol. 2007;27:147–156. doi: 10.1128/MCB.00867-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LC, Veverka V, Böhm M, Schmedt T, Choong PT, Muskett FW, et al. Structure of the C-terminal MA-3 domain of the tumour suppressor protein Pdcd4 and characterization of its interaction with eIF4A. Oncogene. 2007;26:4941–4950. doi: 10.1038/sj.onc.1210305. [DOI] [PubMed] [Google Scholar]
- Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, et al. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci USA. 2008;105:3274–3279. doi: 10.1073/pnas.0712235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Cho YH, Sohn SY, Choi JM, Kim A, Kim YC, et al. Cyrstal structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci USA. 2009;106:3148–3153. doi: 10.1073/pnas.0808275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, et al. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J. 2009;28:274–285. doi: 10.1038/emboj.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LC, Strong SL, Ferlemann E, Oka O, Muskett FW, Veverka V, et al. Structure of the tandem MA-3 region of Pdcd4 protein and characterization of its interactions with eIF4A and eIF4G: molecular mechanisms of a tumor suppressor. J Biol Chem. 2011;286:17270–17280. doi: 10.1074/jbc.M110.166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Wedeken L, Waters LC, Carr MD, Klempnauer K-H. Pdcd4 directly binds the coding region of c-myb mRNA and suppresses its translation. Oncogene. 2011;30:4864–4873. doi: 10.1038/onc.2011.202. [DOI] [PubMed] [Google Scholar]
- Wedeken L, Singh P, Klempnauer K-H. Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA. J Biol Chem. 2011;286:42855–42862. doi: 10.1074/jbc.M111.269456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Goto S, Nakashima W, Ura Y, Abe SI. Loss of programmed cell death 4 induces apoptosis by promoting the translation of procaspase-3 mRNA. Cell Death Differ. 2012;19:573–581. doi: 10.1038/cdd.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- Chen LY, Chen JD. Daxx silencing sensitizes cells to multiple apoptotic pathways. Mol Cell Biol. 2003;23:7108–7121. doi: 10.1128/MCB.23.20.7108-7121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson JS, Bader D, Kuo F, Kozak C, Lder P. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 1999;13:1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Huang YS, Jeng JC, Kuo HY, Chang CC, Chao TT, et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol Cell. 2006;24:341–354. doi: 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Wethkamp N, Hanenberg H, Funke S, Suschek CV, Wetzel W, Heikaus S, et al. Daxx-beta and Daxx-gamma, two novel splice variants of the transcriptional co-repressor Daxx. J Biol Chem. 2011;286:19576–19588. doi: 10.1074/jbc.M110.196311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puto LA, Reed JC. Daxx represses RelB target promoters via DNA methyltransferase recruitment and DNA hypermethylation. Genes Dev. 2008;22:998–1010. doi: 10.1101/gad.1632208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Pei H, Watson DK, Papas TS. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene. 2000;19:745–753. doi: 10.1038/sj.onc.1203385. [DOI] [PubMed] [Google Scholar]
- Lehembre F, Muller S, Pandolfi PP, Dejean A. Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene. 2001;20:1–9. doi: 10.1038/sj.onc.1204063. [DOI] [PubMed] [Google Scholar]
- Lin DY, Fang HI, Ma AH, Huang YS, Pu YS, Jenster G, et al. Negative modulation of androgen receptor transcriptional activity by Daxx. Mol Cell Biol. 2004;24:10529–10541. doi: 10.1128/MCB.24.24.10529-10541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lin DY, Fang HI, Chen RH, Shih HM. Daxx mediates the small ubiquitin-like modifier-dependent transcriptional repression of Smad4. J Biol Chem. 2005;280:10164–10173. doi: 10.1074/jbc.M409161200. [DOI] [PubMed] [Google Scholar]
- Kuo HY, Chang CC, Jeng JC, Hu HM, Lin DY, Maul GG, et al. SUMO modification negatively modulates the transcriptional activity of CREB-binding protein via the recruitment of Daxx. Proc Natl Acad Sci USA. 2005;102:16973–16978. doi: 10.1073/pnas.0504460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethkamp N, Klempnauer K-H. Daxx is a transcriptional repressor of CCAAT/enhancer-binding protein beta. J Biol Chem. 2009;284:28783–28794. doi: 10.1074/jbc.M109.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HM, Chang CC, Kuo HY, Lin DY. Daxx mediates SUMO-dependent transcriptional control and subnuclear compartmentalization. Biochem Soc Trans. 2007;35:1397–1400. doi: 10.1042/BST0351397. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang X, Wu X, Rui Y, Liu W, Wang J, et al. Daxx cooperates with the Axin/Hipk2/p53 complex to induce cell death. Cancer Res. 2007;67:66–74. doi: 10.1158/0008-5472.CAN-06-1671. [DOI] [PubMed] [Google Scholar]
- Tang J, Qu LK, Zhang J, Wang W, Michaelson JS, Degenhardt YY, et al. Critical role for Daxx in regulating Mdm2. Nat Cell Biol. 2006;8:855–862. doi: 10.1038/ncb1442. [DOI] [PubMed] [Google Scholar]
- Song MS, Song SJ, Kim SY, Oh HJ, Lim DS. The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J. 2008;27:1863–1874. doi: 10.1038/emboj.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Qu L, Pang M, Yang X. Daxx is reciprocally regulated by Mdm2 and Hausp. Biochem Biophys Res Commun. 2010;393:542–545. doi: 10.1016/j.bbrc.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455:813–817. doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140:678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drané P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev. 2010;24:1253–1265. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci USA. 2010;107:14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidtkamp-Peters S, Lenser T, Negorev D, Gerstner N, Hofmann TG, Schwanitz G, et al. Dynamics of component exchange at PML nuclear bodies. J Cell Sci. 2008;121:2731–2743. doi: 10.1242/jcs.031922. [DOI] [PubMed] [Google Scholar]
- D'Orazi G, Cecchinelli B, Bruno T, Manni I, Higashimoto Y, Saito S, et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- Winter M, Sombroek D, Dauth I, Moehlenbrink J, Scheuermann K, Crone J, et al. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat Cell Biol. 2008;10:812–824. doi: 10.1038/ncb1743. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhou J, Peres L, Riaucoux F, Honoré N, Kogan S, et al. A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell. 2005;7:143–153. doi: 10.1016/j.ccr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Calzado MA, Renner F, Roscic A, Schmitz ML. HIPK2: a versatile switchboard regulating the transcription machinery and cell death. Cell Cycle. 2007;6:139–143. doi: 10.4161/cc.6.2.3788. [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Prodosmo A, Siepi F, Soddu S. HIPK2: a multitalented partner for transcription factors in DNA damage response and development. Biochem Cell Biol. 2007;85:411–418. doi: 10.1139/O07-071. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Vousden KH. P53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Cancer. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Mathew R, Karandza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Maurice MM, Boutell C, Teunisse AF, Ovaa H, Abraham TE, et al. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol Cell. 2005;18:565–576. doi: 10.1016/j.molcel.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Muromoto R, Sugiyama K, Yamamoto T, Oritani K, Shimoda K, Matsuda T. Physical and functional interactions between Daxx and TSG101. Biochem Biophys Res Commun. 2004;316:827–833. doi: 10.1016/j.bbrc.2004.02.126. [DOI] [PubMed] [Google Scholar]
- Hofmann TG, Möller A, Sirma H, Zentgraf H, Taya Y, Dröge W, et al. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat Cell Biol. 2002;4:1–10. doi: 10.1038/ncb715. [DOI] [PubMed] [Google Scholar]
- Mink S, Haenig B, Klempnauer K-H. Interaction and functional collaboration of p300 and C/EBPbeta. Mol Cell Biol. 1997;17:6609–6617. doi: 10.1128/mcb.17.11.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.