Abstract
αA-crystallin is abundant in the lens of the eye and acts as a molecular chaperone by preventing aggregation of denaturing proteins. We previously found that chemical modification of the guanidino group of selected arginine residues by a metabolic α-dicarbonyl compound, methylglyoxal (MGO), makes human αA-crystallin a better chaperone. Here, we examined how the introduction of additional guanidino groups and modification by MGO influence the structure and chaperone function of αA-crystallin. αA-crystallin lysine residues were converted to homoarginine by guanidination with o-methylisourea (OMIU) and then modified with MGO. LC-ESI-mass spectrometry identified homoargpyrimidine and homohydroimidazolone adducts after OMIU and MGO treatment. Treatment with 0.25 M OMIU abolished most of the chaperone function. However, subsequent treatment with 1.0 mM MGO not only restored the chaperone function but increased it by ~40% and ~60% beyond that of unmodified αA-crystallin, as measured with citrate synthase and insulin aggregation assays, respectively. OMIU treatment reduced the surface hydrophobicity but after MGO treatment, it was ~39% higher than control. FRET analysis revealed that αA-crystallin subunit exchange rate was markedly retarded by OMIU modification, but was enhanced after MGO modification. These results indicate a pattern of loss and gain of chaperone function within the same protein that is associated with introduction of guanidino groups and their neutralization. These findings support our hypothesis that positively charged guanidino group on arginine residues keeps the chaperone function of αA-crystallin in check and that a metabolic α-dicarbonyl compound neutralizes this charge to restore and enhance chaperone function.
Keywords: αA-crystallin, chaperone, homoarginine, homoargpyrimidine, homohydroimidazolone
Alpha-crystallin is a major structural protein in the lens of the eye. It is a polydisperse protein, usually occurring in aggregates of 40 subunits with a molecular weight ~800 kDa (1). The polymeric aggregate consists of both αA- and αB-crystallins, usually in a stable ratio of 3:1 (1). The αA- and αB-crystallins both belong to a family of proteins known as small heat-shock proteins, or sHsps, which includes Hsp27 (2, 3). The sHsps have three distinct structural domains. The inner core domain, or α-crystallin domain, is about 80 amino acids in length (4). This is flanked by an N-terminal domain that varies in length and sequence. The C-terminal domain is flexible without a rigid structure and is believed to be responsible for the solubility of many sHsps, including α-crystallin (5).
Although sHsps are resident ‘housekeeping’ proteins, they are synthesized in higher amounts in response to thermal, oxidative and other stresses in order to prevent cell damage (6, 7). Their molecular chaperone activity is one mechanism through which sHsps provide defence; in addition, they are anti-apoptotic (6–8). The chaperone function of α-crystallin was first demonstrated by Horwitz (9) and subsequently confirmed by a number of other investigators (10–12). The chaperone function enables α-crystallin to inhibit aggregation of denaturing proteins, and alteration of this process has been linked to certain diseases characterized by protein aggregates, including cataract formation and Alzheimer’s disease (13, 14). Experimental evidence from congenital dominant mutations of α-crystallin (15–17) or from post-translational modifications (18–20) indicates that compromised chaperone function can lead to cataract formation.
The hydrophobic regions of α-crystallin are thought to be sites of chaperone–substrate interaction that are essential for chaperone function (21–23). Several of these substrate binding sites have been identified (21–24), notably at amino acids 12–21 and 71–78 in αA-crystallin and 9–20, 28–34, 43–58 and 75–82 in αB-crystallin. Recent studies suggest that arginine residues, either within the protein interaction sites or elsewhere within the protein, dictate the chaperone function of both αA- and αB-crystallins (15, 25–27). Thampi and Abraham (28) found that cleavage of 11 C-terminal residues of rat αA-crystallin, including Arg-163, decreased chaperone function. The R116C mutation in αA-crystallin cripples its chaperone activity and promotes cataract formation in the human lens (15, 29) as does over-expression of R116C in the mouse lens (30). An R120G mutation in αB-crystallin similarly compromises the chaperone function and leads to cataract formation (25). Other mutations, such as R49C, also appear to be linked to cataract formation (31).
Amid these loss-of-chaperone effects relating to arginine residues, several studies suggest that a gain of such function is associated with deletion or modification of arginine residues in α-crystallin. Pasta et al. (32) showed that deletion of the 20SRLFDQFFG28 sequence, and thus R21, in αA-crystallin made it a better chaperone. More recently, deletion of 54FLRAPSWF61 sequence was reported to improve chaperone function (33). Our laboratory demonstrated that replacement or modification of specific arginine residues with methylglyoxal (MGO), a metabolic α-dicarbonyl, improved chaperone function of αA-crystallin, presumably by modification of R21, R49 and R103 to argpyrimidine (34). We observed similar but lesser effects with other arginine-modifying compounds, such as, phenylglyoxal, 1,2-cyclohexanedione and 2,3-butanedione, which confirmed that modification of guanidino group of arginine residues is responsible for the increased chaperone function of α-crystallin (34). Recently, we reported that replacement of R21 and R103 with alanine by site-directed mutagenesis was equally effective (26) and that deletion of R56 in human αB-crystallin enhances its chaperone function by at least 35% as compared to the native protein (27). Our studies also confirmed that the positive charge on selected arginine residues modulates the chaperone function of α-crystallin.
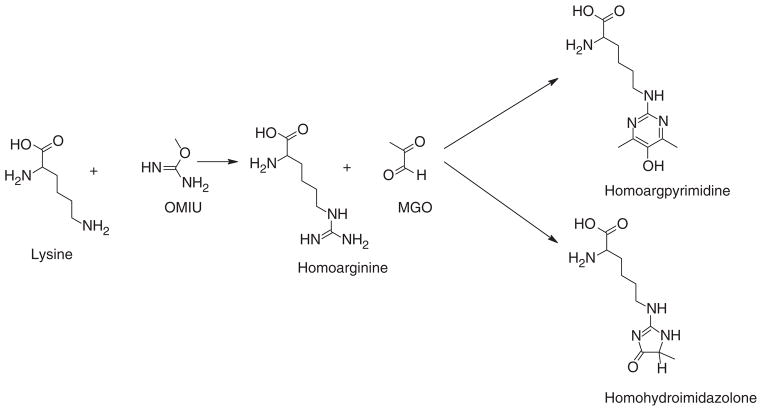
Realization that MGO modification neutralized positive charge on selected arginine residues and enhanced the chaperone function of αA-crystallin prompted us to investigate if we could modulate the chaperone function by introducing additional arginine residues and their modification with MGO. Arginine residues could be introduced by cloning strategies at random places on the protein, but that might change the net charge on the protein and might disrupt its folding pattern as well. Another method is to replace positively charged lysine residues with arginine by site-directed mutagenesis. Since there are seven lysine residues, to replace them one at a time or in combination would require many mutations and therefore could be extremely tedious. The third strategy, the one we adopted in this study, is to introduce homoarginine (similar to arginine in that it carries positive charge on the guanidino group) residues by converting lysine residues to homoarginine by reacting with o-methylisourea (OMIU) (Fig. 1). This approach is simple and straightforward. This method yielded some surprising results: while modification by OMIU almost completely abolished the chaperone function, additional modification by MGO not only restored it, but it made αA-crystallin an even better chaperone than the unmodified protein. We then sought to define the structural changes associated with such dramatic changes in the chaperone function.
Fig. 1. Formation of homoarginine + MGO adducts on αA-crystallin.
Lysine residues are converted to homoarginine by reaction with OMIU. Reaction of homoarginines with MGO produces homoargpyrimidine (HAP) and homohydroimidazolone (HHI) adducts on αA-crystallin.
MATERIALS AND METHODS
Bovine insulin, citrate synthase (CS), dithiothreitol (DTT) and o-methylisourea (OMIU) were obtained from Sigma Chemical Co., St. Louis, MO, USA. Citrate synthase was dialysed against 0.04 M HEPES buffer (pH 7.4) for 24 h before use. 2-p-toluidinylnaphthalene-6-sulphonate (TNS), lucifer yellow iodoacetamide (LYI) and 4-acetamido-4′-((iodoacetyl)amino)-stilbene-2,2′-disulphonic acid (AIAS) were obtained from Molecular Probes (Invitrogen, Carlsbad, CA, USA). All other chemicals were of analytical grade.
Cloning, Expression and Purification of Recombinant αA-Crystallin
The cDNA encoding human αA-crystallin was kindly provided by J. Mark Petrash, Washington University, St. Louis, MO, USA. Cloning, expression, and purification of this protein were done as previously described (26).
Chemical Modification of αA-Crystallin
Modification by OMIU
An aqueous solution of recombinant human αA-crystallin (1.0 mg/ml) was incubated with various concentrations (0 to 1 M) of OMIU at pH 10.5 for 48 h at 4°C as previously described (35). We then dialysed all solutions against 50 mM sodium phosphate buffer (pH 7.4) for 48 h to eliminate excess OMIU.
Modification by MGO
Unmodified and OMIU-modified αA-crystallin (0.5 mg/ml) in 0.05 M sodium phosphate buffer (pH 7.4) were incubated with 1 mM MGO for 2 days at 37°C. We then dialysed all solutions against 50 mM sodium phosphate buffer (pH 7.4) to eliminate any unreacted MGO.
BSA was similarly modified with OMIU (0.25 and 1.0 M) and MGO (1.0 mM) and dialysed.
Assessment of Chaperone Function
Chaperone activity of unmodified and modified αA-crystallin (20 μg each) was measured in 96-microwell plates using a microplate reader (Molecular Devices, Model 190, Sunnyvale, CA, USA). Insulin, 80 μg (0.32 mg/ml), was reduced by freshly prepared DTT (final concentration 20 mM) to break the inter-chain S–S bond; this results in aggregation of the B-chain. Aggregation was measured (at 25°C) in absence and presence of 20 μg (0.08 mg/ml) unmodified and modified αA-crystallin by monitoring light scattering at 400 nm for 1 h (36). Citrate synthase, 15 μg (0.06 mg/ml protein in 0.04 M HEPES buffer, pH 7.4) was heated at 43°C in the presence or absence of 0.75 μg unmodified and modified αA-crystallin, and light scattering was monitored at 360 nm (37). Reactions for both assays were carried out in a total volume of 250 μl.
Amino Acid Analysis
Amino acid analyses were done at the Protein Chemistry Laboratory, Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX, USA. Briefly, unmodified and modified αA-crystallin (treated with 0.1 to 1.0 M OMIU) were hydrolysed with 6 N HCl at 110°C for 20 h. The hydrolysed samples were evaporated to dryness and suspended in 300 μl of Milli-Q water. For the analysis, 20 μl of diluted sample was mixed with 250 μl of 0.4 N borate buffer, and the pH was adjusted to 10.0. Five nanomoles of internal standard was added to all samples, standards and blanks. The assay was calibrated with two 5 nM standards that were not hydrolysed. All samples were analysed on a Hewlett Packard AminoQuant System, which includes automated pre-column derivitization of the hydrolysed primary amino acids with o-phthalaldehyde (OPA) and the secondary amino acids with 9-flouromethyl-chloroformate (FMOC). Derivatized amino acids were separated by reverse phase HPLC on an HP 1090L and detected by photodiode array (UV-DAD).
Synthesis of Homoargpyrimidine
t-BOC-homoargi-nine (0.5 M) and MGO (1.0 M) were dissolved in 5.0 ml 0.2 M sodium phosphate buffer (pH adjusted to 7.4 with 10 N NaOH) and incubated at 50°C for 24 h. The incubation mixture (500 μl) was injected onto a C18 reverse phase semi-preparative column (Vydac, 218TP1010) with a water–acetonitrile gradient system. Solvent A was water with 0.1% trifluoroacetic acid (TFA), and solvent B was 50% acetonitrile in water with 0.1%TFA. The linear gradient program was as follows: 0 to 10 min, 0% B; 10 to 20 min, 30% B; 20 to 35 min, 50% B; 35 to 45 min, 100% B; 45 to 52 min, 100% B; 52 to 63 min, 0% B. The flow rate was 2.0 ml/min. The column effluent was monitored for fluorescence at 385 nm (excitation, 335 nm) with an online fluorescence detector. The fluorescent peak at Rt ~45 min was collected from 10 injections, dried in a Speed Vac concentrator, suspended in 0.5 N HCl and incubated at 50°C for 2 h to remove the t-BOC group. The sample was dried, suspended in 500-μl solvent A and subjected to HPLC as described above. We noted a single homogenous peak at Rt ~28 min, which we collected and lyophilized. The yield was 12 mg. 1H-NMR analysis of this product showed the following signals (CD3OD): δ 3.94 (t, 1 H, J = 6.0 Hz), 3.48 (t, 2 H, J = 7.2 Hz), 2.44 (s, 6 H), 1.97 (m, 2H), 1.45 to 1.75 (2 H). ESI-MS, m/z 269 (M++1). These characteristics are fully compatible with homoargpyrimidine [N6-(5-hydroxy-4,6 dimethylpyrimidin-2-yl-)lysine] (Fig. 1).
HPLC Assay for Argpyrimidine and Homoargpyrimidine
Protein samples (300 μg each) were hydrolysed in 6N HCl at 110°C for 20 h. The acid was evaporated in a Speed Vac system, and the pellet was suspended in 200 μl water and filtered through a 0.45 μm centrifugal filter. The amino acid content of each hydrolysate was estimated with ninhydrin as described (38). The samples were injected onto a C18 reverse phase column (GraceVydac, 218TP54) and separated in a gradient system of water and acetonitrile. Solvent A was water with 0.01 M heptafluorobutyric acid (HFBA), and solvent B was 70% acetonitrile in water with 0.01M HFBA. The solvent program was as follows: 0–39 min, 16% B; 40–50 min, 20% B; 50–60 min, 22% B; 60–62 min, 28% B; 62–71 min, 100% B; 71–80 min, 16% B. We monitored the column eluate with an online fluorescence detector set at excitation/emission wavelengths of 335/385 nm. Under these conditions, argpyrimidine had an Rt of ~28 min, and homoargpyrimidine had an Rt ~45 min. We estimated amounts of argpyrimidine and homoargpyrimidine in our protein samples by comparison with peak areas of synthetic standards.
HPLC-ESI-Mass Spectrometry
Protein reduction, S-alkylation and digestion
Unmodified αA-crystallin (20 μg), αA-crystallin modified by OMIU (0.25 M) or by the combination of 0.25 M OMIU + 1 mM MGO were reduced with DTT for 2 h at 30°C in 20 μl of 1 M Tris–HCl (pH 8.0) containing 8 M urea. The samples were S-alkylated by treating with 2.5 mM iodoacetamide for 30 min at 25°C in the dark. Unreacted reagents were removed by dialysis of the proteins against 0.1% formic acid, and then the samples were lyophilized, re-dissolved in 1 M Tris–HCl (pH 8.0) and digested by the endoproteinase, Asp-N (Roche Applied Science, Indianapolis, IN, USA) for 16 h at 25°C. The enzyme-to-protein ratio was 1:100 (w/w).
Identification of homoarginine, homohydroimidazolone and homo-argpyrimidine
LC–ESI–MS/MS analyses of the Asp-N digests were done with a Q-Star XL quadrupole/time of flight (TOF) mass spectrometer (Applied Biosystems-MDS Sciex, Foster City, CA, USA) coupled to an Agilent 1100 capillary HPLC system (Agilent, Santa Clara, CA, USA) as described previously (39). The data were manually analysed by examining the spectra for non-modified Asp-N peptides as well as for ions with the mass increment predicted from our chemical modifications of the protein (a mass increase of 42 Da for homoarginine, 96 Da for homohydroimidazolone and 122 Da for homo-argpyrimidine). The peptide sequences were confirmed by their product ion spectra.
Tryptophan Fluorescence Measurements
Tryptophan fluorescence of αA-crystallin solutions was measured in a LS-55 Perkin Elmer spectrofluorometer as described previously (26). The excitation wavelength was set to 295 nm, and emission spectra were recorded between 310 and 400 nm. Excitation and emission band passes were 5 nm each. Data were collected at a 0.5 nm wavelength resolution.
CircularDichroism (CD) Spectroscopy
Far- and near-UV CD spectra were recorded at 25°C in a Jasco 810 spectropolarimeter (Jasco, Inc., Japan). The spectra were measured using 1 and 10 mm cells with 0.2 mg/ml protein for far- and 1.0 mg/ml for near-UV CD. Our reported CD spectra comprise an average of five scans, and we used the curve-fitting program CONTINLL to analyse the secondary structure of unmodified, OMIU and OMIU + MGO modified αA-crystallin (40).
TNS Fluorescence Measurements
αA-Crystallin (0.1 mg/ml) was incubated in a methanolic solution of TNS (100 μM) for 2 h at 25°C. Fluorescence of TNS-bound samples was measured between 350 and 520 nm following excitation at 320 nm and recorded at 25°C using a LS-55 Perkin Elmer spectrofluorometer with the excitation and emission band passes set at 5 nm. Data were collected at a 0.5 nm resolution.
Fluorescence Labelling of Recombinant αA-Crystallin with LYI and AIAS
The cysteine residue at position 131 in αA-crystallin was labelled separately with the fluorescent probes AIAS and LYI as described (41). The covalently labelled αA-crystallin was then separated by passage through Sephadex G-25 column (20 × 2.0 cm) equilibrated with buffer (pH 7.5) containing 100 mM NaCl, 2 mM DTT and 50 mM sodium phosphate. The first fluorescence peak, which contained the labelled proteins, was collected and dialysed for 24 h against 50 mM phosphate buffer (pH 7.5). We followed the same procedure to calculate the percentage labelling of AIAS/LYI in αA-crystallin as described in (41).
Measurements of Subunit Exchange Rate
The excitation maxima of AIAS- and LYI-labelled αA-crystallin were found to be 335 nm and 435 nm; the emission maximum of AIAS-labelled protein was 415 nm and that for the LYI-labelled αA-crystallin was 525 nm. Subunit exchange kinetics was measured according to a previously published method (42). Subunit exchange was accomplished by mixing equal volumes of 0.4 mg/ml AIAS-labelled αA-crystallin (unmodified and modified) and 0.4 mg/ml LYI-labelled αA-crystallin (unmodified and modified) at 37°C in 50 mM phosphate buffer (pH 7.5) containing 2 mM DTT and 100 mM NaCl. At various time intervals, 20 μl of the reaction mixture was removed and diluted 100-fold with the same buffer. The fluorescence spectra of the samples were recorded from 360 to 600 nm at room temperature with an LS-55 Perkin Elmer spectrofluorometer after excitation at 335 nm. Both excitation and emission monochromators had a slit width of 5 nm. We measured the intensity at 415 nm and calculated the subunit exchange rate from the following equation:
where F(t) is the fluorescence intensity at 415 nm at various time intervals, F(0) is the fluorescence intensity at 415 nm at t = 0, and k is the subunit exchange rate constant. The constants D1 and D2 were determined using conditions at which D1 + D2 = 1 at t = 0 and D1 is the fluorescence intensity at t = ∝. MGO modification alone of αA-crystallin without fluorescent tags did not affect the LYI emission (see Supplementary Data).
RESULTS
Effect of OMIU and MGO Modifications on Chaperone Function of Human αA-Crystallin
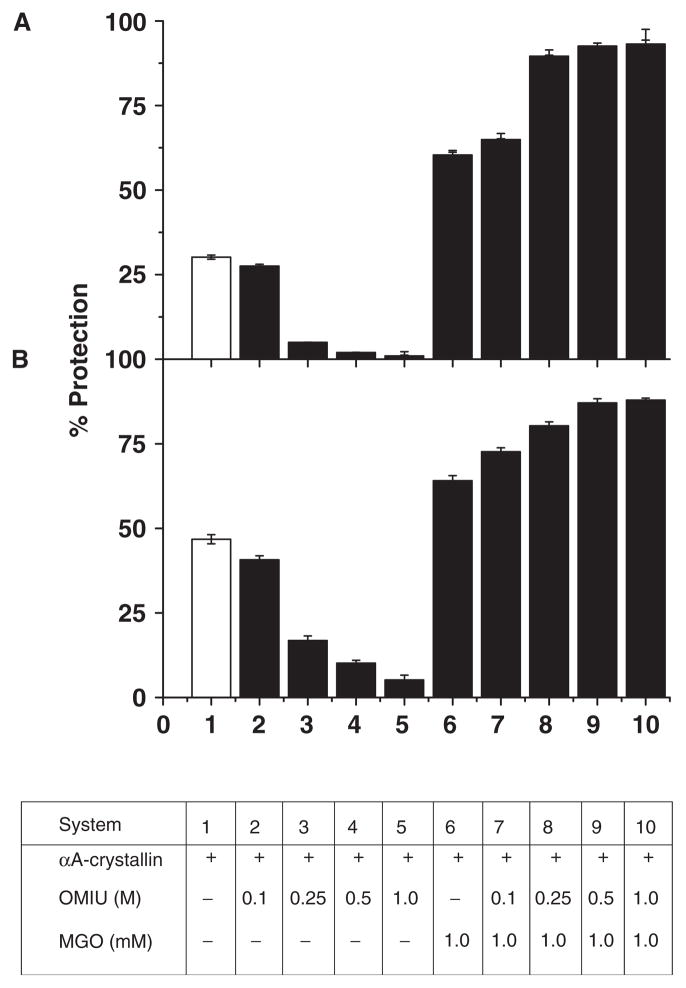
We wanted to determine how homoarginine residues, formed by the reaction of lysine residues and OMIU, and the subsequent modification of these residues by MGO influenced the chaperone function of αA-crystallin. We first determined the capacity of unmodified and modified αA-crystallins to prevent DTT-induced insulin aggregation and heat-induced citrate synthase (CS) aggregation. With a ratio of 1:4 (w/w) of unmodified αA-crystallin to insulin, we found ~30% protection against protein aggregation (Fig. 2A), but with a similar ratio of OMIU-modified (0.25 M) αA-crystallin to insulin, protection declined to ~3% (Fig. 2A). Chaperone function was completely lost when the OMIU concentration was increased to 0.5 M or 1.0 M (Fig. 2A).
Fig. 2. Chaperone function of OMIU and MGO-modified αA-crystallin.
DTT-induced aggregation of 0.32 mg/ml insulin at 25°C (A) and thermal aggregation of 0.06 mg/ml CS at 43°C (B) with and without αA-crystallins. Chaperone function of unmodified αA-crystallin (bar 1) and αA-crystallin incubated with various concentrations of OMIU: 0.1 M (bars 2 and 7), 0.25 M (bars 3 and 8), 0.5 M (bars 4 and 9), 1.0 M (bars 5 and 10) for 48 h at 4°C followed by incubation with 1.0 mM MGO (bars 7 to 10) at 37°C for 48 h. The chaperone function of αA-crystallin incubated with 1.0 mM MGO for 48 h is shown in bar 6. The chaperone:substrate ratio (w/w) was 1:4 and 1:20 for insulin and CS aggregation assays, respectively. Each bar represents the average of three assays.
Surprisingly, subsequent modification of OMIU-treated αA-crystallin with 1 mM MGO not only restored but also markedly improved its chaperone function (Fig. 2A). At a chaperone-to-substrate (insulin) ratio of 1:4, as above, MGO-modified αA-crystallin (no OMIU treatment) provided ~60% protection against insulin-induced aggregation (Fig. 2A). More importantly, MGO strikingly enhanced the chaperone function of OMIU-treated αA-crystallin. This phenomenon was obvious at all concentrations of OMIU (0.25 to 1 M), with an increase of ~90% or more beyond that of the OMIU-modified proteins alone (Fig. 2A).
Assays with CS as the substrate gave similar results. At an αA-crystallin:CS (w/w) ratio of 1:20, protection against CS-induced aggregation was 47%. Protection declined if OMIU-modified αA-crystallin was used; the extent of reduction depended on the concentration of OMIU used to modify the protein (Fig. 2B). As in the insulin assay, further modification of OMIU-treated αA-crystallin with MGO not only salvaged the chaperone function, but also markedly improved it. The ability to protect insulin and CS increased ~2-fold in both 0.5 M OMIU + 1.0 mM MGO and 1.0 M OMIU + 1.0 mM MGO αA-crystallin when compared to the unmodified protein (Fig. 2). Data from these assays confirm that MGO modification of OMIU-treated protein not only re-institutes the chaperone function but also augments it beyond the effect of MGO alone. Treatment of BSA with OMIU and MGO as above failed to show similar effects, suggesting that the effects seen with αA-crystallin are specific (data not shown).
We also investigated the effect of OMIU treatment after MGO modification on the chaperone function of αA-crystallin. Treatment with 0.5 M or 1.0 M OMIU after modification with 1.0 mM MGO resulted nearly 70% decrease of chaperone function in the CS aggregation assay and 10–15% decrease in the insulin aggregation assay compared to the unmodified protein (data not shown). These results suggest that guanidination of lysine residues has a superseding effect over MGO modification at the concentrations of OMIU and MGO used in the present study.
Amino Acid Analysis
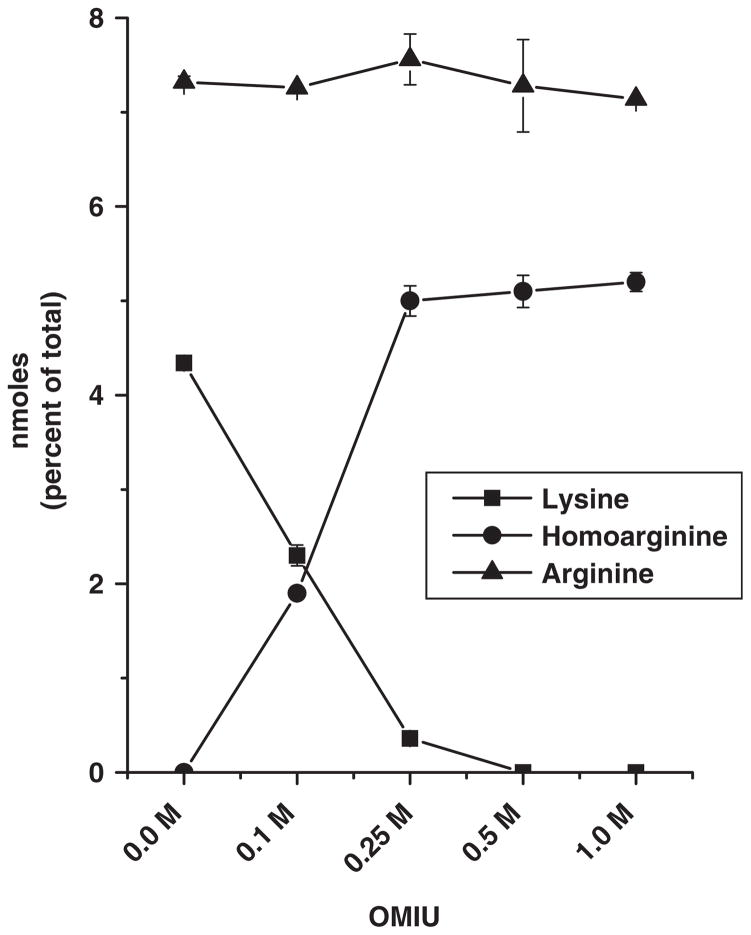
Amino acid analysis was used to determine the extent of lysine modification during reaction of αA-crystallin with OMIU. Treatment with 0.1 M OMIU converted 50% of lysine residues in αA-crystallin to homoarginine. At higher OMIU concentrations (0.5 M and 1.0 M), all the lysine residues were converted to homoarginine. At these concentrations, OMIU did not modify arginine residues (Fig. 3). In control samples (without OMIU) at pH 10.5, the amino acid content was unchanged, ruling out deamidation of asparagine as a possible effect. In light of our findings on altered chaperone function described above, it would seem that introduction of guanidino groups, not modification of lysines, underlies this change in function.
Fig. 3. Content of lysine, arginine and homoarginine in αA-crystallin.
αA-crystallin was modified by incubation with 0.1–1.0 M OMIU at 4°C for 48 h. Amino acids in acid-hydrolysed samples were quantified by amino acid analysis. The assay was calibrated with two 5 nM amino acid standards. Data show mean values ± SD of triplicate measurements.
Identification of Homoargpyrimidine and Argpyrimidine by HPLC
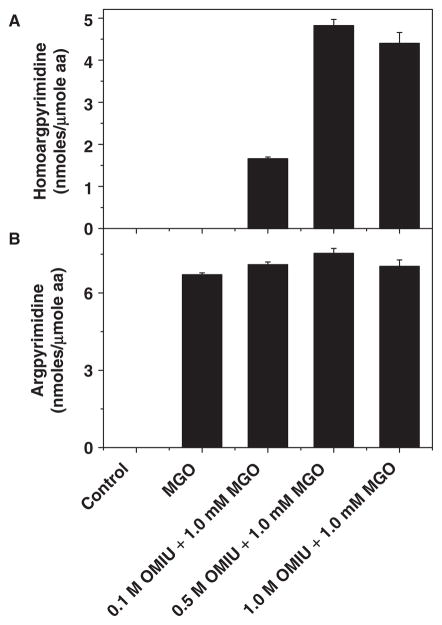
We found that the amount of homoargpyrimidine nearly tripled in 0.5 M OMIU-treated αA-crystallin (from 1.6 to 4.6 nmol/μmol amino acid) compared to the 0.1 M OMIU-treated protein (Fig. 4A). Because all lysines were modified to homoarginine by 0.5 M OMIU (Fig. 3), further increases in the OMIU concentration produced no additional homoargpyrimidine. We also measured argpyrimidine, which is produced by reaction of MGO with arginine residues in αA-crystallin. Treatment with 1.0 mM MGO produced nearly 6 nmol/μmol amino acid argpyrimidine; this effect of 1.0 mM MGO was similar across all concentrations of OMIU (Fig. 4B). These data imply that homoarginine is further converted to homoargpyrimidine by MGO treatment. Although homohydroimidazolone could be another major modification produced by MGO, we did not measure this product because of limitations imposed by our HPLC system. However, as shown below we were able to detect it by mass spectrometry.
Fig. 4. Homoargpyrimidine and argpyrimidine in modified αA-crystallin.
Homoargpyrimidine was identified only in 0.1–1.0 M OMIU modified proteins (4°C for 48 h) and argpyrimidine adduct was identified in MGO (1 mM) and OMIU + MGO modified αA-crystallin (37°C for 48 h). Protein samples (300 μg each) were hydrolysed in 6 N HCl at 110°C for 20 h and products were measured by reversed phase HPLC with an on-line fluorescence detector. Each bar represents the average of three measurements.
Homoarginine, Homoargpyrimidine and Homohydroimidazolone Identified by Mass Spectroscopy
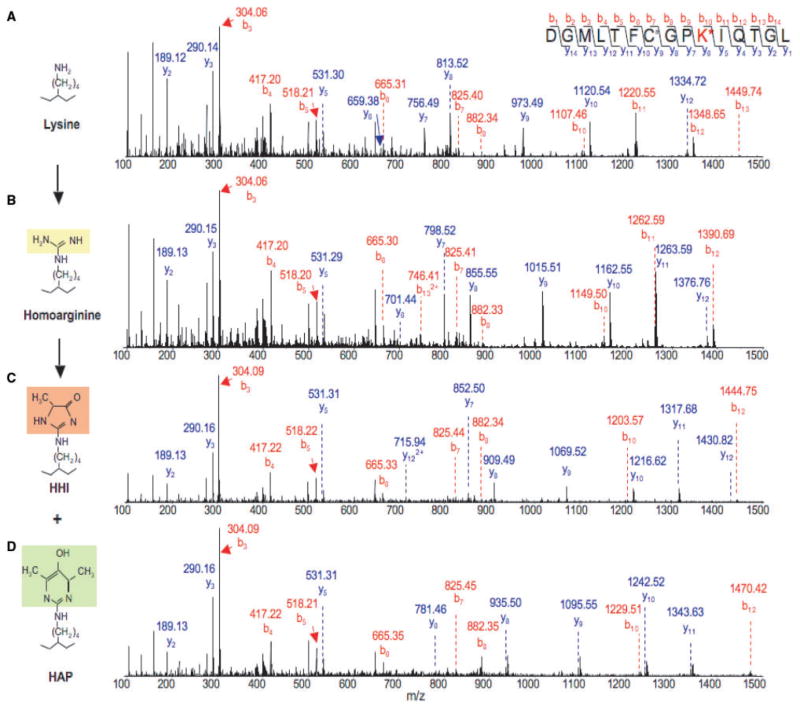
To identify homoarginine, homoargpyrimidine and homohydroimidazolone within αA-crystallin, we digested the unmodified protein as well as protein treated with 0.25 M OMIU or 0.25 M OMIU + 1.0 mM MGO with the endopeptidase, Asp-N, and analysed the resulting peptides by LC–ESI–MS/MS. Table 1 summarizes the results. Treatment with 0.25 M OMIU transformed six of the seven lysine residues to homoarginine; the seventh was not detectable under the conditions used. We noted an increase of 42 Da on the modified lysine residues. This agrees with the amino analysis data (Fig. 3), which indicated all lysine residues were modified by 0.25 M OMIU. Further mass increments of 96 Da and 122 Da accrued when MGO (1.0 mM) converted these six homoarginines to homohydroimidazolone and homoargpyrimidine, respectively (Table 1). LC-MS/MS spectra of the peptides in Table 1 confirmed the presence of these modifications. Of the modified residues detected at sites of the original six lysine residues, six were homohydroimidazolone and three were homoargpyrimidine. The peptide 136DGMLTFCGPKIQTGL150 showed both homoargpyrimidine and homohydroimidazolone adducts. Tandem mass spectrum analysis of this peptide confirmed the sites of modification. Figure 5 shows the MS/MS spectra from unmodified, OMIU-modified and OMIU-modified plus MGO-modified αA-crystallin. The results indicate that the mass increase of 42 Da corresponded to the conversion of Lys145 to homoarginine, the increase of 96 Da corresponded to the conversion of homoarginine to homohydroimidazolone and the increase of 122 Da corresponded to the conversion of homoarginine to homoargpyrimidine. Together, these data suggest that hydroimidazolones are the dominant modifications induced by MGO in αA-crystallin. Previous studies also showed higher hydroimidazolone concentrations relative to argpyrimidine in MGO-modified proteins (43). We concluded that the increased chaperone function of αA-crystallin results from formation of homohydroimidazolone and homoargpyrimidine adducts within the protein structure.
Table 1.
LC-MS/MS results of Asp-N cleaved unmodified, OMIU-modified and OMIU + MGO-modified αA-crystallin.
| No | Sequence | αA-crystallin (Da) | αA-crystallin + OMIUa (Da) | αA-crystallin + OMIU + MGOb (Da) | Modification Adducts by b |
|---|---|---|---|---|---|
| 2–23 | DVTIQHPWFKRTLGPFYPSRLF | 2704.42 (2704.43)c | 2746.47 (2746.45) | 2800.52 (2800.46) | HHI |
| 69–75 | DKFVIFL | 880.52 (880.51) | 992.54 (992.53) | 976.57(976.54) | HHI |
| 76–83 | DVKHFSPE | 1053.53 (1053.49) | HHI | ||
| 1079.55 (1079.50) | HAP | ||||
| 76–90 | DVKHFSPEDLTVKVQ | 1740.92 (1740.90) | 1782.96 (1782.93) | ||
| DVKHFSPEDLTVKVQ | 1824.99 (1824.95) | ||||
| 84–90 | DLTVKVQ | 843.49 (843.48) | 897.53 (897.49) | HHI | |
| 923.55 (923.51) | HAP | ||||
| 91–104 | DDFVEIHGKHNERQ | 1722.80(1722.81) | 1764.86 (1764.83) | 1818.92 (1818.84) | HHI |
| 136–150 | DGMLTFCGPKIQTGL | 1636.84 (1636.80) | 1678.85 (1678.82) | 1732.87 (1732.83) | HHI |
| 1758.94 (1758.84) | HAP | ||||
| 35–57 | DLLPFLSSTISPYYRQSLFRTVL | 2715.53 (2715.46) | 2795.54 (2795.49) | HAP |
HHI, homohydroimidazolone; HAP, homoargpyrimidine.
Mass increase of 42 Da.
Mass increase of 96 Da for HHI and 122 Da for HAP in samples modified with 0.25 M OMIU + 1.0 mM MGO.
Observed mass and calculated mass in parenthesis.
Fig. 5. LC-MS/MS-spectroscopy detection of MGO adducts on OMIU-modified and unmodified αA-crystallin.
LC-MS/MS spectra of 135DGMLTFCGPKIQTGL150 unmodified (A), OMIU-modified (B), OMIU + MGO-modified αA-crystallin (C and D). Cysteines were alkylated by treatment with idoacetamide. Precursor ions (doubly charged) subjected to MS/MS analyses were m/z 819.42 (A), 840.44 (B), 867.43 (C) and 880.45 (D), respectively. Highlighted portions of structure indicate the adducts that are derived from modifications.
Effect of OMIU and MGO Modification on Surface Hydrophobicity of αA-Crystallin
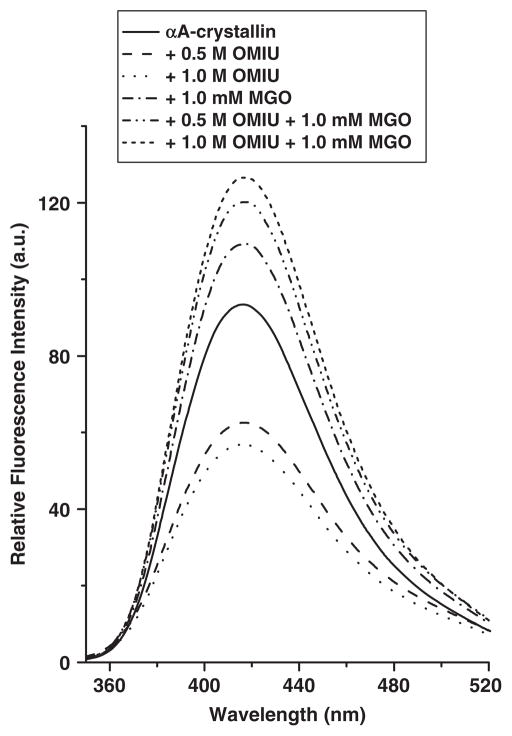
The surface hydrophobicity and chaperone function of α-crystallin are strongly correlated (12, 44–46). Earlier studies showed that structural perturbation influences exposure of hydrophobic residues and consequently, the chaperone function of α-crystallin (24, 45–47). To determine whether the formation of homoarginine, homohydroimidazolone and homoargpyrimidine accompanies altered surface hydrophobicity, we probed unmodified and modified proteins with the hydrophobic probe, 2-(p-toludino) naphthalene-6-sulphonic acid, sodium salt (TNS). The fluorescence intensity of this reagent increases when it binds to hydrophobic regions of a protein (15, 20). Figure 6 shows the intense fluorescence of TNS [emission maximum (λmax) at 432 nm] when bound to unmodified αA-crystallin. We found that neither the OMIU-modification nor OMIU + MGO modification altered the λmax. However, the fluorescence intensity of TNS bound to αA-crystallin modified by 0.5 M or 1.0 M OMIU was reduced by ~32% and ~38%, respectively, as compared to the unmodified protein. Subsequent modification of these two proteins by MGO (1.0 mM) resulted in an increase in fluorescence intensity (~30% and ~39%) above that of unmodified αA-crystallin. We also observed that modification by 1.0 mM MGO alone increased the fluorescence intensity of TNS bound to αA-crystallin by ~20% when compared to the unmodified protein. These findings suggest that formation of homo-hydroimidazolone and homoargpyrimidine enhanced the fluorescence intensity and thus the hydrophobicity of the protein. Based on these results, we conclude that the enhanced chaperone function of αA-crystallin with OMIU and MGO modifications results from an increase in surface hydrophobicity.
Fig. 6. Fluorescence spectra of TNS-bound αA-crystallin.
The protein concentration was 0.1 mg/ml; the TNS concentration was 100 μM. The excitation wavelength was 320 nm. Fluorescence spectra of different samples at 25°C were recorded from 350 to 520 nm at a 0.5 nm interval.
Effect of OMIU and MGO Modification on the Secondary and Tertiary Structure of αA-Crystallin
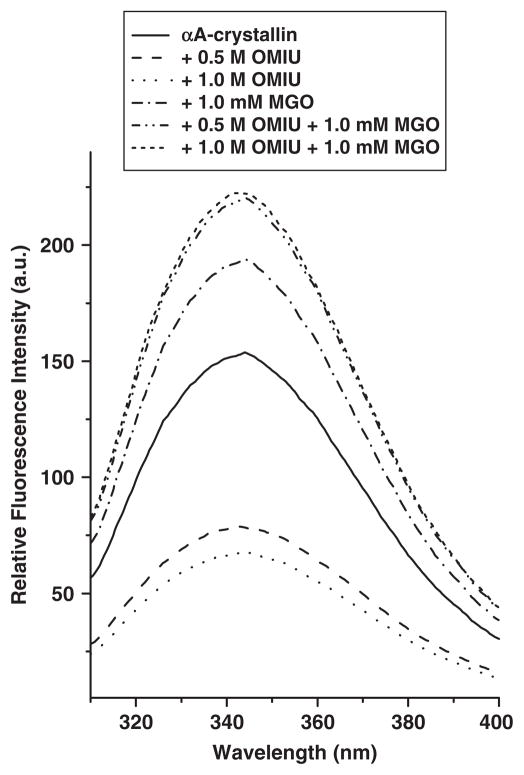
Earlier studies indicate that structural perturbation of α-crystallin leads to altered chaperone function (12, 24, 26, 27, 44, 45). To determine if structural perturbation dictated the functional changes in αA-crystallin, we utilized the intrinsic fluorescence (due to the presence of tryptophan residues), near- and far-UV CD spectroscopy. Intrinsic fluorescence spectra indicated significant differences between the unmodified and modified proteins (Fig. 7). The fluorescence emission maximum (λmax) of unmodified αA-crystallin was 344 nm. Although λmax did not change in either OMIU-modified or OMIU + MGO-modified proteins. However, the fluorescence intensity decreased ~1.5-fold in proteins modified with 0.5 M and 1.0 M OMIU compared to unmodified αA-crystallin. Modification with MGO increased the fluorescence intensity in all protein preparations. The increase was 28% in the protein modified with 1.0 mM MGO (without OMIU), and ~54% in OMIU + MGO modified proteins. These changes in fluorescence intensity suggest that the tryptophan micro-environment is affected by OMIU and MGO treatments.
Fig. 7. Intrinsic fluorescence spectra of OMIU and MGO-modified αA-crystallin.
Fluorescence spectra of unmodified and modified proteins (0.1 mg/ml) were recorded at 25°C from 310 to 400 nm with a LS 55 Perkin Elmer spectrofluorometer (bandwidth of 5 nm for excitation and emission modes). The excitation wavelength was set to 295 nm.
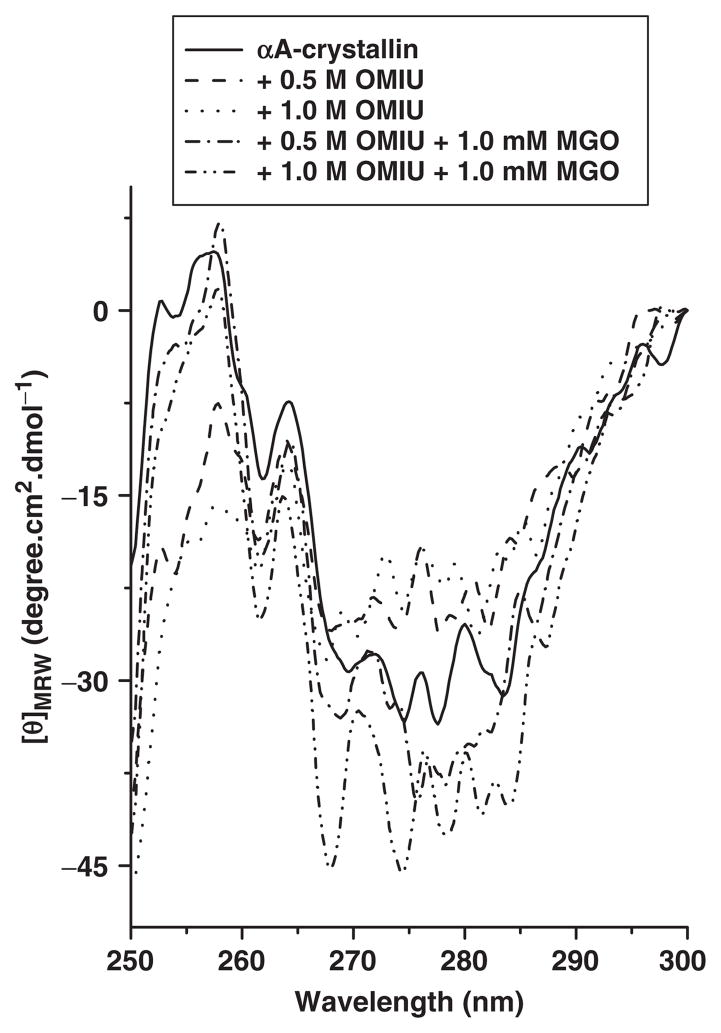
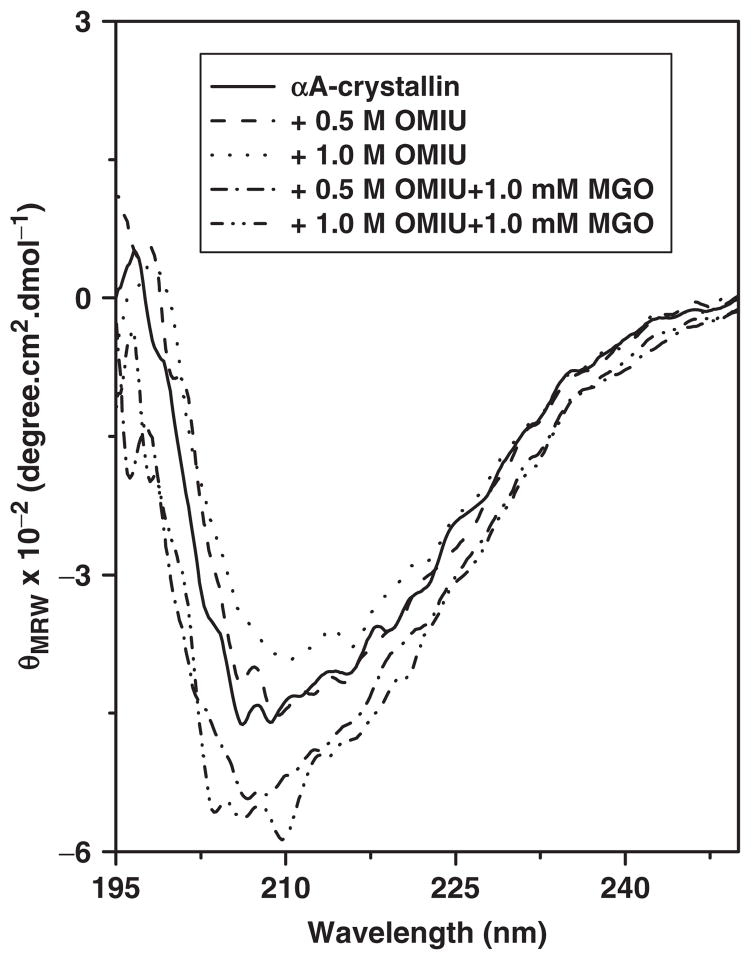
We also studied perturbation on the tertiary level with near-UV CD spectroscopy (Fig. 8). The near-UV CD spectrum profile obtained for unmodified αA-crystallin is consistent with previously published results (26, 48). The microenvironment of phenylalanine was unaffected by protein modifications introduced by treatment with OMIU or OMIU + MGO. Because differences existed between the unmodified and modified proteins in the region of 270–290 nm (Fig. 8), we assume that the tyrosine and tryptophan microenvironments were perturbed. Together, these results imply that MGO and OMIU treatments perturb the tertiary structure differently. Perturbation by MGO presumably leads to variation in hydrophobic sites on the protein surface that ultimately controls the chaperone function of αA-crystallin.
Fig. 8. Near-UV CD spectra of OMIU and MGO-modified αA-crystallin.
Spectra were collected using a Jasco J810 spectropolarimeter with a 10 mm path length CD cell. The protein concentration was 1.0 mg/ml. Each spectrum was the average of five scans.
We determined the secondary structure of unmodified, OMIU-modified and OMIU + MGO modified αA-crystallin by far-UV CD spectroscopy (Fig. 9). The CONTINLL program was used to estimate the secondary structural elements (40). We found that β-sheet content of αA-crystallin did not change by modifications (data not shown) and concluded that neither OMIU nor OMIU + MGO markedly perturbed the secondary structure.
Fig. 9. Far UV CD spectra of OMIU and MGO modified αA-crystallin.
Spectra from protein samples (0.2 mg/ml in 10 mM phosphate buffer, pH 7.2) were recorded with a 1.0 mm path length cell. Data interval was 0.2 nm. Each spectrum was the average of five scans.
Effect of OMIU and MGO Modifications on the Subunit Exchange Rate of αA-Crystallin
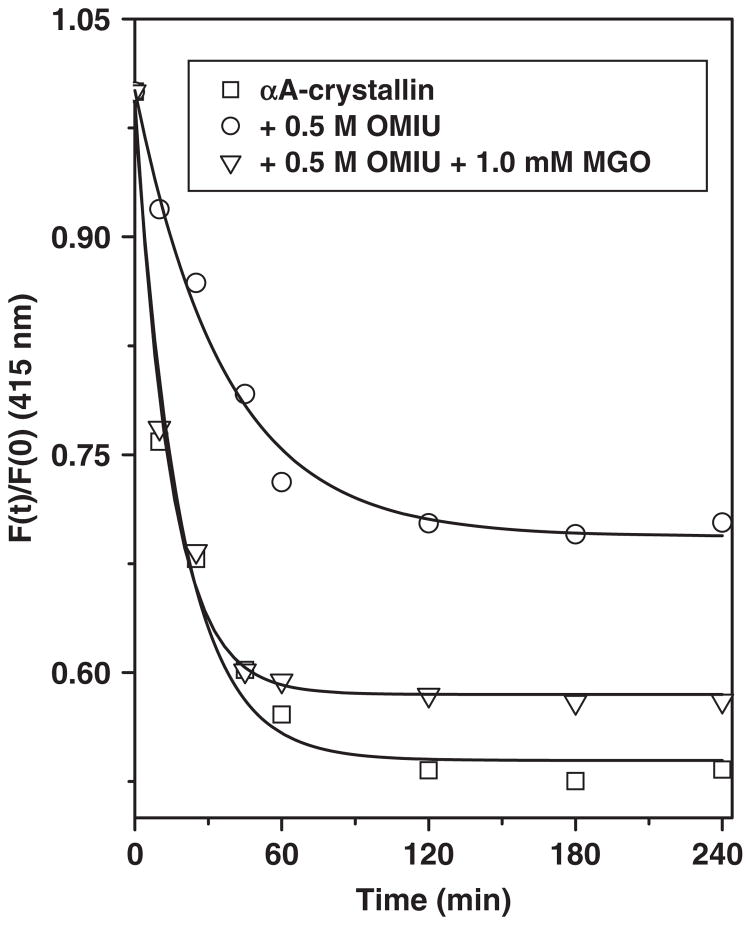
Many sHSPs, including α-crystallin, exchange subunits between their oligomers (42, 49–51). We used the fluorescence resonance energy transfer (FRET) technique to monitor the effect of OMIU and OMIU + MGO modifications on subunit exchange of human αA-crystallin. Of the two cysteine residues of human αA-crystallin, Cys131 is fully exposed whereas Cys142 is completely buried. When we compared the percentage labelling of αA-crystallin with the FRET probes, 4-acetamido-4′-((iodoacetyl)amino)-stilbene-2,2′-disulphonic acid (AIAS) and lucifer yellow iodoacetamide (LYI), we found ~1 mol of probe to be covalently bound to 1 mol of αA-crystallin subunit, suggesting covalent modification of the fully exposed Cys131 residue. When we mixed 0.4 mg/mL AIAS-labelled and 0.4 mg/ml LYI-labelled proteins at 37°C, we observed a time-dependent decrease in AIAS emission intensity at 415 nm along with a concomitant increase in LYI fluorescence intensity at 525 nm. We plotted the decrease in AIAS fluorescence intensity as a function of time (Fig. 10) to obtain the subunit exchange rate constant (k) according to the equation shown in the MATERIALS AND METHODS. The subunit rate constant for unmodified αA-crystallin was 0.061 min−1, which agrees with previously reported value (42). The k-value for the protein modified by 0.5 M OMIU was 0.032 min−1, which suggests that introduction of a guanidino group reduces the subunit exchange rate by ~48%. Subsequent MGO modification, which presumably neutralizes the guanidine groups, restored the subunit exchange rate (k = 0.063 min−1). Our findings clearly indicate that the introduction of guanidino groups and subsequent neutralization of those groups by MGO have opposing effects on the dynamics of the subunit assembly of αA-crystallin. Several studies suggest that the dynamic properties of subunit assembly in α-crystallin are important for its chaperone activity (20, 42, 52), whereas others have not found such a correlation (53–55). Notwithstanding these differences, our findings indicate a strong correlation between subunit exchange and the chaperone function of αA-crystallin.
Fig. 10. Effect of modification on subunit exchange properties of αA-crystallin.
Subunit exchange between AIAS-labelled and LYI-labelled αA-crystallin was measured at 37°C. An equal amount (0.4 mg/ml) of AIAS-labelled and LYI-labelled αA-crystallin was mixed together at 37°C and decrease in relative fluorescence intensity at 415 nm was determined. The subunit exchange rate was calculated using the equation F(t)/F(0) = D1 + D2e−kt. The subunit exchange rate constant for unmodified, OMIU and OMIU + MGO modified αA-crystallin was 0.061 min−1, 0.032 min−1 and 0.063 min−1, respectively.
DISCUSSION
The purpose of this study was to investigate how the additionally introduced guanidino groups and their reaction with MGO modulate the chaperone function of αA-crystallin. We previously found that modification of discrete arginine residues in αA-crystallin by MGO improved its chaperone ability and that replacement of MGO-modifiable arginine residues by site-directed mutagenesis with neutral alanine duplicated the effects of MGO (26, 34). These studies led to our hypothesis that the positive charge on the guanidino groups of selected arginine residues controls the chaperone function of αA-crystallin and their removal, either by reaction with MGO or replacement by site-directed mutagenesis, makes αA-crystallin a better chaperone.
Indeed, we found that conversion of lysine residues to homoarginine residues by treatment with OMIU causes αA-crystallin to lose chaperone function, which suggests that introduction of additional guanidino groups is detrimental to the chaperone function. Additionally, this treatment reduced the subunit exchange rate and surface hydrophobicity of the protein. The altered function is not likely due to the removal of positive charge on lysine because previous studies show either no or only partial loss of chaperone function due to chemical modification of lysine residues in α-crystallin (34, 56, 57). Taken together, these observations indicate that introduction of guanidino groups rather than modification of lysine residues causes the decrease in chaperone function.
What we found most remarkable was that treatment with MGO restored the chaperone function of OMIU-treated αA-crystallin. In fact, the gain in chaperone function was consistently greater than that which could be achieved by treating αA-crystallin directly with MGO (without prior OMIU treatment). This strongly suggests that conversion of homoarginine to homohydroimidazolone and homoargpyrimidine at positions 11, 70, 78, 88, 99 and 145 makes αA-crystallin a stronger chaperone.
Alpha-crystallin has been shown to assist in re-folding of several denatured enzymes in vitro (44, 58, 59). Whether the OMIU and OMIU+MGO treatments would decrease or enhance αA-crystallin’s ability to assist in such re-folding is currently being investigated.
Based on these findings, we suggest that conversion of lysine to homoarginine reduces the chaperone function and that neutralization of a guanidino group on homoarginine residues after MGO treatment enhances the chaperone function of αA-crystallin. Most lysine residues in αA-crystallin are exposed on the protein surface, so they are accessible to aqueous solvents. Conversion of lysines to more polar homoarginines might result in subtle structural perturbation leading to burial of the hydrophobic pockets responsible for the chaperone function, eventually leading to decreased chaperone function. Establishment of van der Waal’s contacts between homoarginines and other amino acids could also restrict substrate binding. Yet another possibility is that protonated guanidino groups on homoarginines could form salt bridges with amino acids, such as glutamic acid and aspartic acid, through guanidinium-carboxylate ion pairs. Whatever the underlying mechanism, decreased accessibility of the substrate-binding hydrophobic regions for target proteins seems to be involved in reducing chaperone function. Loss of chaperone function when α-crystallin is chemically cross-linked also has been reported (57), supporting our notion that decreased flexibility, and consequently a decrease in substrate-binding sites, reduces the chaperone function of OMIU-modified αA-crystallin. That the TNS-binding studies showing reduced surface hydrophobicity reinforces this view. Reaction of the homoarginine guanidino group with MGO might collapse existing salt bridges to expose substrate-binding hydrophobic sites. The end result would be enhanced chaperon function. Our finding of a substantial increase in TNS binding after MGO treatment indicates this possibility.
Our results support the hypothesis that the guanidino group on selected arginine residues in αA-crystallin controls the chaperone function of the protein. If the arginines are chemically modified or removed by site-directed mutagenesis, the protein gains chaperone function. It is debatable whether such a gain of function is beneficial for the lens. The α-crystallin–target protein association in the lens would likely be irreversible because there are no other chaperones to aid in the dissociation of the α-crystallin–substrate complex. A recent study by Koteiche and Mchaourab (60) showed that during chaperoning of proteins, α-crystallin induced unfolding of nascent proteins, causing them to aggregate as well. Thus, even though modification of selected arginine residues by MGO with a gain in chaperone function might seem beneficial, it could prove detrimental to the lens because of the enhanced irreversible α-crystallin–substrate complex formation that could lead to greater light scattering.
Supplementary Material
Acknowledgments
This study was supported from National Institutes of Health (NIH) grants R01EY-016219 and R01EY-09912 (RHN), P30EY-11373 (Visual Sciences Research Center of CWRU), Research to Prevent Blindness (RPB), NY, Ohio Lions Eye Research Foundation and Carl F. Asseff, M.D. Professorship to R.H.N. The authors thank Michael Zagorski and Krzysztof Palczewski of Case Western Reserve University, Cleveland, OH for use of CD spectropolarimeter and the fluorescence spectrofluorometer.
Abbreviations
- sHsps
small heat shock proteins
- DTT
dithiothreitol
- CS
citrate synthase
- OMIU
o-methylisourea
- MGO
methylglyoxal
- TNS
2-(p-toludino) naphthalene-6-sulphonic acid, sodium salt
- FRET
fluorescence resonance energy transfer
- LC-ESI-MS
liquid chromatography-electrospray ionization mass spectrometry
- AIAS
4-acetamido-4′-((iodoacetyl)amino)-stilbene-2,2′-disulophonic acid
- LYI
lucifer yellow iodoacetamide
References
- 1.Spector A, Li LK, Augusteyn RC, Schneider A, Freund T. Crystallin. The isolation and characterization of distinct macromolecular fractions. Biochem J. 1971;124:337–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin–small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 3.de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 4.Caspers GJ, Leunissen JA, de Jong WW. The expanding small heat-shock protein family, and structure predictions of the conserved “alpha-crystallin domain”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- 5.Smulders R, Carver JA, Lindner RA, van Boekel MA, Bloemendal H, de Jong WW. Immobilization of the C-terminal extension of bovine alphaA-crystallin reduces chaperone-like activity. J Biol Chem. 1996;271:29060–29066. doi: 10.1074/jbc.271.46.29060. [DOI] [PubMed] [Google Scholar]
- 6.Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RJ. The molecular chaperone concept. Semin Cell Biol. 1990;1:1–9. [PubMed] [Google Scholar]
- 8.Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaenicke R, Creighton TE. Protein folding: junior chaperones. Curr Biol. 1993;3:234–235. doi: 10.1016/0960-9822(93)90342-l. [DOI] [PubMed] [Google Scholar]
- 11.Merck KB, Groenen PJ, Voorter CE, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- 12.Reddy GB, Das KP, Petrash JM, Surewicz WK. Temperature-dependent chaperone activity and structural properties of human alphaA- and alphaB-crystallins. J Biol Chem. 2000;275:4565–4570. doi: 10.1074/jbc.275.7.4565. [DOI] [PubMed] [Google Scholar]
- 13.Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279:3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 14.Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, Wood DE, Sahagan BG. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging. 2003;24:397–413. doi: 10.1016/s0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 15.Bera S, Thampi P, Cho WJ, Abraham EC. A positive charge preservation at position 116 of alpha A-crystallin is critical for its structural and functional integrity. Biochemistry. 2002;41:12421–12426. doi: 10.1021/bi0204140. [DOI] [PubMed] [Google Scholar]
- 16.Cobb BA, Petrash JM. Structural and functional changes in the alpha A-crystallin R116C mutant in hereditary cataracts. Biochemistry. 2000;39:15791–15798. doi: 10.1021/bi001453j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47:1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii N, Hiroki K, Matsumoto S, Masuda K, Inoue M, Tanaka Y, Awakura M, Akaboshi M. Correlation between the loss of the chaperone-like activity and the oxidation, isomerization and racemization of gamma-irradiated alpha-crystallin. Photochem Photobiol. 2001;74:477–482. doi: 10.1562/0031-8655(2001)074<0477:cbtlot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Srivastava OP. Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin. J Biol Chem. 2004;279:44258–44269. doi: 10.1074/jbc.M405648200. [DOI] [PubMed] [Google Scholar]
- 21.Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone alpha-crystallin: identification of binding sites in alpha B-crystallin. Biochem Biophys Res Commun. 1997;239:217–222. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- 22.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry. 2005;44:14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- 24.Smith JB, Liu Y, Smith DL. Identification of possible regions of chaperone activity in lens alpha-crystallin. Exp Eye Res. 1996;63:125–128. doi: 10.1006/exer.1996.0100. [DOI] [PubMed] [Google Scholar]
- 25.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–4577. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas A, Goshe J, Miller A, Santhoshkumar P, Luckey C, Bhat MB, Nagaraj RH. Paradoxical effects of substitution and deletion mutation of Arg56 on the structure and chaperone function of human alphaB-crystallin. Biochemistry. 2007;46:1117–1127. doi: 10.1021/bi061323w. [DOI] [PubMed] [Google Scholar]
- 28.Thampi P, Abraham EC. Influence of the C-terminal residues on oligomerization of alpha A-crystallin. Biochemistry. 2003;42:11857–11863. doi: 10.1021/bi030129w. [DOI] [PubMed] [Google Scholar]
- 29.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CD, Kymes S, Petrash JM. A transgenic mouse model for human autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2006;47:2036–2044. doi: 10.1167/iovs.05-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 32.Pasta SY, Raman B, Ramakrishna T, Rao Ch M. Role of the conserved SRLFDQFFG region of alpha-crystallin, a small heat shock protein. Effect on oligomeric size, subunit exchange, and chaperone-like activity. J Biol Chem. 2003;278:51159–51166. doi: 10.1074/jbc.M307523200. [DOI] [PubMed] [Google Scholar]
- 33.Sharma KK, Santhoshkumar P. Deletion of residues 54–61(FLRAPSWF) in alpha B-crystallin leads to decreased oligomeric mass with increased chaperone-like activity. Invest Ophthalmol Vis Sci. 2005;46:E. Abstract 3486. [Google Scholar]
- 34.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 35.Engler DA, Campion SR, Hauser MR, Cook JS, Niyogi SK. Critical functional requirement for the guanidinium group of the arginine 41 side chain of human epidermal growth factor as revealed by mutagenic inactivation and chemical reactivation. J Biol Chem. 1992;267:2274–2281. [PubMed] [Google Scholar]
- 36.Bhattacharyya J, Das KP. Alpha-crystallin does not require temperature activation for its chaperone-like activity. Biochem Mol Biol Int. 1998;46:249–258. doi: 10.1080/15216549800203762. [DOI] [PubMed] [Google Scholar]
- 37.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J Biol Chem. 2001;276:47094–47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao KC, Palamalai V, Dunlevy JR, Miyagi M. Peptidyl-Lys metalloendopeptidase-catalyzed 18O labeling for comparative proteomics: application to cytokine/lipolysaccharide-treated human retinal pigment epithelium cell line. Mol Cell Proteomics. 2005;4:1550–1557. doi: 10.1074/mcp.M500150-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Provencher SW, Glockner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 41.Biswas A, Das KP. Differential recognition of natural and nonnatural substrate by molecular chaperone alpha-crystallin-A subunit exchange study. Biopolymers. 2007;85:189–197. doi: 10.1002/bip.20630. [DOI] [PubMed] [Google Scholar]
- 42.Bova MP, Ding LL, Horwitz J, Fung BK. Subunit exchange of alphaA-crystallin. J Biol Chem. 1997;272:29511–29517. doi: 10.1074/jbc.272.47.29511. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280:5724–5732. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- 44.Biswas A, Das KP. Role of ATP on the interaction of alpha-crystallin with its substrates and its implications for the molecular chaperone function. J Biol Chem. 2004;279:42648–42657. doi: 10.1074/jbc.M404444200. [DOI] [PubMed] [Google Scholar]
- 45.Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 46.Raman B, Ramakrishna T, Rao CM. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995;365:133–136. doi: 10.1016/0014-5793(95)00440-k. [DOI] [PubMed] [Google Scholar]
- 47.Rao CM, Raman B, Ramakrishna T, Rajaraman K, Ghosh D, Datta S, Trivedi VD, Sukhaswami MB. Structural perturbation of alpha-crystallin and its chaperone-like activity. Int J Biol Macromol. 1998;22:271–281. doi: 10.1016/s0141-8130(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 48.Sun TX, Das BK, Liang JJ. Conformational and functional differences between recombinant human lens alphaA- and alphaB-crystallin. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 49.Sun TX, Akhtar NJ, Liang JJ. Subunit exchange of lens alpha-crystallin: a fluorescence energy transfer study with the fluorescent labeled alphaA-crystallin mutant W9F as a probe. FEBS Lett. 1998;430:401–404. doi: 10.1016/s0014-5793(98)00707-8. [DOI] [PubMed] [Google Scholar]
- 50.van den Oetelaar PJ, van Someren PF, Thomson JA, Siezen RJ, Hoenders HJ. A dynamic quaternary structure of bovine alpha-crystallin as indicated from intermolecular exchange of subunits. Biochemistry. 1990;29:3488–3493. doi: 10.1021/bi00466a010. [DOI] [PubMed] [Google Scholar]
- 51.Bova MP, Huang Q, Ding L, Horwitz J. Subunit exchange, conformational stability, and chaperone-like function of the small heat shock protein 16.5 from Methanococcus jannaschii. J Biol Chem. 2002;277:38468–38475. doi: 10.1074/jbc.M205594200. [DOI] [PubMed] [Google Scholar]
- 52.Srinivas V, Raman B, Rao KS, Ramakrishna T, Rao Ch M. Arginine hydrochloride enhances the dynamics of subunit assembly and the chaperone-like activity of alpha-crystallin. Mol Vis. 2005;11:249–255. [PubMed] [Google Scholar]
- 53.Sreelakshmi Y, Santhoshkumar P, Bhattacharyya J, Sharma KK. AlphaA-crystallin interacting regions in the small heat shock protein, alphaB-crystallin. Biochemistry. 2004;43:15785–15795. doi: 10.1021/bi048151s. [DOI] [PubMed] [Google Scholar]
- 54.Bera S, Abraham EC. The alphaA-crystallin R116C mutant has a higher affinity for forming heteroaggregates with alphaB-crystallin. Biochemistry. 2002;41:297–305. doi: 10.1021/bi011010v. [DOI] [PubMed] [Google Scholar]
- 55.Sreelakshmi Y, Sharma KK. Recognition sequence 2 (residues 60–71) plays a role in oligomerization and exchange dynamics of alphaB-crystallin. Biochemistry. 2005;44:12245–12252. doi: 10.1021/bi051005h. [DOI] [PubMed] [Google Scholar]
- 56.Horwitz J, Huang Q, Ding L. The native oligomeric organization of alpha-crystallin, is it necessary for its chaperone function? Exp Eye Res. 2004;79:817–821. doi: 10.1016/j.exer.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Sharma KK, Ortwerth BJ. Effect of cross-linking on the chaperone-like function of alpha crystallin. Exp Eye Res. 1995;61:413–421. doi: 10.1016/s0014-4835(05)80136-8. [DOI] [PubMed] [Google Scholar]
- 58.Biswas A, Das KP. Alpha-crystallin assisted refolding of enzyme substrates: optimization of external parameters. Protein J. 2007;26:247–255. doi: 10.1007/s10930-006-9066-8. [DOI] [PubMed] [Google Scholar]
- 59.Muchowski PJ, Clark JI. ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin. Proc Natl Acad Sci USA. 1998;95:1004–1009. doi: 10.1073/pnas.95.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koteiche HA, McHaourab HS. Mechanism of a hereditary cataract phenotype. Mutations in alphaA-crystallin activate substrate binding. J Biol Chem. 2006;281:14273–14279. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.