Abstract
1. CYP2S1 is an evolutionarily conserved, mainly extra-hepatic member of the CYP2 family and proposed to be regulated by the aryl hydrocarbon receptor (AhR).
2. The present study explores AhR's regulation of CYP2S1 in male Sprague Dawley rats using PCB126 (3,3',4,4',5-pentachlorobiphenyl), the most potent AhR agonist among the PCBs. Additionally, CYP2S1 expression was examined after treatments with the classic CYP-inducers β-naphthoflavone (β-NF, AhR activator), phenobarbital (PB, CAR activator) and dexamethasone (Dex, PXR activator). CYP2S1 and CYP1A1/2, CYP1B1, CYP2B and CYP3A mRNAs were measured in liver, lung, spleen, stomach, kidney, and thymus at different time points.
3. Constitutive CYP2S1 was expressed at comparable levels to other CYPs with the highest expression levels in stomach, kidney and lung. CYP2S1 mRNA was only non-significantly elevated by β-NF in liver tissues. PCB126 did not increase CYP2S1 mRNA in any organ and at any time point examined despite a significant induction of CYP1 genes. PCB126 reduced CYP2S1 mRNA by 40% (not significant) from the 7th post-exposure day in thymus. PB and Dex had no effect on CYP2S1 mRNA levels.
4. These observations show that in this model CYP2S1 is not, or only weakly, regulated by AhR and not induced by CAR or PXR activators.
INTRODUCTION
Cytochrome P450 2S1 (CYP2S1) is a newly identified member of the cytochrome P450 2 family. It is found primarily in extra-hepatic tissues (Rylander et al., 2001) and is restricted to epithelial cell types (Saarikoski et al., 2005). In humans, CYP2S1 is highly expressed in the respiratory tract, digestive tract, lymphocytes and spleen (Rylander et al., 2001, Smith et al., 2003). In the mouse, CYP2S1 is one of the most widely and highly expressed CYPs in all adult tissues except liver (Choudhary et al., 2003). In the rat, CYP2S1 mRNA levels are detected in most tissues, following similar patterns as reported in mouse and human (Deb and Bandiera, 2009).
The higher expression of CYP2S1 in the extra-hepatic tissues, like lung, stomach, GI-tract and skin, organs representing major routes of exposure to exogenous compounds, may suggest the enzyme's involvement in non-hepatic metabolism of xenobiotics (Ding and Kaminsky, 2003). Recently it was demonstrated that CYP2S1 has the ability to biotransform several carcinogens and oxygenated eicosanoids, suggesting its roles in both pathological and physiological processes (Bui et al., 2010, Bui and Hankinson, 2009, Nishida et al.). The promoter regions of both human and mouse CYP2S1 gene contain several xenobiotic responsive elements (XREs) identical to those found in the promoter regions of the known AhR gene battery which includes CYP1A1/2, CYP1B1 and others. It was shown that CYP2S1 mRNA is induced by 2,3,7,8-tetrachlorodibenzodioxin (TCDD) in the mouse hepatoma cell line Hepa-1 and in mouse tissues, which has been confirmed in vitro to be mediated by conventional AhR-ARNT (AhR nuclear translocator) interaction (Rivera et al., 2002). Recently, Deb and Bandiera (2010) reported that CYP2S1 mRNA levels were increased dose-dependently in the lung, liver and kidney of rats following TCDD-exposure, while protein levels were only increased in the lung by TCDD at 50 μg/kg body weight (b.w.). Other AhR agonists, such as 3-methylcholanthrene and benzo[a]pyrene, had no effect on protein levels of CYP2S1 in lung, kidney or liver either (Deb and Bandiera, 2009). In addition, little is known about the timeline of the presumed induction. As a result, more studies were called for to investigate CYP2S1's response to AhR agonists using other compounds and additional time points.
PCB126 (3,3',4,4',5-pentachlorobiphenyl) is one of the 209 congeners in the family of polychlorinated biphenyls (PCBs), which were manufactured commercially and widely used due to their chemical stability. However, their production was later banned because of their bioaccumulation in the environment and adverse health effect to humans, some of which are mediated through AhR activation. PCB126 is the most potent AhR agonist in the PCB family with one tenth of the potency of TCDD (Bandiera et al., 1982, Safe, 1990), a position reflected in TEF schemes (Van den Berg et al., 2006).
The present study was conducted to investigate the expression of CYP2S1 mRNA in different organs in male Sprague Dawley rats at different time points after a single treatment with PCB126. In addition, the effect of classical inducers which activate CYPs through 3 different nuclear receptors on CYP2S1 expression in liver and lung of rats was analyzed. The mRNA levels of CYP1A1, CYP1A2, CYP1B1, CYP2B and CYP3A1 were determined for comparison of the effects.
MATERIALS AND METHODS
Chemicals and reagents
RNeasy Mini Kit™ and the RNase Free DNase Set were purchased from Qiagen Inc. (Valencia, CA, USA). High Capacity cDNA Reverse Transcription Kit™ and Power SYBR Green Master Mix™ were purchased from Applied Biosystems by Life Technologies Corporation (Carlsbad, CA, USA). PCR primers were synthesized by Integrated DNA Technologies Inc (Coralville, IA, USA). PCB126 was synthesized and characterized as described (Lai et al., 2010).
Animals
Male Sprague Dawley rats (4 weeks old for the PCB126 study; 6–8 weeks old for classical inducers study) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Rats were housed in polycarbonate cages (2 rats per cage for PCB126 study and 3 rats per cage for classical inducers study) with ad libitum access to water and a commercial rodent diet (7013 Teklad Rodent Diet, Harlan). The facility was maintained at a temperature of around 22°C and a 12h light-dark cycle. Rats were allowed to acclimatize to the animal care facility for 5 days before the treatments. All animal experiments were performed in accordance with the University of Iowa policy and federal regulations for humane treatment and safe use of vertebrate animals in research, and approved by the Institutional Animal Care and Use Committee.
Animal treatment and tissue collection
For the PCB126 time study, rats were randomly divided into 6 groups with 4 or 5 animals per group. One group of animals received no treatment. The four PCB groups received one intraperitoneal (ip) injection of PCB126 (dissolved in corn oil; 5 μmol/kg b.w.) on days 1, 4, 8, or 10 of the experiment. The solvent control group received one i.p. injection of 5 ml corn oil/kg b.w. on day 4. On day 11 of the experiment all rats were weighed and killed by carbon dioxide asphyxiation followed by cervical dislocation. Blood and organs, i.e. liver, lung, spleen, thymus, stomach and kidneys, were harvested immediately after sacrifice; liver and thymus weight were determined; all organs were snap frozen in liquid nitrogen and stored at −80 °C until further analysis.
For the classical inducers study, rats were randomly divided into four groups with 3 animals each. One group received i.p. injections of corn oil (5ml/kg b.w.) on days 1 and 4 as the negative control; one group received phenobarbital at 400 μmol/kg b.w. dissolved in saline on days 4, 5, and 6; the other two groups received either dexamethasone (50 mg/kg b.w.) or β-naphthoflavone (100 μmol/kg b.w.), both dissolved in corn oil, on days 3, 4, 5, and 6. This exposure scheme was adapted from Schramm and coworkers (Schramm et al., 1985). On day 7, all rats were sacrificed and liver, lung, and thymus harvested as described above.
Total RNA extraction
Total RNA from tissue samples was isolated using an RNeasy Mini Kit™ following the manufacturer's instructions. An on-column DNase digestion was performed to further remove residue genomic DNA contaminants. The quantity and quality of RNA was determined by the absorbance at 260nm (A260) and the ratio between A260 and A280 in 10mM Tris buffer at pH 7.0.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
For each sample, 2.5 μg total RNA was reverse-transcribed into cDNA in a 25 μl reaction volume using the High Capacity RT Kit™ following the manufacturer's instructions. The primers used for PCR amplification of each gene are listed in Table 1. All primer pairs have been evaluated by standard curves and melting curves to ensure PCR efficiency and specificity. The PCR efficiency for different genes ranged between 80% and 100% for all the samples tested, but was similar for each primer pair across different organs. The efficiency for CYP2S1 was between 86% and 92%, slightly higher for the liver. On every PCR plate standard curves were included for each primer pair. For calculations the “relative standard curve method” was used to account for the differences in PCR efficiencies. Additionally, the final PCR reaction mixture was cleaned up and sequenced to confirm the identity of the product as CYP2S1. Furthermore, another primer pair closer to the 5' end for CYP2S1 gene was also tested which gave the same mRNA levels as the one reported in this study. Real-time PCR was carried out using Power SYBR Green Master Mix™ according to manufacturer's procedure and an Eppendorf Realplex2 Master Cycler (Hamburg, Germany). Each 20 μl reaction well contained 1–100 ng cDNA depending on the organ and gene in question and 900 nM of each primer of the appropriate primer pair. Quantification was achieved employing the Relative Standard Curve method, i.e. the relative standard curves on each plate are used to calculate the relative amount of a CYP gene and the β-actin housekeeping gene before the ratio of the two is taken for each sample and compared to the ratios for the controls.
Table 1.
Primer pairs used in qRT-PCR experiments
| Gene | Accession No. | Sequence (5'-3') | Product length | Reference |
|---|---|---|---|---|
| CYP2S1 | NM_001107495 | Forward: ACAGACAGGGACCACAGACC | 112 bp | Designed using Primer 3 |
| Reverse: GGACTTGTAGGCAGCCTGAG | ||||
| CYP1A1 | NM_012540 | Forward: CCATGACCAGGAACTATGGG | 341 bp | (Deb and Bandiera, 2010) |
| Reverse: TCTGGTGAGCATCCAGGACA | ||||
| CYP1A2 | NM_012541 | Forward: GTGAGAACTACAAAGACAACGGTG | 94 bp | (Vondracek et al., 2006) |
| Reverse: GTGACTGTTTCAAATCCAGCTCC | ||||
| CYP1B1 | NM_012940 | Forward: CTTGGCCATTGATCGGAAA | 66 bp | (Dewa et al., 2009) |
| Reverse: CAAGGCGAGCGAAGTACAAGT | ||||
| CYP2B1/2 | NM_001134844 | Forward: TGGTGGAGGAACTGCGGAAATC | 66 bp | (Saito et al., 2010) |
| XM_001062335 | Reverse: TGATGCACTGGAAGAGGAAGGT | |||
| CYP3A1 | NM_013105 | Forward: GGAAATTCGATGTGGAGTGC | 329 bp | (Nagata et al., 1999) |
| Reverse: AGGTTTGCCTTTCTCTTGCC | ||||
| β-actin | NM_031144 | Forward: CAGCCTTCCTTCCTGGGTATG | 247 bp | (Deb and Bandiera, 2010) |
| Reverse: TAGAGCCACCAATCCACACAG |
Data analysis
Dunnett's test was used to determine the significance of differences in the experiments using data analysis software of SAS (version 9.1.3 of the SAS System for Windows. Copyright 2000–2004 SAS Institute Inc.). A p value of less than or equal to 0.05 was considered statistically significant. In addition, Dixon's test was used to identify outliers in all the data using statistical computing environment of R (version2.12.1, R Development Core Team, Vienna, Austria, 2010). Data points with p values of less than 0.05 were considered outliers. Only 1.6% (10 of over 600) data point qualified as outliers and only 2 of them were from CYP2S1 data (a lung corn oil control sample and a day 1 liver 2S1 sample). Presence or absence of these data points did not change the significance of the results by Dunnett's test.
RESULTS
Relative expression of CYP genes in various organs
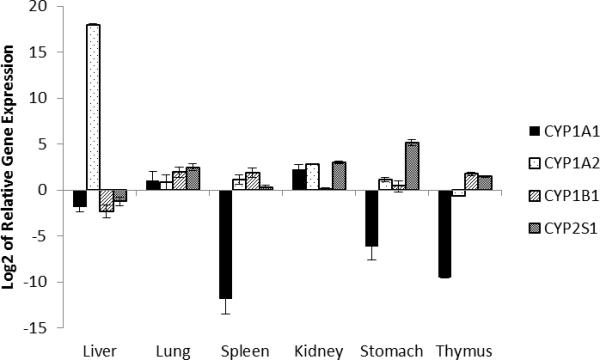
The relative expressions of CYP1A1, CYP1A2, CYP1B1 and CYP2S1 in the different organs of the corn oil control are displayed in Fig.1. CYP2S1 mRNA was detected in all the organs examined, with by far the highest relative quantity in stomach, followed by kidney > lung > thymus > liver ~ spleen. Compared to the other three CYPs, the CYP2S1 mRNA level was by far the major CYP form in the stomach and still the highest in lung and kidney.
Fig.1.
Relative expression of CYPs in various organs (n=3, Mean±SE) in the solvent control animals of the time-response study. Expression data of each gene as determined by the relative standard curves was normalized to that of the house keeping gene β-actin. The relative data were then adjusted according to the relative quantity of β-actin in each organ and normalized to the expression of CYP1A1 level in the liver for ease of comparison.
Similar to CYP2S1, CYP1B1 had generally higher expression levels in extra-hepatic organs compared to the liver. Highest levels were found in lung, spleen, and thymus; CYP1B1 mRNA levels surpassed all other three CYP mRNA levels in spleen and thymus. CYP1A2, on the other hand, was by far the predominant CYP in liver with mRNA levels more than 105 higher than those of the other CYP forms. Finally, CYP1A1 mRNA levels were the lowest among the four CYP genes in most organs except lung and kidney. In thymus, stomach, and spleen CYP1A1 was barely or not detectable.
PCB126 time course study
The purpose of this study was to analyze the changes in the expression of various CYP forms in different organs over a period of 10 days following a single ip injection of the strong AhR agonist PCB126.
Final body, liver and thymus weights
Injection of 5 μmol/kg b.w. PCB126 resulted in a decreased body weight of animals 7 and 10 days after injection and in increased liver weight compared to control animals on day 3 and 7 (Table 2). PCB126 caused a time-dependent increase of relative liver weight which reached its plateau at day 7 post exposure, an almost 40% increase that was maintained through day 10. The relative thymus weights decreased in a time-dependent manner until they reached one third of the normal weight on day 7 and remained at this level at day 10. These data are consistent with the visual observation of evidently enlarged livers and involuted thymuses in PCB126-treated rats at the time of necropsy.
Table 2.
Body and organ weights of rats 1 to 10 days (D 1 – D 10) after a single ip injection of 5 μmol/kg PCB126 (Mean ± SD).
| Final body weight (g) | Liver weight (g) | Liver/body weight × 100 | Thymus weight (g) | Thymus/body weight × 1000 | |
|---|---|---|---|---|---|
| No treatment | 207.8 ± 4.0 | 9.88 ± 0.74 | 4.75 ± 0.28 | 0.66 ± 0.04 | 3.15 ± 0.18 |
| Corn Oil | 199.8 ± 8.7 | 9.75 ± 0.59 | 5.31 ± 0.31 | 0.61 ± 0.09 | 2.80 ± 0.48 |
| D 1 | 198.2 ± 14.0 | 10.52 ± 0.35 | 5.89 ± 0.22 | 0.63 ± 0.09 | 2.13 ± 0.35 |
| D 3 | 197.3 ± 9.7 | 12.01 ± 0.78* | 6.42 ± 0.22** | 0.51 ± 0.07* | 1.61 ± 0.25 |
| D 7 | 171.0 ± 12.4** | 12.19 ± 1.28* | 6.77 ± 0.46** | 0.17 ± 0.02** | 1.08 ± 0.054** |
| D 10 | 153.5 ± 15.8** | 10.45 ± 1.85 | 6.25 ± 0.53** | 0.17 ± 0.07** | 1.45 ± 0.34** |
Statistical significance:
p< 0.05
p<0.01
CYP expression after exposure to PCB126
The relative mRNA levels of CYP1A1, CYP1A2, CYP1B1 and CYP2S1 at day 1, 3, 7 and 10 after PCB126 injection are shown in Fig. 2 to Fig. 5. It should be noted that in these figures for each organ and cytochrome form the mRNA levels are shown in relation to the related untreated control. Thus the different scales of the y-axes reflect the change of CYP expression in that organ, not the level of mRNA.
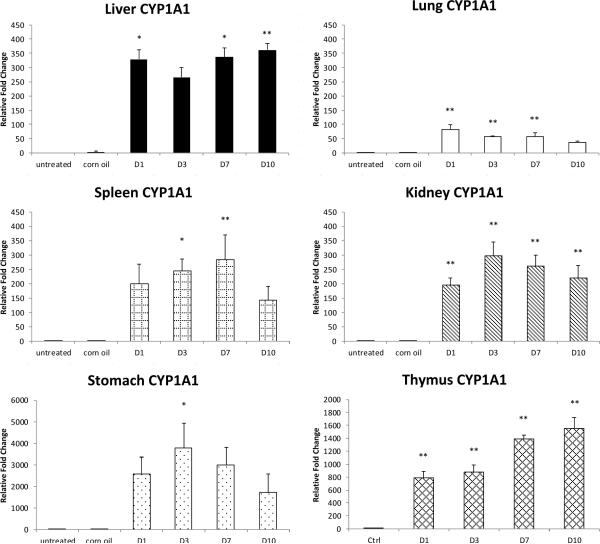
Fig.2.
CYP1A1 relative expression in various organs at day 1 – 10 (D1 – D10) after PCB126 treatment. mRNA levels were first normalized to that of the house keeping gene β-actin in the same sample and then compared to control sample of the organ. Results are presented as mean±SEM (n= 4 or 5). **: P<0.01; *: P<0.5
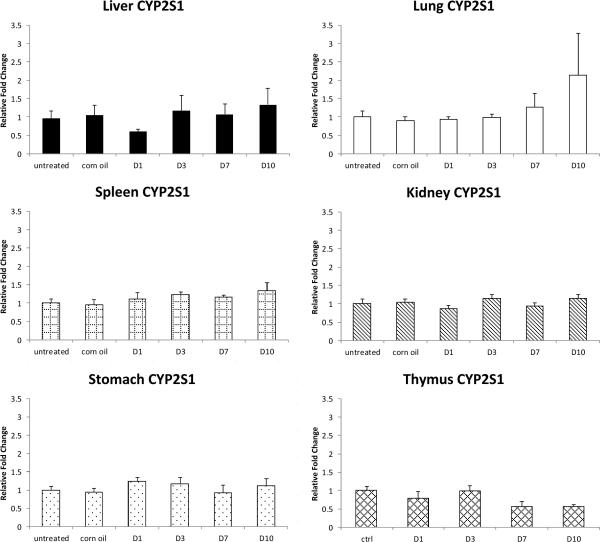
Fig. 5.
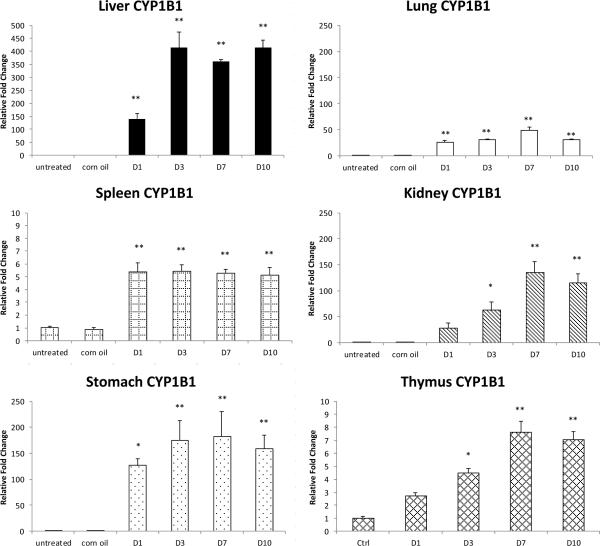
CYP2S1 expression relative to the controls in the same organ after PCB126 treatment. Results are mean±SEM (n= 4 or 5). **: P<0.01; *: P<0.5
CYP1A1 mRNA was highly increased (up to 6,000 times compared to control) in all the organs examined even as early as 24 hours after PCB126 exposure commenced (Fig. 2). Highest fold changes were observed with stomach and thymus, two organs with extremely low baseline levels of CYP1A1; however, in the stomach the induction reached its peak at day 3 and started to fall back quickly afterwards, whereas the CYP1A1 mRNA levels continued to increase with length of exposure in the thymus.
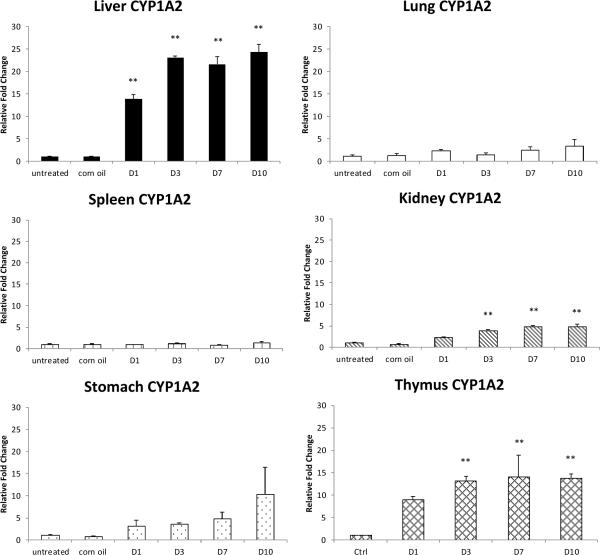
The strongest induction of CYP1A2 expression, as much as 25 fold, was seen in liver, where its baseline expression already far exceeds those of the other CYPs or that one in the other organs (Fig. 3). The induction was visible from day 1 of exposure and plateaued from day 3 to day 10. An increase in expression was also observed in kidney and thymus, although to a lesser extent. In the stomach, spleen and lung, however, the increase was only seen after long exposure and was not statistically significant.
Fig. 3.
CYP1A2 relative expression in various organs at different time points post exposure to PCB126. Data are mean SEM (n= 4 or 5). **: P<0.01; *: P<0.5
CYP1B1 mRNA levels were increased by PCB126 in all organs tested from day 1 or 3 to day 10 of exposure (Fig. 4). The time-course of induction differed between the different organs, with a 5-fold induction seen from days 1–10 in the spleen compared to a time-dependent increase in induction to day 7 in kidney and thymus, for example. Compared to the induction in extra-hepatic organs, CYP1B1 mRNA was most highly increased in liver, similar to CYP1A2.
Fig.4.
Fig.4. CYP1B1 expression relative to the controls in the same organ after PCB126 treatment. Results are mean±SEM (n= 4 or 5). **: P<0.01; *: P<0.5
Unlike the known AhR-regulated genes CYP1A1, CYP1A2 and CYP1B1, CYP2S1 mRNA levels were not increased by PCB126-treatment in any of the organs examined (Fig. 5). In contrast, CYP2S1 mRNA was decreased by around 40% in thymus starting from day 7 post exposure, and although not statistically significant, this repressive effect of PCB126 was stable and even more evident at day 10.
Classic inducer study
To address the question whether CYP2S1 expression in lung and liver is induced through nuclear receptor pathways, rats were treated with the classical CYP inducers β-NF, PB, and Dex which act through binding/activation of the nuclear receptors AhR, CAR, and PXR, respectively. To verify the activation of these receptors, CYP2B and CYP3A mRNA analysis were included in these studies. To optimize CYP induction, animals received 3 (PB) or 4 (β-NF, Dex) ip injections on consecutive days and were sacrificed one day after the last injection, resulting in a maximum exposure time of 3 and 4 days, respectively.
Final body, liver, thymus and lung weights
As shown in Table 3, final body weights of the rats were significantly reduced only in the Dex-treated group. Thymus weights were slightly (not statistically significant) decreased only in the AhR agonist β-NF-treated group, similar to what was seen with PCB126-treated rats on day 3, although to a lower extent. The weight of the lungs was not significantly changed by any of these treatments.
Table 3.
Body and organ weights of rats treated with classical CYP inducers.
| Final body weight (g) | Liver/body weight × 100 | Thymus/body weight × 1000 | Lung/body weight × 1000 | |
|---|---|---|---|---|
| Corn Oil | 281 ± 10.5 | 3.49 ± 0.44 | 1.47 ± 0.14 | 4.72 ± 0.43 |
| β-NF | 281 ± 1.94 | 4.15 ± 0.16 | 1.30 ± 0.11 | 4.94 ± 0.78 |
| PB | 278 ± 32.7 | 4.53 ± 0.82 | 1.43 ± 0.11 | 4.79 ± 0.82 |
| Dex | 232 ± 12.8* | 6.18 ± 0.50** | 1.52 ± 0.02 | 5.09 ± 0.40 |
Statistical significance:
p< 0.05
p<0.01
Relative expression of CYP genes in rat liver
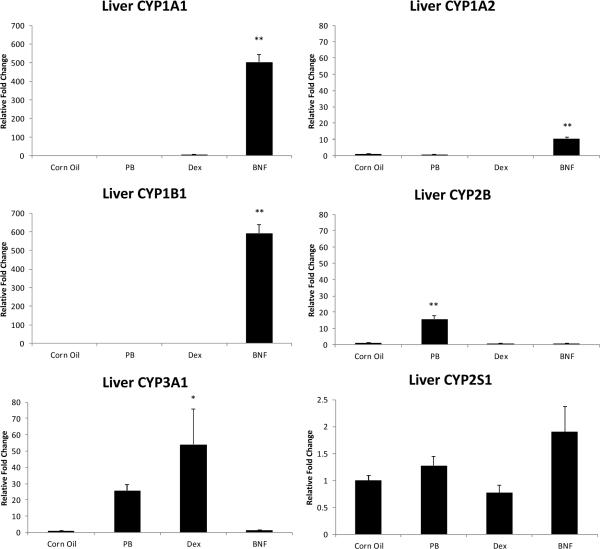
Similar to PCB126-treated rats, the classical AhR agonist β-NF strongly increased the mRNA levels of CYP1A1 (~500 fold), 1A2 (~11 fold) and 1B1 (~600 fold) relative to the control (Fig.6). The magnitude of induction was comparable to that by PCB126 (CYP1A1 ~350 fold; 1A2 ~25fold and 1B1 ~400 fold) on days 3–10. As expected, CYP2B mRNA was increased about 15 fold by PB treatment and CYP3A1 mRNA was increased by both PB (not significant) and Dex treatment with Dex having a higher efficacy. CYP2S1 mRNA was increased by β-NF treatment, but only to less than 2 fold which was not statistically significant. Other classical inducers failed to elicit any significant changes to the expression of this CYP isoform.
Fig.6.
Liver cytochrome P450 enzyme mRNA levels after treatment with classical CYP inducers. Results are mean±SEM (n= 3). **: P < 0.01; *: P < 0.5. CYP3A1 was significant only with a 2 sample t-test, not Dunnett's or One-Way-ANOVA.
Relative expression of CYP genes in rat lung
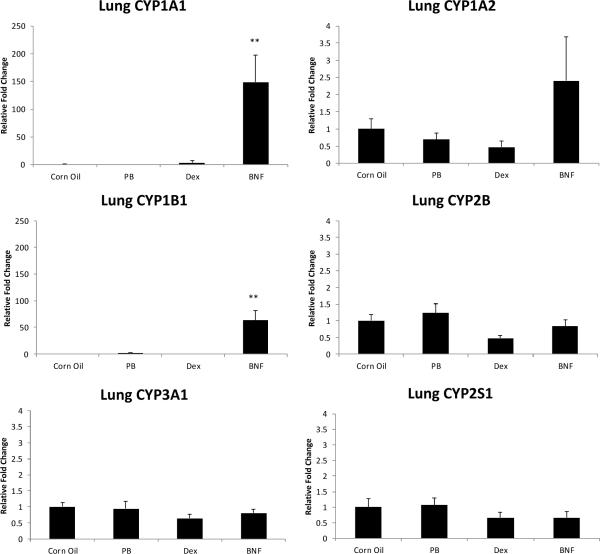
The AhR gene battery CYP1A1, 1A2 and 1B1 was induced by β-NF in the lung of the male rats to a similar extent as by PCB126 on day 7 (Fig. 7), although the induction of CYP1A2 was not statistically significant in lung. The mRNA level of CYP2B was only slightly and not significantly increased and no effect on CYP3A1 was visible in PB animals in the lung. Similarly Dex did not induce CYP3A1 expression, and even appeared to have decreased (not statistically significant) CYP2B and CYP3A1 mRNA levels in the lung. The mRNA levels of CYP2S1, on the other hand, were not significantly changed by any of the classical inducers.
Fig.7.
Lung cytochrome P450 enzyme mRNA levels after treatment with classical CYP inducers. Results are mean±SEM (n= 3). **: P < 0.01; *: P < 0.5
DISCUSSION
In this study we examined the baseline mRNA levels of four cytochrome P450 forms, CYP1A1, 1A2, 1B1 and 2S1, and the changes in these levels over time 1 – 10 days after a single injection of PCB126 at a dose known to elicit a strong Ah-receptor mediated responses (Lai et al., 2010). We also tested the mRNA expression of these enzymes as well as those of CYP2B and CYP3A1 after exposure to classical CYP-inducers β-NF, PB and Dex which act through the different nuclear receptor pathways of AhR, CAR (constitutive androstane receptor) and PXR (pregnane × receptor), respectively.
All three major Ah receptor-regulated cytochrome P450 genes, CYP1A1, 1A2, and 1B1, were expressed in all organs tested at expression levels consistant with the literature reports, i.e. very high levels of CYP1A2 in the liver, very low expression of CYP1A1 in most organs except kidneys, and overall low expression of 1B1 (Sesardic et al., 1990, Bhattacharyya et al., 1995). PCB126 induced these classical AhR-responsive genes in most of these organs. This is in agreement with the effects of TCDD (Badawi et al., 2000, Deb and Bandiera, 2010). In our studies CYP1A1, a minor form in many organs, was highly induced in all organs from days 1 to 10 after PCB126 injection, although CYP1A1 induction levels already started to decline in lung, spleen, kidney and stomach at later time points while continuing to increase in thymus. Similar strong and long lasting effects of TCDD on the CYP1A1 (EROD) activity in the spleen and thymus of rats were reported (Stephen et al., 1997). With PCB126 CYP1B1 showed a similar expression pattern and increase as CYP1A1, although the increases in mRNA levels were comparatively small in thymus and spleen, the two lymphatic organs tested. CYP1A2 showed the smallest changes in mRNA levels after PCB126 treatment and, despite a time-dependent increase, no significant induction in spleen, lung or stomach CYP1A2 binds and sequesteres dioxin and PCB126 in the liver of mice and rats, which could have caused the lower induction of CYP1A2 in extra-hepatic organs (Diliberto et al., 1997, Chen et al., 2003). It is important to note that these data are depicted as the relative level of each gene for each organ and thus do not directly reflect the actual copy number of any gene transcript.
Our major goal in this study was, however, to learn more about a relatively new cytochrome form, CYP2S1, which was first described in 2001 (Rylander et al., 2001). We detected CYP2S1 mRNA in rat liver, lung, spleen, stomach, kidney and thymus. This is consistent with the previous findings in male and female Sprague Dawley rats (Deb and Bandiera, 2010), although these authors observed the highest level of CYP2S1 mRNA in the lung, but the highest level of CYP2S1 protein in the stomach.
CYP2S1 was reported to be inducible by AhR agonists such as TCDD and 3-MC (Deb and Bandiera, 2010). PCB126 is also a strong Ah-receptor agonist (Bandiera et al., 1982). The observed hepatomegaly and thymus atrophy in the current experiment are typical signs of AhR activation by treatments such as TCDD (Harris et al., 1973) or PCB126 (Lai et al., 2010). Moreover, the induction of the AhR-regulated genes CYP1A1, CYP1A2 and CYP1B1 by PCB126 in almost all organs examined also confirms the activation of the AhR as generally accepted (Nebert et al., 2004). However, we found that relative CYP2S1 mRNA levels were not increased at day 1, 3, 7 and 10 after a single i.p. dose of 5 μmol/kg b.w. PCB126 in liver, lung, spleen, stomach, kidney or thymus. Deb et. al. reported an up to 7-fold, dose-dependent increase of CYP2S1 mRNA levels in the lung, and a 3.6- and 2.7-fold increase in liver and kidney, respectively, after a single i.p. injection of 1–100 μg/kg (3–300 nmol/kg) b.w TCDD (Deb and Bandiera, 2010). These changes are relatively small compared to CYP1A1 and 1B1. TCDD is about 10 times more potent as Ah receptor agonist than PCB126 (Bandiera et al., 1982, Safe, 1994). We used a 5 μmol/kg dose of PCB126 which is comparable to the highest (0.3 μmol/kg) dose of TCDD used by Deb and Bandiera. Both compounds are highly persistent and their effects persevere. Thus, it is unlikely that an insufficient dose of PCB126 is the explanation for the lack of CYP2S1 induction by PCB126. In addition, in the study by Deb and Bandiera relative protein levels of CYP2S1 were only increased in lung and only at the dose of 150 nmol TCDD/kg b.w. (Deb and Bandiera, 2010). Other AhR agonists such as 3-MC and benzo[a]pyrene failed to produce any effect on the protein expression, despite an about 3-fold increase in mRNA in the liver by 3-MC-treatment, consistent with our findings on CYP2S1 mRNA. As a result, the authors concluded that CYP2S1 was only weakly regulated by the AhR pathway in rats (Deb and Bandiera, 2009, Deb and Bandiera, 2010), which might explain the fact that we did not observe an induction of CYP2S1 mRNA by PCB126 in rats. Considering these mostly negative results regarding CYP2S1 protein increases, our own negative findings with respect to mRNA increases, and the limited specificity of the available antibodies (Bandiera, personal communication) we believe that the possibility of changes in CYP2S1 protein levels in our experiments is extremely unlikely. Due to the lack of a specific CYP2S1 activity assay suitable for tissue homogenates, we were unable to determine the change in enzyme activity of CYP2S1 in our study.
To find an explanation for the unresponsiveness of SD rats we examined the gene regulatory region 10,000 bp upstream of the rat CYP2S1 gene provided from the University of California, Santa Cruz, Genome Browser for regulatory sequences. The rat upstream region contains three overlapping XRE core sequences 5'-CACGCN(A/C)-3', almost identical to the three overlapping XRE core sequences found in the mouse CYP2S1 promoter region (Rivera et al., 2007). Nevertheless, the presence or lack of an XRE in the promoter region alone does not necessarily dictate the inducibility of the gene by AhR agonists. Rivera and coworker clearly demonstrated the AhR-dependency of CYP2S1 induction by dioxin in a mouse hepatoma (HePa-1) cell line, but they did not find CYP2S1 induction in two human hepatoma cell lines (HepG2 and Hep3B) that produce AhR-mediated CYP1A induction after dioxin exposure (Rivera et al., 2002, Rivera et al., 2007). In addition, Rivera et. al. 2002 reported that the EC50 for CYP2S1 mRNA induction by dioxin is 10 times higher than that for CYP1A1 in cultured mouse Hepa-1 cells. Although this cannot explain our negative findings with CYP2S1 since we observed maximal CYP1A1/2 activity induction with as little as 0.2 μmol/kg b.w. in male SD rats (unpublished results), it indicates a significant difference in inducibility of CYP1A1 and 2S1 by the AhR pathway. The lack of changes in CYP2S1 mRNA in rats could be due to other factors, for example, to regulation by microRNAs, as was recently shown for human CYP1B1 (Tsuchiya et al., 2006).
To further examine the gene regulation of CYP2S1, a group of classical CYP inducers, PB, Dex, and β-NF, was used. These classical inducers bind to different receptors and induce different CYP forms. PB is a prototype of a CAR agonist. Upon activation, the nuclear receptor CAR binds to the DNA fragment known as PB-responsible enhancer module (Honkakoski et al., 1998) and induces the expression of CYP2B genes in the liver (Kawamoto et al., 1999). In addition, it is also responsible for the induction of many other CYP isoforms including CYP2A, CYP2C, CYP2H and CYP3A (Sueyoshi and Negishi, 2001). Dex is a synthetic steroid and is known to activate the glucocorticoid receptor (Lan et al., 1984) as well as the orphan nuclear receptor PXR, thereby inducing the expression of CYP3A family genes (Kliewer et al., 1998, Zhang et al., 1999, Mei et al., 2004, LeCluyse, 2001). Unlike PCB126, the AhR agonist β-NF significantly change any organ weights, probably because of the short exposure time (3 days) and it's fast metabolic elimination (McKillop and Case, 1991). PB also did not influence organ weights. Dex, on the other hand, cuased hepatomegaly, which is consistent with previous reports (Crunkhorn et al., 2004). Our observation regarding constitutive CYP2B and CYP3A1 expression in liver, CYP2B and 3A1 induction by PB and CYP3A1 induction by DEX in the liver, but lack of induction in the lung, which is attributed to low constitutive levels of CAR and PXR in the lung of rats compared to the liver (Chirulli et al., 2005, Nannelli et al., 2008), is in agreement with the literature. In addition, neither of these compounds produced a significant increase in CYP2S1 mRNA in the liver or lung of treated rats; if anything Dex may decrease CYP2S1 levels in these organs, although the decrease was not statistically significant. These findings confirm that CYP2S1 gene expression is not regulated through the CAR or PXR pathway. As expected, β-NF induced CYP1A1/2 and 1B1 in liver and lung. Although we found a 2-fold increase of CYP2S1 mRNA in the liver of rats exposed to β-NF, this was not statistically significant due to small number of animals (n=3) and relatively big variations between them. The fact that β-NF seemed to have weakly induced CYP2S1 in the liver while PCB126 hasn't probably results from the different exposure scheme of the animals: PCB126 was given in a single dose while β-NF was applied in four doses on four consecutive days. The higher extent of AhR activation is also reflected by the fold-induction of CYP1A1 in livers, which was around 350 fold and 500 fold by PCB126 and β-NF treatment respectively. Nevertheless, we could not find any induction of CYP2S1 in the lungs of the same animals. This is in agreement with studies of CYP2S1 in pigs, which found that the mRNA levels of this enzyme did not increase in midbrain after exposure to β-NF (Nannelli et al., 2009). On the other hand, TCDD and 3-MC, two other AhR agonists, caused a strong increase in CYP2S1 mRNA in the lungs of treated rats and a small but significant induction at high concentrations in the liver (TCDD only, 3-MC was negative) (Deb and Bandiera, 2009). We also compared the liver and lung mRNA levels of CYP2S1 in the corn oil control groups of the PCB126 time-response study (younger rats) with those in the classical inducers' study (older rats) and found no age-related differences in CYP2S1 expression. It is also worth noting that microarray analysis of liver tissue from TCDD-exposed rats or mice, and mice or human liver cells in vitro, usually did not show any changes in CYP2S1 expression (Boverhof et al., 2006, Dere et al., 2006, Kim et al., 2009). Thus the regulation of CYP2S1 through the AhR pathway may be weak and/or only visible under specific conditions. An interesting working hypothesis in this context could be that higher competition for the AhR and/or ARNT in the liver could be one of the reasons for the more robust effect of AhR agonists on CYP2S1 induction in lung and stomach.
CONCLUSIONS
In summary, we found CYP2S1 gene expression in all organs investigated in male SD rats, but no increase in mRNA levels after treatment with the potent AhR agonist PCB126, despite significant induction of other AhR-regulated genes and typical morphological changes in the liver and thymus attesting to AhR activation. However, we did observe that relative expression of CYP2S1 was non-significantly increased by β-NF in the liver and reduced by PCB126 treatment in the thymus at later time points, suggesting a tissue specific, persistent, secondary response of this enzyme to the treatment. The transcriptional regulation of CYP2S1 is clearly more complicated than the AhR-driven process seen with other isoforms.
ACKNOWLEDGMENTS
The authors thank Dr. Gregor Luthe, Feodor Lynen fellow of the A. von Humbold Foundation, for the synthesis of PCB 126, Dr. Stelvio Bandiera for helpful discussions and comments.
FUNDING This work was supported by the Iowa Superfund Research Program grant from the National Institute of Environmental Health Sciences (P42 ES013661). Research funds were also available from the Environmental Health Sciences Research Center (P30 ES 05605).
Footnotes
These studies were presented in part at the Society of Toxicology Annual Meeting in Washington, DC, March 6–10, 2011, Abstract # 1854
CONFLICT OF INTEREST All of the authors declare that there are no conflicts of interest in this study.
REFERENCES
- BADAWI AF, CAVALIERI EL, ROGAN EG. Effect of chlorinated hydrocarbons on expression of cytochrome P450 1A1, 1A2 and 1B1 and 2- and 4-hydroxylation of 17beta-estradiol in female Sprague-Dawley rats. Carcinogenesis. 2000;21:1593–9. [PubMed] [Google Scholar]
- BANDIERA S, SAFE S, OKEY AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–77. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYYA KK, BRAKE PB, ELTOM SE, OTTO SA, JEFCOATE CR. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem. 1995;270:11595–602. doi: 10.1074/jbc.270.19.11595. [DOI] [PubMed] [Google Scholar]
- BOVERHOF DR, BURGOON LD, TASHIRO C, SHARRATT B, CHITTIM B, HARKEMA JR, MENDRICK DL, ZACHAREWSKI TR. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 2006;94:398–416. doi: 10.1093/toxsci/kfl100. [DOI] [PubMed] [Google Scholar]
- BUI PH, HANKINSON O. Functional characterization of human cytochrome P450 2S1 using a synthetic gene-expressed protein in Escherichia coli. Mol Pharmacol. 2009;76:1031–43. doi: 10.1124/mol.109.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUI PH, IMAIZUMI S, BEEDANAGARI SR, REDDY ST, HANKINSON O. Human Cytochrome P450, CYP2S1, Metabolizes Cyclooxygenase - and Lipoxygenase - Derived Eicosanoids. Drug Metab Dispos. 2010 doi: 10.1124/dmd.110.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN JJ, CHEN GS, BUNCE NJ. Inhibition of CYP 1A2-dependent MROD activity in rat liver microsomes: an explanation of the hepatic sequestration of a limited subset of halogenated aromatic hydrocarbons. Environ Toxicol. 2003;18:115–9. doi: 10.1002/tox.10107. [DOI] [PubMed] [Google Scholar]
- CHIRULLI V, LONGO V, MARINI S, MAZZACCARO A, FIORIO R, GERVASI PG. CAR and PXR expression and inducibility of CYP2B and CYP3A activities in rat and rabbit lungs. Life Sci. 2005;76:2535–46. doi: 10.1016/j.lfs.2004.09.042. [DOI] [PubMed] [Google Scholar]
- CHOUDHARY D, JANSSON I, SCHENKMAN JB, SARFARAZI M, STOILOV I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- CRUNKHORN SE, PLANT KE, GIBSON GG, KRAMER K, LYON J, LORD PG, PLANT NJ. Gene expression changes in rat liver following exposure to liver growth agents: role of Kupffer cells in xenobiotic-mediated liver growth. Biochem Pharmacol. 2004;67:107–18. doi: 10.1016/j.bcp.2003.09.001. [DOI] [PubMed] [Google Scholar]
- DEB S, BANDIERA SM. Characterization and expression of extrahepatic CYP2S1. Expert Opin Drug Metab Toxicol. 2009;5:367–80. doi: 10.1517/17425250902865586. [DOI] [PubMed] [Google Scholar]
- DEB S, BANDIERA SM. Characterization of a new cytochrome P450 enzyme, CYP2S1, in rats: its regulation by aryl hydrocarbon receptor agonists. Toxicology. 2010;267:91–8. doi: 10.1016/j.tox.2009.10.025. [DOI] [PubMed] [Google Scholar]
- DERE E, BOVERHOF DR, BURGOON LD, ZACHAREWSKI TR. In vivo-in vitro toxicogenomic comparison of TCDD-elicited gene expression in Hepa1c1c7 mouse hepatoma cells and C57BL/6 hepatic tissue. BMC Genomics. 2006;7:80. doi: 10.1186/1471-2164-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWA Y, NISHIMURA J, JIN M, KAWAI M, SAEGUSA Y, HARADA T, SHIBUTANI M, MITSUMORI K. Molecular expression analysis of beta-naphthoflavone-induced hepatocellular tumors in rats. Toxicol Pathol. 2009;37:446–55. doi: 10.1177/0192623309335062. [DOI] [PubMed] [Google Scholar]
- DILIBERTO JJ, BURGIN D, BIRNBAUM LS. Role of CYP1A2 in hepatic sequestration of dioxin: studies using CYP1A2 knock-out mice. Biochem Biophys Res Commun. 1997;236:431–3. doi: 10.1006/bbrc.1997.6973. [DOI] [PubMed] [Google Scholar]
- DING X, KAMINSKY LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–73. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- HARRIS MW, MOORE JA, VOS JG, GUPTA BN. General biological effects of TCDD in laboratory animals. Environ Health Perspect. 1973;5:101–9. doi: 10.1289/ehp.7305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONKAKOSKI P, ZELKO I, SUEYOSHI T, NEGISHI M. The nuclear orphan receptor CAR-retinoid × receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–8. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMOTO T, SUEYOSHI T, ZELKO I, MOORE R, WASHBURN K, NEGISHI M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–22. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S, DERE E, BURGOON LD, CHANG CC, ZACHAREWSKI TR. Comparative analysis of AhR-mediated TCDD-elicited gene expression in human liver adult stem cells. Toxicol Sci. 2009;112:229–44. doi: 10.1093/toxsci/kfp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLIEWER SA, MOORE JT, WADE L, STAUDINGER JL, WATSON MA, JONES SA, MCKEE DD, OLIVER BB, WILLSON TM, ZETTERSTROM RH, PERLMANN T, LEHMANN JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- LAI I, CHAI Y, SIMMONS D, LUTHE G, COLEMAN MC, SPITZ D, HASCHEK WM, LUDEWIG G, ROBERTSON LW. Acute toxicity of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–23. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAN NC, KARIN M, NGUYEN T, WEISZ A, BIRNBAUM MJ, EBERHARDT NL, BAXTER JD. Mechanisms of glucocorticoid hormone action. J Steroid Biochem. 1984;20:77–88. doi: 10.1016/0022-4731(84)90192-4. [DOI] [PubMed] [Google Scholar]
- LECLUYSE EL. Pregnane × receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–9. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- MCKILLOP D, CASE DE. Mutagenicity, carcinogenicity and toxicity of beta-naphthoflavone, a potent inducer of P448. Biochem Pharmacol. 1991;41:1–7. doi: 10.1016/0006-2952(91)90003-n. [DOI] [PubMed] [Google Scholar]
- MEI Q, RICHARDS K, STRONG-BASALYGA K, FAUTY SE, TAYLOR A, YAMAZAKI M, PRUEKSARITANONT T, LIN JH, HOCHMAN J. Using real-time quantitative TaqMan RT-PCR to evaluate the role of dexamethasone in gene regulation of rat P-glycoproteins mdr1a/1b and cytochrome P450 3A1/2. J Pharm Sci. 2004;93:2488–96. doi: 10.1002/jps.20102. [DOI] [PubMed] [Google Scholar]
- NAGATA K, OGINO M, SHIMADA M, MIYATA M, GONZALEZ FJ, YAMAZOE Y. Structure and expression of the rat CYP3A1 gene: isolation of the gene (P450/6betaB) and characterization of the recombinant protein. Arch Biochem Biophys. 1999;362:242–53. doi: 10.1006/abbi.1998.1030. [DOI] [PubMed] [Google Scholar]
- NANNELLI A, CHIRULLI V, LONGO V, GERVASI PG. Expression and induction by rifampicin of CAR- and PXR-regulated CYP2B and CYP3A in liver, kidney and airways of pig. Toxicology. 2008;252:105–12. doi: 10.1016/j.tox.2008.08.004. [DOI] [PubMed] [Google Scholar]
- NANNELLI A, ROSSIGNOLO F, TOLANDO R, ROSSATO P, LONGO V, GERVASI PG. Effect of beta-naphthoflavone on AhR-regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and antioxidant enzymes in various brain regions of pig. Toxicology. 2009;265:69–79. doi: 10.1016/j.tox.2009.09.010. [DOI] [PubMed] [Google Scholar]
- NEBERT DW, DALTON TP, OKEY AB, GONZALEZ FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–50. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- NISHIDA CR, LEE M, DE MONTELLANO PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol Pharmacol. 78:497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVERA SP, SAARIKOSKI ST, HANKINSON O. Identification of a novel dioxin-inducible cytochrome P450. Mol Pharmacol. 2002;61:255–9. doi: 10.1124/mol.61.2.255. [DOI] [PubMed] [Google Scholar]
- RIVERA SP, WANG F, SAARIKOSKI ST, TAYLOR RT, CHAPMAN B, ZHANG R, HANKINSON O. A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of cytochrome P4502S1 by both dioxin and hypoxia. J Biol Chem. 2007;282:10881–93. doi: 10.1074/jbc.M609617200. [DOI] [PubMed] [Google Scholar]
- RYLANDER T, NEVE EP, INGELMAN-SUNDBERG M, OSCARSON M. Identification and tissue distribution of the novel human cytochrome P450 2S1 (CYP2S1) Biochem Biophys Res Commun. 2001;281:529–35. doi: 10.1006/bbrc.2001.4390. [DOI] [PubMed] [Google Scholar]
- SAARIKOSKI ST, RIVERA SP, HANKINSON O, HUSGAFVEL-PURSIAINEN K. CYP2S1: a short review. Toxicol Appl Pharmacol. 2005;207:62–9. doi: 10.1016/j.taap.2004.12.027. [DOI] [PubMed] [Google Scholar]
- SAFE S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- SAFE SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- SAITO K, KOBAYASHI K, MIZUNO Y, FUKUCHI Y, FURIHATA T, CHIBA K. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonists induce constitutive androstane receptor (CAR) and cytochrome P450 2B in rat primary hepatocytes. Drug Metab Pharmacokinet. 2010;25:108–11. doi: 10.2133/dmpk.25.108. [DOI] [PubMed] [Google Scholar]
- SCHRAMM H, ROBERTSON LW, OESCH F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol. 1985;34:3735–9. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- SESARDIC D, COLE KJ, EDWARDS RJ, DAVIES DS, THOMAS PE, LEVIN W, BOOBIS AR. The inducibility and catalytic activity of cytochromes P450c (P450IA1) and P450d (P450IA2) in rat tissues. Biochem Pharmacol. 1990;39:499–506. doi: 10.1016/0006-2952(90)90056-q. [DOI] [PubMed] [Google Scholar]
- SMITH G, WOLF CR, DEENI YY, DAWE RS, EVANS AT, COMRIE MM, FERGUSON J, IBBOTSON SH. Cutaneous expression of cytochrome P450 CYP2S1: individuality in regulation by therapeutic agents for psoriasis and other skin diseases. Lancet. 2003;361:1336–43. doi: 10.1016/S0140-6736(03)13081-4. [DOI] [PubMed] [Google Scholar]
- STEPHEN FD, DRAHUSHUK AT, OLSON JR. Cytochrome P450 1A1 induction in rat lymphoid tissues following in vivo and in vitro exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin requires protein kinase C. Toxicology. 1997;124:39–51. doi: 10.1016/s0300-483x(97)00128-5. [DOI] [PubMed] [Google Scholar]
- SUEYOSHI T, NEGISHI M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–43. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA Y, NAKAJIMA M, TAKAGI S, TANIYA T, YOKOI T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–8. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- VAN DEN BERG M, BIRNBAUM LS, DENISON M, DE VITO M, FARLAND W, FEELEY M, FIEDLER H, HAKANSSON H, HANBERG A, HAWS L, ROSE M, SAFE S, SCHRENK D, TOHYAMA C, TRITSCHER A, TUOMISTO J, TYSKLIND M, WALKER N, PETERSON RE. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VONDRACEK J, SVIHALKOVA-SINDLEROVA L, PENCIKOVA K, KRCMAR P, ANDRYSIK Z, CHRAMOSTOVA K, MARVANOVA S, VALOVICOVA Z, KOZUBIK A, GABELOVA A, MACHALA M. 7H-Dibenzo[c,g]carbazole and 5,9-dimethyldibenzo[c,g]carbazole exert multiple toxic events contributing to tumor promotion in rat liver epithelial 'stem-like' cells. Mutat Res. 2006;596:43–56. doi: 10.1016/j.mrfmmm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- ZHANG H, LECULYSE E, LIU L, HU M, MATONEY L, ZHU W, YAN B. Rat pregnane × receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]