Abstract
Rationale
Signal initiation by the HDL receptor scavenger receptor class B, type I (SR-BI), which is important to actions of HDL on endothelium and other processes, requires cholesterol efflux and the C-terminal transmembrane domain (CTTM). The CTTM uniquely interacts with plasma membrane (PM) cholesterol.
Objective
The molecular basis and functional significance of SR-BI interaction with plasma membrane cholesterol are unknown. We tested the hypotheses that the interaction is required for SR-BI signaling, and that it enables SR-BI to serve as a plasma membrane cholesterol sensor.
Methods and Results
In studies performed in COS-M6 cells, mutation of a highly-conserved CTTM glutamine to alanine (SR-BI-Q445A) decreased PM cholesterol interaction with the receptor by 71% without altering HDL binding or cholesterol uptake or efflux, and it yielded a receptor incapable of HDL-induced signaling. Signaling prompted by cholesterol efflux to methyl-β-cyclodextrin (CD) was also prevented, indicating that PM cholesterol interaction with the receptor enables it to serve as a PM cholesterol sensor. Using SR-BI-Q445A, we further demonstrated that PM cholesterol sensing by SR-BI does not influence SR-BI-mediated reverse cholesterol transport to the liver in mice. However, the PM cholesterol sensing does underlie apolipoprotein B intracellular trafficking in response to postprandial micelles or CD in cultured enterocytes, and it is required for HDL activation of eNOS and migration in cultured endothelial cells and HDL-induced angiogenesis in vivo.
Conclusion
Through interaction with plasma membrane cholesterol, SR-BI serves a PM cholesterol sensor, and the resulting intracellular signaling governs processes in both enterocytes and endothelial cells.
Keywords: Endothelium, enterocyte, nitric oxide synthase, reverse cholesterol transport, scavenger receptor BI
INTRODUCTION
Scavenger receptor class B, type I (SR-BI) is a high affinity receptor for high density lipoprotein cholesterol (HDL), and it also binds low density lipoprotein cholesterol (LDL), very low density lipoprotein cholesterol (VLDL), and phospholipids1. The classical function of SR-BI is to mediate the selective uptake of HDL cholesterol by cells, primarily in the form of cholesteryl esters (CE). SR-BI also mediates the bidirectional flux of unesterified cholesterol and phospholipids between lipoproteins and cell plasma membranes1. The binding of HDL to hepatic SR-BI and the selective uptake of cholesterol that ensues underlies the delivery of extrahepatic cholesterol to the liver in the process known as reverse cholesterol transport (RCT)2. SR-BI and the related receptor CD36 share a hairpin-like membrane topology, with the mid-portion of the protein that resides extracellularly anchored to the plasma membrane by two transmembrane domains adjacent to short N- and C-terminal cytoplasmic domains1, 3.
Along with its classical function of mediating cholesterol and phospholipid movement between its ligands and cells, SR-BI initiates signaling in certain cell types. In endothelial cells the binding of HDL to SR-BI activates endothelial NO synthase (eNOS)4. eNOS activation by HDL attenuates monocyte-endothelial cell adhesion, thereby playing a major role in the anti-inflammatory capacity of the lipoprotein5. HDL stimulation of eNOS entails sequential activation of Src kinase(s), PI3 kinase, Akt kinase and Erk1/2 MAPK, with Akt phosphorylation of Ser1179 of eNOS causing enzyme activation6. The HDL/SR-BI tandem also stimulates endothelial cell migration7. In endothelium HDL/SR-BI signaling requires the adapter protein PDZK1, which directly binds to the C-terminus of SR-BI and couples it to Src kinase(s)8. Signaling by SR-BI also occurs in enterocytes in response to apical exposure to postprandial micelles (PPM), which are involved in the delivery of dietary lipids as triglyceride-rich lipoproteins (TRL). PPM cause SR-BI-dependent activation of Erk1/2 and p38MAPK that leads to the trafficking of apolipoprotein B from the apical region of the cell to basolateral secretory domains where it participates in TRL assembly and secretion9.
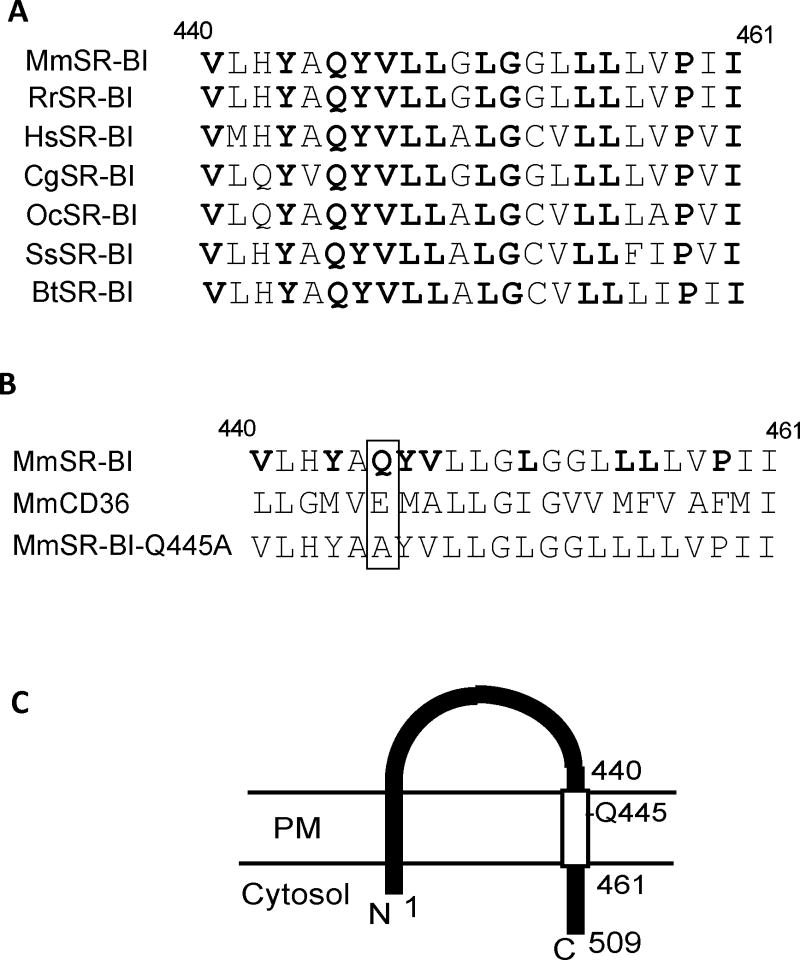
There are three primary characteristics of SR-BI required for signal initiation by the receptor: (1) its ability to invoke cholesterol flux, (2) its C-terminal cytoplasmic domain that binds PDZK1, and (3) its C-terminal transmembrane domain (CTTM), which uniquely interacts with plasma membrane cholesterol10. The molecular basis and functional significance of SR-BI interaction with plasma membrane cholesterol are unknown. In the present investigation, we tested the hypotheses that the interaction is required for SR-BI signaling, and that it enables SR-BI to serve as a plasma membrane cholesterol sensor. The SR-BI CTTM that binds plasma membrane cholesterol lacks sequence homology with known cholesterol binding domains such as those within the sterol sensing proteins HMG-CoA reductase, SREBP cleavage–activating protein (SCAP) and Niemann-Pick C1 (NPC1)11–15. However, in select cholesterol binding proteins such as NPC1, glutamine (Q) forms hydrogen bonds with the 3β-hydroxyl group of cholesterol to mediate direct binding14, 15, and within the SR-BI CTTM there is a highly conserved glutamine that is lacking in the CTTM of CD36, which does not interact with plasma membrane cholesterol 10 (Fig. 1). We therefore tested the primary hypotheses and their implications by studying a point mutant of SR-BI in which the glutamine at position 445 was substituted by alanine (SR-BI-Q445A) (Fig. 1B,C).
Figure 1. Structure of the SR-BI C-terminal transmembrane (CTTM) domain and the SR-BI-Q445A point mutant.
(A) Sequence alignment of amino acids in the CTTM domain of SR-BI homologs (residues 440-461) from mouse (Mus musculus, Mm, Q61009, Swiss-Prot), rat (Rattus norvegius, Rr, P97943, Swiss-Prot), human (Homo sapien, Hs, Q8WTUO, Swiss-Prot), Chinese hamster (Cricetulus griseus, Cg, Q60417, Swiss-Prot), rabbit (Oryctolagus cuniculus, Oc, AAP40266.1, Pubmed), pig (Sus scrofa, Ss, Q85QC1, Swiss-Prot) and bovine (Bos Taurus, Bt, O18824, Swiss-Prot). Fully conserved residues are in bold. (B) Comparison of murine SR-BI and CD36 CTTM domains. Conserved residues of SR-BI not shared with CD36 are shown in bold. The sequence of the Q445A mutant form of mouse SR-BI is also shown, and the location of the point mutation is highlighted by the box. (C) Depiction of the topography of the SR-BI protein on the plasma membrane (PM) and its N- and C-termini. The open rectangle identifies the CTTM domain (residues 440-461), with the position of Q445 indicated.
METHODS
Cell culture, transfection and mutagenesis
COS-M6, bovine aortic endothelial cells (BAEC) and Caco-2/TC7 cells were used as previously described8–10. COS-M6 cells lack endogenous SR-BI, thereby providing a model system in which forms of SR-BI can be expressed for structure-function experiments 10. Transient transfections were performed using Lipofectamine 2000 (Invitrogen)6, and unless otherwise stated, studies were performed 48h post-transfection. Although transfected receptor is abundant at greater levels than are endogenously expressed, prior studies of mechanisms of SR-BI function in endothelium using this approach yielded parallel findings for endogenous versus exogenous receptor whenever such comparisons were possible 10. To study the impact of SR-BI versus SR-BI-Q445A in Caco-2/TC7 cells, stable cell lines were created by cotransfection with the puromycin resistance gene-containing pPUR vector (BD Biosciences) and selection with 10 μg/ml puromycin (Sigma-Aldrich); experiments were performed on well-differentiated cells on semi-permeable filters 15–20d after seeding9. Relative levels of expression of SR-BI and SR-BI-Q445A were evaluated by real time-Q-PCR16, using cyclophilin transcript abundance as an internal control. mSR-BI-Q445A was generated by PCR-based site-directed mutagenesis (Stratagene) using the entire open reading frame of murine SR-BI in pcDNA 3.1 as template, and resulting sequences confirmed by DNA sequencing.
HDL preparation
Human HDL prepared by density gradient ultracentrifugation was provided by Drs. J. Goldstein and M. Brown, UT Southwestern17. The HDL was isolated from healthy volunteers after a 12h fast and the HDL subfraction (density range 1.12–1.2181 g/ml) was obtained and used in all experiments. The HDL was stored at 4° C prior to use. The concentration used refers to the total 3 protein concentration, with the vast majority of HDL protein being apoA-I.
Assessments of intracellular and global cholesterol homeostasis
Cell HDL binding, cholesterol efflux and selective uptake, and SR-BI plasma membrane cholesterol binding were evaluated using previously-described methods10, 18. In mice, plasma total cholesterol was measured enzymatically and lipoprotein analysis was performed using fast protein liquid chromatography (FPLC) gel filtration and quantification of fraction cholesterol content19.
Protein abundance and interaction
Cell fractionation to assess SR-BI subcellular localization, immunoblot analyses including for the evaluation of eNOS, c-Src, and p38MAPK phosphorylation, and co-immunoprecipitations to evaluate interaction between SR-BI and HA-tagged PDZK1 (PDZK1HA) or c-Src were done as described6, 9, 20, 21.
eNOS activation and cell migration assays
Radiolabelled arginine-to-citrulline conversion by intact cells and scratch assays were done as previously reported7.
Adenovirus generation and administration
Recombinant adenoviruses were constructed by in vitro cre/loxP-mediated recombination, propagated in 911 cells, purified, and particles/ml were quantified, and the adenoviruses were administered to mice by intravenous injection as outlined previously22–25. These and all other in vivo procedures were approved by the Institutional Animal Care and Use Committees.
Macrophage reverse cholesterol transport (RCT)
In vivo measurement of macrophage reverse cholesterol transport was conducted in mice using 3H-cholesterol labeled foam cells as previously reported26.
Immunofluorescence and confocal microscopy
Immunofluorescence and confocal analyses in enterocytes (Caco-2/TC7 cells) were performed as described9, 27. The postprandial micelles (PPM) used to invoke responses in the enterocytes were lipid micelles with a composition resembling that of human postprandial duodenal micelles. They were prepared as previously described9.
In vivo angiogenesis assays
The impact of SR-BI versus SR-BI-Q445A on HDL-induced angiogenesis was evaluated in vivo in mice using subcutaneously-placed 20 ul silicone cylinders containing matrix and fibroblast growth factor-228, 29.
Additional methods are provided in the Online Supplement.
RESULTS
SR-BI-Q445A and cellular cholesterol homeostasis
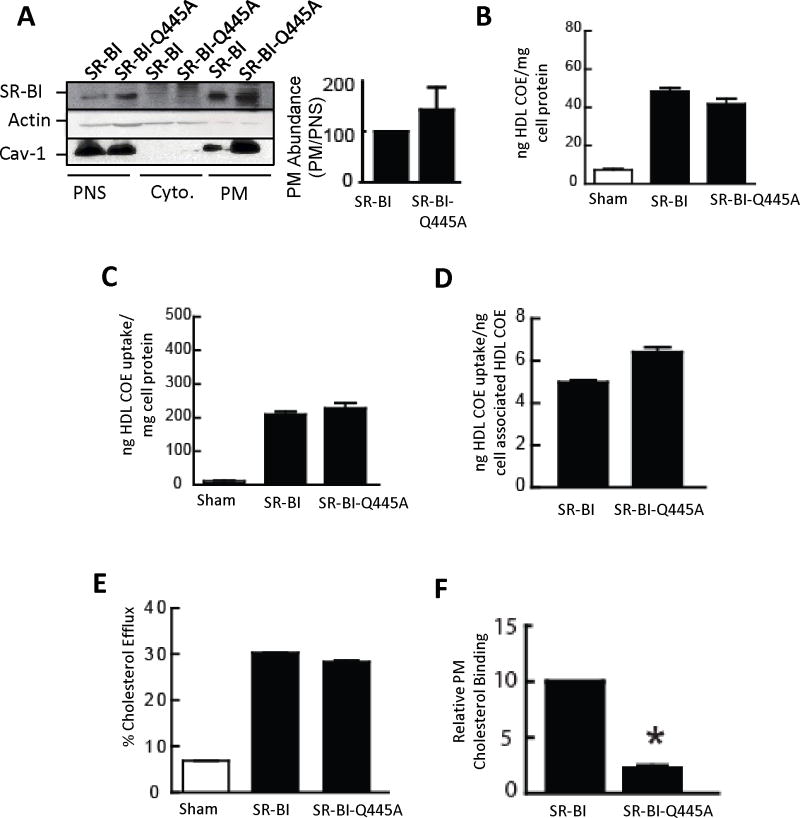
The plasma membrane targeting of WT SR-BI and SR-BI-Q445A, which is necessary for the receptor to mediate cholesterol and phospholipid flux, were compared in transfected COS-M6 cells by subfractionation and immunoblot analysis (Fig. 2A). Quantification of receptor abundance in plasma membrane relative to postnuclear supernatant revealed that SR-BI-Q445A localization to plasma membrane was similar to that of WT receptor.
Figure 2. SR-BI-Q445A targets normally to plasma membrane and mediates normal HDL binding and cholesterol uptake and efflux, but has attenuated interaction with plasma membrane cholesterol.
(A) COS-M6 cells were transfected with WT SR-BI or SR-BI-Q445A, postnuclear supernatant (PNS), cytoplasm (Cyto) and plasma membranes (PM) were isolated, and SR-BI localization was evaluated by immunoblotting using actin (cytoplasmic marker) and caveolin-1 (PM marker) Ab. The graph depicts PM abundance relative to abundance in the PNS, normalized to values for WT SR-BI. (B-D) COS-M6 cells transfected with sham plasmid or cDNA encoding WT SR-BI or SR-BI-Q445A were incubated at 37°C for 1.5 h with [125I]Dilactitol tyramine-[3H]-cholesterol oleolyl ether (COE)-HDL (10 μg HDL protein/ml), and processed to determine cell associated HDL COE (B), selective HDL COE uptake (C) and selective uptake efficiency (D). (E) To evaluate cholesterol efflux, transfected COS-M6 cells were loaded with 3H-cholesterol for 24h, washed, and HDL was added as a cholesterol acceptor for 4h. (F) Plasma membrane cholesterol binding by SR-BI was evaluated in similarly-transfected COS-M6 cells using photoactivatable 3H-cholesterol. Results are expressed relative to PM cholesterol binding to WT SR-BI. In A-F, values are mean±SEM, n=3, *p<0.05 vs WT SR-BI.
The functions of WT SR-BI and SR-BI-Q445A in cellular cholesterol homeostasis were tested in transfected COS-M6 cells. WT SR-BI and SR-BI-Q445A displayed similar HDL binding (Fig. 2B), selective HDL CE uptake (Fig. 2C), selective uptake efficiency (Figure 2D), and cholesterol efflux to HDL (Fig. 2E). In contrast, compared with WT SR-BI, photocholesterol binding to SR-BI-Q445A was decreased by 71% (Fig. 2F). Thus, SR-BI-Q445A has normal targeting to plasma membrane, HDL particle binding and cholesterol efflux and selective uptake efficiency, but attenuated interaction with plasma membrane cholesterol.
SR-BI-plasma membrane cholesterol interaction and PDZK1
Direct SR-BI interaction with PDZK1 via its cytoplasmic C-terminal tail is required for SR-BI-initiated signal transduction10, 30. To determine if SR-BI interaction with plasma membrane cholesterol influences receptor protein-protein interaction with PDZK1, coimmunoprecipitation was performed in COS-M6 cells expressing either WT SR-BI or SR-BI-Q445A and C-terminally HA-tagged PDZK1 (PDZK1HA). In cells expressing WT SR-BI and PDZK1HA, antibody to HA yielded coimmunoprecipitation of SR-BI (Online Fig. IA) and antibody to SR-BI yielded coimmunoprecipitation of PDZK1HA (Online Fig. IB). Similarly, in cells expressing SR-BI-Q445A and PDZK1HA, antibody to HA yielded coimmunoprecipitation of SR-BI-Q445A (Online Fig. IC) and antibody to SR-BI yielded coimmunoprecipitation of PDZK1HA (Online Fig. ID). Thus, the interaction of plasma membrane cholesterol with SR-BI does not influence receptor interaction with PDZK1.
SR-BI-plasma membrane cholesterol interaction and receptor signaling
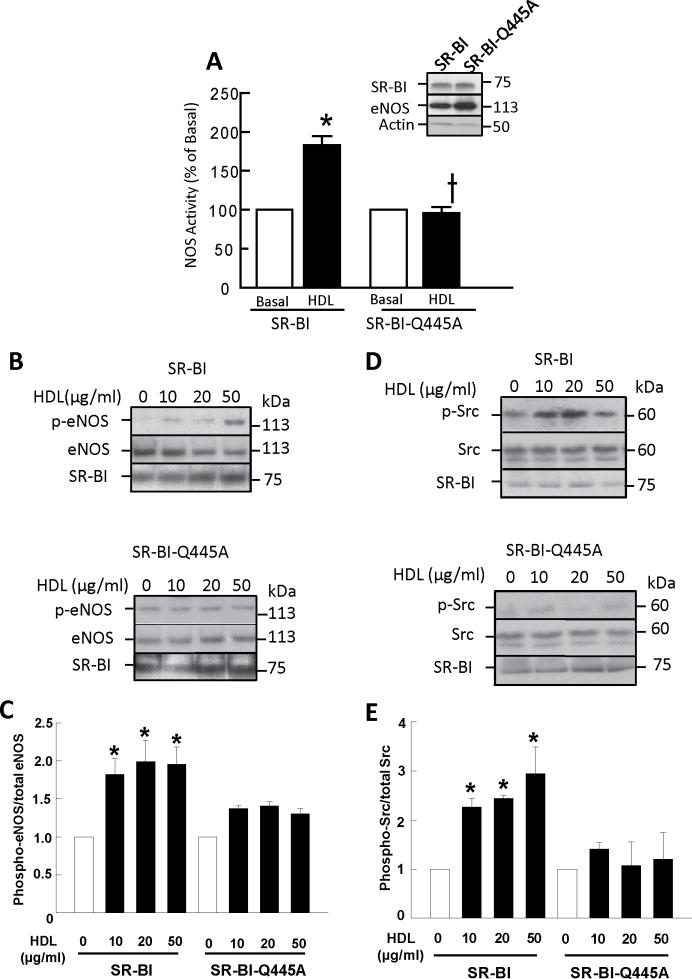
Since SR-BI-Q445A has attenuated interaction with plasma membrane cholesterol but normal capacity to bind HDL, to mediate cholesterol flux, and to interact with PDZK1, the mutant allows discrete determination of the role of plasma membrane cholesterol interaction in signaling by the receptor. We evaluated HDL-mediated activation of eNOS in transfected COS-M6 cells, which provides a robust means to detect amplified downstream processes. As seen previously4, eNOS activity was increased 2-fold by HDL in cells co-expressing WT SR-BI and eNOS (Fig. 3A). However, eNOS activation by HDL did not occur in cells expressing SR-BI-Q445A. Similarly, treatment of WT SR-BI-expressing cells with HDL caused eNOS Ser1179 phosphorylation (Fig. 3B,C), whereas it did not occur in SR-BI-Q445A expressing cells.
Figure 3. Loss of SR-BI interaction with plasma membrane cholesterol attenuates signaling by the receptor to eNOS and Src.
(A) COS-M6 cells were cotransfected with cDNAs encoding eNOS and either WT SR-BI or SR-BI-Q445A. Twenty-four h later eNOS activation was assessed in intact cells by measuring 14C-L-arginine to 14C-L-citrulline conversion during 15 min incubations in the absence (basal) or presence of HDL (50 μg/ml). Values are mean±SEM, n=16, *p<0.05 vs basal, †p<0.05 vs WT SR-BI. Inset shows SR-BI and eNOS abundance. (B-E) COS-M6 cells similarly transfected with eNOS and WT SR-BI or SR-BI-Q445A were treated with varying concentrations of HDL for 20 min, and eNOS Ser1179 phosphorylation was evaluated by immunoblot analyses using anti-phospho-eNOS and anti-eNOS Ab (B). c-Src Tyr416 phosphorylation was also evaluated by immunoblot analysis using anti-phospho-Src and anti-Src Ab (D). Summary data for eNOS phosphorylation and Src phosphorylation are provided in C and E, respectively (values are mean±SEM, n=4, *p<0.05 vs basal).
Since the most proximal signaling event known to be initiated by HDL/SR-BI is Src kinase activation6, we determined if SR-BI-Q445A initiates HDL-induced Src Tyr419 phosphorylation. Whereas WT SR-BI invoked Src phosphorylation in response to HDL (Fig. 3D,E), SR-BI-Q445A did not. We further determined whether the interaction of SR-BI with Src is altered by the Q445A mutation by coimmunoprecipitation. WT SR-BI and SR-BI-Q445A displayed comparable interaction with Src (Online Fig. IE). Thus, although the capacity to bind plasma membrane cholesterol does not influence the interaction between Src and SR-BI, it is required for SR-BI to initiate signaling in response to HDL.
SR-BI-plasma membrane cholesterol interaction and cholesterol sensing
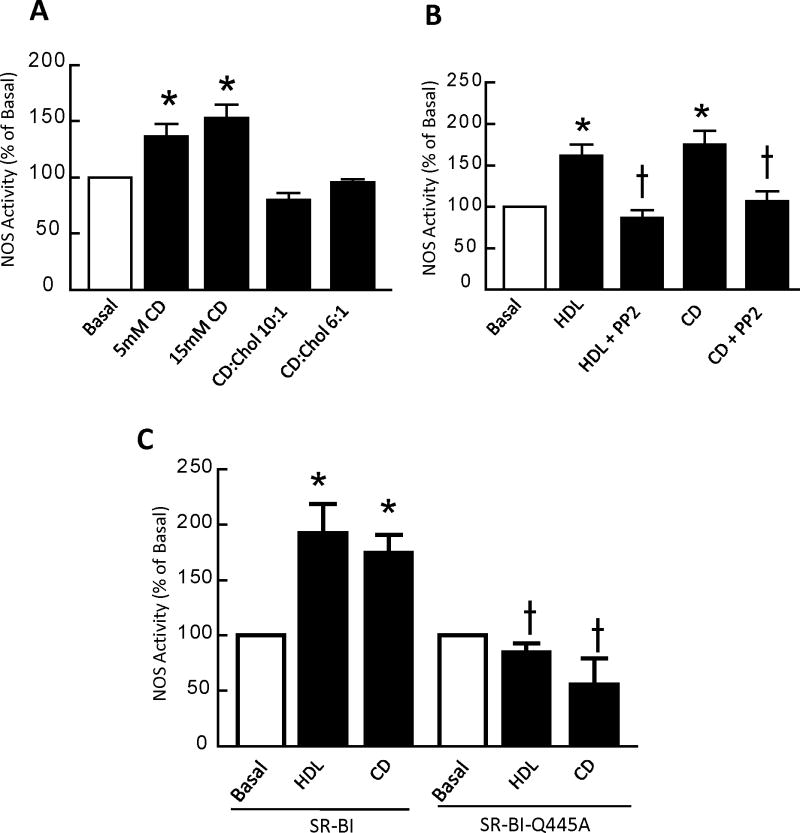
We previously demonstrated that cholesterol-free methyl-β-cyclodextrin (CD) activates signaling in an SR-BI-dependent manner10. Since CD removes plasma membrane cholesterol and does not require cell surface receptors to serve as a cholesterol acceptor31, this prior observation suggests that SR-BI may be a plasma membrane cholesterol sensor. To characterize this further, signaling to eNOS upon manipulation of cholesterol flux with CD was evaluated in COS-M6 cells expressing the enzyme and WT SR-BI. Whereas cholesterol-free CD stimulated eNOS, cholesterol-containing CD did not (Fig. 4A), and both HDL and cholesterol-free CD activation of eNOS were prevented by the Src kinase inhibitor PP2 (Fig. 4B). We then used SR-BI-Q445A to determine if SR-BI interaction with plasma membrane cholesterol is required for signaling in response to cholesterol efflux to CD (Fig. 4C). Whereas both HDL-stimulated and CD-stimulated eNOS activation occurred in cells expressing WT receptor, neither occurred in SR-BI-Q445A-expressing cells. Thus, the interaction of SR-BI with plasma membrane cholesterol is required for the receptor to initiate signaling in response to cholesterol movement.
Figure 4. Loss of SR-BI interaction with plasma membrane cholesterol attenuates plasma membrane cholesterol sensing.
(A) COS-M6 cells were cotransfected with cDNAs encoding WT SR-BI and eNOS, and 24h later NOS activation in response to cholesterol-free methyl-β-cyclodextrin (CD) or cholesterol-laden CD were compared by measuring 14C-L-arginine to 14C-L-citrulline conversion in intact cells during 15 min incubations. (B) In similarly transfected COS-M6 cells, NOS activation was evaluated in the absence (basal) or presence of HDL (50 μg/ml) or 15 mM CD, with or without addition of the Src family kinase inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d]pyrimidine) at a final concentration of 1 μM. (C) In COS-M6 cells cotransfected with cDNAs encoding eNOS and either WT SR-BI or SR-BI-Q445A, NOS activity was evaluated during 15 min incubations performed in the absence (basal) or presence of HDL (50 μg/ml) or 15 mM CD. In A-C, values are mean±SEM, n=8, *p<0.05 vs basal, †p<0.05 vs no PP2 (in B), or †p<0.05 vs WT SR-BI (in C). All findings were replicated in 3 independent experiments.
SR-BI plasma membrane cholesterol sensing and hepatic receptor function
To determine the role of plasma membrane cholesterol sensing by SR-BI in the function of the receptor in liver, adenoviral constructs were created harboring WT SR-BI (AdSR-BI) or SR-BI-Q445A (AdSR-BI-Q445A). Male C57BL/6 mice were injected with empty control adenovirus (AdNull) or AdSR-BI or Ad-SR-BI-Q445A and allowed to recover. AdSR-BI or AdSR-BI-Q445A injection resulted 7d later in a 6-fold increase in hepatic SR-BI protein expression (Online Fig. IIA). In mice injected with AdSR-BI, plasma total cholesterol was decreased by 68% on day 3 compared to levels in mice receiving AdNull (Online Fig. IIB), mirroring prior observations22, 32. Total cholesterol was similarly lowered by 69% on day 3 in mice injected with AdSR-BI-Q445A. By day 7, total cholesterol rose in both AdSR-BI-injected and Ad-SR-BI-Q445A-injected mice to levels similar to those in control mice. FPLC analysis of lipoproteins in mice injected with AdSR-BI revealed reduced HDL cholesterol on day 3, with persistently low HDL cholesterol on day 7 accompanied by an increase in VLDL cholesterol (Online Fig. IIC, left panel). This mimicks prior findings22, 32. Similarly, mice receiving AdSR-BI-Q445A displayed lower HDL cholesterol on day 3 and day 7, with an increase in VLDL cholesterol levels on Day 7 (Online Fig. IIC, right panel).
To directly measure how PM cholesterol sensing by SR-BI in the liver influences RCT, in vivo measurements of macrophage RCT were performed following IV injection of AdNull, AdSR-BI or AdSR-BI-Q445A in male mice. Measurements of plasma 3H-cholesterol recovery revealed a progressive increase in plasma 3H-cholesterol in AdNull-injected mice (Online Fig. IIIA); in contrast, AdSR-BI and AdSR-BI-Q445A injection markedly lowered 3H-cholesterol recovery in the plasma to a similar degree. Liver 3H-cholesterol recovery, biliary 3H-cholesterol and 3H-bile acid secretion, and 3H-cholesterol recovery in the feces were equally increased by liver overexpression of wild-type SR-BI or SR-BI-Q445A (Online Fig. IIIB-E, respectively). Thus, cholesterol transfer from macrophages to feces was similar for the two forms of receptor. Taken together, these results indicate that plasma membrane cholesterol sensing does not influence the hepatic role of SR-BI in reverse cholesterol transport.
SR-BI plasma membrane cholesterol sensing in enterocytes
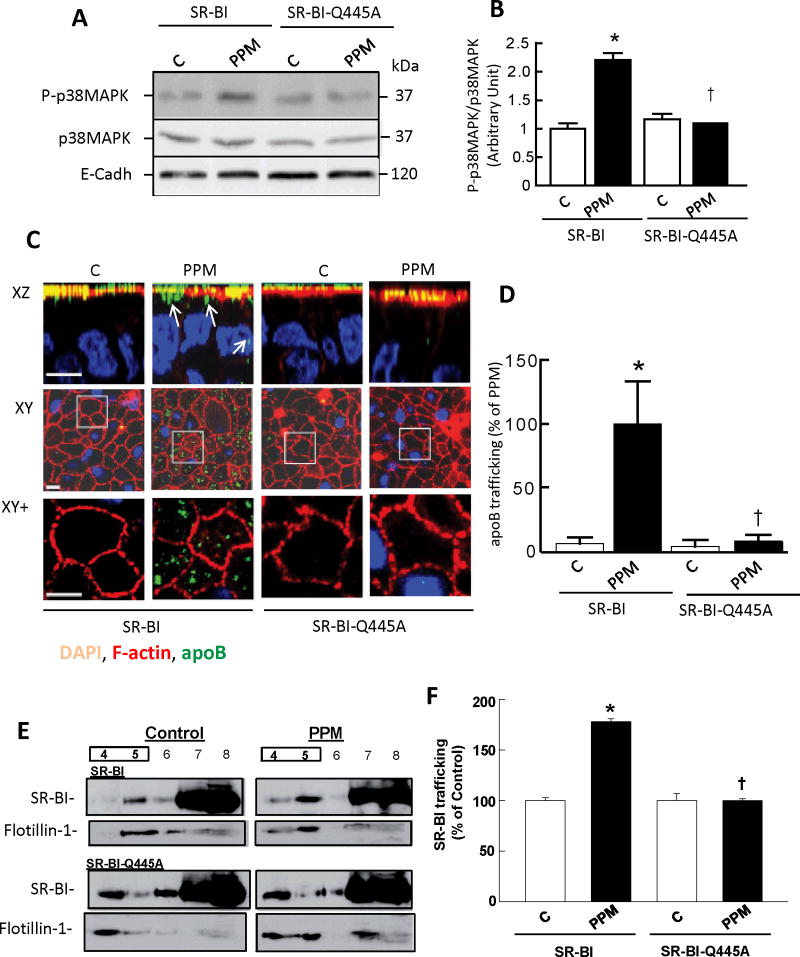
In enterocytes, PPM-induced signaling and apolipoprotein B trafficking involved in the delivery of dietary lipids as TRL are fully prevented by BLT-19, which blocks SR-BI-mediated lipid transfer to and from the receptor’s membrane lipid environment33, 34. To determine if SR-BI plasma membrane cholesterol sensing occurs in enterocytes, Caco-2/TC7 cells were stably transfected with WT SR-BI or SR-BI-Q445A. Both cell lines expressed abundant exogenous SR-BI (data not shown). Upon PPM treatment, p38MAPK activation was observed in wild type SR-BI-expressing cells, but not in SR-BI-Q445A-expressing cells (Fig. 5A,B). We further analyzed PPM-induced movement of apoB, and found that in WT SR-BI transfected cells, apoB trafficks normally to intracellular compartments after PPM supply9. However, apoB is maintained in the apical compartment despite PPM application in SR-BI-Q445A-expressing cells (Fig. 5C,D). These findings indicative of a dominant-negative effect of SR-BI-Q445A reveal that SR-BI interaction with plasma membrane cholesterol is required for PPM-induced signaling and the functional response of apoB trafficking away from the apical surface in enterocytes.
Figure 5. Loss of SR-BI plasma membrane cholesterol sensing blunts PPM-induced p38MAPK activation and apoB trafficking in enterocytes.
(A) Caco-2/TC7 cells were stably transfected with cDNA encoding mouse SR-BI or SR-BI-Q445A and incubated in absence (control, C) or presence of PPM for 10 min. Cell lysates were analyzed by immunoblotting using phospho-p38MAPK (P-p38MAPK) Ab. Total p38MAPK and E-cadherin (E-cadh) were used as loading controls. Summary data is provided in B (n=3, mean±SEM, *p<0.05 vs control, †p<0.05 vs SR-BI). (C) Experimental groups described in A were incubated in absence or presence of PPM for 20 min and apolipoprotein B distribution (apoB; green) was analyzed by confocal microscopy. F-actin (red) is used for visualization of brush border domain and DAPI (blue) for nucleus. For each condition, XZ projections and XY acquisitions 6 μm below the apical plane (Intracellular) are presented, as well as enlarged views of the fields designated by white squares (XY+). The trafficking of apoB from the apical surface is visualized by its resulting presence in the intracellular compartment (see also arrows in XZ plane). Bar = 5 μm. (D) Summary data (mean±SEM) for apoB fluorescence quantified in −6 μm XY sections (10 fields from 3 coverslips/condition). Mean value for PPM-treated SR-BI-expressing cells is set at 100%, *p<0.05 vs control, †p<0.05 vs SR-BI. (E, F) PPM cause redistribution of SR-BI but not SR-BI-Q445A to lipid rafts. Caco-2/TC7 cells stably expressing SR-BI or SR-BI-Q445A were incubated in the absence (control) or presence of PPM for 20 min, cell lysates were applied to a 5–40% sucrose gradient and eleven fractions were collected. Fractions 4 to 8 were analyzed by immunoblotting with antibodies against SR-BI or flotillin-1 (marker for raft fractions 4, 5). (F) Relative abundance of SR-BI in raft fractions versus all fractions, normalized to abundance in control-treated cells (n=3, mean±SEM, *p<0.05 vs control, †p<0.05 vs SR-BI).
There is evidence that SR-BI function, particularly in the initiation of intracellular signaling, depends on its localization in plasma membrane caveolae/lipid rafts4, 35. In enterocytes PPM exposure causes increased trafficking of SR-BI to caveolae/lipid rafts9. Since the basis for PPM-stimulated trafficking of SR-BI to rafts in enterocytes is unknown, the impact of PPM on receptor trafficking was evaluated in wild-type SR-BI versus SR-BI-Q445A-expressing cells in sucrose gradient experiments9. In SR-BI expressing cells that received no micelles in the apical medium (Fig. 5E, upper left panel), there was an abundance of SR-BI in non-raft/high sucrose density cell fractions (fractions 7,8) compared with low sucrose density raft fractions (fractions 4,5; flotillin-1 serves as marker). In response to the application of PPM, less SR-BI was found in the non-raft fractions and more SR-BI was found in the raft fractions (Fig. 5E, upper right panel). Summary data for 3 independent experiments revealed that there was a 78% increase in raft-associated SR-BI in response to PPM (Fig. 5F). In contrast, in SR-BI-Q445A-expressing enterocytes there was no change in the partitioning of the receptor in response to PPM (Fig. 5E, lower panels, and Fig. 5F). These collective findings indicate that plasma membrane cholesterol sensing by SR-BI occurs in enterocytes, and that it is required for enterocyte responses to PPM. In addition, there is a role for plasma membrane cholesterol binding in PPM-induced changes in SR-BI abundance in enterocyte rafts.
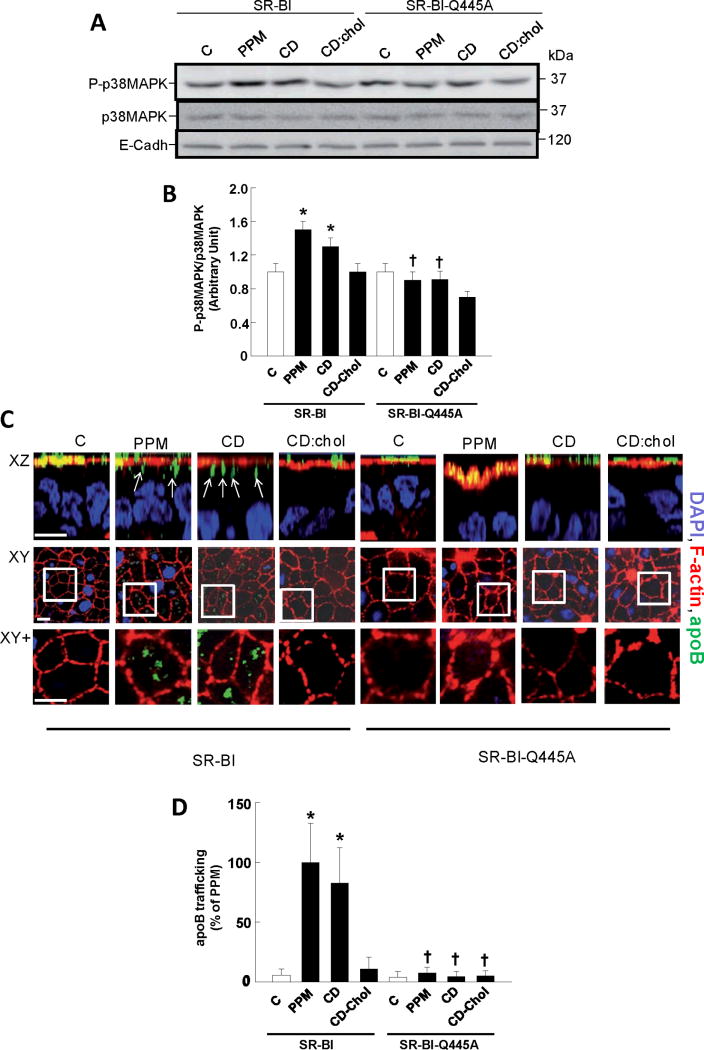
To further determine if SR-BI senses cholesterol movement from the plasma membrane in enterocytes, additional experiments were performed with CD and cholesterol-containing CD. In cells expressing wild-type receptor, p38MAPK was activated by both PPM and CD but not by CD-cholesterol (Fig. 6A,B) In parallel, PPM and CD caused equal trafficking of apoB from the apical compartment, whereas CD-cholesterol had no effect (Fig. 6C,D). In contrast, neither PPM or CD activated p38MAPK or invoked apoB trafficking in cells expressing SR-BI-Q445A. Thus, SR-BI interaction with plasma membrane cholesterol underlies the capacity of the receptor to recognize alterations in plasma membrane cholesterol in enterocytes.
Figure 6. Interaction with cholesterol enables SR-BI to sense changes in plasma membrane cholesterol in enterocytes.
(A) Differentiated wild-type SR-BI and SR-BI-Q445A-transfected Caco-2/TC7 cells were incubated for 10 min in the absence (control, C) or presence of PPM, 10 mM cholesterol-free methyl-β-cyclodextrin (CD) or cholesterol-laden CD (CD:Chol 6:1). Cell lysates were analyzed by immunoblotting using phospho-p38MAPK (P-p38MAPK) Ab. Total p38MAPK and E-cadherin (E-cadh) were used as loading controls. Summary data is provided in B (n=3, mean±SEM, *p<0.05 vs control, †p<0.05 vs SR-BI). (C) Experimental groups described in A were incubated in absence or presence of PPM, CD or CD:Chol for 20 min and apolipoprotein B distribution (apoB; green) was analyzed by confocal microscopy. F-actin (red) is used for visualization of brush border domain and DAPI (blue) for nucleus. For each condition, XZ projections and XY acquisitions 6 μm below the apical plane (Intracellular) are presented, as well as enlarged views of the fields designated by white squares (XY+). The trafficking of apoB from the apical surface is visualized by its resulting presence in the intracellular compartment (see also arrows in XZ plane). Bar = 5 μm. (D) Summary data (mean±SEM) for apoB fluorescence quantified in −6 μm XY sections (10 fields from 3 coverslips/condition). Mean value for PPM-treated SR-BI-expressing cells is set at 100%, *p<0.05 vs control, †p<0.05 vs SR-BI.
SR-BI plasma membrane cholesterol sensing in endothelial cells
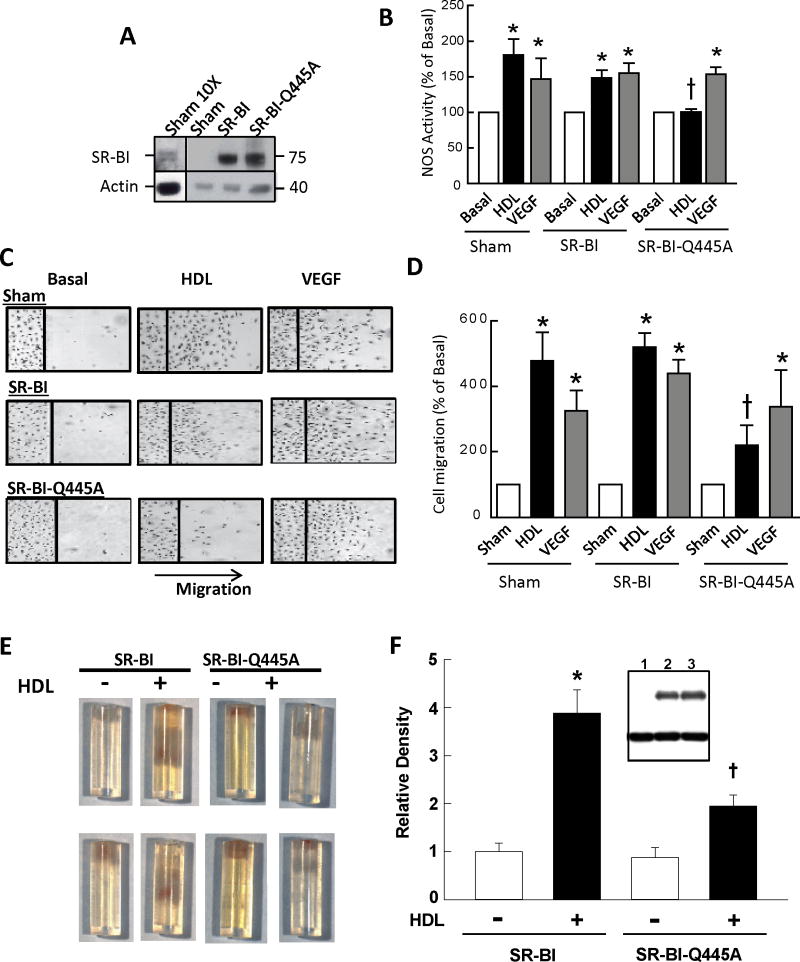
To determine the role of SR-BI plasma membrane cholesterol sensing in endothelial cells, BAEC were transfected with sham plasmid or cDNA encoding WT SR-BI or SR-BI-Q445A, and responses to HDL were tested 24 or 48h later. Equal expression of exogenous receptors was attained (Fig. 7A). Sham-transfected BAEC and cells transfected with WT SR-BI displayed similar eNOS activation by HDL, and comparable stimulation was invoked by the control eNOS agonist VEGF (Fig. 7B). In contrast, the introduction of SR-BI-Q445A prevented eNOS activation by HDL, whereas enzyme stimulation by VEGF was unaffected. Sham-transfected cells and cells transfected with WT receptor displayed migratory responses to HDL and VEGF that were similar (Fig. 7C,D). In contrast, although VEGF-induced migration did not change with the introduction of SR-BI-Q445A, there was 72% attenuation of migration activated by HDL.
Figure 7. Loss of SR-BI plasma membrane cholesterol sensing blunts HDL stimulation of eNOS, endothelial cell migration, and in vivo angiogenesis.
(A) BAEC were transfected with sham plasmid or cDNA encoding WT SR-BI or SR-BI-Q445A, and receptor abundance was evaluated 48h later by immunoblot analysis. Panel on left displays abundance of endogenous SR-BI when the protein load is increased 10-fold. (B) In the experimental groups described in A, NOS activity was evaluated in intact cells by measuring 14C-L-arginine to 14C-L-citrulline conversion during 15 min incubations in the absence (basal) or presence of HDL (50 μg/ml) or VEGF (100ng/nl). (C,D) In the experimental groups described in A, endothelial cell migration was evaluated in scratch assays during 24h treatments with media alone (basal), HDL (100 μg/ml) or VEGF (100ng/nl). In B and D, values are mean±SEM, n=4–5, *p<0.05 vs basal, †p<0.05 vs SR-BI. (E,F) In vivo evaluation of HDL-induced angiogenesis in matrix-containing cylinders placed subcutaneously in male C57BL/6 mice. Microvessel ingrowth in response to HDL (100 ug/ml) was compared in cylinders into which Ad-SR-BI versus Ad-SR-BI-Q445A was introduced at the time of matrix preparation. (E) Two example cylinders per study group (opening at top) with blood-filled microvessels within the matrix. (F) Summary data for endothelial cell density, expressed relative to density in control-treated cylinders. Values are mean±SEM, n=8 for control cylinders and 14–16 for HDL-treated cylinders, *p<0.05 vs basal, †p<0.05 vs SR-BI. Inset in F shows comparable efficacy of adenovirus-based expression of SR-BI and SR-BI-Q445A in cultured endothelial cells (lanes 1–3 are AdNull, AdSR-BI, and AdSR-BI-Q445A, respectively, upper blot is SR-BI, lower blot is actin).
To determine if SR-BI plasma membrane cholesterol sensing governs endothelial cell behavior in vivo, we established a model system for the study of HDL/SR-BI modulation of angiogenesis in mice that is independent of hepatic or systemic actions of the lipoprotein or its receptor. The model entails subcutaneous placement of matrix- and fibroblast growth factor-2-containing 20 ul silicone cylinders open at one end in C57BL/6 male mice at 8 weeks of age. The cylinders were harvested 14 days later and endothelial cell abundance in the defined volume of matrix was determined by staining the cells with FITC-lectin and quantification by fluorescence28, 29. First we demonstrated that the addition of HDL (100 ug/ml) into the FGF-2 containing matrix causes a 5.5±1.0-fold (n=6, p<0.05) increase in endothelial cell abundance, indicating a robust stimulation of angiogenesis. We then determined that HDL activation of angiogenesis was similar in cylinders containing AdNull versus AdSR-BI (data not shown), indicating that the provision of additional wild-type receptor does not affect the process. This in vivo finding is identical to what we observed with overexpression of AdSR-BI in cultured endothelial cells (Fig. 7C,D). After confirming equal ability to overexpress wild-type versus mutant receptor in infected endothelial cells (Fig. 7F, inset), the impact of HDL was compared in cylinders into which AdSR-BI or AdSR-BI-Q445A was introduced at the time of matrix preparation. Fig. 7E shows two example cylinders per study group (open at top) with blood-filled microvessels within the matrix, and summary data for endothelial cell density is in Fig. 7F. Whereas HDL caused a 4-fold increase in endothelial cell density in the presence of Ad-SR-BI, the response was decreased by more than half in the presence of Ad-SR-BI-Q445A. Thus, SR-BI-Q445A had a specific dominant-negative effect on endothelial cell behaviors invoked by HDL both in cell culture and in vivo. As such, SR-BI plasma membrane cholesterol sensing is required for the known potential cardiovascular protective actions of HDL in endothelium.
DISCUSSION
In the present studies, the novel capacity of SR-BI to interact with plasma membrane cholesterol and its implications on receptor function have been interrogated. We discovered that glutamine (Q) 445 in the SR-BI CTTM is critically involved in the interaction of the receptor with plasma membrane cholesterol. Importantly, alanine substitution of Q445 yielded a receptor with normal capacities to bind HDL and to mediate cholesterol flux, and with normal interactions with PDZK1 and Src, which are necessary for intracellular signaling initiated by SR-BI. Having retained these critical features, with SR-BI-Q445A it was possible to demonstrate that the interaction with plasma membrane cholesterol is required for HDL-induced signaling by the receptor. Thus, an important new feature of SR-BI enabling it to serve diverse functions has been identified.
In prior studies we observed that in cells devoid of SR-BI, intracellular signaling does not occur upon treatment with CD, which removes plasma membrane cholesterol and does not require cell surface receptors to serve as a cholesterol acceptor31; in contrast, the introduction of SR-BI yields cells that display signaling in response to CD10. In the present work we demonstrate that whereas intracellular signaling occurs with CD treatment of cells expressing WT SR-BI, cells expressing SR-BI-Q445A, which has attenuated interaction with plasma membrane cholesterol, are unresponsive. These findings indicate that SR-BI is a plasma membrane cholesterol sensor, and that its capacity to interact with plasma membrane cholesterol underlies this unique function. SR-BI now joins the list of known sterol sensing proteins, which includes SCAP, Insig HMG Co-A reductase, LXR (liver X receptors) and NPC1,236. However, in contrast to these known sterol sensing proteins, SR-BI is the first identified plasma membrane cholesterol sensor and the first sterol sensing protein to regulate kinase signaling.
By overexpressing SR-BI-Q445A in the mouse liver, we found that plasma membrane cholesterol sensing is most likely not operative in the role of hepatic SR-BI in global cholesterol homeostasis or in macrophage RCT. This observation indicates that the classical function of SR-BI to mediate cholesterol movement between its ligands and cells is sufficient for the receptor in hepatocytes to participate in RCT. However, in studies in enterocytes, in which SR-BI enables the sensing of PPM involved in the delivery of dietary lipids as TRL9, using SR-BI-Q445A we discovered that plasma membrane cholesterol sensing by the receptor is required for PPM-induced signaling and apoB intracellular movement. We also found that plasma membrane cholesterol sensing is necessary for PPM to promote the recruitment of SR-BI to plasma membrane caveolae/lipid rafts in enterocytes. In addition, we discovered that plasma membrane cholesterol sensing is critically involved in important functions of SR-BI in endothelial cells. In particular, we found that both HDL activation of eNOS that increases the production of the atheroprotective signaling molecule NO4 and HDL stimulation of endothelial cell migration that is critical to the maintenance of intimal layer integrity7 require SR-BI plasma membrane cholesterol sensing. Moreover, to determine if SR-BI plasma membrane cholesterol sensing governs endothelial cell behavior in vivo, we devised a means to study HDL/SR-BI modulation of angiogenesis in mice that is independent of hepatic or systemic actions of the lipoprotein or its receptor. Using the new model system, we found that plasma membrane cholesterol sensing is required for HDL-induced angiogenesis in vivo. Thus, we have shown in two different cell types, in response to two different lipid complexes (HDL and PPM), in cell culture as well as in mice, that SR-BI plasma membrane lipid sensing mediates cellular responses of relevance to cardiovascular and metabolic health and disease. The differences in the impact of the SR-BI-Q445A mutant in enterocytes and endothelium versus in the liver further suggests that the sensing of changes in plasma membrane cholesterol is relevant to SR-BI action particularly in cell types in which an important function of the receptor entails the regulation of intracellular signaling. These functions could potentially be further interrogated in vivo by the creation of an SR-BI-Q445A knock-in mouse.
Evaluations of the actions of HDL on endothelium have received attention recently as potential means to interrogate the functional characteristics of HDL37–42. Differences in HDL function may be more informative than simple assessments of HDL cholesterol abundance in our attempts to better understand the cardiovascular impact of HDL, to assign cardiovascular disease risk, and to interpret the findings of trials testing HDL-targeted therapies43, 44. The model system that we have devised to study HDL-induced angiogenesis in mice in a manner that avoids hepatic or systemic actions of the lipoprotein may provide a valuable means to directly evaluate endothelial cell responses to minute quantities of patient-derived HDL in vivo. As importantly, through genetic manipulation within the 20 ul cylinder or by use of the cylinder in genetically-modified mice, the molecular basis for direct actions of HDL on endothelium can be interrogated to greater depth in vivo.
In addition to its utility in revealing the existence of cholesterol sensing by SR-BI and the functional implications of that sensing in endothelium and enterocytes, further studies of SR-BI-Q445A will allow greater elucidation of the mechanisms that underlie SR-BI signaling. This includes future studies aimed at determining how the sensing of cholesterol movement causes SR-BI to activate kinases, with the activation of Src kinases being the most proximal known signaling event6. The interaction between SR-BI and plasma membrane cholesterol may underlie possible conformational changes that occur in the receptor upon cholesterol movement, perhaps resulting in dynamic alterations in yet-to-be-discovered interactions between the receptor and other transmembrane or intracellular signaling molecules. The SR-BI-Q445A mutant also provides a means to segregate cellular responses caused specifically by SR-BI-mediated cholesterol or phospholipid movement from those that require SR-BI-mediated signaling. Further study of plasma membrane cholesterol sensing by SR-BI will enhance our basic understanding of the role of the receptor in cardiovascular health and disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Signal initiation by the HDL receptor scavenger receptor class B, type I (SR-BI) is important to actions of HDL on endothelium and other processes.
Signaling by SR-BI requires cholesterol flux and the C-terminal transmembrane domain of this hairpin-looped plasma membrane receptor.
What New Information Does The Article Contribute?
A highly-conserved glutamine residue in the C-terminal transmembrane domain binds plasma membrane cholesterol.
Plasma membrane cholesterol interaction with SR-BI enables it to serve as a plasma membrane cholesterol sensor.
Whereas plasma membrane cholesterol sensing is irrelevant to SR-BI function in hepatocytes, it is required for SR-BI functions in the enterocytes and the endothelium.
In addition to transporting cholesterol from peripheral tissues to the liver for excretion, HDL has direct action on numerous cell types that influence cardiovascular and metabolic health. Many of these actions are mediated by the hairpin-looped plasma membrane receptor SR-BI, and our understanding of non-hepatic functions of SR-BI is limited. We discovered that a highly-conserved glutamine residue in SR-BI binds plasma membrane cholesterol, and that this interaction enables the receptor to serve as a plasma membrane cholesterol sensor. In contrast to other known sterol sensing proteins, SR-BI is the first identified plasma membrane cholesterol sensor and the first sterol sensing protein to regulate kinase signaling. We found that although plasma membrane cholesterol sensing is irrelevant to SR-BI function in hepatocytes, it is required for SR-BI functions in enterocytes and endothelium. Future studies of this unique feature of SR-BI will increase our understanding of non-hepatic functions of the receptor and HDL.
Acknowledgments
The authors thank Jessica Mullens, Mohamad Ahmed, Yolanda F. Darlington and Beatrice Riveau for technical assistance.
SOURCES OF FUNDING
The work was supported by NIH grants HL058888 (P.W.S.), HL063768 and HL058012 (M.C. and the late Dr. David Williams), HL096166 (J.M.B.), Groupe Lipides Nutrition (V.C.), and the American Heart Association (South-Central Affiliate #0825031F to S.S.). The project was also supported by the Crystal Charity Ball Center for Pediatric Critical Care Research and the Lowe Foundation.
Nonstandard Abbreviations
- CE
cholesteryl esters
- CTTM
C-terminal transmembrane domain
- eNOS
endothelial NO synthase
- HDL
high density lipoprotein cholesterol
- LDL
low density lipoprotein cholesterol
- NPC1
Niemann-Pick C1
- PDZK1HA
HA-tagged PDZK1
- PM
plasma membrane
- PPM
postprandial micelles
- RCT
reverse cholesterol transport
- SCAP
SREBP cleavage-activating protein
- SR-BI
scavenger receptor, class B, type I
- TRL
triglyceride-rich lipoproteins
- VLDL
very low density lipoprotein cholesterol
- VSM
vascular smooth muscle
Footnotes
DISCLOSURES
Margery Connelly, Ph.D is an employee of Johnson & Johnson.
References
- 1.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–7. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–58. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–9. [PubMed] [Google Scholar]
- 4.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–7. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 5.Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovasc Res. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 7.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Saddar S, Seetharam D, Chambliss KL, Longoria C, Silver DL, Yuhanna IS, Shaul PW, Mineo C. The Scavenger Receptor Class B Type I Adaptor Protein PDZK1 Maintains Endothelial Monolayer Integrity. Circ Res. 2008;102:480–7. doi: 10.1161/CIRCRESAHA.107.159079. [DOI] [PubMed] [Google Scholar]
- 9.Beaslas O, Cueille C, Delers F, Chateau D, Chambaz J, Rousset M, Carriere V. Sensing of dietary lipids by enterocytes: a new role for SR-BI/CLA-1. PLoS ONE. 2009;4:e4278. doi: 10.1371/journal.pone.0004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–77. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua X, Nohturfft A, Goldstein JL, Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 1996;87:415–26. doi: 10.1016/s0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–68. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- 14.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–24. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–75. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 16.Carriere V, Vidal R, Lazou K, Lacasa M, Delers F, Ribeiro A, Rousset M, Chambaz J, Lacorte JM. HNF-4-dependent induction of apolipoprotein A-IV gene transcription by an apical supply of lipid micelles in intestinal cells. J Biol Chem. 2005;280:5406–13. doi: 10.1074/jbc.M408002200. [DOI] [PubMed] [Google Scholar]
- 17.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 18.Connelly MA, Klein SM, Azhar S, Abumrad NA, Williams DL. Comparison of class B scavenger receptors, CD36 and scavenger receptor BI (SR-BI), shows that both receptors mediate high density lipoprotein- cholesteryl ester selective uptake but SR-BI exhibits a unique enhancement of cholesteryl ester uptake. J Biol Chem. 1999;274:41–7. doi: 10.1074/jbc.274.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–44. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Saddar S, Seetharam D, Chambliss KL, Longoria C, Silver DL, Yuhanna IS, Shaul PW, Mineo C. The Scavenger Receptor Class B Type I Adaptor Protein PDZK1 Maintains Endothelial Monolayer Integrity. Circ Res. 2008;102:480–7. doi: 10.1161/CIRCRESAHA.107.159079. [DOI] [PubMed] [Google Scholar]
- 21.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 22.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–7. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 23.Zeng X, Moore TA, Newstead MW, Hernandez-Alcoceba R, Tsai WC, Standiford TJ. Intrapulmonary expression of macrophage inflammatory protein 1alpha (CCL3) induces neutrophil and NK cell accumulation and stimulates innate immunity in murine bacterial pneumonia. Infect Immun. 2003;71:1306–15. doi: 10.1128/IAI.71.3.1306-1315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki K, Barker C, Danthinne X, Imperiale MJ, Nabel GJ. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med. 1999;5:224–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Janssens SP, Bloch KD, Nong Z, Gerard RD, Zoldhelyi P, Collen D. Adenoviral-mediated transfer of the human endothelial nitric oxide synthase gene reduces acute hypoxic pulmonary vasoconstriction in rats. J Clin Invest. 1996;98:317–24. doi: 10.1172/JCI118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel E, Demignot S, Chateau D, Chambaz J, Rousset M, Delers F. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol Biol Cell. 2004;15:132–41. doi: 10.1091/mbc.E03-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG. Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol. 2003;162:1431–9. doi: 10.1016/S0002-9440(10)64276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, Popel AS. Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer. 2010;10:29. doi: 10.1186/1471-2407-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikemoto M, Arai H, Feng D, Tanaka K, Aoki J, Dohmae N, Takio K, Adachi H, Tsujimoto M, Inoue K. Identification of a PDZ-domain-containing protein that interacts with the scavenger receptor class B type I. Proc Natl Acad Sci U S A. 2000;97:6538–43. doi: 10.1073/pnas.100114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335:433–40. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiersma H, Nijstad N, Gautier T, Iqbal J, Kuipers F, Hussain MM, Tietge UJ. Scavenger receptor BI facilitates hepatic very low density lipoprotein production in mice. J Lipid Res. 2010;51:544–53. doi: 10.1194/jlr.M000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci U S A. 2002;99:15422–7. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieland TJ, Shaw JT, Jaipuri FA, Duffner JL, Koehler AN, Banakos S, Zannis VI, Kirchhausen T, Krieger M. Identification of the molecular target of small molecule inhibitors of HDL receptor SR-BI activity. Biochemistry (Mosc) 2008;47:460–72. doi: 10.1021/bi701277x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–9. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- 36.Liu JP. New functions of cholesterol binding proteins. Mol Cell Endocrinol. 2009;303:1–6. doi: 10.1016/j.mce.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 38.Persegol L, Verges B, Foissac M, Gambert P, Duvillard L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia. 2006;49:1380–6. doi: 10.1007/s00125-006-0244-1. [DOI] [PubMed] [Google Scholar]
- 39.Persegol L, Foissac M, Lagrost L, Athias A, Gambert P, Verges B, Duvillard L. HDL particles from type 1 diabetic patients are unable to reverse the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia. 2007;50:2384–7. doi: 10.1007/s00125-007-0808-8. [DOI] [PubMed] [Google Scholar]
- 40.Persegol L, Verges B, Gambert P, Duvillard L. Inability of HDL from abdominally obese subjects to counteract the inhibitory effect of oxidized LDL on vasorelaxation. J Lipid Res. 2007;48:1396–401. doi: 10.1194/jlr.M600309-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von EA, Fogelman AM, Luscher TF, Landmesser U. Mechanisms adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horvath T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–22. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 43.Khera AV, Cuchel M, Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mineo C, Shaul PW. PON-dering differences in HDL function in coronary artery disease. J Clin Invest. 2011;121:2545–8. doi: 10.1172/JCI57671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.