Abstract
Telomeres are composed of simple tandem DNA repeats that protect the ends of linear chromosomes from replicative erosion or inappropriate DNA damage response mechanisms. The mammalian Protection Of Telomeres (POT1) protein interacts with single-stranded telomeric DNA and can exert positive and negative effects on telomere length. Of four distinct POT1 homologs in the roundworm Caenorhabditis elegans, deficiency for POT-1 or POT-2 resulted in progressive telomere elongation that occurred because both proteins negatively regulate telomerase. We created a POT-1::mCherry fusion protein that forms discrete foci at C. elegans telomeres, independent of POT-2, allowing for live analysis of telomere dynamics. Transgenic pot-1::mCherry repressed telomerase in pot-1 mutants. Animals deficient for pot-1, but not pot-2, displayed mildly enhanced telomere erosion rates in the absence of the telomerase reverse transcriptase, trt-1. However, trt-1; pot-1 double mutants exhibited delayed senescence in comparison to trt-1 animals, and senescence was further delayed in trt-1; pot-2; pot-1 triple mutants, some of which survived robustly in the absence of telomerase. Our results indicate that POT-1 and POT-2 play independent roles in suppressing a telomerase-independent telomere maintenance pathway but may function together to repress telomerase.
Human somatic cells have finite replicative lifespans and can enter an irreversible cell-cycle arrest, termed senescence, in response to various stresses. Senescence can occur due to progressive shortening of telomeres, which cannot be completely replicated by canonical DNA polymerases (Harley et al. 1990). Telomeres are composed of simple TTAGGG repeats in vertebrates and related sequences in other organisms, such as TTAGGC repeats in Caenorhabditis elegans. To combat telomere erosion, cells can express the enzyme telomerase, which adds de novo telomere repeats to chromosome ends via reverse transcription from an RNA template (Greider and Blackburn 1989). Telomerase is expressed at high levels in germ cells and can be expressed in human somatic cells, but its expression is transient or absent altogether in more differentiated cell types (Kim et al. 1994; Sharma et al. 1995).
The shelterin complex, composed of six mammalian telomere-binding proteins TRF1, TRF2, TIN2, POT1, RAP1, and TPP1, and its associated proteins protect telomeres from nucleases and DNA damage repair mechanisms that can lead to exacerbated telomere shortening or cellular senescence (Diotti and Loayza 2011). Shelterin components maintain telomere homeostasis by positively and negatively regulating telomere length. The double-stranded telomeric DNA-binding proteins TRF1 and TRF2 have been implicated as negative regulators of telomere length, where removal of TRF1 from telomeres or overexpression of TRF2 yielded telomere elongation or erosion, respectively (Smogorzewska et al. 2000; van Steensel and de Lange 1997). TIN2 and TPP1 proteins bridge the interaction between these double-stranded telomere-binding proteins and the single-stranded telomere-binding protein, POT1, and are also considered negative regulators of telomere length as their depletion results in progressive telomere elongation (Kim et al. 1999; Ye and de Lange 2004; Ye et al. 2004).
Human Protection Of Telomeres 1 interacts with single-stranded telomeric DNA via two oligonucleotide/oligosaccharide (OB) folds and is primarily considered a negative regulator of telomere length (Kendellen et al. 2009; Veldman et al. 2004; Ye et al. 2004). However, in numerous studies researchers have revealed roles for POT1 in both telomere elongation and telomere protection. POT1 overexpression (Armbruster et al. 2004; Liu et al. 2004) and mutant or splice-variant POT1 expression (Armbruster et al. 2004; Colgin et al. 2003; Kendellen et al. 2009; Liu et al. 2004; Loayza and De Lange 2003) can elicit telomere elongation. In addition, POT1 can inhibit telomere repeat synthesis in the presence of its binding partner TPP1 but promotes telomerase processivity in vitro in its absence (Kelleher et al. 2005; Wang et al. 2007).
Both mouse Pot1 homologs promote chromosome end protection, as G-strand overhangs lengthen in Pot1b−/− cells, and end-to-end chromosome fusions occur as a result of telomere deprotection in both Pot1a−/− and Pot1b−/− cells (He et al. 2006, 2009; Hockemeyer et al. 2006, 2008; Wu et al. 2006). However, disparate cellular and telomere phenotypes have been reported. For example, fibroblasts derived from Pot1a−/− mice senesced prematurely in one study (Wu et al. 2006) but not in another (Hockemeyer et al. 2006). In addition, Pot1b−/− cells did not prematurely senescence in one study (Hockemeyer et al. 2006), but mouse embryonic fibroblasts overexpressing an OB-fold Pot1b mutant exhibited early-onset senescence in another study (He et al. 2006). Moreover, telomeres from Pot1b−/− cells have been shown to either shorten or stay the same (He et al. 2009; Hockemeyer et al. 2006, 2008), whereas Pot1a−/− cells exhibited telomere elongation (Wu et al. 2006).
The C. elegans genome is predicted to encode four proteins with OB folds homologous to mammalian POT1, including a single protein with an OB1 fold, POT-1, and three proteins with OB2 folds, POT-2, POT-3, and MRT-1 (Figure 1A) (Meier et al. 2009; Raices et al. 2008). Previous work has illustrated that POT-1, also known as CeOB2, and POT-2, also known as CeOB1, can interact with single-stranded telomeric DNA in vitro (Raices et al. 2008). In addition, this study reported elongated telomeres for both pot-1(tm1620) and pot-2(tm1400) mutant strains, although pot-1(tm1620) telomeres were distinctive and appeared similar to those of human cells that maintain their telomeres by a telomerase-independent telomere replication pathway termed alternative lengthening of telomeres (ALT).
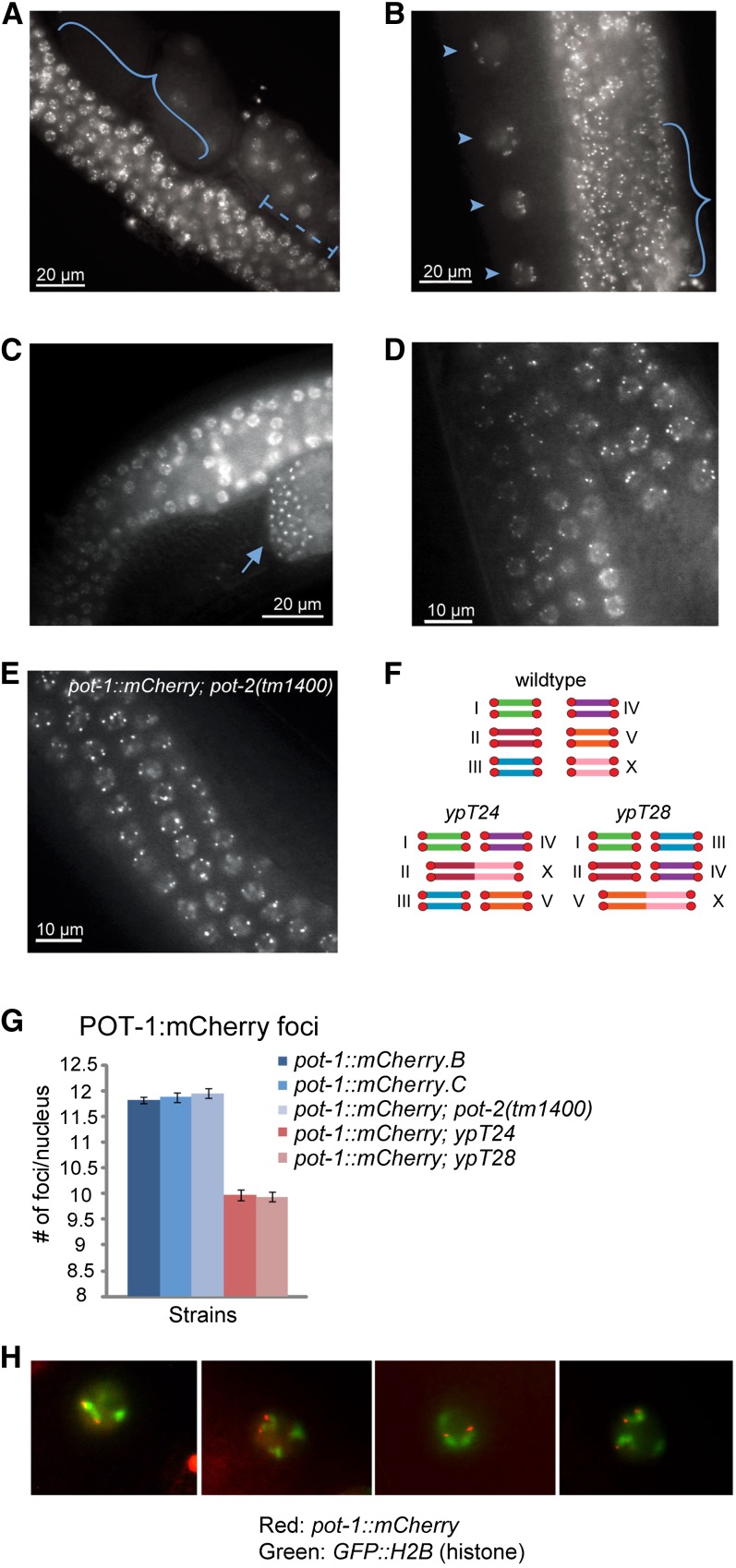
Figure 1 .
POT-1 and POT-2 are negative regulators of telomere replication. (A) A representation of the four POT-1 homologs in C. elegans. Terminal restriction fragment length analysis was performed on DNA collected from consecutive generations of (B) pot-1(tm1620), pot-2(1400), and pot-3(ok1530) single mutants, (C) outcrossed pot-2(1400) and pot-1(tm1620) single mutants, (D) wildtype and pot-1; pot-2 double mutants, (E) outcrossed pot-1::mCherry; pot-1(tm1620) strains, and (F) outcrossed pot-1::mCherry strains. Dashed line to the right of the blots indicates internal telomeric sequences.
We previously demonstrated that one of four POT1 homologs, MRT-1, is necessary for telomerase-mediated telomere repeat addition in vivo (Meier et al. 2009). Here we investigate additional roles for these C. elegans proteins that contain POT1 OB folds by studying telomere dynamics in pot-1 and pot-2 mutants. We illustrate that both POT-1 and POT-2 are negative regulators of telomerase, indicating a similar and previously unknown role for these proteins in C. elegans telomere biology. We develop a pot-1::mCherry transgene that localizes to telomeres and represses telomerase in vivo. Additionally, we demonstrate a unique role for POT-1 in telomere protection and that POT-1 and POT-2 function non-redundantly to repress a telomerase-independent telomere maintenance pathway.
Materials and Methods
Strains
Unless noted otherwise, all strains were cultured at 20° on nematode growth medium plates seeded with Escherichia coli OP50. Strains used include Bristol N2 ancestral, CB61 dpy-5(e61) I, YA1059 trt-1(ok410) I, CB402 unc-55(e402) I, CB193 unc-29(e193) I, YA1197 ypIn2 (Pdaz-1::pot-1::mCherry::tbb-2utr), YA1198 ypIn3 (Pdaz-1::pot-1::mCherry::tbb-2utr), YA1024 pot-2(tm1400) II, CB187 rol-6(e187) II, dpy-17(e164) III, YA1022 pot-1(tm1620) III, unc-32(e189) III, and YA1026 pot-3(ok1530) III.
The pot-1 mutation was outcrossed vs. an outcrossed stock of dpy-17unc-32. pot-2 and pot-1::mCherry lines were outcrossed vs. outcrossed stocks of unc-52 or rol-6, respectively. Freshly isolated homozygous F2 lines were established for analysis.
To create pot-1; pot-2 double mutants, a pot-1; unc-52 double mutant and a pot-2; dpy-17unc-32 triple mutant were first created; phenotypically wild-type F2 progeny of unc-52 / pot-2; pot-1 / dpy-17, unc-32 F1 heterozygotes were selected; and the strains that segregated only phenotypically wild-type F3 progeny were retained for analysis.
To create the trt-1; pot-1 and trt-1; pot-2 double mutants, dpy-5unc-55; pot-1, dpy-5unc-55; pot-2, trt-1; dpy-17unc-32, and trt-1; unc-52 mutants were generated. Phenotypically wild-type F2 progeny of dpy-5unc-55 / trt-1; pot-1 / dpy-17unc-32 or dpy-5unc-55 / trt-1; pot-2 / unc-52 F1 heterozygotes were selected, and strains that segregated only phenotypically wild-type F3 progeny were retained for analysis.
To create trt-1; pot-2; pot-1 triple mutants, trt-1; pot-2; dpy-17unc-32 and trt-1; unc-52; pot-1 triple mutants were first created, phenotypically wild-type F2 progeny were selected from trt-1; pot-2 / unc-52; pot-1 / dpy-17unc-32 heterozygotes, and the strains that segregated only phenotypically wild-type F3 progeny were retained for analysis.
To place the ypIn2 pot-1::mCherry transgene into a pot-1 mutant background, ypIn2; dpy-17unc-32 hermaphrodites were crossed to rol-6 / +; pot-1 / dpy-17unc-32 males, and phenotypically wild-type F2 progeny were singled from rol-6 / ypIn2; pot-1 / dpy-17unc-32 F1, and F2 that segregated only phenotypically wild-type F3 progeny were retained for analysis. ypIn2 pot-1::mCherry was placed into the pot-2 mutant background analogously, where ypIn2 pot-1::mCherry unc-52 hermaphrodites were crossed to rol-6 / +; pot-2 / unc-52 males and phenotypically wildtype F2 progeny were selected.
To create a pot-1::mCherry strain that expressed GFP::Histone H2B from the transgene insertion ruIs32, N2 males were crossed to hermaphrodites of the strain TH32 unc-119ed3; ddIs6[tbg-1::GFP + unc-119(+)]; ruIs32[unc-119(+) pie-1::GFP::H2B] (Desai et al. 2003), and the resultant F1 males were crossed with pot-1::mCherry hermaphrodites. F1 cross-progeny were singled and allowed to self-fertilize, F2 progeny were singled from animals heterozygous for both ruIs32[unc-119(+) pie-1::GFP::H2B] and pot-1::mCherry, and F3 progeny homozygous for both transgenes were selected.
Terminal restriction fragment length analysis
C. elegans genomic DNA was isolated using Gentra Puregene reagents (QIAGEN), digested with HinfI enzyme (NEB), and separated on a 0.6% agarose gel at 1.5 V/cm. Southern blotting was performed using the DIG Wash and Block Buffer Set (Roche) following the manufacturer’s instructions. A telomere probe, corresponding to the C. elegans telomeric repeat TTAGGC, was synthesized and labeled with digoxigenin (DIG)-dUTPs using the PCR DIG Probe Synthesis Kit (Roche) following the manufacturer’s instructions.
Telomere erosion rate calculation
The sizes of individual telomere bands, between 2 and 6 kb, that could be clearly followed for consecutive generations (and were distinct from bands corresponding to neighboring telomeres or to interstitial telomeric tracts) were calculated using semi-log graphs of molecular marker size and distance traveled from the well (Ahmed and Hodgkin 2000; Boerckel et al. 2007; Lowden et al. 2008; Meier et al. 2006, 2009). Data are presented as the mean ± SD.
Transgene construction
All transgene constructs were made using the MosI-mediated single-copy insertion system that allows for the incorporation of a single copy of a transgene into one specific locus in the C. elegans genome (Frokjaer-Jensen et al. 2008). The pot-1::mCherry transgene was constructed using the Invitrogen Gateway Cloning kit using the positive selection marker Cb-unc-119(+), a germline-specific promoter daz-1, full-length genomic pot-1 sequence lacking a stop codon, mCherry sequence, and the tbb-2 3′ UTR. An extrachromosomal array consisting of this construct, Pglh-2::Mos1 transposase, and three fluorescent mCherry negative selection markers was introduced into Mos-1(ttTi5605); unc-119 worms via microinjection into the germline of young adults. Progeny of injected animals were screened for loss of the Unc phenotype and for the presence of mCherry fluorescence, suggesting successful transformation of the injected extrachromosomal array. Lines with successful transformants were further propagated and progeny were screened for loss of coinjection mCherry fluorescence markers but continued rescue of the Unc phenotype, indicating successful integration of the construct. Genomic DNA prepared from these lines was tested by PCR and DNA sequencing to confirm the presence of a single-copy insertion in the Mos-1(ttTi5605) insertion site on chromosome II. The unc-119 mutation was removed from transgenic strains prior to analysis by crossing with rol-6 / + males, singling non-Rol, non-Unc F2 from F1 with Rol F2, choosing F2 that lacked unc-119 homozygotes, and selecting against rol-6. Single-copy transgene insertions were designated ypIn2 (Pdaz-1::pot-1::mCherry::tbb-2utr) and ypIn3 (Pdaz-1::pot-1::mCherry::tbb-2utr), which we refer to below as pot-1::mCherry.B and pot-1::mCherry.C, respectively.
DAPI staining
One-day-old adult worms were soaked in 150 µL of a 400 ng/mL DAPI in ethanol solution for 30 min or until evaporated, rehydrated in 2 mL of M9 solution overnight at 4°, and mounted in 5 µL of fresh NPG/glycerol medium. Chromosome counts were performed under ×100 magnification and a 359 excitation wavelength using a Nikon Eclipse E800 microscope.
POT-1::mCherry foci quantification
Live 1-day-old adult worms were mounted onto 2% agarose pads in 5 µL of tetramisole and Z stacks were taken within 2 hr of mounting under ×100 magnification and a 595-nm excitation wavelength using a Nikon Eclipse E800 microscope. Foci from individual nuclei were quantified by manually scanning through compiled Z stacks.
C-circle quantification
The C-circle amplification assay was performed as previously described (Henson et al. 2009) with the following modifications: (1) the 96-nucleotide oligomer control was generated with a C. elegans telomeric sequence (5′ CCCATATCACTAA(GCCTAA)12CCTCAATTCCC 3′); (2) the DNA was resolved on an agarose gel and normalized by ethidium bromide staining, and amplified DNA was dot blotted onto a neutral nylon membrane (GE Healthcare Life Sciences) and probed with a telomeric G strand (TTAGGC)3 oligo conjugated to DIG at 37°; and (3) the membrane was washed as described for the DIG Wash and Block Buffer Set (Roche) at 37° and developed with ECF reagent (GE Life Sciences). Fluorescence signals were collected with a Typhoon Trio scanner (GE Life Sciences) and quantified with ImageQuant TL software (GE Life Sciences) using edge subtraction.
Results
POT-1 and POT-2 are negative regulators of telomere extension in vivo
We obtained strains harboring the deletions pot-1(tm1620) or pot-2(tm1400) from Shohei Mitani and verified the presence of homozygous deletions in these strains using the polymerase chain reaction. Southern blotting revealed long telomeres for genomic DNA isolated from pot-1 or pot-2 mutant strains that were propagated for varying numbers of generations (Figure 1B). In contrast, the pot-3(ok1530) deletion did not have an overt effect on telomere length (Figure 1B). Outcrossing of pot-1 or pot-2 mutations for 15 generations as heterozygotes, followed by isolation of homozygous mutant pot-1 or pot-2 strains, revealed normal telomere lengths in early generations followed by progressive telomere elongation (Figure 1C). Therefore, the telomere elongation phenotypes caused by pot-1 and pot-2 mutations are recessive and can be eliminated if the mutations are maintained as heterozygotes.
Telomeres from pot-1 and pot-2 mutant strains had qualitatively similar dynamics, suggesting that POT-1 and POT-2 may perform similar functions at telomeres. The rapid appearance of smeary, long telomeres, assessed from numerous, outcrossed lines of pot-1 and pot-2 single mutants or pot-2; pot-1 mutants, precluded measurement of telomere elongation rates with errors of <100 bp/generation, although telomere elongation was qualitatively similar among the three genotypes (Figure 1, C and D; supporting information, Figure S1).
To confirm that the pot-1(tm1620) mutation was responsible for the telomere elongation phenotype of outcrossed pot-1 strains, single-copy transgenes designed to express wild-type POT-1 fused to a fluorescent mCherry protein at its C terminus were created, outcrossed nine times, and crossed into a pot-1(tm1620) background that had been outcrossed 30 times. In contrast to pot-1(tm1620) mutants (Figure 1C), telomere lengths in independent pot-1::mCherry; pot-1(tm1620) strains remained constant over many generations (Figure 1E), indicating that the progressive telomere elongation phenotype of pot-1(tm1620) mutants is caused by the pot-1 deletion rather than a tightly linked mutation. Moreover, telomeres did not progressively lengthen or shorten for independently outcrossed pot-1::mCherry strains in a wildtype pot-1 background, indicating that the POT-1::mCherry fusion protein does not perturb the ability of endogenous POT-1 to regulate telomere length (Figure 1F). However, bulk telomere length was slightly longer than wildtype for strains containing a pot-1::mCherry transgene (Figure 1, D and F).
POT-1 foci at C. elegans telomeres in vivo
Live imaging of animals possessing pot-1::mCherry transgenes revealed strong punctate POT-1::mCherry foci within the nuclei of sperm, some oocytes, and at the nuclear periphery throughout the rest of the germline (Figure 2, A−C). POT-1::mCherry foci could be robustly quantified in meiotic pachytene nuclei near the bend of germline arms, where the six homologous chromosomes of C. elegans are synapsed and in late stages of meiotic recombination (Dernburg et al. 1998). Analysis of independent pot-1::mCherry transgene insertions, pot-1::mCherry.B and pot-1::mCherry.C, revealed approximately 12 foci per pachytene nucleus (11.8 ± 0.1; 11.9 ± 0.1; Figure 2G), which could plausibly correspond to chromosome termini of the six paired homologous chromosomes (Figure 2, D and F). Slightly fewer than the expected mean number of telomeric foci were observed, likely due to telomeres that were occasionally near one another within a nucleus, precluding them from being distinguished as distinct foci.
Figure 2 .
POT-1:mCherry localizes to telomeres as punctate foci independent of POT-2. Live imaging of pot-1::mCherry strains revealed germline-specific expression, including meiotic nuclei (A, B; solid brackets), mitotic nuclei (A; dashed brackets), oocytes (B; arrowheads), and sperm (C; arrow). (D) Representative image of pot-1::mCherry. (E) Representative image of pot-1::mCherry; pot-2(tm1400). (F) A representation of six C. elegans chromosomes in wild-type and in two strains harboring end-to-end chromosomal fusions (ypT24 and ypT28). Red circles at chromosome termini represent telomeres. (G) POT-1:mCherry foci were quantified in the meiotic nuclei of two independent wild-type strains, pot-1::mCherry.B (n = 83) and C (n = 54), in a pot-2(tm1400) mutant strain (n = 56), and in the strains ypT24 (n = 30) and ypT28 (n = 51). The close paring of both sister chromatids and homologous chromosomes in C. elegans oocytes precludes the resolution of telomeres from distinct homologous chromosomes and instead reveals 12 or 10 telomeric spots in wildtype or fusion strains, instead of 24 or 20, respectively. (H) Representative images of live pot-1::mCherry; GFP::histone animals demonstrate POT-1::mCherry localization at chromosome ends.
Immunoprecipitation experiments have previously shown that a POT-1::HA fusion protein can interact with telomeric DNA in C. elegans (Raices et al. 2008). To confirm that POT-1::mCherry foci occurred at telomeres, we used the well-characterized end-to-end chromosome fusions ypT24 and ypT28, which were isolated from C. elegans strains that were deficient for telomerase and then crossed onto telomerase-positive genetic backgrounds. These chromosome fusions were created from two chromosome ends that had lost all (TTAGGC)n telomeric repeat sequences as well as several thousand base pairs of subtelomeric DNA prior to being joined together (Lowden et al. 2008, 2011). Strains homozygous for ypT24 and ypT28 X-autosome chromosome fusions harbor five homologous chromosomes and 10 chromosome termini (Figure 2F), and these chromosome fusions can be stably maintained in C. elegans due to the presence of holocentric chromosomes. ypT24 and ypT28 chromosome fusions were crossed with pot-1::mCherry to create pot-1::mCherry; ypT24 and pot-1::mCherry; ypT28 strains, and quantification of POT-1 fluorescent foci in these strains revealed approximately 10 meiotic foci per nucleus (10 ± 0.1; 9.9 ± 0.1; Figure 2G), indicating that POT-1:mCherry foci correspond to discrete chromosome termini that are not clustered in meiotic pachytene nuclei. In addition, POT-1::mCherry foci were observed at termini of some condensed chromosomes in diakinesis-stage oocyte nuclei, where chromosomes were marked by histone H2B::GFP expression (Figure 2H). However, oocyte nuclei displayed reduced numbers of POT-1::mCherry foci in comparison with pachytene nuclei, and high-resolution images of POT-1::mCherry foci at both ends of a bivalent were not observed in oocytes. POT-1::mCherry expression became attenuated and more diffuse in the oocyte closest to the spermatheca.
Our results demonstrate that POT-1 localizes to telomeres as small, quantifiable, nuclear domains and is unlikely to form foci at any other segment of the C. elegans genome. To our knowledge, this is the first demonstration that stable genome rearrangements can be employed to show that a telomere binding protein specifically interacts with telomeres in vivo. Telomere clustering has been reported as chromosomes pair during meiosis (Bass et al. 1997; Cooper et al. 1998; Scherthan et al. 1996; Yamamoto and Hiraoka 2001), a process that occurs in transition zone nuclei of the C. elegans germline. POT-1::mCherry foci became diffuse and were rarely discrete at this stage of germ cell development (Figure S2), possibly due to rapid chromosome movements as chromosomes pair.
In contrast to previous results suggesting different functions for POT-1 and POT-2 (Raices et al. 2008), the lack of a qualitative additive telomere elongation phenotype for pot-1; pot-2 double mutants suggested a common function for their gene products (Figure 1D; Figure S1). Therefore, we assessed whether the telomeric localization of POT-1 was affected by POT-2 by quantifying POT-1::mCherry foci in live pot-1::mCherry; pot-2(tm1400) animals. Approximately 12 foci per meiotic pachytene nucleus were observed when pot-2 was mutant (12 ± 0.1), and the POT-1::mCherry localization pattern was qualitatively similar throughout the germline in wild-type and pot-2 mutant backgrounds (Figure 2, E and G). Thus, POT-2 did not have an obvious effect on the telomeric localization of POT-1.
The distal portion of the C. elegans germline is composed of a population of proliferating mitotic cells (Cinquin et al. 2010). In contrast to cells arrested in meiotic pachytene, quantification of POT-1::mCherry foci in mitotic nuclei revealed an average of 18.9 foci per nucleus (± 2 SD). We observed a broader range of foci per nucleus in the mitotic region (16−23, n = 31), in part due to the smaller size and denser clustering of the nuclei, which precluded the more precise resolution of POT-1::mCherry foci that was possible in large pachytene nuclei. As some mitotic nuclei displayed less than 24 POT-1::mCherry spots, weak telomere clustering is likely to occur in mitotic cells of C. elegans. However, the presence of 20−23 spots in some nuclei suggested that mitotic telomere clustering could either be transient or could vary with the cell cycle. Our pot-1::mCherry transgene was only expressed in germ cells, because it was driven by the germ cell−specific pgl-3 promoter. Future analysis of telomere behavior in somatic cell types may be an interesting line of investigation.
POT-1 and POT-2 repress telomerase activity at telomeres
To ascertain whether the telomere elongation phenotype of pot-1 and pot-2 mutants is mediated by telomerase, we crossed the pot-1 and pot-2 mutations into a telomerase-deficient background by constructing double and triple mutants with a null allele of the telomerase reverse transcriptase, trt-1(ok410). Telomeres shortened progressively in the absence of telomerase and pot-1, pot-2 or both pot-1 and pot-2 (Figure 3, A−C). Therefore, telomere elongation of pot-1 or pot-2 single mutants depends on telomerase activity.
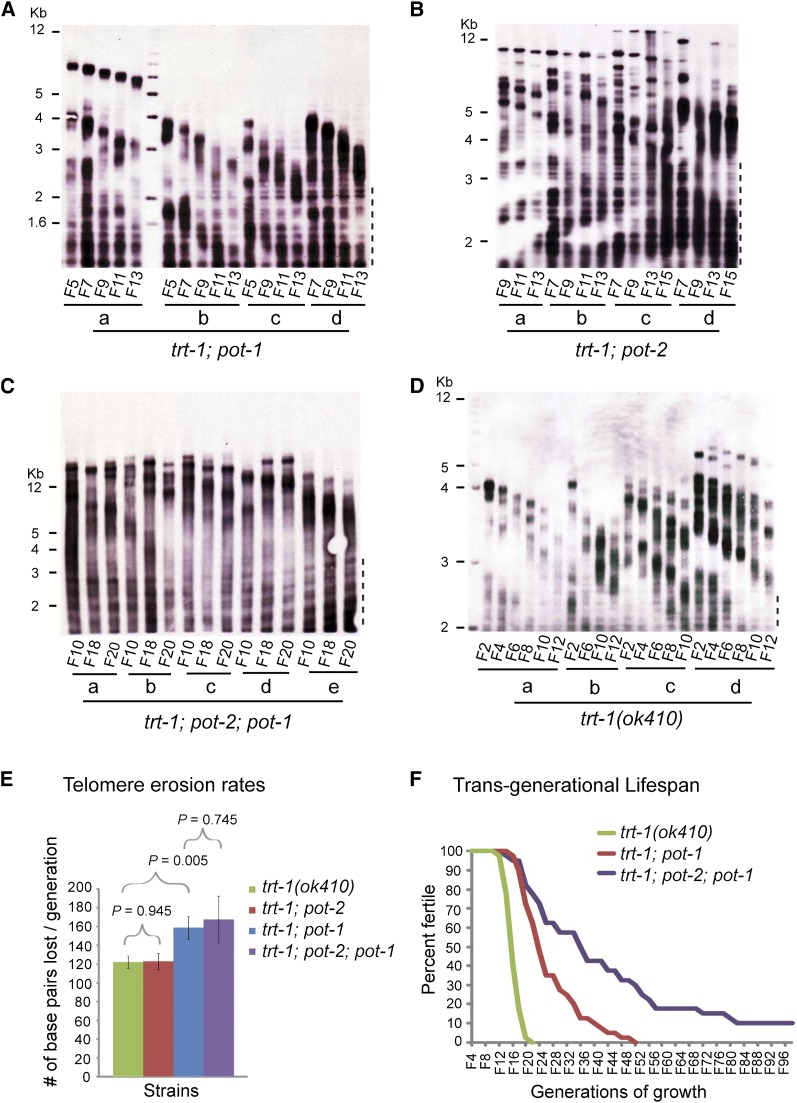
Figure 3 .
POT-1 and POT-2 negatively regulate telomerase-mediated telomere repeat addition. DNA collected from consecutive generations of (A) trt-1; pot-1, (B) trt-1; pot-2, (C) trt-1; pot-1; pot-2, and (D) trt-1 mutant strains was submitted to terminal restriction fragment length analysis. (E) To measure shortening rates, telomeres that were 2−6 kb in size and could be accurately scored for changes in length were examined. Error bars represent the SEM, and P values were determined by the Student’s t-test. (F) Six animals per strain (n = 40) of the indicated genotypes were passaged weekly until sterility, where one week indicates two generations of growth. Dashed line to the right of the blots indicates internal telomeric sequences.
In the absence of telomerase, canonical DNA polymerases cannot maintain telomere length, resulting in a loss of telomere sequence with each cell division. Previous analysis of strains that are deficient for independent alleles of trt-1, for mutations in three additional C. elegans genes that are required for telomerase-mediated telomere maintenance, or for double mutants corresponding to trt-1 and the former genes, has revealed consistent rates of telomere erosion of ~120 bp per generation for every genotype (Ahmed and Hodgkin 2000; Boerckel et al. 2007; Lowden et al. 2008; Meier et al. 2006, 2009). We asked whether POT-1 or POT-2 affected the rate of telomere erosion in the absence of telomerase by quantifying telomere shortening for trt-1; pot-1 and trt-1; pot-2 double mutants, for trt-1; pot-2; pot-1 triple mutants and for trt-1 single mutant controls. An enhanced telomere erosion rate was observed for trt-1; pot-1 mutants (159 ± 12 bp/generation) in comparison to trt-1 single mutants (122 ± 6 bp/generation; Figure 3D) or trt-1; pot-2 double mutants (123 ± 9 bp/generation; Figure 3E). Therefore, POT-1, but not POT-2, protects telomeres from exacerbated erosion in the absence of telomerase. Moreover, trt-1; pot-2; pot-1 telomeres shortened at a similar rate (168 ± 9 bp/generation) to trt-1; pot-1 telomeres, indicating that POT-2 does not have an obvious telomere protection function in the absence of POT-1 (Figure 3E).
To study the effects of the modestly exacerbated telomere erosion observed in pot-1 mutant strains that are deficient for telomerase, the onset of sterility (senescence) was quantified for trt-1; pot-1 double mutants and trt-1; pot-2; pot-1 triple mutants (n = 40 independent lines per strain). Both trt-1; pot-1 double mutants (26.8+/−1.3 generations) and trt-1; pot-2; pot-1 triple mutants (≥44 ± 3.6 generations) exhibited longer trans-generational lifespans (the number of generations until sterility) in comparison to trt-1 mutant controls (16.7 ± 0.4 generations; P < 0.001; Student’s t-test), despite their faster rates of telomere erosion (Figure 3F). In addition, trt-1; pot-2; pot-1 triple mutants exhibited a significantly longer average trans-generational lifespan than the trt-1; pot-1 double mutants (P < 0.001; Student’s t-test), and some did not senesce (Figure 3F). Thus, although POT-1 mildly represses telomere shortening in the absence of telomerase, deficiency for pot-1 or both pot-1 and pot-2 failed to enhance the onset of senescence in the absence of telomerase. This increased trans-generational lifespan is consistent with recent reports that either POT-1 or POT-2 can suppress the telomerase-independent telomere maintenance mechanism termed ALT (Cheng et al. 2012; Lackner et al. 2012).
It has been previously reported that strains deficient for pot-1 or pot-2 with long telomeres display high levels of circular telomeric DNA (Raices et al. 2008). Further, mammalian cells that use the ALT telomere maintenance pathway possess high levels of telomeric C-circles, an established marker of ALT (Henson et al. 2009), and C. elegans trt-1; pot-1 ALT strains display increased levels of telomeric C-circles in comparison with wild type (Lackner et al. 2012). We observed wild-type levels of telomeric C-circles in early-generation pot-2 strains with short telomeres, and large 5- to 7-fold increases in C-circles in late-generation pot-2 strains with long telomeres (Figure 4). Previously established trt-1; pot-2 ALT strains with telomeres of normal lengths (Cheng et al. 2012) displayed little or no increases in C-circle formation, indicating that the high levels of C-circles in late-generation pot-2 strains could require the presence of extremely long telomeres and possibly the activity of the telomerase reverse transcriptase. Consistent with the notion that telomerase could contribute to C-circle formation, trt-1; pot-1 ALT strains with either short or very long telomeres displayed modestly elevated levels of C-circles (Figure 4C). Our data suggest that elevated C-circle levels could contribute to ALT, although the greatest levels of C-circles were observed for late-generation pot-2 that possess long telomeres and were wild type for telomerase.
Figure 4 .
Telomeric C-circle levels increase in late-generation pot-2 mutants. (A) A representative dot blot of a C-circle assay that was performed with DNA collected from multiple, independent mutant animals that were ALT, had grown for multiple generations (>20, “late”), or had grown for few generations (<6, “early”). (B) Quantification of C-circle assay amplification signals from (A). (C) Quantification of a C-circle assay with DNA from wild type, from late-generation pot-2 single mutants with long telomerase, and from a variety of ALT strains that harbored short or long telomeres.
Discussion
POT1 is a multifunctional telomere capping protein, and the presence of four C. elegans genes with homology to human POT1 OB folds provides an opportunity to further elucidate the functions of POT1 in telomere biology. Here we show that C. elegans POT-1 and POT-2 single-stranded telomere-binding proteins negatively regulate telomerase-mediated telomere repeat addition. In addition, abrogation of telomerase activity in pot-1 or pot-2 mutants resulted in progressive telomere erosion. In vitro studies have previously shown that POT-1 preferentially interacts with single-stranded G-rich telomeric DNA, whereas POT-2 interacts with single-stranded C-rich telomeric DNA (Raices et al. 2008), suggesting that these proteins may play distinct roles at telomeres. Qualitatively similar telomere elongation dynamics for pot-1 and pot-2 mutants, and for pot-1; pot-2 double mutants, suggest that these proteins may function in a similar manner to repress telomerase. Our data do not allow us to distinguish whether POT-1 and POT-2 function at distinct steps to repress telomerase or if this occurs via a POT-1/POT-2 heterodimer that possesses both OB1 and OB2 folds and could structurally resemble canonical POT1 proteins (Figure 5A).
Figure 5 .
Model for interactions of POT-1 and POT-2 with telomerase and ALT. (A) POT-1 and POT-2 may repress telomerase-mediated telomere length maintenance via independent functions in the same process (left) or as a heterodimer (right). (B) In the absence of telomerase, POT-1 and POT-2 independently repress ALT-mediated telomere maintenance. (C) Early-generation telomerase mutants typically possess telomeres of normal lengths (top right), but deficiency for trt-1 and pot-1 (middle right) or for both pot-1 and pot-2 (bottom right) may initiate a rapid yet transient telomerase-independent telomere maintenance process that extends telomeres (length of double-stranded telomeric duplex is not drawn to scale), thereby allowing for an extended number of generations prior to senescence. A subset of trt-1; pot-2; pot-1 triple mutants (bottom right) may activate an ALT-mediated telomere maintenance pathway, thereby allowing for immortalization in later generations.
We provide evidence for a distinct role for POT-1 in C. elegans telomere biology, as deficiency for pot-1 but not pot-2 modestly enhanced the rate of telomere erosion in trt-1 mutants, suggesting a telomere capping function of POT-1. POT-1 is the sole C. elegans protein with an OB1 fold (Figure 1A) and can interact with non-terminal segments of single-stranded telomeric oligonucleotides in vitro (Raices et al. 2008). Thus, POT-1 may be well positioned to prevent resection of the 5′ end of the C-rich strand of the telomere. Consistent with our observations, mammalian POT1 has been shown to protect the 5′ end of the telomeric C-strand, which could be subjected to aberrant processing or resection in the absence of POT-1 (Hockemeyer et al. 2005). In contrast, the OB2 fold of POT-2 is predicted to interact with the 3′ end of single-stranded telomeric overhangs (Raices et al. 2008), which could be less relevant to protection or processing of telomeres in the absence of telomerase.
Deficiency for pot-1 delayed the senescence phenotype of telomerase mutants, even though a modestly faster rate of telomere erosion occurred when pot-1 was deficient. Recent independent reports have indicated that deficiency for pot-1 or pot-2 can promote the telomerase-independent telomere maintenance pathway ALT in trt-1 mutants (Cheng et al. 2012; Lackner et al. 2012). POT-1 has been previously shown to repress C-circle formation (Raices et al. 2008), a bona fide marker of ALT, and here we show the same function for POT-2 (Figure 4), suggesting that DNA replication intermediates relevant to ALT may occur in animals lacking either of these proteins. We previously observed an ALT phenotype that allows telomerase mutants to escape senescence indefinitely, but only when hundreds of animals were transferred weekly (Cheng et al. 2012). In the present study, we transferred only 6 animals once a week, which we expected might preclude the onset of a full-blown ALT phenotype. We observed temporary extension of trans-generational lifespan for trt-1 strains deficient for pot-1. However, a subset of trt-1; pot-2; pot-1 triple mutants strains survived indefinitely when 6 larvae were transferred (Figure 3F). This indefinite survival phenotype, in conjunction with a longer trans-generational lifespan of trt-1; pot-2; pot-1 triple mutants in comparison to trt-1; pot-1 double mutants (Figure 3F), is consistent with our previous observation that POT-1 and POT-2 have independent roles in repressing a telomerase-independent telomere replication pathway (Figure 5B; Cheng et al. 2012), which may become fully engaged to robustly drive ALT in small populations of animals when both pot-1 and pot-2 are deficient. We observed that early generation trt-1; pot-1 or trt-1; pot-2; pot-1 mutants had longer initial telomere lengths than trt-1 single mutants (Figure 3, A, C, and D), so initial telomere length may be largely responsible for the extended trans-generational lifespans of the double or triple mutants. Given that well-outcrossed pot-1 or pot-2 mutations with short telomere lengths were employed to establish these strains, we speculate that creation of trt-1 strains that are deficient for pot-1 or for both pot-1 and pot-2 promotes a telomerase-independent ALT-like pathway that rapidly extends telomeres in early generations (Figure 5C), but then dissipates allowing late-onset senescence to occur in most strains (Figure 3F).
The modest and progressive effects of deficiency for pot-1 or pot-2 are at odds with rapid and severe telomere phenotypes that occur in the presence of telomerase for S. pombe pot1 mutants (Baumann and Cech 2001), for P. patens pot1 mutants (Shakirov et al. 2010), and for expression of C-terminally truncated Pot2 in Arabidopsis (Shakirov et al. 2005; Surovtseva et al. 2007). More modest effects have been observed when Pot1 was abrogated in mammalian cells (Hockemeyer et al. 2006; Veldman et al. 2004). The four C. elegans POT1 homologs may each possess one or more functions of ancestral POT1, allowing their roles in telomere biology to be studied in detail. Finally, our pot-1::mCherry transgene allows for chromosome termini to be observed in living worms and may provide a useful tool for future studies of telomere and chromosome biology in C. elegans.
Supplementary Material
Acknowledgments
We thank A. Desai for TH32, the National Bioresource Project for the Experimental Animal C. elegans (Shohei Mitani) for pot-1(tm1620) and pot-2(tm1400), and members of the Ahmed laboratory for discussion and critical reading of the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). This research was supported by NIH grant GM066228 (to S.A.).
Footnotes
Communicating editor: M. C. Zetka
Literature Cited
- Ahmed S., Hodgkin J., 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403: 159–164 [DOI] [PubMed] [Google Scholar]
- Armbruster B. N., Linardic C. M., Veldman T., Bansal N. P., Downie D. L., et al. , 2004. Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1. Mol. Cell. Biol. 24: 3552–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass H. W., Marshall W. F., Sedat J. W., Agard D. A., Cande W. Z., 1997. Telomeres cluster de novo before the initiation of synapsis: a three-dimensional spatial analysis of telomere positions before and during meiotic prophase. J. Cell Biol. 137: 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Cech T. R., 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Boerckel J., Walker D., Ahmed S., 2007. The Caenorhabditis elegans Rad17 homolog HPR-17 is required for telomere replication. Genetics 176: 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Shtessel L., Brady M. M., Ahmed S., 2012. Caenorhabditis elegans POT-2 telomere protein represses a mode of alternative lengthening of telomeres with normal telomere lengths. Proc. Natl. Acad. Sci. USA 109: 7805–7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinquin O., Crittenden S. L., Morgan D. E., Kimble J., 2010. Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc. Natl. Acad. Sci. USA 107: 2048–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin L. M., Baran K., Baumann P., Cech T. R., Reddel R. R., 2003. Human POT1 facilitates telomere elongation by telomerase. Curr. Biol. 13: 942–946 [DOI] [PubMed] [Google Scholar]
- Cooper J. P., Watanabe Y., Nurse P., 1998. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature 392: 828–831 [DOI] [PubMed] [Google Scholar]
- Dernburg A. F., McDonald K., Moulder G., Barstead R., Dresser M., et al. , 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398 [DOI] [PubMed] [Google Scholar]
- Desai A., Rybina S., Muller-Reichert T., Shevchenko A., Hyman A., et al. , 2003. KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev. 17: 2421–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotti R., Loayza D., 2011. Shelterin complex and associated factors at human telomeres. Nucleus 2: 119–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C., Davis M. W., Hopkins C. E., Newman B. J., Thummel J. M., et al. , 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H., 1989. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337: 331–337 [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W., 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460 [DOI] [PubMed] [Google Scholar]
- He H., Multani A. S., Cosme-Blanco W., Tahara H., Ma J., et al. , 2006. POT1b protects telomeres from end-to-end chromosomal fusions and aberrant homologous recombination. EMBO J. 25: 5180–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Wang Y., Guo X., Ramchandani S., Ma J., et al. , 2009. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol. Cell. Biol. 29: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. D., Cao Y., Huschtscha L. I., Chang A. C., Au A. Y., et al. , 2009. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat. Biotechnol. 27: 1181–1185 [DOI] [PubMed] [Google Scholar]
- Hockemeyer D., Sfeir A. J., Shay J. W., Wright W. E., de Lange T., 2005. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24: 2667–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D., Daniels J. P., Takai H., de Lange T., 2006. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell 126: 63–77 [DOI] [PubMed] [Google Scholar]
- Hockemeyer D., Palm W., Wang R. C., Couto S. S., de Lange T., 2008. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 22: 1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher C., Kurth I., Lingner J., 2005. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Mol. Cell. Biol. 25: 808–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendellen M. F., Barrientos K. S., Counter C. M., 2009. POT1 association with TRF2 regulates telomere length. Mol. Cell. Biol. 29: 5611–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., et al. , 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Kaminker P., Campisi J., 1999. TIN2, a new regulator of telomere length in human cells. Nat. Genet. 23: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner D. H., Raices M., Maruyama H., Haggblom C., Karlseder J., 2012. Organismal propagation in the absence of a functional telomerase pathway in Caenorhabditis elegans. EMBO J. 31: 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Safari A., O’Connor M. S., Chan D. W., Laegeler A., et al. , 2004. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 6: 673–680 [DOI] [PubMed] [Google Scholar]
- Loayza D., De Lange T., 2003. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423: 1013–1018 [DOI] [PubMed] [Google Scholar]
- Lowden M. R., Meier B., Lee T. W., Hall J., Ahmed S., 2008. End joining at Caenorhabditis elegans telomeres. Genetics 180: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowden M. R., Flibotte S., Moerman D. G., Ahmed S., 2011. DNA synthesis generates terminal duplications that seal end-to-end chromosome fusions. Science 332: 468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B., Clejan I., Liu Y., Lowden M., Gartner A., et al. , 2006. trt-1 is the Caenorhabditis elegans catalytic subunit of telomerase. PLoS Genet. 2: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B., Barber L. J., Liu Y., Shtessel L., Boulton S. J., et al. , 2009. The MRT-1 nuclease is required for DNA crosslink repair and telomerase activity in vivo in Caenorhabditis elegans. EMBO J. 28: 3549–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M., Verdun R. E., Compton S. A., Haggblom C. I., Griffith J. D., et al. , 2008. C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell 132: 745–757 [DOI] [PubMed] [Google Scholar]
- Scherthan H., Weich S., Schwegler H., Heyting C., Harle M., et al. , 1996. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J. Cell Biol. 134: 1109–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirov E. V., Surovtseva Y. V., Osbun N., Shippen D. E., 2005. The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Mol. Cell. Biol. 25: 7725–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirov E. V., Perroud P. F., Nelson A. D., Cannell M. E., Quatrano R. S., et al. , 2010. Protection of Telomeres 1 is required for telomere integrity in the moss Physcomitrella patens. Plant Cell 22: 1838–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. W., Sokoloski J. A., Perez J. R., Maltese J. Y., Sartorelli A. C., et al. , 1995. Differentiation of immortal cells inhibits telomerase activity. Proc. Natl. Acad. Sci. USA 92: 12343–12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A., van Steensel B., Bianchi A., Oelmann S., Schaefer M. R., et al. , 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surovtseva Y. V., Shakirov E. V., Vespa L., Osbun N., Song X., et al. , 2007. Arabidopsis POT1 associates with the telomerase RNP and is required for telomere maintenance. EMBO J. 26: 3653–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B., de Lange T., 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385: 740–743 [DOI] [PubMed] [Google Scholar]
- Veldman T., Etheridge K. T., Counter C. M., 2004. Loss of hPot1 function leads to telomere instability and a cut-like phenotype. Curr. Biol. 14: 2264–2270 [DOI] [PubMed] [Google Scholar]
- Wang F., Podell E. R., Zaug A. J., Yang Y., Baciu P., et al. , 2007. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445: 506–510 [DOI] [PubMed] [Google Scholar]
- Wu L., Multani A. S., He H., Cosme-Blanco W., Deng Y., et al. , 2006. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126: 49–62 [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Hiraoka Y., 2001. How do meiotic chromosomes meet their homologous partners?: lessons from fission yeast. Bioessays 23: 526–533 [DOI] [PubMed] [Google Scholar]
- Ye J. Z., de Lange T., 2004. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat. Genet. 36: 618–623 [DOI] [PubMed] [Google Scholar]
- Ye J. Z., Hockemeyer D., Krutchinsky A. N., Loayza D., Hooper S. M., et al. , 2004. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18: 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.