Abstract
Hyperforin, a lipophilic constituent of medicinal herb St John's wort, has been identified as the main active ingredient of St John's wort extract for antidepressant action by experimental and clinical studies. Hyperforin is currently known to activate transient receptor potential canonical (subtype) 6 (TRPC6) channel, increase the phosphorylated CREB (p-CREB), and has N-methyl-𝒟-aspartate receptor-antagonistic effect that convert potential neuroprotective effects in vitro. However, the protective effects of hyperforin on ischemic stroke in vivo remain controversial and its neuroprotective mechanisms are still unclear. This study was designed to examine the effects of intracerebroventricular (ICV) injection of hyperforin on transient focal cerebral ischemia in rats. Hyperforin, when applied immediately after middle cerebral artery occlusion (MCAO) onset, significantly reduced infarct volumes and apoptotic cells, and also increased neurologic scores at 24 hours after reperfusion accompanied by elevated TRPC6 and p-CREB activity and decreased SBDP145 activity. When MEK or CaMKIV activity was specifically inhibited, the neuroprotective effect of hyperforin was attenuated, and we observed a correlated decrease in CREB activity. In conclusion, our results clearly showed that ICV injection of hyperforin immediately after MCAO onset blocked calpain-mediated TRPC6 channels degradation, and then to stimulate the Ras/MEK/ERK and CaMKIV pathways that converge on CREB activation, contributed to neuroprotection.
Keywords: acute stroke, animal models, calcium channels, focal ischemia, neuroprotection
Introduction
Stroke is the third leading cause of death after cardiovascular disease and cancer. Brain injury after focal cerebral ischemia is the most common cause of stroke. Treatment with recombinant tPA, a thrombolytic agent, is the only approved therapy for acute stroke; however, very few stroke patients benefit from this intervention.1, 2 Many stroke victims are still left with long-term disability. Therefore, the need for developing an effective treatment for acute stroke remains very important.
Cerebral ischemia caused excessive glutamate released, exposure to high levels of glutamate leads to overactivation of N-methyl-𝒟-aspartate receptors (NMDARs), which causes Ca2+ overload and then leads to calpain activation.3, 4, 5 The activation of calpain leads to proteolysis of transient receptor potential canonical (subtype) 6 (TRPC6) channels.6 The TRPC channels are widely expressed in the brain.7, 8 Previous studies have provided evidence that TRPC6 channels have a critical role in promoting neuronal survival against ischemic stroke.6, 9 Influx of Ca2+ through TRPC6 channels increases CREB phosphorylation through both the Ras/MEK/ERK and CaM/CaMK pathways, contribute to CREB activation and promotes neuronal survival.9 However, activation of NMDARs and calpain leads to TRPC6 degradation and contributes to neuronal damage in cerebral ischemia.6 Therefore, maintained phosphorylation of CREB through blocking calpain proteolysis of TRPC6 to preserve neuronal survival could be a new therapeutic strategy against cerebral ischemic stroke.
Hyperforin, a lipophilic constituent of St John's wort with a phloroglucinol structure, is considered as the main active ingredient of St John's wort extract for antidepressant action.10, 11 Besides its antidepressant activity, hyperforin also exerts potent antibacterial, antiinflammatory, antioxidant, and antitumoral effects.12, 13, 14 Preliminary results suggest that hyperforin has NMDAR antagonistic and potential neuroprotective effect in vitro.15 Hyperforin is currently known to activate TRPC6 channels.16 When chronically applied, hyperforin promotes neurite extension in the neuronal cell line PC12 through activation of TRPC6 channels.17 Hyperforin is also identified to increase the phosphorylation of CREB.18
The purpose of this study was to determine whether intracerebroventricular (ICV) injection of hyperforin has neuroprotective effects in vivo. We also sought to verify whether hyperforin administered by ICV injection improves the neurologic status of middle cerebral artery occlusion (MCAO) rats through the inhibition of calpain-mediated TRPC6 proteolysis and the subsequent activation of CREB via the Ras/MEK/ERK and CaM/CaMKIV pathways; and also to provide theoretical evidence for the treatment of cerebral ischemic stroke for stroke patients during the acute or subacute period.
Materials and methods
Animals and Focal Cerebral Ischemia
Male Sprague-Dawley rats weighing 200 to 250 g were purchased from Hunan weasleyg scene of experimental animals Co., LTD. All experimental protocols and animal handling procedures were performed in accordance with the National Institutes of Health (NIH, USA) guidelines for the use of experimental animals and the experimental protocols were approved by the committee of experimental animals of Tongji Medical College. The animals were maintained at temperature of 23±1°C with 50±10% relative humidity in the Experimental Animal Center. Rats were allowed free access to food and water in a 12-hour light/dark cycle. The animals were anesthetized with Chloral hydrate (400 mg/kg, intraperitoneally). Transient focal cerebral ischemia was induced by intraluminal occlusion of the right middle cerebral artery (MCA) for 2 hours. The right carotid artery was exposed to separate the external carotid artery and the internal carotid artery, external carotid artery was occluded at the level the MCA branches out, a 4-0 monofilament nylon suture (Beijing Sunbio Biotech Co Ltd, Beijing, China) with a rounded tip was introduced through the internal carotid artery, when a slight resistance was felt, the threading was stopped. Two hours later, the filament was gently removed for the reperfusion (reperfusion confirmed by laser Doppler). Sham-operated rats were manipulated in the same way, but the MCA was not occluded. The body temperature was measured by inserting a thermometric probe into the rectum of the rat, and the temperature was maintained at 37.5±0.5°C during the operation by using a heating pad and a heat controller. Regional cerebral blood flow (rCBF) was monitored by laser-Doppler flowmeter (Periflux system 5000, PERIMED, Stockholm, Sweden) before, during, and after MCAO, as well as before death. Abrupt reduction in rCBF by ∼75% to 90% indicated a successful occlusion of the MCA. Rats in which ipsilateral blood flow was not reduced to <20% of the baseline after placement of the intraluminal filament and whose ipsilateral blood flow was not rapidly restored during reperfusion were excluded from subsequent experiments (∼10% in each group). In a separate experiment, physiologic parameters (cranial temperature, arterial pH, Pa𝒞𝒪2, and Pa𝒪2) were monitored and analyzed in six additional rats. Arterial blood samples were taken 5 minutes before ischemia (baseline), 60 minutes after ischemia, 6, 12, and 24 hours after reperfusion for blood gas analysis.
Experimental Groups and Drug Administration
Rat ICV injection was performed under anesthesia in a stereotaxic instrument using a microsyringe pump. A scalp incision was made and burr hole was opened in the right parietal skull, 1.8 mm lateral, and 1.0 mm posterior to the bregma. A syringe was inserted into the brain to a depth of 4.2 mm below the cortical surface. Drugs or vehicle was injected slowly (0.5 μL/min) into the right ventricle. The needle was kept in place for 10 seconds to allow diffusing into the ventricle.
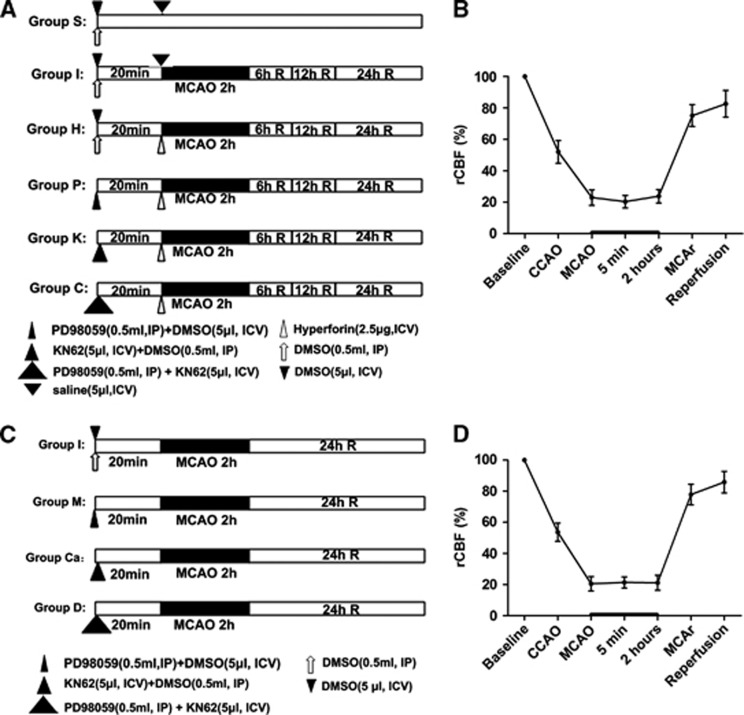
All treatments were administered in a blinded manner. The rats were randomly divided into six groups, and each group was again divided into three subgroups (n=12 per subgroup) according to the time of reperfusion after ischemia (6, 12, and 24 hours after reperfusion) (Figure 1A). The experimental groups and subgroups were as follows: (1) sham operation (group S; subgroups S6, S12, and S24); (2) MCAO (group I; subgroups I6, I12, and I24); (3) ischemia combined with hyperforin treatment (group H; subgroups H6, H12, and H24); (4) ischemia combined with hyperforin plus PD98059 (MEK inhibitor) treatment (group P; subgroups P6, P12, and P24); (5) ischemia combined with hyperforin plus KN62 (CaMKIV inhibitor) treatment (group K; subgroups K6, K12, and K24), and (6) ischemia combined with hyperforin plus PD98059 and KN62 treatment (group C; subgroups C6, C12, and C24).
Figure 1.
Experimental protocol used to determine the effect of hyperforin after ischemia and reperfusion. (A) The rats were randomly divided into six groups, and each group was again divided into three subgroups according to the time of reperfusion after ischemia (6, 12, and 24 hours after reperfusion) (n=3 rats for each). (C) Another 36 rats were randomly divided into four groups. (B, D) Change in regional cerebral blood flow (rCBF) in rats. Right common carotid artery occlusion (CCA) reduced CBF to∼50% of the baseline, and additional middle cerebral artery (MCA) occlusion (MCAO) further decreased rCBF to∼20%. Group S (subgroups S6, S12, and S24): sham operation; group I (subgroups I6, I12, and I24): MCAO; group H (subgroups H6, H12, and H24): ischemia combined with hyperforin treatment; group P (subgroups P6, P12, and P24): ischemia combined with hyperforin plus PD98059 (MEK inhibitor) treatment; group K (subgroups K6, K12, and K24): ischemia combined with hyperforin plus KN62 (CaMKIV inhibitor) treatment; group C (subgroups C6, C12, and C24): ischemia combined with hyperforin plus PD98059 and KN62 treatment; group M: ischemia combined with PD98059 treatment; group Ca: ischemia combined with KN62 treatment; group D: ischemia combined with PD98059 plus KN62 treatment. R: reperfusion; MCAr, MCA remove. DMSO, dimethyl sulfoxide; IP, intraperitoneally; ICV, intracerebroventricular.
Another 36 rats were randomly divided into four groups (n=9 per group) (Figure 1C): (1) MCAO (group I); (2) ischemia combined with PD98059 treatment (group M); (3) ischemia combined with KN62 treatment (group Ca); and (4) ischemia combined with PD98059 plus KN62 treatment (group D).
Hyperforin (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in saline. KN62 (1 μg/ μL; Sigma-Aldrich, St Louis, MO, USA) or PD98059 (1.5 mg/mL; Sigma-Aldrich) was prepared in 1% dimethyl sulfoxide. Hyperforin (0.5 μg/μL; 5 μL) or saline (5 μL) was injected slowly (0.5 μL/min) into the right ventricle immediately after MCAO onset. KN62 (5 μL) or dimethyl sulfoxide (5 μL) was injected into the right ventricle 20 minutes before the operation. PD98059 (0.5 mL, intraperitoneally) or dimethyl sulfoxide (0.5 mL, intraperitoneally) was also administered to rats 20 minutes before the operation.
Terminal Deoxynucleotidyl Transferase Nick-End Labeling and Quantification of Apoptosis
To evaluate the influence of hyperforin on neuronal survival under ischemic conditions, terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) staining analysis was used to detect apoptotic cell death at 24 hours after reperfusion. The TUNEL staining was performed with a kit (Roche Molecular Biochemicals, Mannheim, Germany). The TUNEL-positive nuclei with chromatin condensation and fragmented nuclei were considered as probable apoptotic cells. The total number of TUNEL-positive neurons in the ipsilateral hemisphere was counted in three different fields for each section by an investigator who was blinded to the studies by light microscopy at × 200 magnification.
Infarct Volume Measurement
For measurements of infarct volume at 24 hours after cerebral artery reperfusion, rats were killed and the brains were rapidly removed and mildly frozen at −20°C for 10 minutes to keep the morphology intact during slicing. In brief, brains were sliced into five serial 2 mm coronal sections and incubated in a 2% 2,3,5-triphenyltetrazolium chloride (TTC; Amresco, Solon, OH, USA) for 15 minutes at 37°C and then transferred into a 4% paraformaldehyde in phosphate buffer for fixation at 4°C. The infarcted tissue remained unstained (white), whereas normal tissue was stained red. The extent of ischemic infarction was traced and the integrated volume was calculated using Image J software (NIH, USA). The areas of the infarcted tissue and the areas of both hemispheres were calculated for each brain slice. To compensate for the effect of brain edema, the corrected infarct volume was calculated as follows: percentage of corrected infarct volume={[Total lesion volume−(ipsilateral hemisphere volume−contralateral hemisphere volume)]/contralateral hemisphere volume} × 100.
Neurologic Scoring
The neurologic behavior of rats was scored at 24 hours after reperfusion by an investigator who was unaware of animal grouping. An 18-point scale of neurologic deficit scores was used for evaluation of neurologic behavior.19 The scale was based on the following six tests: (1) spontaneous activity (0 to 3 points); (2) symmetry in the movement of four limbs (0 to 3 points); (3) forepaw outstretching (0 to 3 points); (4) climbing (1 to 3 points); (5) body proprioception (1 to 3 points); and (6) response to vibrissae touch (1 to 3 points). The six individual test scores were summed up at the end of the evaluation (minimum score, 3; maximum score, 18).
Lysis and Protein Content Determination
All the rats were killed by decapitation at 6, 12, and 24 hours after reperfusion and the infarct side of cortex (from an area between 3 and 6 mm posterior to the frontal pole) was harvested. The tissues were immediately frozen in liquid nitrogen and stored at −80°C.
Total protein extraction was performed according to a commercially available kit (KGP250; Nanjing Keygen Biotech Co. Ltd., Nanjing, China). Total protein extracts were for protein determination and analysis by western blotting for TRPC6 and aII-spectrin. Nuclear protein extraction was performed according to the ProteoJET cytoplasmic and nuclear protein extraction kit (Fermentas International, Glen Burnie, MD, USA). Nuclear protein extracts were for analysis by western blotting for phosphor-CREB. Protein concentrations were also determined using the BCA protein assay.
Western Blot Analysis
Equal amounts of total protein extracts or nuclear protein extracts were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) by electrophoresis, and membranes were blocked with 5% nonfat milk in TBST (0.1% Tween 20 in TBS) for 1 hour at room temperature. Membranes were then incubated overnight at 4°C with mouse monoclonal anti-aII-spectrin (1:1,000; Enzo Life Sciences, Plymouth Meeting, PA, USA), rabbit polyclonal anti-TRPC6 (1:1,000; Abcam, Cambridge, UK), rabbit monoclonal anti-p-CREB (1:1,000; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal anti-Lamin B1 (1:500; Bioworld, Minneapolis, MN, USA), or mouse monoclonal anti-GAPDH antibody (1:100; Proteintech Group, Inc., Wuhan, Hubei, China) followed by horse radish peroxidase-conjugated goat anti-mouse IgG antibody (1:3,000; Proteintech Group, Inc.) or anti-rabbit IgG antibody (1:5,000; Proteintech Group, Inc.). Labeled proteins were detected with the ChemiDocXRS+chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA). Protein bands were quantified by Image Lab image acquisition and analysis software (Bio-Rad).
Quantum Dots-based Immunofluorescence Staining
At 6, 12, and 24 hours after reperfusion, the rats were anesthetized and intracardially perfused with cold saline 250 mL followed by 4% paraformaldehyde 100 mL in 0.1 mol/L phosphate buffer, pH 7.4. Brains were removed and immersed overnight in 4% paraformaldehyde. The brain was blocked and embedded in paraffin. Paraffin-embedded brains were cut into 4 μm-thick sections according to standard procedures. Paraffin sections (n=3 for each group) were incubated overnight with antibodies against TRPC6 (1:100; Abcam) and p-CREB (1:100; Cell Signaling Technology) at 4°C after being blocked with 2% bovine serum albumin. Then, the samples were incubated with a biotinylated secondary antibody for 30 minutes at 37°C, and rinsed 3 × with TBST (5 minutes). Paraffin sections were incubated with streptavidin-conjugated QDs605 (1:100, Wuhan Jiayuan Quantum Dot Co, Ltd., Wuhan, Hubei, China) after being blocked with 2% bovine serum albumin. The nuclei of cells were stained with 4′,6-diamidino-2-phenylindole. TRPC6- and p-CREB-positive cells were measured at × 200 per visual field in the cortex, three visual fields per section, and three brain sections. Fluorescent signals were detected with a fluorescence microscope (BX51; Olympus, Tokyo, Japan). The acquisition and quantitative analysis of images was performed with multispectral imaging system (Nuance Fx, CRi, Hopkinton, MA, USA).
Statistical Analyses
For all quantitative analysis of data, measures were made with the experimenter blind to the animals' treatment group. GraphPad Prism (version 5 for Windows, San Diego, CA, USA) software was used for all statistical analyses. All values are expressed as means±s.e.m. One-way analysis of variance followed by Newman–Keuls Multiple Comparison Test was performed for statistical comparison of several groups. The unpaired Student's t-test was used for comparison of two groups. Statistical significance was accepted at P<0.05.
Results
Physiologic Parameters
No statistical significance was noted among different time points for any of the physiologic parameters including cranial temperature and blood gas (Table 1). Monitoring of rCBF ensured successful MCAO (Figures 1B and 1D).
Table 1. Physiologic parameters, mean±s.d. (n=6).
| Time point | Temperature (°C) | Pa𝒪2 (mm Hg) | Pac𝒪2 (mm Hg) | Arterial pH |
|---|---|---|---|---|
| Baseline | 37.3±0.2 | 98.2±6.9 | 35.7±4.7 | 7.35±0.05 |
| Ischemia, 60 minutes | 37.1±0.3 | 97.5±5.1 | 37.9±5.9 | 7.39±0.03 |
| Reperfusion, 6 hours | 37.5±0.1 | 94.9±7.1 | 38.2±4.2 | 7.35±0.12 |
| Reperfusion, 12 hours | 37.4±0.2 | 91.6±5.8 | 39.5±3.6 | 7.32±0.13 |
| Reperfusion, 24 hours | 37.7±0.1 | 93.2±6.5 | 38.8±2.9 | 7.34±0.08 |
Pa𝒪2, arterial oxygen tension; Pa𝒞𝒪2, arterial carbon dioxide tension.
Hyperforin Significantly Reduced Apoptotic Cell Death in Ipsilateral Cortex
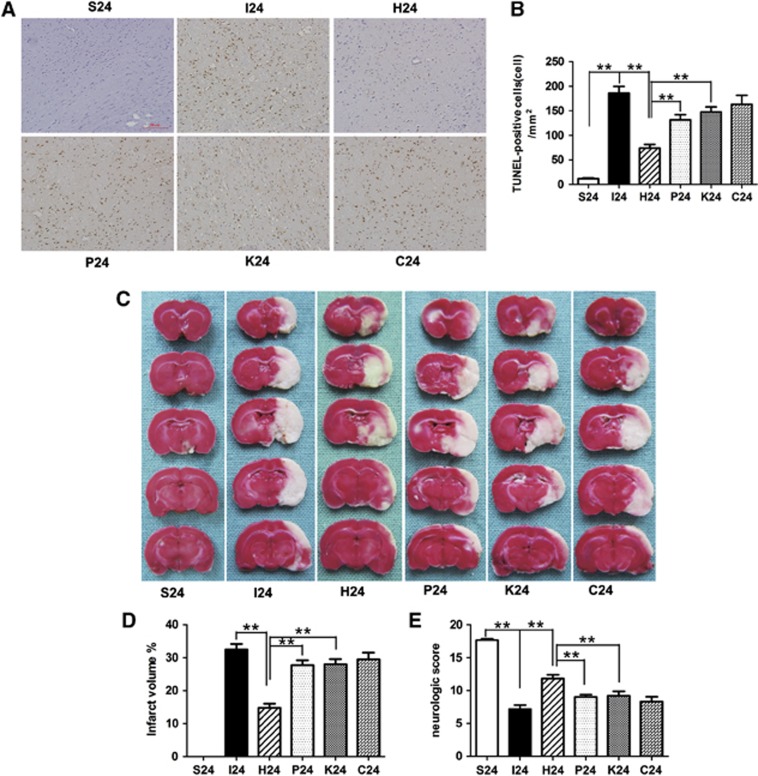
In the TUNEL assay, a large number of TUNEL-positive cells were observed in the right cortex of rats subjected to ischemia and reperfusion injury at 24 hours after reperfusion, whereas very few TUNEL-positive cells were visible in the sham-operated group. It is important to emphasize that hyperforin, when applied immediately after MCAO onset, significantly reduced apoptotic cell death in the right cortex at 24 hours after reperfusion (P<0.01; Figure 2). Application of hyperforin plus PD98059 or KN62, the number of TUNEL-positive cells was significantly increased compared with hyperforin-treated group (P<0.01 and P<0.01, respectively). There was no significant difference between group P, group K, and group C (P>0.05). Our results revealed a negative correlation between the levels of TRPC6 proteins and the numbers of TUNEL-positive cells during reperfusion.
Figure 2.
Effect of hyperforin on the apoptotic cell death, infarct volumes, and neurologic scores after 2 hours of transient middle cerebral artery occlusion (MCAO). (A) Representative photomicrographs of terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) staining of the ischemic cortex ( × 200). (B) Quantitative analysis showed that hyperforin treatment reduced the number of TUNEL-positive cells in the ischemic cortex; application of PD98059 or KN62, the number of TUNEL-positive cells was significantly increased. (C) Representative 2,3,5-triphenyltetrazolium chloride staining of the cerebral infarct in the rat brain (n=6). (D) Statistical analysis of the percentage of infarct volume was determined for each study group. Hyperforin-treated rats significantly reduced infarct volumes by 55% compared with the MCAO group. Application of PD98059 or KN62 alone, the infarct volume was significantly increased compared with hyperforin-treated group. (E) Quantification of neurologic scores at 24 hours after reperfusion (n=6). Hyperforin-treated animals had significantly greater scores than the MCAO group. PD98059 or KN62 administrated 20 minutes before the operation blocked hyperforin-induced functional recovery. Bars represent mean±s.e.m. Scale bar, 20 μm. **P<0.01.
Hyperforin Significantly Reduced Infarct Volumes in Ipsilateral Ischemic Hemispheres
After ischemia/reperfusion injury, white-stained infarct area was prominent in MCAO group, and almost the entire MCA territory appeared infarcted. By contrast, hyperforin-treated rats significantly reduced infarct volumes by 55% compared with MCAO group at 24 hours after reperfusion (14.77±1.28% versus 32.48±1.68%, P<0.01; Figures 2C and 2D). Application of PD98059 or KN62 alone, the infarct volumes were significantly increased compared with hyperforin-treated group (14.77±1.28% versus 27.72±1.50%, P<0.01; 14.77±1.28% versus 27.97±1.63%, P<0.01).
Hyperforin Promoted Functional Recovery
Sham-operated rats did not have any deficits. Statistical analysis confirmed that hyperforin-treated animals had significantly greater scores than MCAO group at 24 hours after reperfusion (P<0.01; Figure 2E). While treated with PD98059 or KN62, the neurologic scores were significantly decreased compared with hyperforin-treated group (P<0.01 and P<0.01, respectively).
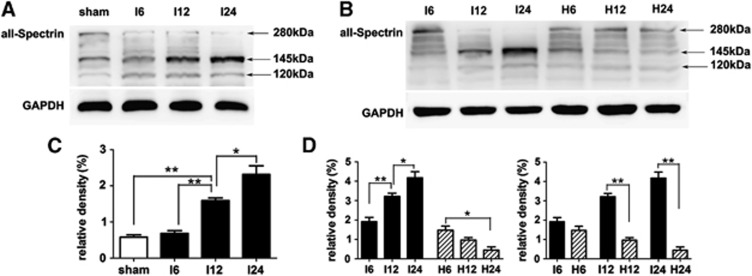
Hyperforin Blocked Calpain-Specific aII-spectrin Breakdown Products (SBDP145) Formation
Sham-operated group presented litter SBDP145. Quantitative analysis confirmed that the protein levels of SBDP145 in MCAO group were gradually increased during the experimental time course (Figures 3A and 3C). Compared with sham-operated group, the protein levels of SBDP145 in MCAO group were significantly increased as early as 12 hours (P<0.01); this significant increase was also presented at 24 hours (P<0.01). When MCAO rats were treated with hyperforin, the protein levels of SBDP145 were significantly decreased at 12 hours (P<0.01) and 24 hours (P<0.01); and in the hyperforin-treated group, the protein levels of SBDP145 were progressively reduced during the experimental time course (Figures 3B and 3D).
Figure 3.
Activation of calpain was confirmed by aII-spectrin proteolysis at 6, 12, and 24 hours after reperfusion. (A, B) Western blot analysis of calpain-specific aII-spectrin breakdown products (SBDP145). Total protein extracts were prepared from the ipsilateral cortex. Identical amounts of protein (50 μg) were applied to each lane of the SDS-PAGE gel (6% polyacrylamide gel). GAPDH was used as an internal reference. A 145-kDa band corresponding to SBDP145 protein was clearly detected. (C, D) Densitometric analysis of the protein levels of SBDP145 in the ipsilateral cortex (n=3). Values are mean±s.e.m. *P<0.05; **P<0.01.
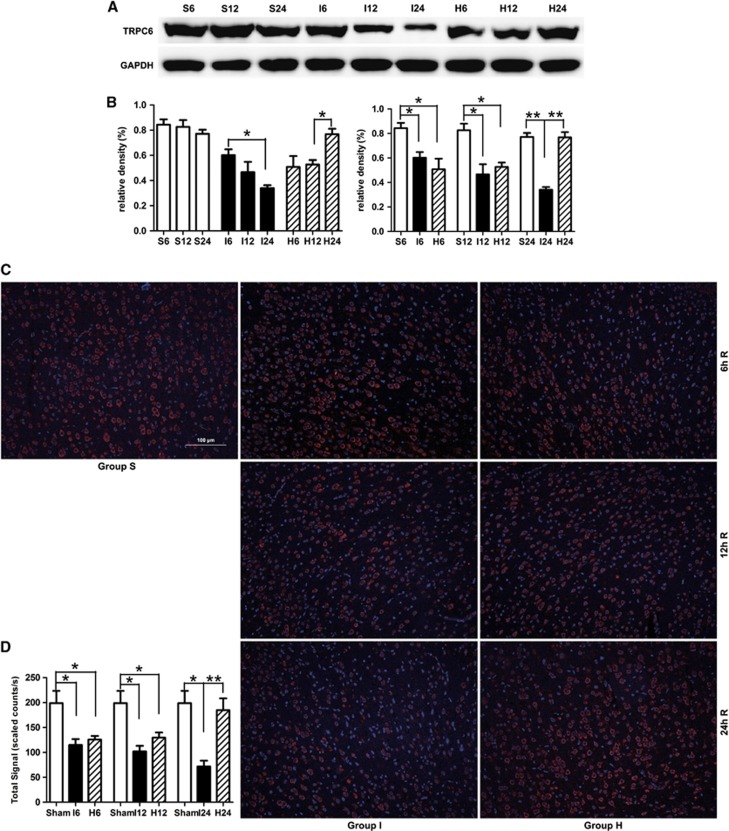
Hyperforin Inhibited Calpain-Mediated TRPC6 Channels Degradation
In MCAO group, TRPC6 protein level was also gradually decreased during the experimental time course (Figures 4A and 4B). Compared with sham-operated group, TRPC6 level in MCAO group was significantly decreased at 6 hours (P<0.05), 12 hours (P<0.05), and 24 hours (P<0.01) after reperfusion. In the hyperforin-treated group, TRPC6 level recovered to that of the sham-operated group at 24 hours; TRPC6 level at 24 hours was significantly higher than that at 12 hours (P<0.05). Hyperforin significantly reduced TRPC6 degradation induced by ischemia compared with MCAO group only at 24 hours (P<0.01). Immunofluorescence analysis showed cytomembrane staining pattern of TRPC6 in neurons of cerebral cortex (Figures 4C and 4D), and the immunofluorescence analysis got the similar results with the western blot analysis.
Figure 4.
Effect of hyperforin on the levels of TRPC6 at 6, 12, and 24 hours after reperfusion. (A) Western blot analysis of transient receptor potential canonical (subtype) 6 (TRPC6). Total protein extracts were prepared from the ipsilateral cortex. Identical amounts of homogenate protein (50 μg) were applied to each lane of the SDS-PAGE gel (8% polyacrylamide gel). GAPDH was used as an internal reference. A 106-kDa band corresponding to TRPC6 protein was clearly detected. (B) Densitometric analysis of the protein levels of TRPC6 in the ipsilateral cortex (n=3). (C) TRPC6 immunoreactivity ( × 200). All nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Staining is present within cell membrane. Scale bar: 100 μm. (D) Quantification of the levels of TRPC6 in the ipsilateral cortex (n=3). Values are mean±s.e.m. *P<0.05; **P<0.01.
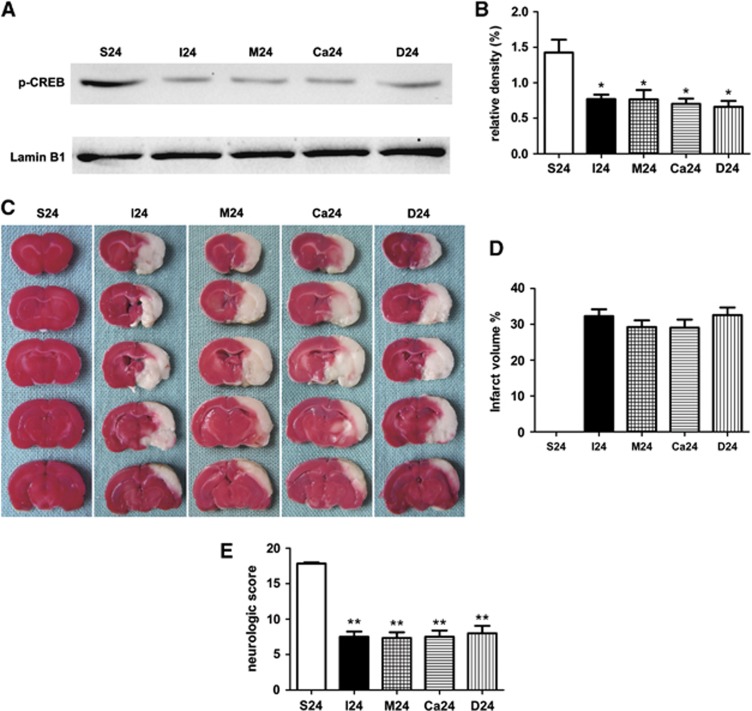
PD98059 or KN62 Had No Effect on Ischemic Stroke in Rats at 24 Hours After Reperfusion
To study the effect of PD98059 or KN62 alone in the stroke rats, PD98059 or KN62 was administrated 20 minutes before the operation (Figure 1C). Interestingly, application of PD98059 or KN62, no statistical significance was noted in the protein levels of p-CREB in MCAO rats (Figures 5A and 5B). There was also no significant difference between the four groups during the whole process of ischemia and reperfusion through measuring the infarct volumes and neurologic scores (Figures 5C–5E).
Figure 5.
Effect of PD98059 or KN62 in the stroke rats at 24 hours after reperfusion. (A, B) Western blot analysis of phosphorylated CREB (p-CREB) (n=3). Nuclear protein extracts were prepared from the ipsilateral cortex. Quantification of p-CREB assessed by western blot analysis was normalized to the expression level of Lamin B1. (C, D) Representative 2,3,5-triphenyltetrazolium chloride (TTC) staining of the cerebral infarct in the rat brain (n=6). (E) Quantification of neurologic scores (n=6 rats for each). Values are mean±s.e.m. *P<0.05; **P<0.01.
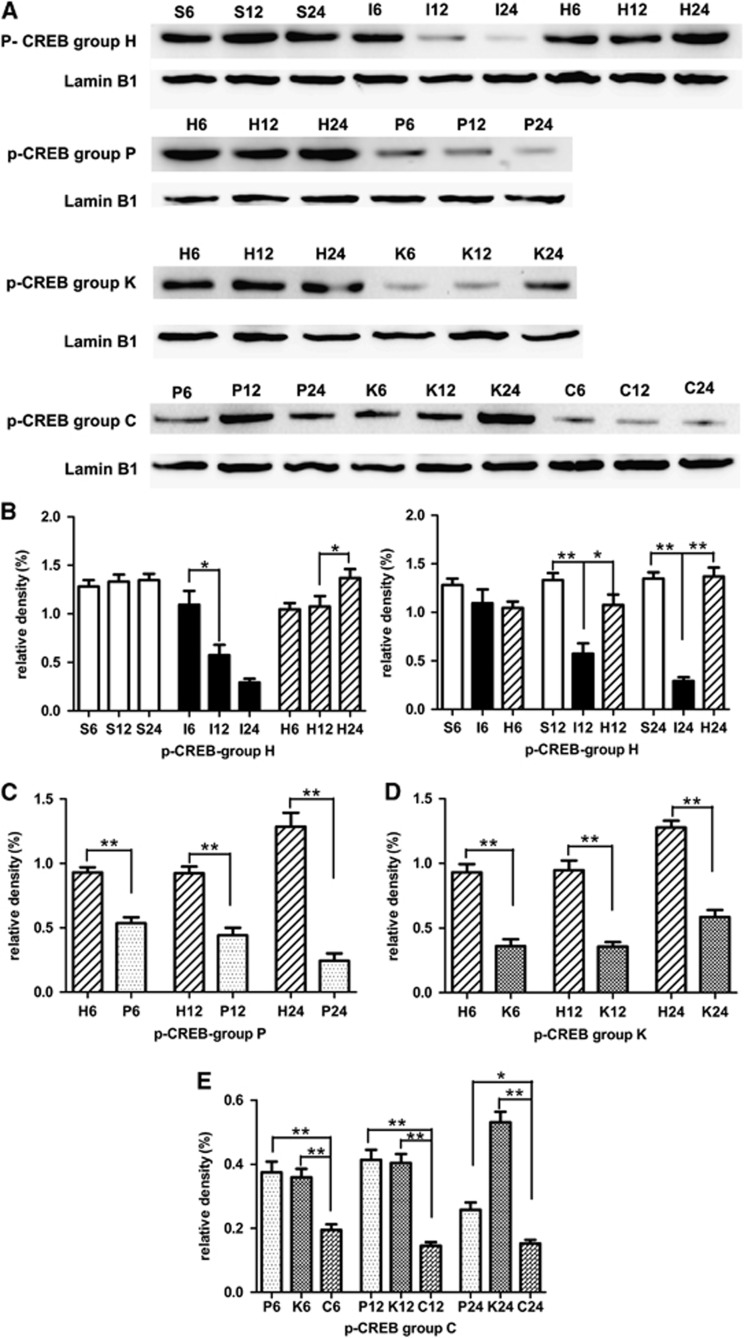
Hyperforin Maintained Phosphorylation of CREB through Blocking TRPC6 Degradation
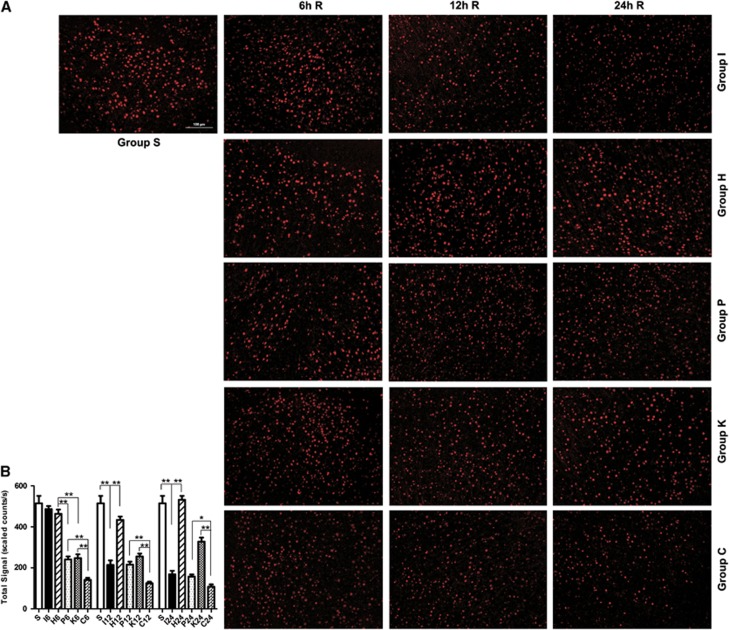
In MCAO group, p-CREB was gradually decreased during the experimental time course (Figures 6A and 6B). Compared with sham-operated group, p-CREB in MCAO group was significantly decreased at 12 hours (P<0.01) and 24 hours (P<0.01) after reperfusion. In the hyperforin-treated group, the protein levels of p-CREB reached a peak level at 24 hours after reperfusion. Compared with MCAO group, p-CREB in the hyperforin-treated group significantly increased at 12 hours (P<0.05) and 24 hours (P<0.01). As expected, administration with PD98059 20 minutes before the operation, the protein levels of p-CREB were significantly decreased compared with hyperforin-treated group at 6 hours (P<0.01), 12 hours (P<0.01), and 24 hours (P<0.01; Figures 6A and 6C). Application of KN62 20 minutes before the operation, the protein levels of p-CREB were significantly decreased at 6 hours (P<0.01), 12 hours (P<0.01), and 24 hours (P<0.01; Figures 6A and 6D) compared with hyperforin-treated group. Application of hyperforin plus PD98059 and KN62, the protein levels of p-CREB were significantly lower than group P at 6 hours (P<0.01), 12 hours (P<0.01), or 24 hours (P<0.05; Figures 6A and 6E); and were also significantly lower than group K at 12 hours (P<0.01), 24 hours (P<0.01), and 48 hours (P<0.01). Immunofluorescence analysis showed nuclear staining pattern of TRPC6 in neurons of cerebral cortex, and the immunofluorescence analysis got the similar results with the western blot analysis (Figure 7).
Figure 6.
Effect of hyperforin treatment on the protein levels of phosphorylated CREB (p-CREB) at 6, 12, and 24 hours after reperfusion. (A) Western blot analysis of p-CREB. Nuclear protein extracts were prepared from the ipsilateral cortex. Identical amounts of homogenate protein (60 μg) were applied to each lane of the SDS-PAGE gel (10% polyacrylamide gel). Lamin B1 was used as an internal reference. A 43-kDa band corresponding to p-CREB protein was clearly detected. (B–E) Densitometric analysis of the protein levels of p-CREB in the ipsilateral cortex (n=3). Values are mean±s.e.m. *P<0.05; **P<0.01.
Figure 7.
Effect of hyperforin treatment administrated immediately after middle cerebral artery occlusion (MCAO) onset on the immunoreactivity of phosphorylated CREB (p-CREB) at 6, 12, and 24 hours after reperfusion. (A) p-CREB immunoreactivity ( × 200). Staining was present within cell nucleus. (B) Quantification of the levels of p-CREB in the ipsilateral cortex (n=3). Values are mean±s.e.m. Scale bar, 100 μm. *P<0.05; **P<0.01.
Discussion
Our results clearly showed that hyperforin, when applied immediately after MCAO onset, significantly decreased infarct volumes by 55%, reduced apoptotic cell death, and enhanced functional recovery at 24 hours after reperfusion. One previous study has provided evidence that hyperforin has NMDAR-antagonistic and potential neuroprotective effect in vitro.15 However, these protective effects of hyperforin in our in vivo study were in contradiction with the observations of Kumar et al,15 who showed that hyperforin failed to reduce infarct volumes in MCAO models in mice. One possible explanation for hyperforin's lack of effect in the in vivo model is that hyperforin, administered intraperitoneally, cannot reach a sufficient concentration in the brain. In our study, hyperforin was directly injected into the right ventricle. Unlike intravenous and intraperitoneal routes, ICV injection of hyperforin could rapidly attenuate ischemic cerebral injury within 24 hours after reperfusion reflected by decreased infarct volumes and apoptotic cell death and enhanced functional recovery, suggesting that ICV injection of hyperforin could be a high efficient method for treating cerebral ischemia injury. This rapid effect of hyperforin could be because of ICV route, and this is possible that ICV injection of hyperforin could quickly reach a high concentration of the drug in the brain. However, we have not yet performed detailed pharmacokinetic studies. Therefore, further pharmacokinetic studies are needed to confirm our speculation in the future.
Although several mechanisms, including excitotoxicity, peri-infarct depolarizations, ionic imbalance, oxidative and nitrosative stresses, and apoptosis3, 20, 21 have played some roles in the pathogenesis of ischemic neuronal death, the intracellular Ca2+ overload is still the most critical. The NMDAR, the important excitatory neurotransmitter receptor in the brain, has been reported as the pivotal player for the Ca2+ overload in response to cerebral ischemia. Calpains are intracellular Ca2+-dependent nonlysosomal neutral cysteine proteases. Cytosolic Ca2+ overload through NMDAR can lead to calpain activation.5 Under physiologic conditions, calpain activity is likely to be reversibly stimulated by transient localized increases in cytosolic Ca2+, and calpain activity is tightly regulated by the specific endogenous inhibitor calpastatin.22 The increase in cytosolic Ca2+ that occurs during brain ischemia and reperfusion overwhelms endogenous regulatory systems resulting in pathologic calpain activity. Previous studies have shown that calpain activity is increased by focal cerebral ischemia,23, 24 and calpain inhibitors provide varying degrees of neuroprotection in animal models.24, 25 aII-spectrin is an abundant cytoskeletal protein that is specifically cleaved by calpains into 150/145-kDa fragments. The calpain-specific aII-spectrin breakdown products of 145 kDa (SBDP145) results from sequential calpain cleavage of aII-spectrin to generate SBDP150, followed by cleavage to remove an additional 5 kDa.26, 27 The intension of calpain activation is reflected by the protein levels of SBDP145. This characteristic makes aII-spectrin cleavage be a useful tool to evaluate the activity of calpains.28, 29 In our study, sham-operated rats presented very litter SBDP145 while the MCAO rats had high levels of SBDP145 in the cortical regions of the ipsilateral hemisphere in the first 24 hours after injury. Hyperforin treatment significantly reduced SBDP145 formation and made it recover to the level of the sham-operated group at 24 hours after reperfusion. Taken together, these observations suggested that hyperforin, when applied immediately after MCAO onset, inhibited calpain activation, and induced resistance to ischemia and reperfusion injuries. However, in our study, it is not clear whether hyperforin suppressed calpain activation directly or suppressed calpain activation through inhibition of NMDAR.
The canonical transient receptor potential channels (TRPCs) are nonselective cation channels that are expressed in a variety of multicellular organisms with different functions.30 TRPC3 and TRPC6 are involved in brain-derived neurotrophic factor-mediated growth cone turning, neuron survival, and spine formation.9, 31 TRPC6 also promoted dendritic growth via the CaMKIV-CREB-dependent pathway.32 One previous study has provided evidence that TRPC6 was specifically degraded by calpain in transient ischemia and this degradation occurred before and during the neuronal cell death.6 Inhibition of calpain proteolysis of TRPC6 protected animals from ischemic brain damage.6 Therefore, TRPC6 channels have a critical role in promoting neuronal survival against focal cerebral ischemia and the decrease in the protein levels or activity of TRPC6 channels contributes to ischemic brain injury.6 Our results showed that the levels of TRPC6 proteins in MCAO group were greatly decreased at 6 hours after reperfusion and the reduction in TRPC6 protein levels remained prominent at 12 and 24 hours after reperfusion, in support of the observations of Du et al.6 In our study, hyperforin treatment significantly reduced TRPC6 degradation induced by ischemia at 24 hours after reperfusion. As mentioned above, hyperforin treatment also significantly inhibited calpain activity at 24 hours after reperfusion. In addition, hyperforin-treated rats significantly reduced infarct volumes and apoptotic cell death, and enhanced functional recovery at 24 hours after reperfusion. Therefore, our results indicated that inhibition of calpain proteolysis of TRPC6 by hyperforin protected rats from ischemic brain damage.
The CREB is expressed in all cells in the brain and is a member of a family of proteins that function as transcription factors. Extracellular stimulation activates CREB by phosphorylation of serine-133 in CREB.33 The CREB is a major mediator of neurotrophin-induced transcription.34 In neurons, CREB influences the expression of a variety of genes including brain-derived neurotrophic factor and c-fos.35 The CREB activation was a critical event in neuroprotection against ischemic injury.36, 37 In cortical neurons, entry of Ca2+ results in Ca2+-dependent activation of ERK and CaMKIV,38 which in turn activate CREB transcriptional pathways to promote neuronal survival.9, 32, 39 Our study clearly showed that the protein levels of p-CREB were significantly increased in the hyperforin-treated group and recovered to the level of the sham-operated group at 24 hours after reperfusion. When MEK or CaMKIV activity was inhibited by PD98059 or KN62, the neuroprotective effect of hyperforin was attenuated and correlated with decreased CREB activity levels. In addition, while treated with PD98059 or KN62 20 minutes before the operation, the apoptotic cell death and infarct volumes significantly increased, suggesting that the neuroprotective effect of hyperforin was attenuated. These results indicated that hyperforin failed to exert its neuroprotective effects if Ras/MEK/ERK-CREB or CaMKIV-CREB pathway was blocked. However, in our study, it is not clear whether activation of TRPC6 by hyperforin can also have the same effect as inhibition of NMDAR by hyperforin.
Overexpressing TRPC6 markedly increased CREB phosphorylation and CREB-dependent transcription.9 Blocking TRPC6 degradation maintained phosphorylation of CREB and greatly prevent ischemic brain injury.6 In our study, hyperforin significantly reduced TRPC6 degradation induced by ischemia at 24 hours after reperfusion. Hyperforin also enhanced the protein levels of p-CREB through Ras/MEK/ERK-CREB and CaMKIV-CREB pathways. Taken together, our data suggested that hyperforin, when administrated immediately after MCAO onset, blocked calpain-mediated TRPC6 channels degradation, and allowed elevation of [Ca2+]i to stimulate the Ras/MEK/ERK and CaMKIV pathways that converge on CREB activation, contributed to neuroprotection at 24 hours after reperfusion. These results clearly showed that the activation of CREB was a pivotal downstream effector for the neuronal protective effect of TRPC channels.
In conclusion, our results suggest that ICV injection of hyperforin can greatly attenuated ischemic brain damage, and hyperforin exerts its neuroprotective effects by inhibition of calpain proteolysis of TRPC6, and then increasing CREB phosphorylation through Ras/MEK/ERK and CaM/CaMKIV pathways. Therefore, acute ICV administration of hyperforin after cerebral ischemia as a neuroprotective treatment could exert rapid and effective neuroprotective effect. Although routine ICV treatment is technically difficult in stroke patients and may also cause some complications, such as infection, our study provides theoretical support for ICV administration of hyperforin in the treatment of cerebral ischemic stroke during the acute or subacute period.
The authors declare no conflict of interest.
References
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing. Trends Neurosci. 1999;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- Caplan LR. Treatment of patients with stroke. Arch Neurol. 2002;59:703–707. doi: 10.1001/archneur.59.5.703. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120:3480–3492. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rozio M, Ekalle-Soppo CB, Guiso G, Morazzoni P, Caccia S. Role of hyperforin in the antidepressant-like activity of Hypericum perforatum extracts. Psychopharmacology (Berl) 2002;164:423–428. doi: 10.1007/s00213-002-1229-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Filippov V, Lishko P, Maximyuk O, Noldner M, Krishtal O. Hyperforin attenuates various ionic conductance mechanisms in the isolated hippocampal neurons of rat. Life Sci. 1999;65:2395–2405. doi: 10.1016/s0024-3205(99)00506-8. [DOI] [PubMed] [Google Scholar]
- Medina MA, Martinez-Poveda B, Amores-Sanchez MI, Quesada AR. Hyperforin: more than an antidepressant bioactive compound. Life Sci. 2006;79:105–111. doi: 10.1016/j.lfs.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Quiney C, Billard C, Salanoubat C, Fourneron JD, Kolb JP. Hyperforin, a new lead compound against the progression of cancer and leukemia. Leukemia. 2006;20:1519–1525. doi: 10.1038/sj.leu.2404301. [DOI] [PubMed] [Google Scholar]
- Borrelli F, Izzo AA. Herb-drug interactions with St John's wort (Hypericum perforatum): an update on clinical observations. AAPS J. 2009;11:710–727. doi: 10.1208/s12248-009-9146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Mdzinarishvili A, Kiewert C, Abbruscato T, Bickel U, van der Schyf CJ, et al. NMDA receptor-antagonistic properties of hyperforin, a constituent of St John's Wort. J Pharmacol Sci. 2006;102:47–54. doi: 10.1254/jphs.fp0060378. [DOI] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, et al. Hyperforin--a key constituent of St John's wort specifically activates TRPC6 channels. FASEB J. 2007;21:4101–4111. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators--identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–377. doi: 10.1124/mol.109.057513. [DOI] [PubMed] [Google Scholar]
- Gibon J, Deloulme JC, Chevallier T, Ladeveze E, Abrous DN, Bouron A. The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int J Neuropsychopharmacol. 2012;. pp. 1–10. [DOI] [PubMed]
- Garcia JH, Liu KF, Ye ZR, Gutierrez JA.Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats Stroke 1997282303–2309.discussion 2310. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Cavanaugh K, Eveleth D, Carriero DL, Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab. 1995;15:969–979. doi: 10.1038/jcbfm.1995.123. [DOI] [PubMed] [Google Scholar]
- Yao H, Ginsberg MD, Eveleth DD, LaManna JC, Watson BD, Alonso OF, et al. Local cerebral glucose utilization and cytoskeletal proteolysis as indices of evolving focal ischemic injury in core and penumbra. J Cereb Blood Flow Metab. 1995;15:398–408. doi: 10.1038/jcbfm.1995.50. [DOI] [PubMed] [Google Scholar]
- Hong SC, Goto Y, Lanzino G, Soleau S, Kassell NF, Lee KS. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke. 1994;25:663–669. doi: 10.1161/01.str.25.3.663. [DOI] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, et al. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319 (Part 3:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference? by kevin K.W. Wang, Vol. 23, pp. 20–26. Trends Neurosci. 2000;23 (59 doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;14:3934–3944. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Reyn CR, Spaethling JM, Mesfin MN, Ma M, Neumar RW, Smith DH, et al. Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J Neurosci. 2009;29:10350–10356. doi: 10.1523/JNEUROSCI.2339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, et al. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121:2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Hata R, Gass P, Mies G, Wiessner C, Hossmann KA. Attenuated c-fos mRNA induction after middle cerebral artery occlusion in CREB knockout mice does not modulate focal ischemic injury. J Cereb Blood Flow Metab. 1998;18:1325–1335. doi: 10.1097/00004647-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Walton MR, Dragunow I. Is CREB a key to neuronal survival. Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Barker PA. Something old, something new: BDNF-induced neuron survival requires TRPC channel function. Nat Neurosci. 2007;10:537–538. doi: 10.1038/nn0507-537. [DOI] [PubMed] [Google Scholar]