Abstract
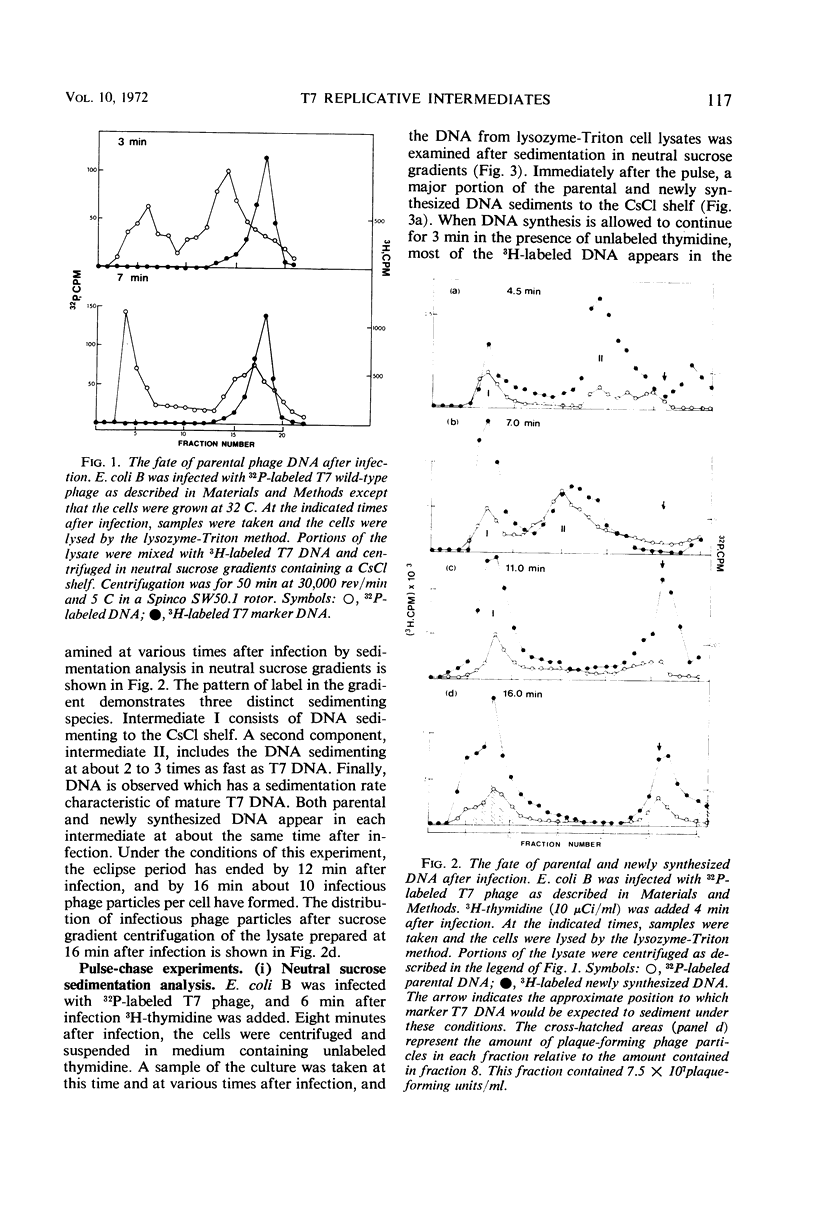
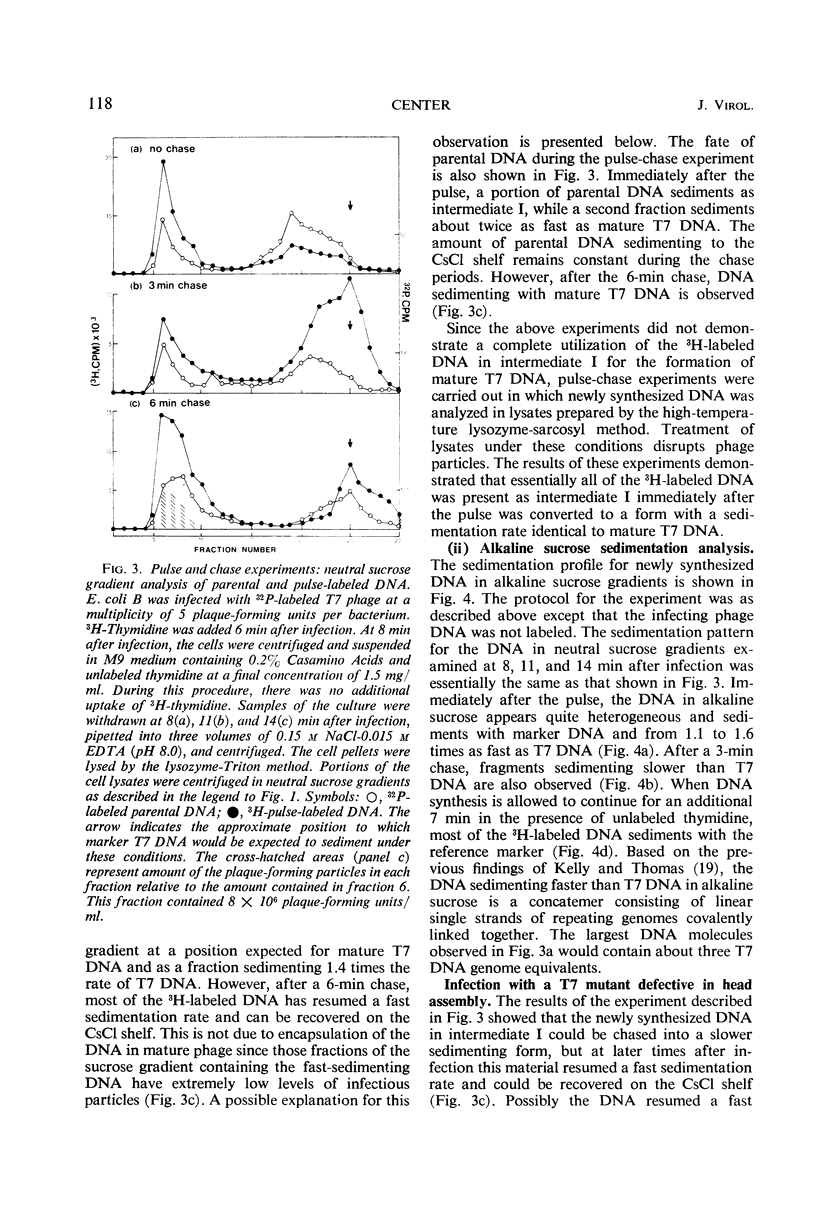
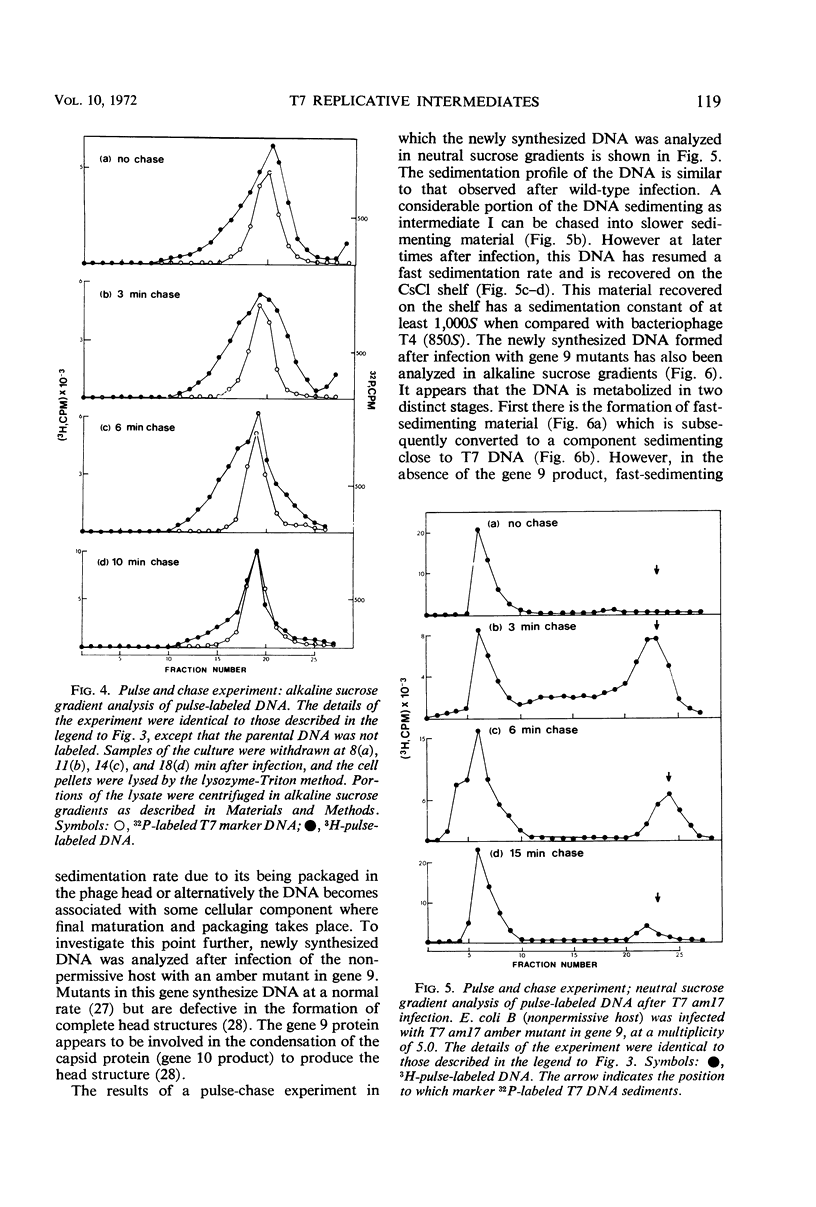
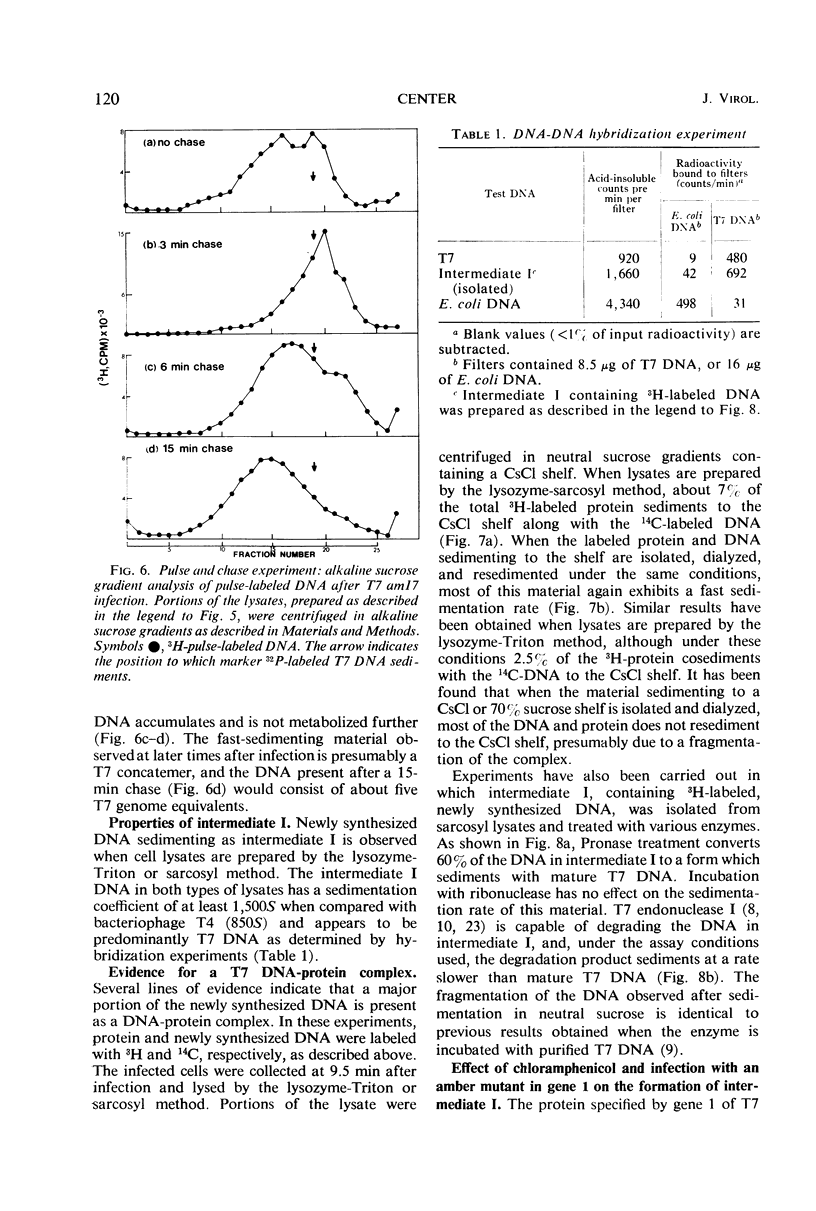
After infection with bacteriophage T7, parental and newly synthesized deoxyribonucleic acid (DNA) exhibit an extremely fast sedimentation rate in neutral sucrose gradients. This fast-sedimenting component (intermediate I) has a sedimentation constant of about 1,500S and contains T7 DNA as determined by DNA-DNA hybridization experiments. Pulse-chase experiments indicate that the fast-sedimenting material is metabolically active and serves as a precursor to the formation of T7 DNA. Intermediate I contains about 2.5 to 7% of the total 3H-labeled protein formed between 3 and 9.5 min after T7 infection. Treatment of intermediate I with Pronase results in the release of the DNA from the complex. At early times after infection, a second intermediate (intermediate II) can be detected which contains both parental and newly synthesized DNA sedimenting slower than intermediate I but 2 to 3 times as fast as mature T7 DNA. Intermediates I and II containing parental DNA are formed after infection of the nonpermissive host with an amber mutant in gene 1, a gene whose expression is necessary for the synthesis of most T7 proteins. The two intermediates are also observed when infection with T7 wild type is carried out in the presence of chloramphenicol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Lyn G., Gefter M., Hurwitz J. The enzymatic repair of DNA, II. Characterization of phage-induced sealase. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1996–2003. doi: 10.1073/pnas.58.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Burton A. J. Intracellular development of bacteriophage phi-R. II. Fractionation of replicative form deoxyribonucleic acid associated with rapidly sedimenting host cell components. J Virol. 1970 Oct;6(4):455–462. doi: 10.1128/jvi.6.4.455-462.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S. Bacteriophage T7-induced endonuclease II. Purification and properties of the enzyme. J Biol Chem. 1972 Jan 10;247(1):146–156. [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1970 Dec 10;245(23):6285–6291. [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. II. Specificity of the enzyme toward single- and double-stranded deoxyribonucleic acid. J Biol Chem. 1970 Dec 10;245(23):6292–6299. [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Hallick L., Boyce R. P., Echols H. Membrane association by bacteriophage lambda-DNA: possible direct role of regulator gene N. Nature. 1969 Sep 20;223(5212):1239–1242. doi: 10.1038/2231239a0. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., LaRue K. Variations in sedimentation patterns among deoxyribonucleic acids synthesized after infection of Escherichia coli by different amber mutants of bacteriophage T7. J Virol. 1969 Feb;3(2):278–281. doi: 10.1128/jvi.3.2.278-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Masamune Y., Frenkel G. D., Richardson C. C. A mutant of bacteriophage T7 deficient in polynucleotide ligase. J Biol Chem. 1971 Nov 25;246(22):6874–6879. [PubMed] [Google Scholar]
- Miller R. C., Jr, Kozinski A. W. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. V. Further studies on the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):490–501. doi: 10.1128/jvi.5.4.490-501.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D. Bacteriophage T7 endonuclease. I. Properties of the enzyme purified from T7 phage-infected Escherichia coli B. J Biol Chem. 1971 Jan 10;246(1):209–216. [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salivar W. O., Gardinier J. Replication of bacteriophage lambda DNA associated with the host cell membrane. Virology. 1970 May;41(1):38–51. doi: 10.1016/0042-6822(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Hausmann R. Integration of two sets of T7 mutants. Virology. 1969 Nov;39(3):587–588. doi: 10.1016/0042-6822(69)90106-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]


