Abstract
Dishevelled (Dsh or Dvl) is an important signaling protein, playing a key role in Wnt signaling and relaying cellular information for several developmental pathways. Dsh is highly conserved among metazoans and has expanded into a multigene family in most bilaterian lineages, including vertebrates, planarians, and nematodes. These orthologs, where explored, are known to have considerable overlap in function, but evidence for functional specialization continues to mount. We performed a comparative analysis of Dsh across animals to explore protein architecture and identify conserved and divergent features that could provide insight into functional specialization with an emphasis on invertebrates, especially nematodes. We find evidence of dynamic evolution of Dsh, particularly among nematodes, with taxa varying in ortholog number from one to three. We identify a new domain specific to some nematode lineages and find an unexpected nuclear localization signal conserved in many Dsh orthologs. Our findings raise questions of protein evolution in general and provide clues as to how animals have dealt with the complex intricacies of having a protein, such as Dsh, act as a central messenger hub connected to many different and vitally important pathways. We discuss our findings in the context of functional specialization and bring many testable hypotheses to light.
Keywords: dishevelled, protein evolution, Nematoda, Wnt, C. elegans
Dishevelled (Dsh or Dvl) is a multifunctional phosphoprotein originally discovered in Drosophila and named for its disruptions in hair and bristle polarity (Fahmy and Fahmy 1959; Klingensmith et al. 1994). Dsh plays a key role in Wnt signaling, thus affecting cell proliferation, migration, polarity, terminal differentiation, and the self-renewal of stem cells (Boutros and Mlodzik 1999; Gao and Chen 2010; Sugimura et al. 2012; Tauriello et al. 2012). Deregulation of pathway components is associated with multiple human diseases (Luo et al. 2007). Wnt signaling has evolved to act in multiple pathways, broadly divided into the canonical/β-catenin−dependent pathway and the noncanonical/β-catenin−independent pathway, with Dsh acting in a key role, relaying signals from receptors to downstream effectors (Gao and Chen 2010). Several components of the Wnt signaling pathway, including Frizzled, GSK3, and β-catenin, can be found in protozoans, but it is not until the emergence of Metazoa that a complete Wnt pathway can be found (Holstein 2012). The early branching metazoan lineage Porifera has only the major components of the canonical pathway, with critical noncanonical pathway components arising subsequently in eumetazoan lineages (Adamska et al. 2007, 2010; Kusserow et al. 2005). Thus, although Wnt signaling is conserved across Metazoa from sponges to humans, it seems that this pathway’s origin and the original role of Dsh lies in the canonical/β-catenin−dependent pathway, with noncanonical signaling developing later. Figure 1 summarizes the evolution of Dsh across animals. including the loss and gain of orthologs, paralogs, and protein domains.
Figure 1 .
A cladogram showing key features during the evolution of Dsh among animals including the origin of Dsh and lineage-specific paralogs as well as the gain or loss of protein domains.
In the current model of the canonical pathway, Wnt signals are received by a Frizzled (Fz) and the LRP5/6 coreceptor complex, leading to the recruitment of Dsh and Axin to the cell membrane. This recruitment results in the inactivation and dissolution of the β-catenin destruction complex, allowing for the nuclear translocation of β-catenin, where it interacts with members of the TCF/Lef transcription family to regulate gene expression (Wharton 2003). Noncanonical signaling encompasses several different pathways that do not necessarily lead to the activation of β-catenin, of which the best understood is the planar cell polarity (PCP) pathway. PCP signaling is responsible for the polarization of cells along an epithelial sheet. The core components of this pathway include the transmembrane proteins Fz, Flamingo (Fmi), and Strabismus (Stbm), as well as the cytoplasmic proteins Diego (Dgo), Prickle (Pk), and Dsh (Seifert and Mlodzik 2007). In general PCP signaling relies on complex interactions between these core components that lead to their asymmetric enrichment and distribution within a cell. For example, during polarization in the Drosophila wing two distinct protein complexes antagonize each other and localize to opposite ends of the cell: a Fz-Dsh-Dgo complex becomes enriched at the distal end of each cell, whereas a Stbm-Pk complex concentrates proximally (Simons and Mlodzik 2008).
The literature establishes the archetypal Dsh protein to contain three conserved domains: an N-terminal DIX (Dishevelled and Axin) domain, a central PDZ (Post Synaptic Density-95, Discs Large, and Zonula occludens-1) domain, and a C-terminal DEP (Dishevelled, EGL-10, Pleckstrin) domain (Figure 2) (Gao and Chen 2010; Penton et al. 2002). In addition to these three conserved domains, Dsh is known to contain a basic region that precedes the N-terminus of the PDZ domain as well as a proline-rich region that includes an SH3 binding motif located between the PDZ and DEP domains (Figure 2). Both of these regions are thought to be conserved in most Dsh orthologs and have functional significance. There is a fourth domain reported to be conserved in Dsh, the DSV or Dishevelled domain, although its functional significance is rarely discussed (Figure 2). The Dsh protein contains approximately 15% serine and threonine residues, many of which are phosphorylated; however, the functional significance of these residues has been questioned (Yanfeng et al. 2011). Expansions of Dsh among metazoan lineages seem common and variable, with most vertebrates containing three Dsh homologs (although the chicken Gallus gallus has two), whereas insects have only one (Gao and Chen 2010; Gray et al. 2009; Klingensmith et al. 1994; Sweetman et al. 2008). The nematode Caenorhabditis elegans has three Dsh homologs, and the planarian Schmidtea mediterranea has two (Figure 1) (Gurley et al. 2008; Ruvkun and Hobert 1998). Table 1 lists the taxa and Dsh ortholog abbreviations used throughout the text.
Figure 2 .

Diagram showing the archetypal Dsh protein with conserved domains and motifs. From left to right: the DIX domain, the DSV or dishevelled domain, the basic region, the PDZ domain, the SH3 binding motif, which is often referred to as the proline-rich region, and the DEP domain.
Table 1. Dsh abbreviations.
| Species | Abbreviation(s) |
|---|---|
| Nematoda | |
| Meloidogyne incognita | Minc-dsh-1, Minc-mig-5 |
| Meloidogyne hapla | Minc-dsh-1, Minc-mig-5 |
| Panagrellus redivivus | Pred-dsh-1, Pred-mig-5 |
| Bursaphelenchus xylophilus | Bxyl-dsh-1, Bxyl-mig-5 |
| Steinernema carpocapsae | Scar-dsh-1, Scar-mig-5 |
| Caenorhabditis briggsae | Cbri-dsh-1, Cbri-dsh-2, Cbri-mig-5 |
| Caenorhabditis remanei | Crem-dsh-1, Crem-dsh-1, Crem-mig-5 |
| Caenorhabditis brenneri | Cbre-dsh-1, Cbre-dsh-2, Cbre-mig-5 |
| Caenorhabditis elegans | Cele-dsh-1, Cele-dsh-2, Cele-mig-5 |
| Caenorhabditis japonica | Cjap-dsh-1, Cjap-dsh-2, Cjap-mig-5 |
| Caenorhabditis angaria | Cang-dsh-1, Cang-mig-5 |
| Pristionchus pacificus | Ppac-dsh-1, Ppac-mig-5 |
| Brugia malayi | Bmal-dsh-1, Bmal-mig-5 |
| Ascaris suum | Asuu-dsh-1, Asuu-mig-5 |
| Trichinella spiralis | Tspi-dsh-1 |
| Arthropoda | |
| Nasonia vitripennis (parasitoid wasp) | Nvit-dsh |
| Tribolium castaneum (red flour beetle) | Tcas-dsh |
| Drosophila melanogaster (fruit fly) | Dmel-dsh |
| Platyhelminthes | |
| Schmidtea mediterraneaa (planaria) | Smed-dsh-1, Smed-dvl-2 |
| Chordata | |
| Xenopus tropicalis | Xtro-Dvl1, Xtro-Dvl2, Xtro-Dvl3 |
| Mus musculus | Mmus-Dvl1, Mmus-Dvl2, Mmus-Dvl3 |
| Homo sapiens | Hsap-Dvl1, Hsap-Dvl2, Hsap-Dvl3 |
| Ciona intestinalisa (sea squirt) | Cint-Dvl |
| Cnidaria | |
| Clytia hemisphaericaa (jellyfish) | Chem-Dvl |
| Porifera | |
| Amphimedon queenslandicaa (sponge) | Aque-Dvl |
All taxa analyzed along with their taxonomic phylum and the abbreviations used to represent their Dsh orthologs.
Dsh orthologs for these taxa were taken from Genbank rather than identified through whole genome searches.
With the ever-increasing amount of genomic data available for analysis, we leveraged the currently available data to study the evolution of Dsh across animals with an emphasis on nematodes. In addition to exploring the potential conservation of the three C. elegans Dsh homologs among nematodes, we were interested in identifying conserved or divergent protein features that correlate with the known functional divergence between Dsh orthologs observed in several animal taxa and that could provide hypotheses about the evolution of Dsh. For example, the planarian Dsh paralogs, Smed-dvl-1 and Smed-dvl-2, appear to be functionally specialized such that only Smed-dvl-2 is thought to be involved in β-catenin−dependent signal transduction, suggesting underlying physical differences in these proteins that have not yet been linked to their divergent function (Almuedo-Castillo et al. 2011). Similarly, the function of Dsh orthologs seems to have diverged among vertebrates, where Dvl1 and Dvl2, but not Dvl3, are necessary to mediate the Wnt-dependent signals that control neural crest specification in Xenopus, but in murines it is thought that Dvl2 and Dvl3 function in neural crest development whereas Dvl1 apparently does not (Etheridge et al. 2008; Gray et al. 2009; Hamblet et al. 2002; Lijam et al. 1997; Monsoro-Burq et al. 2005). It is still not known whether Dsh’s role in neural crest development is through the canonical or noncanonical pathways or both (Etheridge et al. 2008). In our study we found that Dishevelled is a highly conserved protein that has undergone dynamic evolution across metazoans and variation in protein architecture provides clues about its functional roles in β-catenin−dependent and −independent pathways.
Materials and Methods
Orthology analyses
To study the evolution of Dishevelled, we used the available predicted protein datasets from WormBase release WS225 (www.wormbase.org) for the following species: Brugia malayi, Caenorhabditis elegans, Caenorhabditis angaria, Caenorhabditis japonica, Caenorhabditis brenneri, Caenorhabditis remanei, Caenorhabditis briggsae, Meloidogyne hapla, Pristionchus pacificus, and Trichinella spiralis. We also included the Ascaris suum, Bursaphelenchus xylophilus, and Meloidogyne incognita predicted proteome data sets from WormBase release WS229. For outgroup and comparative analysis, we used the predicted protein datasets of Arabidopsis thaliana (vGNOMON 7/9/07), Drosophila melanogaster (v10/30/11), Homo sapiens (v9/7/11), Mus musculus (v3/4/11), Nasonia vitripennis (v1.2), Saccharomyces cerevisiae (v2/3/11), and Tribolium castaneum (vTcas 3.0) genome projects, obtained from the National Center for Biotechnology Information/National Institutes of Health repository (ftp://ftp.ncbi.nih.gov/genomes). Pre-released proteomes for Panagrellus redivivus and Steinernema carpocapsae also were used from manuscripts in preparation (A. R. Dillman, A. Mortazavi, M. Macchietto, C. F. Porter, and H. Goodrich-Blair, unpublished data; Srinivasan et al. 2013; Dillman et al. 2012) Dsh orthologs from the jellyfish Clytia hemisphaerica (AFI99114.1), the planarian Schmidtea mediterranea (Smed-DVL-1 ADZ58511.1 and Smed-DVL-2 ADZ58512.1), the frog Xenopus tropicalis (DVL1 NP_001116886.1, DVL2 NP_001072660.1, and DVL3 NP_01116929.1), the sponge Amphimedon queenslandica (XP_003384321), and the tunicate Ciona intestinalis (NP_001027754.1) were acquired from GenBank (http://blast.ncbi.nlm.nih.gov/).
Version 1.4 of the OrthoMCL pipeline was used to cluster proteins from the proteomes into families of orthologous genes (http://www.orthomcl.org) (Li et al. 2003). To identify orthologs of Dsh across animals, we ran OrthoMCL using the full proteomes of C. elegans, P. redivivus, T. spiralis, N. vitripennis, D. melanogaster, T. castaneum, M. musculus, H. sapiens, S. cerevisiae, and A. thaliana. To identify orthologs across Nematoda, we ran OrthoMCL using the full proteomes of B. malayi, A. suum, P. pacificus, C. elegans, B. xylophilus, M. hapla, M. incognita, P. redivivus, S. carpocapsae, T. spiralis, with N. vitripennis as an outgroup. To identify orthologs within Caenorhabditis we ran OrthoMCL using the full proteomes of C. angaria, C. briggsae, C. brenneri, C. japonica, C. remanei, and C. elegans. All orthology analyses were run using OrthoMCL version 1.4 with default settings and the BLAST parameters recommended in the OrthoMCL documentation (Li et al. 2003).
Domain analysis
Each identified Dsh ortholog was analyzed for protein domains using the SMART protein domain analysis website (http://smart.embl-heidelberg.de), used in normal mode (Letunic et al. 2012). All additional options (outlier homologs, PFAM domain, signal peptides, internal repeats, and intrinsic protein disorder) were turned on for the analysis. The full protein sequences and identified domains are available in the Supporting Information, File S1.
Sequence alignment, phylogenetics, and selection detection
Sequence alignments were made using all of the amino acid sequence from the beginning of the PDZ domain to the end of the DEP domain because these were the only identified domains conserved across all of the animal taxa we evaluated. Protein sequence alignments of this region were made using the online MUSCLE service (http://www.ebi.ac.uk/Tools/msa/muscle) (Edgar 2004). These protein alignments were then replaced with the appropriate nucleotide sequences using the RevTrans server (http://www.cbs.dtu.dk/services/RevTrans), which preserves the alignment obtained from the amino acid sequences but replaces each amino acid with the user-supplied protein coding nucleotides (Wernersson and Pedersen 2003). Coding sequence for the proteomes was downloaded along with the proteomes from the sites listed previously, although the sequences for most of the nematodes we used could also be acquired from WormBase (www.wormbase.org). Two separate alignments were made using this method, one that included the Dsh orthologs across animals, including T. castaneum, N. vitripennis, D. melanogaster, M. musculus, H. sapiens, T. spiralis, A. suum, B. malayi, P. pacificus, C. elegans, S. carpocapsae, B. xylophilus, P. redivivus, M. hapla, M. incognita, and the jellyfish C. hemisphaerica. The other alignment focused on Dsh orthologs within caenorhabditid nematodes, using genes from C. elegans, C. angaria, C. japonica, C. brenneri, C. remanei, C. briggsae, with the intracellular parasite T. spiralis and the parasitoid wasp N. vitripennis as outgroups. Alignments were then shaded to reflect sequence conservation using GeneDoc (http://www.nrbsc.org/gfx/genedoc) (Nicholas et al. 1997).
The nucleotide alignments were then evaluated for the best-fit model of evolution using jModelTest2 (http://code.google.com/p/jmodeltest2) (Darriba et al. 2012; Guindon and Gascuel 2003). For the alignment across animals, the corrected Akaike information criterion, the Bayesian inference criterion, and the decision theory criterion all selected the GTR+I+G model of evolution, with a p-invar = 0.0640 and a gamma shape parameter of 0.9840. The analysis of the Caenorhabditis alignment resulted in the GTR+G model being chosen by all criteria, with a gamma shape parameter of 0.5140.
Following model selection, maximum likelihood (ML) analyses with 1000 bootstraps were done using the RAxML BlackBox server (http://phylobench.vital-it.ch/raxml-bb) (Stamatakis et al. 2008). New technology parsimony analyses were done using TNT (http://www.cladistics.com/aboutTNT.html) (Goloboff 1999; Nixon 1999). Maxtrees was set to 10,000. A new technology, random driven search was performed using ratchet, drift, and tree fusing options. A bootstrap analysis of 1000 was performed by resampling.
Selection was detected using two methods. First, the alignment files of the protein-coding nucleotide sequences were uploaded into MEGA 5.05 (http://www.megasoftware.net) (Tamura et al. 2011). The selection analysis option in MEGA, which estimates selection for each codon using HyPhy, was used. Our ML analysis served as the guide tree, and the ML statistical method was chosen using the GTR model, as selected by jModelTest2. All sites were used in the analysis. Following the MEGA analysis of selection, we used the HyPhy package as implemented by the Datamonkey adaptive evolutionary server (http://www.datamonkey.org). Alignment files with the ML phylogenetic analysis written into them were uploaded using the codon data type and the universal genetic code. We used the recommended meme method in our analyses, setting the options to estimate the global dN/dS value and to average encountered ambiguities in the consensus sequence (Murrell et al. 2012). We set the level of significance at P = 0.1.
Results
Dishevelled conservation and expansion among animals
We evaluated the conservation and potential expansion of Dsh by using cluster analysis of seventeen whole proteomes, including vertebrates, insects, nematodes, and a fungal and plant proteome as outgroups (see Materials and Methods). We found no evidence of Dsh or Dsh-like genes outside Metazoa. It was previously known that D. melanogaster and potentially all insects have one Dsh (Dmel-dsh), the model nematode C. elegans has three Dsh homologs (Cele-dsh-1, Cele-dsh-2, and Cele-mig-5), the planarian Schmidtea mediterranea has two (Smed-dvl-1 and Smed-dvl-2), and most vertebrates have three (Dvl1, Dvl2, and Dvl3; Figure 1). We found three distinct clusters of Dsh genes, the largest included all of the insect orthologs (Dmel-dsh, Nvit-dsh, Tcas-dsh; one copy in each insect proteome), all nematode dsh-1 orthologs, and the vertebrate orthologs of Dvl1 and Dvl3 (Mmus-Dvl1, Mmus-Dvl-3, Hsap-Dvl1, and Hsap-Dvl3). A second cluster included exclusively nematode mig-5 genes, whereas the vertebrate Dvl2 orthologs (Mmus-Dvl2 and Hsap-Dvl2) formed their own cluster, apparently having no orthologs outside vertebrates. Cele-dsh-2 remained an unclustered orphan in this broad analysis.
Using the three C. elegans Dsh homologs (Cele-dsh-1, Cele-dsh-2, and Cele-mig-5) as queries, we found that only Cele-dsh-1 has orthologs outside of Nematoda, being highly conserved across metazoans, with all insects and nematodes having only one strict ortholog, and vertebrates having two, Dvl1 and Dvl3. Among the nematode genera in this analysis, C. elegans is unique in having three Dsh homologs. In addition to having an ortholog of dsh-1, most nematodes also have an ortholog of mig-5, but none of the nematodes in this analysis have orthologs of Cele-dsh-2. Unlike the rest of the nematodes we studied, T. spiralis, which is in the basal clade 2 of Nematoda, retains only a single Dsh ortholog (Tspi-dsh-1) (Figure 3) (Holterman et al. 2006). This result shows that Dsh has experienced dynamic evolution within Nematoda, with extant taxa possessing one, two, or three Dsh homologs.
Figure 3 .
A schematic representation of the division of the phylum Nematoda into clades, with the 12-clade designation after Holterman et al. 2006 (Holterman et al. 2006) and the five-clade designation after Blaxter et al. 1998 (Blaxter et al. 1998) in Roman numerals. Blaxter clades are encompassed in colored boxes.
In evaluating the relationships among Dsh genes across animals, we included known Dsh homologs from organisms for which we did not perform whole-genome analyses (C. hemisphaerica, S. mediterranea, and X. tropicalis). We found that each of the three vertebrate Dsh orthologs shares ancestry and that the planarian S. mediterranea and the nematode T. spiralis Dshs (Smed-dvl-1, Smed-dvl-2, and Tspi-dsh-1) are not very similar to other Dsh homologs (Figure 4). The rest of the nematode Dsh orthologs in this analysis formed two distinct clades, with the mig-5 orthologs forming one clade, and the dsh-1 orthologs forming the other (Figure 4). Although we did not find any proteins with significant similarity to Cele-dsh-2 in our broad clustering analysis, the phylogenetic analyses place it in the clade containing all nematode dsh-1 orthologs (Figure 4). These C. elegans paralogs, Cele-dsh-1 and Cele-dsh-2, are approximately 130 kb apart on chromosome II, have the same orientation, and form a conserved gene cluster with a recombination frequency of 0.61%. Together with the phylogenetic analyses, this suggests that Cele-dsh-2 is a diverging duplication of Cele-dsh-1 and is specific to C. elegans or perhaps the Caenorhabditis lineage.
Figure 4 .
Phylogenetic analysis of Dsh orthologs across animals based on the protein coding nucleotide alignment from the N-terminus of the PDZ domain through the C-terminus of the DEP domain. The ML tree (rooted with the outgroup C. hemisphaerica) is shown. For each node, ML bootstrap support values (1000 replicates) are above the nodes whereas parsimony bootstrap values (1000 replicates) are written below. Support values ≤70 are not shown.
To evaluate the apparent expansion of Dsh among caenorhabditids and determine the origin of dsh-2, we performed a cluster analysis of whole caenorhabditid proteomes, including C. angaria, C. japonica, C. elegans, C. brenneri, C. remanei, and C. briggsae. The relationships among Caenorhabditis nematodes are becoming increasingly refined as more species are described (Kiontke et al. 2011). Our analysis includes members of both the Elegans and Drosophilae supergroups within the Caenorhabditis genus, and resulted in three unsurprising clusters of Dsh genes: dsh-1, dsh-2, and mig-5. We found that all caenorhabditids have dsh-1 and mig-5 orthologs but that C. angaria lacks a dsh-2 ortholog, suggesting that only members of the Elegans supergroup (C. japonica, C. elegans, C. brenneri, C. remanei, and C. briggsae) have dsh-2 orthologs. Furthermore, this clustering analysis revealed the possibility of species-specific expansions. For example, C. angaria appeared to have two potential dsh-1 orthologs, C. japonica appeared to have two dsh-1 orthologs and two dsh-2 orthologs, and C. brenneri appeared to have two orthologs each of dsh-1, dsh-2, and mig-5. Detailed protein analyses of this kind rely on the quality of the assemblies and gene predictions of the proteomes used, and the results can often improve the annotations. Despite valiant efforts to inbreed these nematodes before genomic sequencing, the current assemblies of C. brenneri, C. remanei, and C. japonica (WormBase release WS225) are known to have considerable heterozygosity, with some genes being represented by allelic variants (Barrière et al. 2009). Furthermore, the genome assembly for C. angaria is still quite fragmented (Mortazavi et al. 2010), although additional sequencing is ongoing. We explore these genes in more detail in the following section.
The gene relationships among the Caenorhabditis Dsh paralogs is consistent with those recovered using a broader sampling of animal taxa (Figure 4 and Supporting Information, Figure S1): we recapitulate three clades, one for each of the three paralogs with dsh-1 and dsh-2 being more closely related, supporting the notion that dsh-2 is the result of a fairly recent duplication event and has subsequently diverged from dsh-1. This duplication could have occurred after the split between the Elegans and Drosophilae supergroups, or may have occurred earlier and been subsequently lost in C. angaria. More could be inferred about the evolution of Dsh among caenorhabditids from sequencing additional taxa from this genus.
Conservation and diversification of Dsh domain architecture
Next, we wanted to assess the protein domains in Dsh and evaluate the conservation of domain structure across animal evolution among orthologs and paralogs. The SMART database recognizes the DIX, DSV, PDZ, and DEP domains as being approximately 80, 72, 80, and 75 amino acids, respectively, with some variation between species, particularly in the DIX and DSV domains (Figures 2, 5, and 6). The basic region located between DIX and PDZ, as well as the proline-rich region containing an SH3 binding domain located between PDZ and DEP are not recognized by SMART, but they were identifiable by sequence alignment similarity with known sequences (Penton et al. 2002).
Figure 5 .
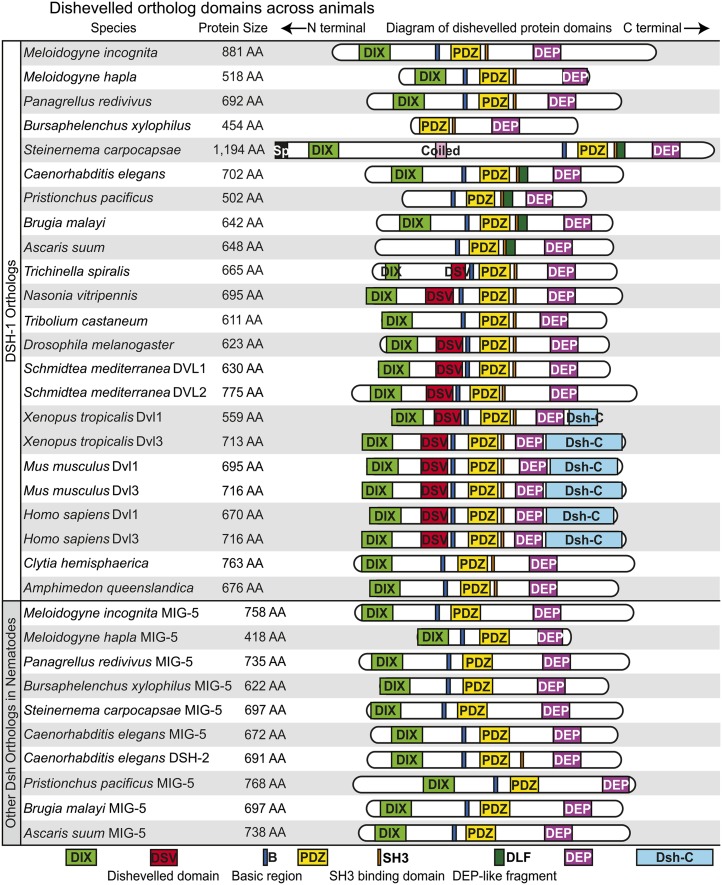
Schematic diagram of Dsh orthologs in selected animal species. Proteins and their domains are drawn in proportion to the number of amino acids they contain.
Figure 6 .
Schematic diagram of Dsh orthologs in sequenced Caenorhabditis species. Proteins and their domains are drawn in proportion to the number of amino acids they contain. †These C. brenneri proteins are thought to be splice isoforms or perhaps allelic variants and not paralogous duplicates. *Cjap-DSH-1a and Cjap-DSH-1b although presently annotated as separate genes, we suggest they are fragments of the same protein rather than two different orthologs of DSH-1. This is not the case with Cjap-DSH-2a and Cjap-DSH-2b, which likely are separate proteins.
We found the PDZ and DEP domains to be the most highly conserved structural components of Dsh across taxa, being present in all taxa from the sponge A. queenslandica to mammals (Figure 5 and File S1). The basic region, just anterior to the PDZ domain, is also highly conserved and only absent from the Bxyl-dsh-1 ortholog, which is truncated. The proline-rich region extends over an approximately 20 amino acid window and contains a class I core SH3 binding motif RxEPV/IR/QP (where x stands for any amino acid), with ligand preference varying around the PxxP core (Penton et al. 2002). Although the proline-rich region is not always conserved, the SH3 binding domain is conserved in the dsh-1 orthologs of all taxa, but is absent in nematode mig-5 orthologs (Figure 5, Figure 6, Figure S2, and Figure S3). We refer to this region as the SH3 binding motif rather than the proline-rich region due to the conservation of the motif across taxa although the area surrounding the motif is not necessarily proline-rich in some nematode taxa. The DIX domain is conserved in nearly all Dsh orthologs but is conspicuously missing from two nematode dsh-1 orthologs; Ppac-dsh-1 and Asuu-dsh-1 (Figure 5). The understudied DSV domain appears to have experienced dynamic evolution, being absent from both sponge and jellyfish taxa and arising in bilaterian taxa (Figure 1, Figure 5, and File S1). The DSV domain is conserved in planaria, vertebrates, and two of the three insect taxa we investigated (Dmel-dsh and Nvit-dsh) but is missing from Tcas-dsh and is absent from all nematode Dsh homologs except Tspi-dsh-1, the only Dsh homolog in the most basal nematode lineage included in our analysis (Figure 3) (Holterman et al. 2006). The Dsh-C domain is vertebrate specific but appears to be truncated in Xtrop-Dvl1 (Figure 5). We found a previously unreported DEP-like fragment (DLF) domain, recognized by the SMART database, and is present and conserved in several nematode species from clades 8, 9, and at least one species, S. carpocapsae, from nematode clade 10 (Figures 3 and 5). The amino acid sequence conservation and codon variation that we detect both in the DSV and DLF domains suggest that these are functionally relevant, despite the current lack of functional data (Figure S2, Figure S3, and File S1). The absence of a recognizable DSV domain in early branching lineages (i.e., A. queenslandica and C. hemisphaerica), and its apparent loss in T. castaneum and all evaluated nematode lineages branching after clade 2 suggest that its conservation among some insects, planarians, and vertebrates has functional significance and should be tested. Similarly, the conservation of DLF among clades 8, 9, and at least one clade 10 nematode (S. carpocapsae), suggest that it too has functional significance.
The domain architecture of Dsh orthologs within the Caenorhabditis genus is more dynamic than that observed across a broader sampling of animals, likely facilitated by the presence of three Dsh orthologs (Figures 5 and 6). The PDZ and DEP domains are the most highly conserved across caenorhabditid orthologs, with both only being absent from Cjap-dsh-1a and Cang-dsh-1b. The DEP domain is missing from Cang-mig-5, and PDZ and DEP are separated between the Cjap-dsh-2a and Cjap-dsh-2b (Figure 6 and Figure S3). The basic region is also highly conserved and is present in all orthologs with protein sequence N-terminal to the PDZ domain (Figure 6). The SH3 binding motif is conserved in dsh-1 and dsh-2 orthologs that contain protein sequence C-terminal to the PDZ and/or N-terminal to the DEP domain, but is entirely absent from all mig-5 orthologs (Figure 6 and Figure S3). The newly discovered DLF domain, where present, is between the PDZ and DEP domains, just C-terminal to the SH3 binding motif. The DLF domain is only present in nematode dsh-1 orthologs, and is present in all nematode dsh-1 orthologs that have PDZ and DEP domains except Cbre-dsh-1a, where it is conspicuously missing.
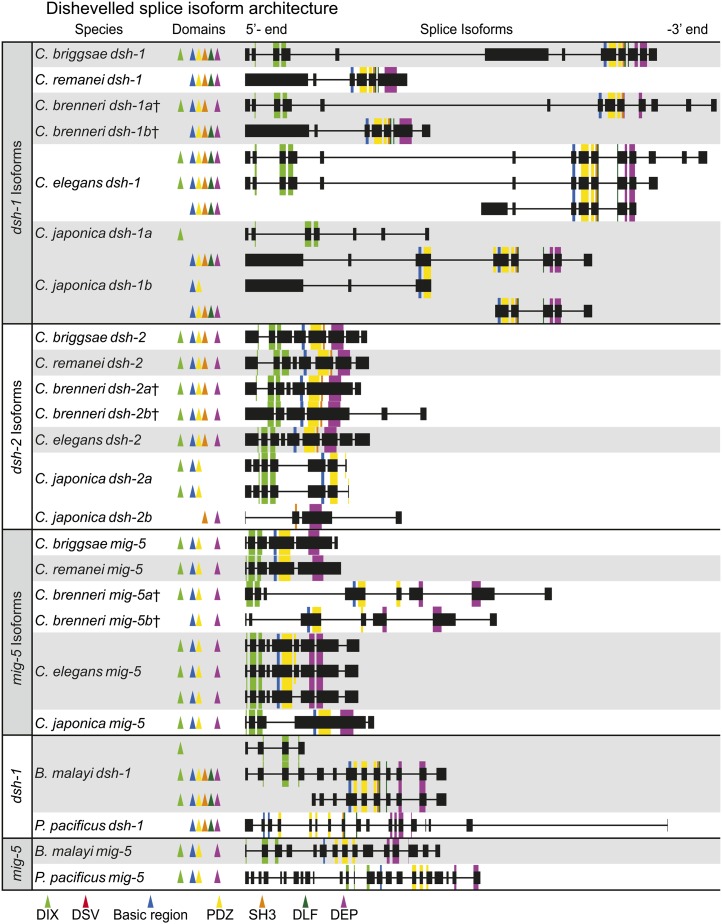
We investigated the splice isoforms of all three Dsh paralogs within Caenorhabditis and used B. malayi and P. pacificus for outgroup comparison (Figure 7). There is conserved isoform architecture among these species for all three paralogs, although no species has been as thoroughly studied as C. elegans, which has three isoforms of Cele-dsh-1 as well as Cele-mig-5 (Figure 7). For example, all dsh-1 isoforms that have a DIX domain have it split across three exons, whereas the PDZ domain appears to be split across two exons in all caenorabditid taxa except C. japonica, where it might be split across three. In addition to partitioning domains among proteins, as P. pacificus seems to have done with the DIX domain being present in Ppac-dsh-1 and absent from Ppac-mig-5, other taxa can produce isoforms with and without certain domains (e.g., Cele-dsh-1 and Bmal-dsh-1; Figure 7). Too little is known about splice isoforms in the other species to draw strong conclusions from these data, but interesting features of conservation and divergence are apparent. Additionally, this analysis sheds light on the potential paralogs identified within C. japonica and C. brenneri. Cjap-dsh-1a and Cjap-dsh-1b are tandem in the same orientation, with Cjap-dsh-1a being <3 kb upstream from Cjap-dsh-1b, suggesting that these are fragments of the same gene (Figures 6 and 7). However, Cjap-dsh-2a and Cjap-dsh-2b, although still in the same orientation, are >10 kb apart with Cjap-dsh-2b, which has the DEP domain, being upstream of Cjap-dsh-2b, inverting the traditional order of DIX, PDZ, and then DEP, suggesting that these might actually be separate genes, representing a physical partitioning of DIX, PDZ, and the basic region on one protein and the SH3 binding motif and DEP on the other (Figures 6 and 7). The potential paralogs within C. brenneri (Cbre-dsh-1a, Cbre-dsh-1b, Cbre-dsh-2a, Cbre-dsh-2b, Cbre-mig-5a, and Cbre-mig-5b) were each on separate contigs, offering no potential clarification. However, a nucleotide alignment of the PDZ and DEP domains revealed that each paralogous pair has identical nucleotide sequence, suggesting that these are likely allelic variants or splice isoforms (Barrière et al. 2009). Splice isoforms seem particularly likely in cases in which a paralogous pair differ in domain content (e.g., Cbre-dsh-1a, Cbre-dsh-1b, and Cbre-mig-5a, and Cbre-mig-5b).
Figure 7 .
The known Dsh splice isoform architecture for dsh-1, dsh-2, and mig-5 among caenorhabditids. All features (exons, introns, and domains) are drawn in proportion to the number of nucleotides they contain. All isoforms are shown in the same orientation, regardless of their actual orientation in their respective genomes. The known isoforms of Bmal-dsh-1, Bmal-mig-5, Ppac-dsh-1, and Ppac-mig-5 are included at the bottom for outgroup comparison.
The amino acid sequence alignments of Dsh across animals and across caenorhabditids show clear regions of high conservation and other regions with considerable divergence. Across animals, we detect at least two codons that are experiencing strong negative selection (codons 318 and 335) and at least 10 codons that are experiencing diversifying selection (codons 6, 221, 222, 234, 235, 238, 239, 252, 284, and 354; Figure S2). Focusing on caenorhabditids, we detect at least three codons experiencing negative selection (codons 113, 252, and 261) and at least four codons that are experiencing diversifying selection (codons 63, 85, 155, and 193; Figure S3). It is not surprising that areas of functional significance are highly conserved across species, whereas those regions that show considerable divergence or are experiencing diversifying selection may play important roles in the acquisition of novel functions but remain to be functionally tested.
Nuclear transport
In addition to the conserved elements of Dsh shown in Figure 2, there are other motifs, structural components, and phosphorylation sites that affect the function of Dsh. For example, the presence of a nuclear export signal (NES) and a nuclear localization signal (NLS) affect the subcellular distribution of Dsh. A conserved NES has been identified as M/LxxLxL, where mutations in the leucines lead to nuclear localization of Dsh in Xenopus (Itoh et al. 2005). We found this NES to have patchy conservation, being present in Aque-Dvl, Cint-Dvl, and all vertebrate Dsh orthologs, except Xtrop-Dvl1 (Figure S2). It was not present in Chem-Dvl or any insect or nematode Dsh orthologs (Figure S2); however, it was present in Smed-dvl-2 but absent from Smed-dvl-1. Previous studies indicate that Dsh translocates to the nucleus and is actively exported into the cytoplasm, presumably via NLS and NES signals and that blocking the nuclear export by mutating the NES or chemically inhibiting nuclear export leads to nuclear localization of Dsh in vertebrates (Itoh et al. 2005; Torres and Nelson 2000). A NLS sequence was previously identified in vertebrates, flies, and Hydra, and identified as IxLT/VAK (Itoh et al. 2005). We found this NLS to be highly conserved across the taxa in our analyses, being present in the Dsh orthologs of most taxa we examined, but absent in all nematode mig-5 orthologs, and identifiable yet slightly altered in dsh-2 orthologs (Figure S2 and Figure S3). This NLS has been shown to be necessary and sufficient for nuclear translocation of Dsh in vertebrates, although this has not been pursued in invertebrate taxa (Itoh et al. 2005; Torres and Nelson 2000).
Phosphorylation of tyrosine 473
The phosphorylation of Dmel-dsh tyrosine 473 (Y473), located in the DEP domain, is essential for PCP signaling (Yanfeng et al. 2011). The substitution of Dmel-dsh Y473 to phenylalanine (DshY473F) leads to strong PCP specific defects in Drosophila but has no effect on canonical Wnt signaling. It is believed that this site in the DEP domain is phosphorylated by an Abelson family tyrosine kinase (Abl), which is also required for PCP signaling but not canonical Wnt signaling (Singh et al. 2010). We found that Y473 is conserved across all evaluated Dsh orthologs except Mhap-mig-5 and Minc-mig-5 as well as Smed-dvl-1 (Figure S2 and Figure S3). All evaluated organisms have at least one Dsh with a conserved Y473, implying an ancient and essential function across Metazoa, and suggesting another potential mechanism for partitioning the function of Dsh paralogs and/or splice isoforms.
Discussion
The origin of Dsh lies in the common ancestor of Metazoa and likely had the three major functional domains DIX, PDZ, and DEP (Figure 8A). Dsh has experienced dynamic evolution across animal evolution, acquiring new domains and experiencing duplications in several animal lineages. The DSV domain seems to have evolved prior to the bilaterian split and been subsequently lost in some nematode and insect taxa. In no phylum where multiple taxa were examined did we find complete conservation of both domain architecture and number of Dsh orthologs. We have identified many structural features that are conserved and others that are divergent or lineage-specific. These features suggest potential mechanisms for partitioning the various functions of Dsh among isoforms and/or paralogs. We discuss these findings in the context of known functional specializations among invertebrates.
Figure 8 .
Graphical summary of events during the evolution of Dsh mapped onto cladograms. (A) A cladogram of animal evolution with important features of Dsh evolution mapped onto it. (B) Cladogram of nematodes with identified features of Dsh evolution mapped onto it. (C) Cladogram of caenorhabditids with identified features of Dsh evolution mapped onto it.
Dishevelled across nematodes
Nematoda is an ancient animal lineage, originating during the Precambrian or Cambrian explosion more than 500 million years ago (Ayala et al. 1998; Rodriguez-Trelles et al. 2002). With this abundance of evolutionary time, nematodes have evolved to inhabit virtually every habitat known and nearly every ecological niche. The model nematode C. elegans was the first metazoan to have its genome sequenced and is among the most studied and best understood animals on earth (C. elegans Sequencing Consortium 1998). Often what is learned about C. elegans is assumed to be conserved among nematodes, and although this may be largely true for some features, e.g., neuroanatomy and CO2 detection and response (Bumbarger et al. 2007, 2009; Hallem et al. 2011a,b; Hallem and Sternberg 2008; Ragsdale et al. 2009), C. elegans is a derived nematode with many unique features (Blaxter 1998, 2011). We have shown that the number of Dsh homologs varies across nematodes, at least from one to three, but many taxa remain unstudied, especially within the basal clades of the phylum (Figure 8B). Most genera in our study have two Dsh homologs, dsh-1 and mig-5. The acquisition of mig-5 is ancient, occurring sometime after the split of clade 2 and before the split of clade 8, although additional taxon sampling would improve this estimate (Figure 8B). The C. elegans genome encodes three Dsh genes, Cele-dsh-1, Cele-dsh-2, and Cele-mig-5. We have shown that dsh-2 is likely a paralog of dsh-1 and a derived character among Caenorhabditis species, perhaps only among members of the Elegans supergroup (Figure 8C). We identified only one Dsh ortholog in T. spiralis, Tspi-dsh-1, and find that among nematodes, it has unique similarity to insect Dsh as it is the only nematode Dsh known to have a DSV domain (Figures 5 and 6).
The domain architecture among nematode Dshs is variable and suggests potential mechanisms of functional divergence. We have discovered a novel Dep-like fragment domain that is present and highly conserved in half of the 10 nematode taxa we examined (Figures 5, 6, and 8B). The domain architecture of mig-5 is conserved, having the same structural features (DIX, PDZ, DEP, and the basic region) in all taxa (except Cang-mig-5, which is missing DEP), while dsh-1 orthologs are more diverse (Figures 5 and 6). Asuu-dsh-1 and Ppac-dsh-1 lack the DIX domain, Bxyl-dsh-1 lacks the DIX domain and the basic region while Scar-dsh-1 seems to have acquired a signal peptide and a coiled domain that is unknown in any other Dsh homologs. Finally, we detected a conserved NLS in all nematode dsh-1 orthologs (and both Cbre-dsh-2 orthologs), suggesting that these proteins may be translocated to the nucleus, as has been shown in vertebrates. It is worth noting that the presence of an NLS and a basic region, features that are broadly conserved in Dsh orthologs across animals, are hallmarks of transcription factors, although this possibility has not been experimentally explored (Grove et al. 2009).
Although there are many examples in C. elegans of the functional overlap of Dsh paralogs, there are also known specializations for each. For example, B-cell polarity in males is controlled by Wnt signaling, where Cele-mig-5 defective males have altered B-cell daughter size (Herman et al. 1995; Sawa et al. 1996; Wu and Herman 2006). Neither Cele-dsh-1 nor Cele-dsh-2 affects the polarity of the B cell as single mutants and neither enhances the phenotype of the Cele-mig-5 mutant, showing specialization of Cele-mig-5 in this pathway (Wu and Herman 2006). The divergence of Dsh function in C. elegans can also be seen in the outgrowth of neurites from RME head motor neurons. In this pathway, Cele-DSH-1 physically interacts with Ror/CAM-1 to transmit the Wnt/CWN-2 signal to downstream components enabling neurite outgrowth (Song et al. 2010). The binding activity of Cele-DSH-1 to Ror/CAM-1 lies in its PDZ and DEP domains, whereas the DIX domain is not required for binding. Furthermore, only Cele-dsh-1b, the isoform that lacks the DIX domain (Figure 7; Cele-dsh-1b is the Cele-dsh-1 ‘b’ isoform from WormBase), was shown to express in the RME cells, and Cele-DSH-1b is sufficient to rescue the dsh-1 null phenotype, suggesting that alternative splicing of Dsh can lead to functional specialization within C. elegans (Song et al. 2010). An example of domain specialization within a Dsh homolog can be seen in the asymmetric cell division of the C. elegans ABpl/rpppa neuroblast via a β-catenin independent pathway (Hingwing et al. 2009). Domain analysis has shown that the DIX domain is not required for ABpl/rpppa asymmetric division but that the DEP domain is essential. Hingwing et al. (Hingwing et al. 2009) go on to show that Cele-dsh-2 is involved in the asymmetric divisions of SGP cells along the proximal-distal axis of the developing gonad, which leads to the formation of distal tip cells from distal daughters and an anchor or ventral uterine cell from the proximal daughters. Loss of Cele-dsh-2 results in two proximal daughters. Unlike the asymmetric division of the ABpl/rpppa neuroblast, both the DIX and DEP domains are essential for proper SGP cell division, thus demonstrating the divergent functional roles of domains in a single Dsh ortholog (Hingwing et al. 2009).
We have identified and shown the conservation of a Dep-like fragment domain across all Caenorhabditis dsh-1 orthologs along with Asuu-dsh-1, Bmal-dsh-1, Ppac-dsh-1, and Scar-dsh-1. Furthermore, we have shown that the basic region, DIX, PDZ, DEP, SH3 binding motif, and the NLS are conserved across nematode dsh-1 orthologs (with a few exceptions lacking the DIX domain and the absence of the basic region in Bxyl-dsh-1). We have shown the extreme structural conservation of all mig-5 orthologs, and that these uniformly lack the SH3 binding motif as well as the NLS. The functional relevance of these features and what role, if any, they play in the partitioning of Dsh function would be interesting to explore. These results suggest, for example, that Ppac-dsh-1 and Ppac-mig-5 might have evolved to function in separate pathways and perform at least some non-overlapping functions (Figure 5). The apparent lack of an NES in any nematode or insect Dsh is also striking, especially considering the presence of an NLS among most dsh-1 orthologs. Perhaps nematodes and insects have an alternative and as yet unidentified NES, since these proteins are not reported to be nuclear-specific.
Dishevelled in other invertebrates
S. mediterranea has two orthologs of Dsh, Smed-dvl-1 and Smed-dvl-2 (Gurley et al. 2008). Initial studies in this flatworm have investigated functional specialization of these paralogs. Only Smed-dvl-2 appears to be involved in β-catenin-dependent signaling: phenotypes described after silencing canonical Wnt ligands are reproduced upon the silencing of Smed-dvl-2 (Almuedo-Castillo et al. 2011). Conversely, both Smed-dvl-1 and Smed-dvl-2 transduce the noncanonical signals that control neural connectivity as well as mediolateral patterning of the central nervous system, neither of which involves components of the PCP pathway. Components of the PCP pathway, including Van Gogh and Diversin, have been implicated in the apical positioning of the basal body in epithelial cells. Interestingly, only Smed-dvl-2 has been shown to function alongside these core PCP components. Our domain analysis of planarian Dsh supports these experimental results. Only Smed-dvl-2 contains the NLS and NES sequences, implying its role in β-catenin−dependent signaling. Because Smed-dvl-1 lacks both sequences, we would suggest that it cannot function in a β-catenin−dependent pathway, and this hypothesis is supported experimentally (Almuedo-Castillo et al. 2011). Furthermore, it has been shown that tyrosine473 is essential for PCP signaling. This amino acid is present in Smed-dvl-2, but not Smed-dvl-1, supporting the experimental finding that only Smed-dvl-2 can function in the PCP pathway (Almuedo-Castillo et al. 2011).
Insects have only one copy of Dsh, at least the taxa that have been investigated so far. Significant effort has gone into understanding how Drosophila, with one Dsh ortholog, channels a Wnt signal into distinct pathways. It has been shown that specificity is achieved by the presence or absence of binding partners as well as the subcellular localization of Dsh (Wallingford and Habas 2005). Other work has shown that qualitatively different Fz-Dsh interactions underlie PCP and canonical Wnt signaling (Strutt et al. 2012).
The insect Dshs, Dmel-dsh, Tcas-dsh, and Nvit-dsh, are very similar in architecture. All have a DIX, PDZ, and DEP domain as well as the basic region and SH3 binding motif. Tcas-dsh is the only one that lacks a DSV domain, but this suggests that additional taxon sampling could reveal a broader trend. Interestingly, all insect Dsh proteins have a NLS, but none have the known NES that has been shown in Xenopus. It is currently not known whether invertebrate Dsh translocates to the nucleus, but if Dsh does, it must employ a different export signal than the one found in Xenopus. More work must be done to better understand its localization and transport in invertebrates.
We have discussed the origin and evolution of Dsh in a variety of metazoan lineages, emphasizing a recurring theme of Dsh duplication and expansion in many phyla. The data we have evaluated suggest that Dsh arose in the most recent common ancestor of Metazoa and possessed many of the structural features that have come to characterize Dsh (Figure 2). Most basal lineages within explored phyla appear to have only a single Dsh ortholog, leading us to conclude that the ancestral state of Wnt signaling pathways was built using a single Dsh protein acting as the hub, and has then experienced lineage-specific expansions in many phyla. The deuterostome taxa wherein Dsh has been explored reveal that early branching deuterostome phyla (e.g., Echinodermata and Hemichordata) have only one Dsh ortholog, which is also true of basal chordate lineages like lancelets and sea squirts (Cephalochordata and Urochordata respectively) (Gray et al. 2009). It is noteworthy that as more taxa in a particular phylum are explored, the derived lineages seem to have convergently evolved multiple Dsh orthologs, although there may be exceptions such as insects, where even the more recent lineages seem to use the ancestral strategy of partitioning Dsh function in ways other than protein duplication and subsequent divergence.
As the hub of Wnt signaling, Dsh plays an essential role in animal development and homeostasis. We have shown that Dsh has experienced dynamic evolution across Metazoa, including the acquisition and loss of domains as well as gene duplication in many lineages. Our findings on the divergent and varied architecture of Dsh across taxa provide testable hypotheses about the means of these specializations. The dynamic evolution of Dsh among nematodes both by paralogous duplication and the formation of lineage-specific splice isoforms raises questions of protein evolution and provides clues as to how these organisms have dealt with the complex intricacies of having a protein, like Dsh, act as a central messenger hub connected to so many different and vitally important pathways.
Supplementary Material
Acknowledgments
We thank Byron J. Adams, Geoffrey T. Smith, and Christian A. Grove for their critical reading of the manuscript and Art Vandelay for helpful insights into the details of importing and exporting. This work was supported by a National Institutes of Health (NIH) United States Public Health Service Training Grant (T32GM07616) to A.R.D. and P.J.M., and by the Howard Hughes Medical Institute (with which P.W.S. is an investigator).
Footnotes
Communicating editor: M. Boutros
Literature Cited
- Adamska M., Degnan S. M., Green K. M., Adamski M., Craigie A., et al. , 2007. Wnt and TGF-beta expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE 2: e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska M., Larroux C., Adamski M., Green K., Lovas E., et al. , 2010. Structure and expression of conserved Wnt pathway components in the demosponge Amphimedon queenslandica. Evol. Dev. 12: 494–518 [DOI] [PubMed] [Google Scholar]
- Almuedo-Castillo M., Salo E., Adell T., 2011. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc. Natl. Acad. Sci. USA 108: 2813–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala F. J., Rzhetsky A., Ayala F. J., 1998. Origin of the metazoan phyla: molecular clocks confirm paleontological estimates. Proc. Natl. Acad. Sci. USA 95: 606–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière A., Yang S.-P., Pekarek E., Thomas C. G., Haag E. S., et al. , 2009. Detecting heterozygosity in shotgun genome assemblies: Lessons from obligately outcrossing nematodes. Genome Res. 19: 470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M., 1998. Caenorhabditis elegans is a nematode. Science 282: 2041–2046 [DOI] [PubMed] [Google Scholar]
- Blaxter M., 2011. Nematodes: The worm and its relatives. PLoS Biol. 9: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M. L., De Ley P., Garey J. R., Liu L. X., Scheldeman P., et al. , 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392: 71–75 [DOI] [PubMed] [Google Scholar]
- Boutros M., Mlodzik M., 1999. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83: 27–37 [DOI] [PubMed] [Google Scholar]
- Bumbarger D. J., Crum J., Ellisman M. H., Baldwin J. G., 2007. Three-dimensional fine structural reconstruction of the nose sensory structures of Acrobeles complexus compared to Caenorhabditis elegans (Nematoda: Rhabditida). J. Morphol. 268: 649–663 [DOI] [PubMed] [Google Scholar]
- Bumbarger D. J., Wijeratne S., Carter C., Crum J., Ellisman M. H., et al. , 2009. Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida). J. Comp. Neurol. 512: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans Sequencing Consortium, 1998. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282: 2012–2018 [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D., 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman A. R., Mortazavi A., Sternberg P. W., 2012. Incorporating genomics into the toolkit of nematology. J. Nematol. 44: 191–205 [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C., 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge S. L., Ray S., Li S., Hamblet N. S., Lijam N., et al. , 2008. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 4: e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy O. G., Fahmy M., 1959. New mutants report. Dros. Inf. Serv. 33: 82–94 [Google Scholar]
- Gao C., Chen Y. G., 2010. Dishevelled: The hub of Wnt signaling. Cell. Signal. 22: 717–727 [DOI] [PubMed] [Google Scholar]
- Goloboff P. A., 1999. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics-the International Journal of the Willi Hennig Society 15: 415–428 [DOI] [PubMed] [Google Scholar]
- Gray R. S., Bayly R. D., Green S. A., Agarwala S., Lowe C. J., et al. , 2009. Diversification of the expression patterns and developmental functions of the dishevelled gene family during chordate evolution. Dev. Dyn. 238: 2044–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove C. A., De Masi F., Barrasa M. I., Newburger D. E., Alkema M. J., et al. , 2009. A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell 138: 314–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Gascuel O., 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52: 696–704 [DOI] [PubMed] [Google Scholar]
- Gurley K. A., Rink J. C., Sanchez Alvarado A., 2008. Beta-catenin defines head vs. tail identity during planarian regeneration and homeostasis. Science 319: 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Sternberg P. W., 2008. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105: 8038–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Dillman A. R., Hong A. V., Zhang Y., Yano J. M., et al. , 2011a A sensory code for host seeking in parasitic nematodes. Curr. Biol. 21: 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Spencer W. C., McWhirter R. D., Zeller G., Henz S. R., et al. , 2011b Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet N. S., Lijam N., Ruiz-Lozano P., Wang J. B., Yang Y. S., et al. , 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129: 5827–5838 [DOI] [PubMed] [Google Scholar]
- Herman M. A., Vassilieva L. L., Horvitz H. R., Shaw J. E., Herman R. K., 1995. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell 83: 101–110 [DOI] [PubMed] [Google Scholar]
- Hingwing K., Lee S., Nykilchuk L., Walston T., Hardin J., et al. , 2009. CWN-1 functions with DSH-2 to regulate C. elegans asymmetric neuroblast division in a beta-catenin independent Wnt pathway. Dev. Biol. 328: 245–256 [DOI] [PubMed] [Google Scholar]
- Holstein T. W., 2012. The evolution of the Wnt pathway. Cold Spring Harb. Perspect. Biol. 4: a007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman M., van der Wurff A., van den Elsen S., van Megen H., Bongers T., et al. , 2006. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 23: 1792–1800 [DOI] [PubMed] [Google Scholar]
- Itoh K., Brott B. K., Bae G. U., Ratcliffe M. J., Sokol S. Y., 2005. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K. C., Felix M. A., Ailion M., Rockman M. V., Braendle C., et al. , 2011. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 11: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J., Nusse R., Perrimon N., 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8: 118–130 [DOI] [PubMed] [Google Scholar]
- Kusserow A., Pang K., Sturm C., Hrouda M., Lentfer J., et al. , 2005. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433: 156–160 [DOI] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P., 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40: D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Stoeckert C. J., Roos D. S., 2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13: 2178–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijam N., Paylor R., McDonald M. P., Crawley J. N., Deng C. X., et al. , 1997. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 90: 895–905 [DOI] [PubMed] [Google Scholar]
- Luo J., Chen J., Deng Z. L., Luo X., Song W. X., et al. , 2007. Wnt signaling and human diseases: what are the therapeutic implications? Lab. Invest. 87: 97–103 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Wang E., Harland R., 2005. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8: 167–178 [DOI] [PubMed] [Google Scholar]
- Mortazavi A., Schwarz E. M., Williams B. A., Schaeffer L., Antoshechkin I., et al. , 2010. Scaffolding a Caenorhabditis nematode genome with RNA-seq. Genome Res. 20: 1740–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell B., Wertheim J. O., Moola S., Weighill T., Scheffler K., et al. , 2012. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 8: e1002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas K. B., Nicholas H. B., Deerfield D. W., 1997. GeneDoc: analysis and visualization of genetic variation. EMBnet.news 4: 1–4 [Google Scholar]
- Nixon K. C., 1999. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics 15: 407–414 [DOI] [PubMed] [Google Scholar]
- Penton A., Wodarz A., Nusse R., 2002. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics 161: 747–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale E. J., Ngo P. T., Crum J., Ellisman M. H., Baldwin J. G., 2009. Comparative, three-dimensional anterior sensory reconstruction of Aphelenchus avenae (nematoda: Tylenchomorpha). J. Comp. Neurol. 517: 616–632 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Trelles F., Tarrio R., Ayala F. J., 2002. A methodological bias toward overestimation of molecular evolutionary time scales. Proc. Natl. Acad. Sci. USA 99: 8112–8115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G., Hobert O., 1998. The taxonomy of developmental control in Caenorhabditis elegans. Science 282: 2033–2041 [DOI] [PubMed] [Google Scholar]
- Sawa H., Lobel L., Horvitz H. R., 1996. The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev. 10: 2189–2197 [DOI] [PubMed] [Google Scholar]
- Seifert J. R., Mlodzik M., 2007. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8: 126–138 [DOI] [PubMed] [Google Scholar]
- Simons M., Mlodzik M., 2008. Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 42: 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Yanfeng W. A., Grumolato L., Aaronson S. A., Mlodzik M., 2010. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 24: 2157–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Zhang B., Sun H., Li X., Xiang Y., et al. , 2010. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet. 12: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J., Dillman A. R., Macchietto M. G., Heikkinen L., Lakso M., et al. , 2013. The draft genome and transcriptome of Panagrellus redivivus are shaped by the harsh demands of a free-living lifestyle. Genetics (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J., 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57: 758–771 [DOI] [PubMed] [Google Scholar]
- Strutt D., Madder D., Chaudhary V., Artymiuk P. J., 2012. Structure-function dissection of the frizzled receptor in Drosophila melanogaster suggests different mechanisms of action in planar polarity and canonical Wnt signaling. Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R., He X. C., Venkatraman A., Arai F., Box A., et al. , 2012. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150: 351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman D., Wagstaff L., Cooper O., Weijer C., Munsterberg A., 2008. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev. Biol. 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., et al. , 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M. A., Nelson W. J., 2000. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149: 1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello D. V., Jordens I., Kirchner K., Slootstra J. W., Kruitwagen T., et al. , 2012. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. USA 109: E812–E820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B., Habas R., 2005. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132: 4421–4436 [DOI] [PubMed] [Google Scholar]
- Wernersson R., Pedersen A. G., 2003. RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 31: 3537–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Jr, 2003. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253: 1–17 [DOI] [PubMed] [Google Scholar]
- Wu M., Herman M. A., 2006. A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev. Biol. 293: 316–329 [DOI] [PubMed] [Google Scholar]
- Yanfeng W. A., Berhane H., Mola M., Singh J., Jenny A., et al. , 2011. Functional dissection of phosphorylation of Disheveled in Drosophila. Dev. Biol. 360: 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.