Abstract
Progesterone receptor (PR) mediates the actions of the ovarian steroid progesterone, which together with estradiol regulates gonadotropin secretion, prepares the endometrium for implantation, maintains pregnancy, and differentiates breast tissue. Separation of estrogen and progesterone actions in hormone-responsive tissues remains a challenge. Pathologies of the uterus and breast, including endometrial cancer, endometriosis, uterine fibroids, and breast cancer, are highly associated with estrogen, considered to be the mitogenic factor. Emerging evidence supports distinct roles of progesterone and its influence on the pathogenesis of these diseases. Progesterone antagonizes estrogen-driven growth in the endometrium, and insufficient progesterone action strikingly increases the risk of endometrial cancer. In endometriosis, eutopic and ectopic tissues do not respond sufficiently to progesterone and are considered to be progesterone-resistant, which contributes to proliferation and survival. In uterine fibroids, progesterone promotes growth by increasing proliferation, cellular hypertrophy, and deposition of extracellular matrix. In normal mammary tissue and breast cancer, progesterone is pro-proliferative and carcinogenic. A key difference between these tissues that could explain the diverse effects of progesterone is the paracrine interactions of PR-expressing stroma and epithelium. Normal endometrium is a mucosa containing large quantities of distinct stromal cells with abundant PR, which influences epithelial cell proliferation and differentiation and protects against carcinogenic transformation. In contrast, the primary target cells of progesterone in the breast and fibroids are the mammary epithelial cells and the leiomyoma cells, which lack specifically organized stromal components with significant PR expression. This review provides a unifying perspective for the diverse effects of progesterone across human tissues and diseases.
Introduction
-
Molecular Mechanisms of Progesterone Action
Progesterone receptor
Progesterone receptor ligands
Genome-wide binding of progesterone receptor
-
Normal Endometrium and Progesterone
Stromal-epithelial interactions
Estrogen-induced endometrial epithelial proliferation
Antiproliferative action of progesterone on endometrial epithelium
Paracrine interactions in the human endometrium
-
Endometrial Cancer and Progesterone
Clinical features of endometrial cancer
Therapeutic use of progestins in endometrial cancer
Role of progesterone in endometrial cancer
Role of stroma in endometrial cancer
-
Endometriosis and Progesterone
Clinical features of endometriosis
Roles of estrogen and progesterone in endometriosis
Progesterone resistance in endometriosis
Role of stroma in endometriosis
-
Uterine Fibroids and Progesterone
Clinical aspects of uterine leiomyoma
Roles of estrogen and progesterone in leiomyoma growth
Permissive role of estrogen to enhance progesterone action
Effect of pregnancy on leiomyoma
Therapeutic use of antiprogestins in leiomyoma
Paracrine interactions in leiomyoma
-
Breast Cancer and Progesterone
Clinical evidence for the role of progesterone in the breast
Mechanisms of progesterone action in normal mammary tissue
Progesterone action in breast cancer cells
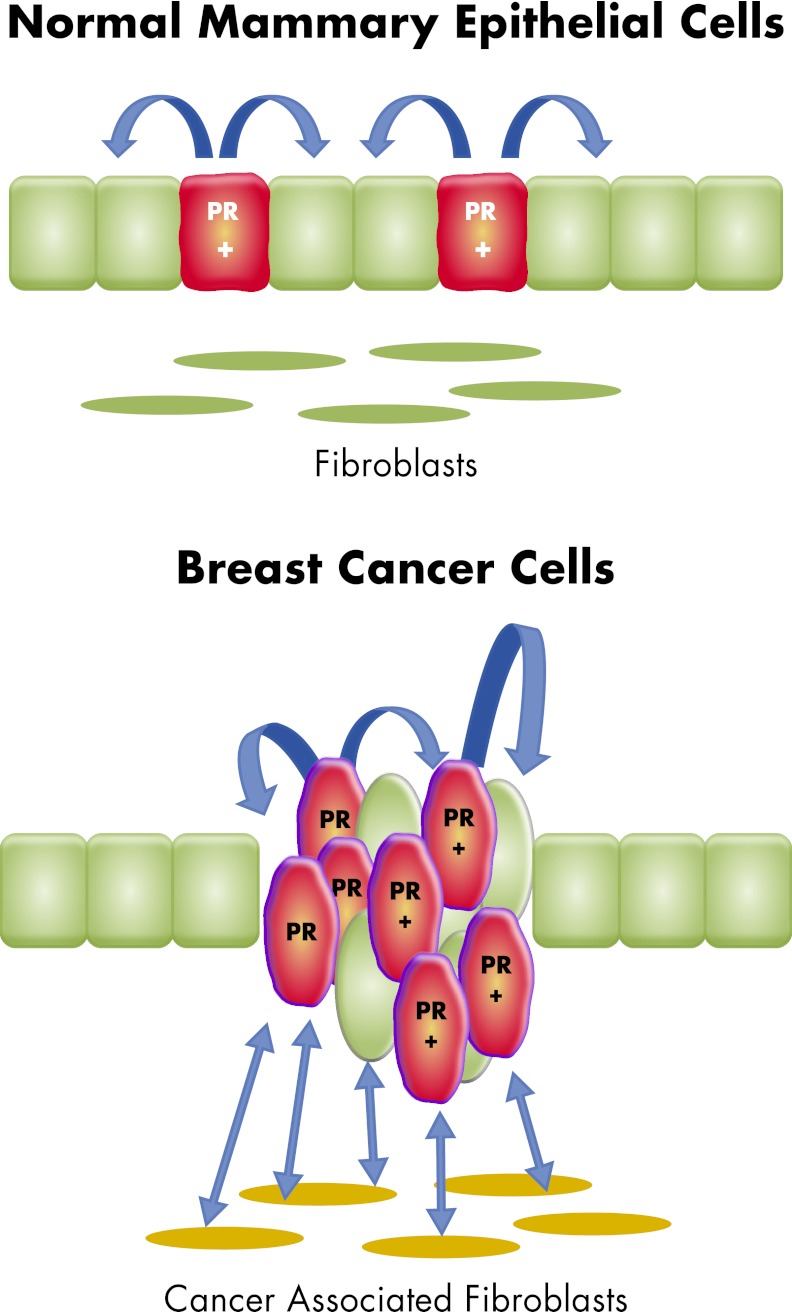
Role of stroma in the breast
Summary and Future Directions
I. Introduction
Progesterone is a steroid hormone that is essential for coordinating normal mammalian female reproductive physiology (1–3). It is secreted primarily by the corpus luteum that develops in the ovary after ovulation. Progesterone affects multiple tissues and organs, including the brain, breast, uterus, ovary, and cervix, as described in detail by Graham and Clarke (3). Progesterone receptor (PR)-null mouse models have demonstrated the necessity of progesterone action for normal reproductive processes, as well as the pleiotropic manner in which progesterone affects different tissues and cell types (2, 4, 5).
The complex and tightly regulated actions of progesterone have been challenging to decipher. Progesterone has diverse effects on reproductive tissues, as well as in different cell types within the same tissue. Furthermore, responses to progesterone are vastly different in normal and diseased target tissues and cells. For example, results from large clinical trials, together with data from animal models, suggest that progesterone and its receptor, PR, promote development and growth of breast cancer and uterine fibroids, whereas progesterone action is protective against the development of estrogen-driven endometrial cancer (6–11). The mechanisms responsible for this striking contrast in progesterone's effects in normal vs. diseased tissues are largely unknown. One plausible explanation may be that the specific microenvironment within target tissues—including locally secreted factors, expressed receptors, and paracrine and autocrine communication—determines the overall effect of progesterone. As an example, PR is primarily expressed in one cell type in the mammary gland and in uterine fibroid smooth muscle cells, and it has an overall protumorigenic effect on these tissues. In contrast, in the endometrium, both epithelial and stromal cells express PR, and although progesterone has distinct actions in each cell type, its actions are modulated by local autocrine and paracrine signaling events between cell types, resulting in an overall preventive effect against estrogen-dependent carcinogenesis of the epithelium (12–14).
The distinctive progesterone/PR-mediated stromal-epithelial interactions, which occur in a uniquely structured endometrial mucosa consisting primarily of multilayered stromal cells lined by a single layer of epithelial cells organized in a pseudoglandular pattern, is contrasted by the breast epithelium that lines true apocrine-type glands buried in an inconspicuously organized adipose stroma (15). These features distinguish endometrial tissue from breast tissue with respect to progesterone action. During the second half of the menstrual cycle, endometrial arterioles containing high concentrations of progesterone of ovarian corpus luteal origin first come in contact with PR-expressing stromal cells, which primarily mediates the actions of circulating progesterone in the neighboring epithelial cells devoid of direct contact with blood vessels (15). It is more challenging to readily recognize this type of a distinct organization of the blood vessels, stroma, and epithelium in breast tissue. This is even less well-defined in the myometrium.
Much of the research thus far has investigated the direct actions of progesterone through PR in isolated normal or diseased breast or endometrial epithelial cells or myometrial/leiomyoma smooth muscle cells. However, the contribution of the stroma and the particular microenvironment within each tissue on its response to progesterone is worth investigation and provides a different perspective on the search for strategies to improve treatment options for various diseases of progesterone target tissues. This review highlights the recent advances in the study of progesterone action in these diseases—including endometrial cancer, endometriosis, uterine leiomyoma, and breast cancer—and the role of stromal paracrine actions in determining the effect of progesterone in some of these pathological tissues.
II. Molecular Mechanisms of Progesterone Action
A. Progesterone receptor
The physiological actions of progesterone are mediated by its interaction with PR, a member of the nuclear hormone superfamily of ligand-activated transcription factors (16, 17). Ligand-occupied PR binds to DNA (18) and recruits coregulatory proteins that activate or repress transcription via interactions with the general transcription apparatus (19–22). PR-dependent transcriptional specificity depends on the availability of PR isoforms and coregulatory factors in target cells (23–26). PR can interact with other transcription factors, such as SP1, AP1, FOXO1 and the p65 subunit of NF-κB (27–31), to modulate transcriptional activity. PR can also interact with Src kinase to activate MAPK signaling (32, 33) and compete for binding to general transcriptional machinery components, thus preventing access of other transcriptional activators in a process known as “squelching” (34, 35). Synthetic PR ligands can affect transcription of PR target genes through selective recruitment or blockade of regulatory cofactors, resulting in the differential regulation of gene expression in various progesterone target tissues.
There are two predominant PR isoforms, PR-A and PR-B, which are transcribed from the same gene by two distinct promoters, with the only difference being that human PR-B is larger than PR-A by an additional 164 amino acids at the amino terminus (36–38). As a result, PR-A and PR-B have distinct transcriptional activities (23, 24, 39–42). Studies in mice with selective ablation of PR isoforms revealed that PR-A is necessary for ovulation and modulates the antiproliferative effects of progesterone in the uterus, and that PR-B is required for normal mammary gland development and function (4, 5). To date, there is no evidence of such selective roles of PR-A and PR-B in human tissues. Recent evidence has suggested the existence of a functional third isoform, designated PR-C, which appears to play a critical role in the onset of parturition (43). The presence of multiple PR isoforms potentially increases the specificity and versatility of hormone action in a particular target tissue.
B. Progesterone receptor ligands
Numerous ligands for PR have been synthesized and used in clinical medicine and research. Ligands with agonistic or antagonistic properties, or both (termed selective PR modulators), interact with PR to activate or repress gene expression in target cells. The progestins, or progesterone agonists, including medroxyprogesterone acetate (MPA), norethindrone acetate, and megestrol acetate, each exhibit a different clinical profile, but all have the ability to repress estrogen-induced endometrial proliferation in vivo (44). Promegestone (R5020) is used as a pure progesterone agonist for in vitro research purposes only.
The antiprogestins, or PR antagonists, oppose the action of progesterone. The most potent known progesterone antagonist is ZK98299 (onapristone). ZK98299 exerts a robust inhibitory effect on progesterone-induced genes and promoters under in vitro conditions (19, 45, 46). In contrast, other antiprogestins, including RU486 (mifepristone), J867 (asoprisnil), CDB4124 (proellex), and CDB2914 (ulipristal acetate), exhibit mixed agonist and antagonist properties both in vitro and in vivo.
RU486 is used primarily for pregnancy termination, but also has utility as treatment for certain gynecological conditions (47, 48). RU486 and J867 permit interaction of the ligand-bound PR with DNA, and thus regulatory sequences of target genes, but alter the capacity of PR to recruit coregulators (49–51). Upon binding to classic progesterone response elements (PREs) that confer progesterone/PR-responsiveness to downstream proximal promoters, RU486 renders the conformation of PR favorable for recruitment of corepressors, thereby antagonizing progesterone action. However, under modulating circumstances such as cotreatment with cAMP analogs or altered coactivator to corepressor ratios, RU486 may function as a progesterone agonist (52, 53). As a further twist, RU486-bound PR, under the same cellular conditions but within different promoter contexts, can repress or enhance transcription of a number of genes (54, 55). RU486, J867, CDB4124, CDB2914, and other compounds in this category that exhibit mixed progesterone antagonist/agonist properties under in vitro and in vivo circumstances are referred to as selective PR modulators (56, 57).
C. Genome-wide binding of progesterone receptor
Genome-wide binding of PR has been demonstrated in both human and mouse tissues. Chromatin immunoprecipitation (ChIP) cloning was used to identify 18 novel binding sites for PR in progesterone-treated primary fibroid smooth muscle cells (58). Specifically, this study identified a novel PR target gene, KLF11, that was significantly up-regulated by RU486 in fibroid cells. Subsequently, ChIP sequencing was used to compare PR binding sites, using RU486 as the ligand, in breast cancer cells and uterine fibroid smooth muscle cells (59). ChIP sequencing revealed 31,457 and 7,034 PR-binding sites in breast cancer and uterine fibroid cells, respectively; 1,035 sites overlapped in both cell types. Based on the chromatin-PR interaction in both cell types, the consensus PRE was refined to G-ACA—TGT-C.
Two striking differences between uterine leiomyoma and breast cancer cells were observed. Interestingly, the cis-regulatory elements for HSF, TEF-1, and C/EBPα and β were statistically enriched at genomic RU486/PR targets in uterine fibroid cells, whereas E2F, FOXO1, FOXA1, and FOXF sites were preferentially enriched in breast cancer cells. Additionally, more than half of the RU486-regulated genes in breast cancer cells, but only 7% of RU486-regulated genes in uterine leiomyoma cells, contained a PR-binding site within 5 kb from their transcription start sites. In leiomyoma cells, 75% of RU486-regulated genes contained a PR-binding site farther than 50 kb from their transcription start sites. ChIP sequencing was also used to define the genome-wide PR cistrome in the murine uterus (60). In this study, 18,432 sites were identified in the uteri of ovariectomized mice after acute progesterone exposure. Using this approach, previously identified PR target genes were found to contain PREs, and novel genes involved in circadian rhythms, as well as Sox17, were identified. These studies highlight the influence of distinct cofactors and/or mechanisms that regulate chromatin accessibility on the recruitment of PR to DNA sites and show that these cofactors and mechanisms differ by tissue and cell type.
III. Normal Endometrium and Progesterone
The endometrium is the lining of the uterus that grows, differentiates, regresses, and regenerates in a cyclical manner in response to ovarian steroid hormones. Estrogen stimulates, whereas progesterone inhibits, endometrial growth. In cells that express ER and PR, the hormones bind to their receptors and can regulate genes directly to regulate endometrial physiology. Additionally, there is strong evidence that paracrine interactions between the epithelial and stromal cells in the endometrium can modulate its response to hormones. The significance of epithelial-stromal tissue interaction in the biology of the uterus has been well established by a series of tissue recombination studies, as well as in transgenic mice.
A. Stromal-epithelial interactions
The first demonstration of the critical role of the stroma in determining the action of steroid hormones on epithelium was in the mouse embryonic mammary gland. Using tissue recombinants consisting of mammary epithelium and stroma derived either from wild-type or mutant androgen-insensitive embryos, apoptosis and regression of the mammary epithelium in response to testosterone secreted from the embryonic testis was found to be mediated via androgen receptor in the mesenchyme (61, 62). Following a similar approach, Cunha and Lung (63) demonstrated that the testosterone-induced organogenesis of the prostate was also dependent on mesenchymal androgen receptor.
In the uterus, estrogen stimulates, whereas progesterone inhibits, epithelial proliferation. Based on the observed androgenic effects in the fetal mammary gland and prostate, it was hypothesized that estrogen and progesterone could control the growth of the endometrial epithelium through epithelial-stromal tissue interactions. The absence of growth stimulation by estrogen in cultured endometrial epithelial cells supported this idea. The introduction of mouse embryonic stem cell culture technology, which led to the generation of mutant mice with genetic disruption of the ER or PR, permitted researchers to perform tissue recombination studies to understand the cell-specific roles of estrogen and progesterone action in the uterus.
B. Estrogen-induced endometrial epithelial proliferation
In 1997, Cooke et al. (64) reported the first tissue recombination study utilizing uteri from estrogen receptor (ER)α-null mice in which ERα lacks the activation function-1 domain (αERKO mice). Epithelium and stroma of neonatal uteri from wild-type and αERKO mice were combined and grafted under the kidney capsule of athymic nude mice. In the study, estradiol treatment stimulated epithelial proliferation only when ERα was expressed in the stromal cells. In contrast, expression of ERα in the epithelial cells was neither essential nor sufficient to mediate estradiol-stimulated proliferation of uterine epithelial cells. This study established the paracrine regulation of endometrial epithelial proliferation by estrogen. Recent studies utilizing conditional ERα knockout mice for uterine epithelium (Esr1flox/flox; Wnt7A-Cre+) (12) and a coculture system for uterine epithelial and stromal cells (65) reconfirmed the essential role of stroma in the regulation of endometrial epithelial proliferation by estradiol.
C. Antiproliferative action of progesterone on endometrial epithelium
Like ERα, PR is expressed in both endometrial epithelial and stromal cells. Therefore, the role of stroma in the antiproliferative action of progesterone on endometrial epithelium was similarly examined in a tissue recombination study utilizing PR knockout (PRKO) mice, which were shown to be insensitive to the inhibitory action of progesterone on estrogen-induced endometrial hyperplasia (13). Epithelial (E) and stromal (S) tissues from neonatal wild-type (WT) and PRKO mice were combined to generate four types of chimeric uteri: WT-E + WT-S; PRKO-E + PRKO-S; WT-E + PRKO-S; and PRKO-E + WT-S. WT-E + WT-S and PRKO-E + PRKO-S uteri served as positive and negative controls, respectively, with the presence or absence of progesterone signaling in both epithelium and stroma. By contrast, WT-E + PRKO-S and PRKO-E + WT-S uteri could respond to progesterone/PR signaling only through epithelial or stromal cells, thus distinguishing signals occurring via the epithelium vs. stroma (Fig. 1). Treatment of all four types of uterine tissue recombinants with estradiol induced DNA synthesis equally in the epithelial cells. However, progesterone inhibited epithelial proliferation in the uterine tissue recombinants composed of wild-type stroma (WT-E + WT-S and PRKO-E + WT-S uteri) but not PRKO-S stroma (PRKO-E + PRKO-S and WT-E + PRKO-S uteri).
Figure 1.
Paracrine regulation of mouse endometrial epithelial proliferation by progesterone (P4). Four types of chimeric uteri were generated with stroma (S) and epithelium (E) from wild-type (Wt) and PRKO uteri. In Wt-E + Wt-S uterus, P4 acts on epithelium through PR in both epithelium and stroma, whereas P4 has no effect on PRKO-E + PRKO-S uterus. P4 action is mediated only by epithelial or stromal PR in the Wt-E + PRKO-S and PRKO-E + Wt-S uteri, respectively. Immunohistochemistry for PR (brown staining) confirms the epithelial/stromal-specific expression of PR. As assessed by incorporation of [3H]thymidine (black dots), estradiol (E2) equally stimulates epithelial proliferation in all four types of uteri. In contrast, P4 inhibits epithelial proliferation only in uterus in which PR was expressed in stroma. Epithelial PR is not necessary for the antiproliferative action of P4 on epithelial cells. [Modified from T. Kurita et al.: Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 139:4708–4713, 1998 (13), with permission. © The Endocrine Society.]
These results clearly indicated that progesterone inhibits the proliferation of endometrial epithelial cells via PR in stromal cells, whereas epithelial PR is not necessary for progesterone inhibition of estradiol-induced epithelial proliferation. Three possible mechanisms were proposed to explain these findings: progesterone blocks stromal production of estradiol-induced mitogenic mediators; progesterone induces paracrine growth inhibitors; and/or PR inhibits ERα action directly via non-transcription-mediated action (13). A recent mouse study by Li et al. (14) demonstrated that progesterone inhibits epithelial proliferation by blocking the production of mitogenic mediators in the stroma. In this study, the antiproliferative action of progesterone on uterine epithelium was mediated by expression of the transcription factor Hand2 (heart and neural crest derivatives expressed 2) in stromal cells. Progesterone induced Hand2 expression in endometrial stroma, and in turn, Hand2 inhibited the expression of several fibroblast growth factor ligands. Fibroblast growth factors act as paracrine mediators to induce proliferation of the epithelium in response to estrogen, and thus, its inhibition by progesterone in the stroma is one mechanism by which epithelial proliferation is decreased. Kurita et al. (66) demonstrated, using tissue recombination, that progesterone inhibits apoptosis of endometrial epithelial cells through PR action in stromal cells. In the mouse uterus, growth and survival of epithelial cells were found to be regulated by progesterone-bound PR in stromal cells, further supporting the role of the endometrial stroma in the pathogenesis of endometrial cancer.
As described above, the importance of stromal PR in progesterone action in the endometrium has been established by tissue recombination studies. Nonetheless, the critical role of epithelial PR in the biology of the uterus should not be discounted. Earlier, a tissue recombination study demonstrated that epithelial PR plays a role in the regulation of lactoferrin expression (67). A recent study utilizing mice devoid of endometrial epithelial PR demonstrated that PR in uterine epithelium is also critical for the antiproliferative action of progesterone (68). In Pgrflox/flox/Wnt7A-Cre+ mice with selective inactivation of endometrial epithelial PR, progesterone failed to inhibit estrogen-induced epithelial proliferation, contradicting previous tissue recombination studies. This inconsistency may be due to differences in methodologies (e.g., tissue recombination vs. cell-selective knockout) or hormone treatment protocols. Interestingly, in this more recent study, the epithelial PR-null endometrium showed defective stromal decidualization owing to the loss of Indian hedgehog expression in epithelial cells (68). Taken together, it is evident that there is a coordinated and intimate interplay between epithelial and stromal cells that is essential for the endometrium to proliferate, remodel, and shed in response to estrogen and progesterone.
D. Paracrine interactions in the human endometrium
Whether the tissue interactions seen in the mouse uterus are similar to those involved in the growth control of human endometrial epithelium has not yet been fully established. A xenografting study involving human endometrium implanted in nude mice demonstrated that the growth kinetics of human and mouse endometrial epithelial cells are significantly different (69). In this study, human endometrial epithelium was grafted under the kidney capsule of nude mice in combination with either mouse or human uterine stromal cells. In the human-mouse chimeric uteri, human endometrial epithelial cells proliferated at a significantly lower rate than mouse epithelial cells under the identical systemic and stromal environment. The human endometrial epithelium required longer exposure to estrogen than the mouse epithelium to reach its peak proliferation rate in the context of either human or mouse uterine stroma. This seems to reflect the significant difference in the physiology of two species: mice progress through the estrous cycle in only 4 d, whereas women take 4 wk to complete a menstrual cycle. In the same study, chimeric uteri were created by grafting human endometrial epithelium into the uterine stroma of ERKO mice (69), the same mutant mice used in the tissue recombination study described above (64). Surprisingly, human endometrial epithelium proliferated in response to estradiol irrespective of the genotype of the mouse stroma, indicating that the activation function-1 domain of ERα in the mouse stromal cells is essential for estradiol-induced proliferation in the mouse, but not the human, endometrial epithelial cells in the chimera. This study revealed only a fundamental difference in the growth control of endometrial epithelium between the mouse and human but did not provide evidence of epithelial-stromal interactions in human uterine tissue. Nonetheless, in vitro coculture studies have shown that stromal cells do play a role in the response of human endometrial epithelium to estradiol and progesterone (70, 71). In one study, primary human endometrial stromal cells supported estradiol-induced proliferation of primary endometrial epithelium by production of IGF-I (70); in another study of three-dimensional coculture systems for endometrial epithelial organoids and stromal cells, epithelial proliferation was induced by estradiol and inhibited by MPA only in the presence of stromal cells (71). Because PR was undetectable in the epithelial cells in MPA-treated organoids, MPA action appeared to be mediated by stromal PR.
Taken together, the existing data obtained from mouse in vivo studies and coculture studies utilizing primary human cells has reasonably established the key role of stromal cells in the hormonal regulation of epithelial proliferation in the normal endometrium.
IV. Endometrial Cancer and Progesterone
A. Clinical features of endometrial cancer
The endometrium is one of the most hormonally responsive tissues in the body and aberrant exposure to hormones can lead to neoplastic changes, including hyperplasia and adenocarcinoma. Endometrial hyperplasia and adenocarcinoma arise from the glands of the endometrium and exhibit distinct histological differences compared with the normal endometrium (Fig. 2). Endometrial hyperplasia is classified as simple or complex, based on the glandular/stromal architectural pattern, and sometimes will exhibit nuclear atypia (72). Women with complex hyperplasia with atypia have the greatest risk of developing cancer. Endometrial cancer can occur in two forms: type 1 is the most common cancer that is classified as being estrogen-dependent, whereas type 2 is not related to estrogen stimulation and usually presents as a higher grade cancer with a poorer prognosis (73). Type 1 endometrial adenocarcinoma occurs in circumstances of chronic exposure to estrogen with insufficient opposing progesterone. Histologically, in a well-differentiated, low-grade cancer, the glands appear numerous and crowded and are usually positioned “back-to-back” (74, 75) (Fig. 2). There is clear stromal invasion, and the glands eventually replace the stroma. The glands exhibit varying degrees of nuclear atypia, mitotic activity, and stratification. As the disease progresses, myometrial invasion occurs, and the tumors lose their gland-like architecture, as depicted in the grade 3 cancer in Fig. 2 (74, 75). It is noteworthy that the stroma is prevalent in both normal and simple hyperplastic endometrium but diminishes as cancer develops and with increasing grade.
Figure 2.
The normal and neoplastic endometrium. Normal proliferative phase endometrium, hyperplasia, grade 1 and grade 3 endometrial adenocarcinoma are shown. Endometrial hyperplasia is characterized by an increase in the epithelium-stroma ratio, irregularities in gland shape, multiple epithelial cell layers, and variation in gland size. Endometrial adenocarcinoma is exhibited by a confluent glandular epithelial pattern in which individual glands appear “back-to-back” with an altered fibroblastic stroma (desmoplastic stromal response). Grade 3 endometrial cancer exhibits more solid growth with a loss in gland-like architecture. Only sheets of malignant epithelial cells are present.
In the United States, endometrial cancer is the most common gynecological malignancy, with 46,470 new cases and 8,120 deaths from the disease in 2011 (76). Both endogenous and exogenous sources of estrogen have been linked to the incidence of type 1 endometrial cancer. Various conditions, including anovulation, polycystic ovarian syndrome, and obesity, lead to high levels of unopposed estrogen exposure (77–79). Exogenous estrogen-only hormone replacement therapy (HRT) and prolonged tamoxifen use can also promote the development of endometrial cancer (77–79). Although the exact mechanisms involved in endometrial carcinogenesis due to chronic estrogen exposure are unclear, it is thought that the pro-proliferative (80, 81) and DNA-damaging (82–85) effects of estrogen and its metabolites, coupled with an insufficient counterbalance by progesterone, promote the hyperproliferation and transformation of cells. Based on the antagonistic role of progesterone to estrogen action, progestins have been used clinically to treat endometrial neoplasias. Women with endometrial hyperplasia and well-differentiated endometrial adenocarcinoma can show a complete response to progestin therapy; however, with increasing severity of the disease, the efficacy of progestins declines. The reasons for this are not known. Given the relatively milder side effects of progestins compared with chemotherapy, strategies to increase the efficacy of progestins for treating endometrial cancer are of great interest, especially for the treatment of patients who wish to preserve fertility after treatment or who are poor surgical candidates.
B. Therapeutic use of progestins in endometrial cancer
In 1961, Kelley and Baker (86) made the initial observation that the treatment of patients with advanced endometrial cancer with progestational agents led to beneficial responses. Kistner et al. (87) showed that the changes that occur in endometrial hyperplasia could be reversed by progestins. Many investigators subsequently confirmed these findings, reporting overall response rates of 15–40% (88–91). Unfortunately, studies investigating the efficacy of progestin therapy in endometrial cancer have been limited to case series and pilot studies. More recent studies have reported higher overall response rates (92–94); however, the recurrence rate upon cessation of progestin therapy poses a significant challenge. Progestins are also used as palliative therapy for recurrent endometrial cancer, and the response rates range from 15 to 20% (95, 96). A Gynecological Oncology Group (GOG) study of women with advanced or recurrent endometrial cancer treated with either 200 or 1000 mg/d oral MPA demonstrated a complete response rate of 17 and 9%, respectively (96). In other GOG studies, patients with advanced endometrial cancer were treated with tamoxifen with alternating weekly cycles of MPA, which resulted in response rates of 27% (97) and 33% (98). The rationale for adding tamoxifen to progestin therapy was based on data showing that tamoxifen acts as an estradiol agonist and increases PR expression in the endometrium (99), whereas progestins alone down-regulate PR, which limits the duration of treatment effects. A recent review of randomized controlled trials of hormonal therapy in adult women with advanced or recurrent endometrial cancer found that low-dose hormonal therapy may have a benefit in terms of overall and progression-free survival compared with high-dose hormonal therapy; however, there was no evidence that hormonal therapy improved the overall or 5-yr disease-free survival rates (100).
Progestins have also been shown to be effective in patients with recurrent low-grade endometrial stromal sarcomas (101–104). Unlike endometrial adenocarcinoma, endometrial stromal sarcomas are extremely rare and arise from the stromal compartment of the endometrium. These tumors express both ER and PR. Reports have shown that these tumors are responsive to progestin therapy and that postoperative treatment with progestins decreased recurrence rates (101, 102, 105, 106).
Given the low incidence of type 2 endometrial cancer, little is generally known about this type of cancer. Type 2 endometrial cancers, which include serous and clear-cell morphologies, are not considered to be hormonally dependent; rather, these tumors are associated with abnormalities of p53 and HER2/neu (78). ER and PR expression is lower in type 2 compared with type 1 endometrial cancer (107, 108). In the GOG study described above, in which women with endometrial cancer were treated with MPA, the group with high-grade tumors (grade 3, papillary serous) had the lowest percentage of responders (96).
From these clinical studies, it is clear that progestin therapy has been and continues to be a viable treatment option for type 1 endometrial cancer. Over the years, however, the response rates have not improved substantially, primarily due to our limited understanding of how progestins are able to regress or prevent progression of these tumors.
C. Role of progesterone in endometrial cancer
The mechanism of action of progesterone in endometrial cancer has been studied in women treated with progestins, in transgenic animals that develop endometrial cancer, in endometrial cancer xenografts in immunocompromised mice, as well as in vitro in endometrial cell lines. These studies have been recently reviewed (109–111). Yet much of the focus of progesterone action in endometrial cancer has been on the tumor cells themselves, and there is very little information regarding the role of stroma in this disease. Because it has been shown that the stroma and the epithelium both respond to progesterone and that the cell types actively communicate in a paracrine manner, it is important that we conduct a more detailed investigation of the role of the stroma in endometrial cancer. Such studies would allow us to understand progesterone action in endometrial cancer from a different perspective and may reveal new strategies for developing more effective therapeutics.
As discussed above, it is well recognized that progesterone promotes differentiation of both the glandular epithelium and the stroma in benign endometrium. In the progestin-treated endometrium in women with endometrial cancer, there are marked histological changes, including a decreased gland-to-stroma ratio, decreased glandular cellularity, decreased to absent mitotic activity, loss of cytological atypia, and a variety of cytoplasmic changes, including mucinous, secretory, squamous, and eosinophilic metaplasia (112). Architectural abnormalities unique to progestin treatment are also observed, such as cribriform and papillary patterns. Kamoi et al. (113) observed swelling of the neoplastic glandular epithelial cells with pale vacuolated cytoplasm and round to oval nuclei, low cuboidal epithelium with or without squamous or morular metaplasia, and lymphoplasmacytic infiltration in response to progestins. In addition, the stromal area exhibited predecidual changes, and vessels were dilated. These histological changes are distinct from the normal endometrial changes that occur during the secretory phase of the cycling endometrium and could be the result of prolonged exposures to supraphysiological doses of progestins, which are different from the endogenous progesterone hormone concentrations. However, the predecidual effects of progestins on the stroma, which also occur during the secretory phase in normal endometrium, indicate that “progestogenic” effects are maintained with progestin therapy. Thus, changes in the stroma could be alternative indicators of progestin responsiveness of the malignant endometrium.
Expression of ER and PR in type 1 endometrial cancer usually signifies that the tumor is well differentiated because expression of these steroid hormone receptors declines in tumors that are poorly differentiated or of higher grade. Clinical response rate, as well as improved overall survival, has been correlated to PR positivity (89, 114). In addition, cancer recurrence has been shown to occur with higher prevalence in PR-negative tumors (89, 114). For reasons that are unclear, however, not all PR-positive endometrial tumors respond to progestins, and some PR-negative tumors do respond to progestins. PR expression in the stromal compartment is not considered during diagnosis. Given that the stromal cells are responsive to progesterone in the normal endometrium, the expression of PR in stromal cells alongside the tumors may be useful. Furthermore, because PR-A is the predominant isoform in the stromal cells of normal endothelium (105), the expression and function of PR-A in the stroma surrounding endometrial tumors should be studied.
Effects of progesterone on endometrial cancer cells have been studied in detail using various established cell lines and xenografts in immunocompromised mice (109–111). Studies have shown that progesterone/progestins do affect the cancer cells directly, causing inhibition of cell growth and invasiveness and increased differentiation and apoptosis, among other effects (115–118). Specific genes induced by progesterone and involved in these processes have been identified, including cyclin D1; matrix metalloproteinase-1, -2, -7, and -9; Ets-1; glycodelin; FOXO1; p21; and p27 (117–125). Using microarray technology, numerous other genes associated with cellular adhesion, cell cycle, apoptosis, immune responses, intracellular protein traffic, and transport have been identified in endometrial cancer cells (115, 117, 126–128). It has been a long-standing challenge to study the biochemical and molecular effects of progesterone in vitro. Responses of cultured cells to progesterone are usually weak, and as a result, cells are manipulated to overexpress PR or treated with supraphysiological concentrations of progestins for prolonged periods of time (115, 116, 129–131). It is unclear why effects of progestins on cultured cells are not robust, although we speculate that the effect of progesterone on the endometrium involves the paracrine action of multiple cell types, producing an environment in vivo that cannot be recreated in vitro with monolayer cultures. The presence of progesterone-responsive stroma, as well as other cell types, around the cancer cells may amplify the physiological effects of progesterone on epithelial tumor cells. On the other hand, it is also possible that the physiological changes in the endometrium observed after progestin therapy in women with endometrial cancer do indeed require supraphysiological doses of progestins for prolonged periods of time (200–1000 mg/d for months).
D. Role of stroma in endometrial cancer
The action of progesterone in the endometrial stroma has been studied predominantly in the normal cycling uterus. PR expression is maintained throughout the menstrual cycle in the stromal cells, and progesterone promotes decidualization of the stroma (132–134). Evidence points to an essential requirement of the stroma in mediating hormonal effects on the normal epithelium (80, 83–85, 101, 106, 135, 136). Comparatively, very little is known on the role of stroma as it pertains to endometrial cancer. Yang et al. (137) demonstrated that human endometrial stromal cells treated with progestin secrete factors that increase 17β-hydroxysteroid dehydrogenase type 2 (HSD17B2) mRNA expression and enzyme activity in the Ishikawa epithelial cell line. In contrast, direct progestin treatment of Ishikawa cells resulted in a much smaller increase in enzyme expression and activity. In another study, Arnold et al. (138) demonstrated that coculture of stromal cells with Ishikawa cells promoted differentiation and expression of glycodelin in Ishikawa cells regardless of hormone treatment. Shi et al. (139) demonstrated that paracrine factors from normal endometrial stromal cells significantly decreased hormone-stimulated activity of PI3K/AKT signaling in Ishikawa cells. Genistein can inhibit the proliferative effects of estradiol on Ishikawa cells through activation of stromal ERβ (140).
The paracrine communication occurring between endometrial stromal and epithelial cells, as it pertains to endometrial cancer, was further demonstrated in transgenic mice, in which the Apc gene encoding APC (adenomatous polyposis coli) protein was knocked out selectively in the stromal cells of the endometrium. APC represses canonical Wnt signaling by promoting degradation of β-catenin; thus, the deletion of Apc should activate canonical Wnt signaling. Upon specific deletion of APC in the stromal cells, endometrial hyperplasia and endometrial carcinogenesis developed (141), demonstrating that dysregulation of the Wnt pathway in stromal cells had dire consequences on the epithelial cells. As more evidence supports the interdependence between stromal cells and epithelial cells, a more detailed analysis of the role of stroma in mediating the inhibitory effects of progesterone on estrogen-driven proliferation of the epithelium is needed. In turn, the influence of endometrial epithelial tumor cells on the stroma would advance our understanding of how stromal cells might promote tumor growth. As endometrial cancer progresses, the stromal and tumor cells will influence each other, and thus the response of stromal cells to hormones, and the subsequent effects on glandular epithelium in the absence of cancer are expected to be different than when cancer is present. As will be discussed in Section VII, this has been shown to be the case in breast cancer, where fibroblasts in the tumor microenvironment can promote tumorigenesis through paracrine effects on the tumor cells (reviewed in Refs. 81, 82, and 132).
Investigation of the stromal response to progestins and the effect of the stroma on endometrial tumors may lead to the discovery of novel markers to predict progestin sensitivity or the identification of key pathways and molecules that could be targeted to improve the efficacy of progestin treatment. This is an emerging area of research that remains relatively unexplored. Progestin therapy continues to be used in the clinic for the management of endometrial cancer, and in terms of its efficacy, there is room for improvement. At a time when the risk factors for endometrial cancer, such as obesity, are becoming more prevalent, it will be important to provide better prevention and treatment strategies.
V. Endometriosis and Progesterone
A. Clinical features of endometriosis
Endometriosis is an inflammatory disease that affects 5–10% of women of reproductive age in the United States (142). The menstrual cycle and the production of ovarian steroids heavily influence its occurrence and symptoms. Endometriosis is defined as the presence of endometrium-like tissue in ectopic sites outside the uterine cavity, primarily on the pelvic peritoneum and ovaries, and is linked to pain during menses and intercourse, and chronic pelvic pain and infertility (142). As cellular and molecular mechanisms in endometriosis are uncovered, this definition continues to evolve to describe a systemic and complex chronic disease with unique features. The classic presentation of pelvic implants associated with pain and infertility may represent a common phenotype that results from diverse anatomic or biochemical aberrations of uterine function (143, 144). As is the case in other chronic disorders, endometriosis is thought to have multifactorial etiology and is inherited in a polygenic manner (145).
Three clinically distinct forms of endometriosis exist: 1) peritoneal endometriosis, with endometriotic implants on the surface of pelvic peritoneum and ovaries; 2) endometriomas, cysts lined by endometrioid mucosa on the ovary; and 3) rectovaginal nodule, a complex solid mass comprised of endometriotic tissue blended with local adipose and fibromuscular tissue and residing between the rectum and vagina. All three forms are possibly variant phenotypes of the same pathological process, i.e., retrograde menstruation of epigenetically abnormal endometrial cells with stem cell properties (146, 147). The common histology of endometriosis is the presence of endometrial stromal and/or epithelial cells, chronic bleeding, and inflammatory changes (Fig. 3). Lesions may occur singly or in combination and are associated with a significantly increased risk of infertility and/or chronic pelvic pain (148, 149). Treatment of infertility is surgical removal of lesions and/or assisted reproductive technology, whereas pain is usually treated with a combination of medical suppression of ovulation and surgery. Peritoneal implants are resected or vaporized by electric current or laser. Ovarian endometriomas and rectovaginal endometriotic nodules, however, are effectively removed only by full dissection.
Figure 3.
Ovarian endometriosis. Ectopic endometriotic tissue in this ovarian hilar region shows endometrioid glands and stroma with hemorrhage and hemosiderin deposition, consistent with endometriosis.
Clinical evidence points to a clear and deleterious effect of uninterrupted ovulatory cycles on the development and persistence of endometriosis (150, 151). Symptoms of endometriosis usually appear after menarche and vanish after menopause (152). Disruption of ovulation with pregnancies, GnRH analogs, oral contraceptives, or progestins reduces pelvic disease and associated pain (151). In line with these observations, basic and clinical research findings indicate major roles of estrogen and progesterone in the pathology of endometriosis. In humans and primate models, estrogen stimulates the growth of endometriotic tissue, whereas aromatase inhibitors that block estrogen formation and antiprogestins are therapeutic (153–155). Expression levels of nuclear receptors for estrogen and progesterone in endometriotic tissue are strikingly different than those in normal endometrium (156–158). Finally, biologically significant quantities of progesterone and estrogen are produced locally via an abnormally active steroidogenic cascade that includes aromatase (159).
In 1927, it was proposed that endometriosis occurs when fragments of menstrual endometrium pass retrograde through the fallopian tubes, then implant and grow on pelvic peritoneal surfaces and ovaries (160). Yet, although more than 90% of women have reflux menstruation into the peritoneal cavity, endometriosis is encountered in only 5–10% of cases. Gene expression profiling of eutopic (intrauterine) endometrium from women with endometriosis vs. that from disease-free women revealed subtle abnormalities, including expression of candidate genes involved in steroid biosynthesis, inflammation, implantation failure, infertility, and progesterone resistance (161–165). One can then envision that a small population of epigenetically abnormal cells, with self-renewal capabilities, may reside in the endometrium of women destined to develop clinically recognizable endometriosis on peritoneal surfaces or the ovary. These epigenetically abnormal cells reach the peritoneal cavity via retrograde menstruation and inappropriately express prosurvival genes such as steroidogenic factor-1 (SF1) or ERβ, which are normally suppressed via promoter methylation in endometrial cells. SF1 and ERβ, in turn, activate the cascade of genes for local estrogen and prostaglandin biosynthesis in endometriotic tissue, giving rise to inflammation, resistance to apoptosis, and persistence of pelvic endometriosis (166).
B. Roles of estrogen and progesterone in endometriosis
Distinct perturbations in basic biological functions such as angiogenesis, immune response, and apoptosis have been found to be altered in favor of survival and replenishment of endometriotic tissue (167–171). These functions were found to be, in part, dependent on estrogen or progesterone action. Excessive formation of estrogen and prostaglandin and the development of progesterone resistance have emerged recently as clinically useful concepts because targeting aromatase in the estrogen biosynthetic pathway, cyclooxygenase-2 in the prostaglandin pathway, or PR significantly reduces or eliminates laparoscopically visible endometriosis and pelvic pain (153, 154, 172, 173). These three critical mechanisms have been linked via specific epigenetic (promoter hypomethylation) defects that cause overexpression of the nuclear receptors SF1 and ERβ (158, 174).
There is agreement among clinicians and researchers that estrogen increases the risk of laparoscopically visible endometriosis and associated pelvic pain. The role of progesterone in the development or persistence of endometriosis, however, has not been well understood for the following reasons. First, the protective role of progesterone in endometrial cancer, an epithelial malignancy, has been inappropriately attributed to endometriosis, which is benign. The basic pathology in endometriosis is not epithelial proliferation but rather increased inflammation and cell survival due to diminished apoptosis or differentiation (171). Paradoxically, progesterone induces a transient proliferation of stromal cells in normal endometrium during the secretory phase. Second, only approximately half of patients who previously received medical or surgical treatment for endometriosis-related pelvic pain derive benefit from progestin therapy (175–177). Progestins likely reduce pain via inhibition or attenuation of ovulation. A direct effect of progestins on endometriotic tissue, however, cannot be excluded. Third, antiprogestins with mixed agonist and antagonist properties reduce endometriosis-associated pelvic pain, possibly more effectively than progestins (53, 178). Lastly, endometriotic tissue produces significant quantities of progesterone and contains strikingly lower levels of PR compared with endometrium (157, 179). These seemingly disparate observations have made it challenging to explain the role of progesterone in the pathology of endometriosis.
C. Progesterone resistance in endometriosis
Estrogen and progesterone are essential and sufficient to control the entirety of endometrial function via regulating the expression of hundreds to thousands of genes throughout the menstrual cycle (180). Indeed, administration of estradiol and progesterone is sufficient to prepare the endometrium for implantation in postmenopausal women undergoing donor embryo transfer (181). Progesterone exposure induces differentiation of endometrial stromal cells and epithelial cells. Molecular markers of progesterone action include increased production of epithelial glycodelin and stromal IGF binding protein-1 and prolactin (180, 182, 183). It has been demonstrated in stromal cells from both eutopic and ectopic tissues of endometriosis that the progesterone response is blunted, in that expression of markers is significantly lower, suggesting progesterone resistance in endometriosis (184–186).
Gene expression profiles characterized by microarray in the endometrium of women with or without endometriosis showed that a number of progesterone target genes are dysregulated during the window of implantation, at which time the endometrium is exposed to the highest levels of progesterone (164, 165). For example, the expression of the prototype progesterone-responsive gene, glycodelin, is lower in the endometrium of women with endometriosis compared with women without endometriosis (165). These findings suggested that eutopic endometrium of women with endometriosis would also exhibit progesterone resistance (165, 187).
Progesterone resistance, originally postulated based on the deficient expression of progesterone-responsive genes in endometriosis, was attributed to extremely low PR levels observed in vivo in this tissue (157). In endometrium, levels of the PR isoforms, PR-B and PR-A, progressively increase during the proliferative phase, peak immediately before ovulation, and diminish after ovulation, suggesting that estradiol stimulates PR levels (157). In contrast, PR-B is undetectable, and PR-A is markedly lower in vivo in simultaneously collected tissues of endometriosis. The mechanism responsible for decreased PR expression in endometriosis is still under investigation. In normal endometrium, estradiol, acting via ERα, stimulates PR expression. ERα levels are significantly lower in endometriosis, whereas ERβ levels are strikingly higher than in normal endometrium. One mechanism for low PR levels in endometriosis has been linked to an altered ERβ:ERα ratio in this tissue (158). In this mechanism, decreased methylation of a CpG island in the promoter of the ERβ gene leads to high levels of expression in endometriotic stromal cells, and hypermethylation silences ERβ expression. ERβ in endometriotic stromal cells occupy the ERα promoter to down-regulate its activity, thus favoring suppression of ERα levels (158). The resulting high ERβ:ERα ratio in endometriotic stromal cells was proposed to increase ERβ binding to the PR promoter and block estradiol induction of PR expression. Collectively, these scientific observations resonated with the clinical concept of progesterone resistance in endometriosis.
D. Role of stroma in endometriosis
In endometriosis, the majority of in vitro studies have used primary stromal cells from ectopic or eutopic tissues. This is due in part to the low yield of epithelial cells that can be obtained from endometrial or endometriotic tissues and their limited growth potential in culture. On the other hand, endometriotic stromal cells proliferate and propagate readily in culture. Studies have demonstrated an insufficient response to progesterone in endometriotic stromal cells, which undoubtedly has consequences in the stromal-epithelial actions that are necessary for adequate hormonal responses of the endometrium. One example of this has been demonstrated for the retinoic acid (RA) pathway, which is activated in stromal cells in normal endometrium in response to progesterone. RA in turn induces the expression of the enzyme HSD17B2 in endometrial epithelial cells in a paracrine fashion (Fig. 4) (188–190). HSD17B2 catalyzes the conversion of biologically potent estradiol to inactive estrone, thereby regulating the levels of estradiol (191–194). Endometriotic stromal cells, however, fail to respond to progesterone and thus do not induce RA (195). This leads to the absence of epithelial HSD17B2 expression and failure to inactivate estradiol to estrone in endometriosis (Fig. 4) (195, 196). Combined with high estradiol production due to aberrant aromatase activity, this additional defect in estradiol metabolism contributes to the abnormally high levels of estradiol in endometriosis (184).
Figure 4.
Proposed model for progesterone and retinoid action in endometrium and endometriosis. The deficient genes and pathways in endometriosis compared with normal endometrium were indicated using arrows and dotted lines. In endometrium, progesterone action is mediated by PR in stromal cells. Blood vessels that transport progesterone (P4) are adjacent to stromal cells. Stromal PR activated by circulating P4 produces a number of paracrine factors including RA. RA and other paracrine factors stimulate differentiation and oppose estradiol (E2)-dependent proliferation in neighboring epithelial cells. Moreover, RA stimulates the enzyme, HSD17B2, which converts biologically active E2 to estrogenically weak estrone (E1). On the other hand, significantly lower stromal PR expression causes deficient formation of RA and other paracrine factors. Consequently, epithelial cells differentiate poorly and do not express HSD17B2, leading to accumulation of E2. The mechanisms for retinoid transport between these two cell types are not well understood. STRA6 in endometrial stromal cells serve as a receptor for the uptake of RBP-bound retinol from the circulation. Retinol is converted to RA, which is then transported to nuclear RARs by the shuttling protein named cellular RA binding protein-2 (CRABP2). RA-RAR enhances differentiation and apoptosis in endometrial stromal cells. PR, in a ligand-independent fashion, induces stromal STRA6 and CRABP2. Deficiency of PR, STRA6, and CRABP2 disrupts this pathway in endometriosis. Moreover, expression of the RA-metabolizing enzymes CYP26B1 and CYP26A1 in stromal and epithelial compartments are perturbed in endometriosis. 4OH-RA, 4-Hydroxy-RA.
Not only are endometriotic stromal cells unable to produce sufficient RA, but it has been demonstrated in vivo that matched biopsies of ovarian endometriosis compared with eutopic endometrium showed striking down-regulation of many genes in the RA pathway, including STRA6 and CRABP2 (197). The RA pathway includes retinol (vitamin A), which is stored in the liver and delivered to other tissues via retinol binding protein (RBP) in circulating blood (198), and a multitransmembrane domain protein called STRA6, which acts as a specific cell surface receptor for RBP (199). STRA6 binds to RBP with high affinity and has robust retinol uptake activity from the retinol-RBP complex. Once retinol enters the cell, retinol and retinal dehydrogenase enzymes catalyze its conversion to transcriptionally active RA. The isomer, all trans (at)-RA, serves as a ligand that regulates transcription through RA receptor (RAR)-α, -β, and -γ and peroxisome proliferator-activated receptor (PPAR)-β/δ nuclear hormone receptors (200, 201). The 9-cis isomer of RA binds to the nuclear receptor retinoid X receptor, which partners with atRA-bound RARs or PPARβ/δ to form heterodimers that are recruited to regulatory regions of specific target genes and modulate their transcriptional rates (201). The enzymes encoded by CYP26A1, -B1, and -C1 control atRA degradation (202, 203).
Transcriptional activation of the nuclear receptor RAR by atRA often leads to inhibition of cell growth. However, in some tissues, atRA promotes cell survival and hyperplasia, activities that are mediated by PPARβ/δ, which in turn induces the expression of prosurvival genes. Partitioning of atRA between the two receptors is regulated by the intracellular lipid-binding proteins CRABP2 and FABP5. These proteins specifically deliver atRA from the cytosol to nuclear RAR and PPARβ/δ, respectively, thereby selectively enhancing the transcriptional activity of their cognate receptors. Consequently, atRA functions through RAR and is a proapoptotic agent in cells with a high CRABP2/FABP5 ratio, but it signals through PPARβ/δ and promotes survival in cells with a low CRABP2/FABP5 ratio (201).
RA induces apoptosis via the CRABP2/RARα pathway in normal endometrial stromal cells, whereas in CRABP2/RARα-deficient endometriotic cells, RA may not exert this beneficial effect (Fig. 4) (197). This favors survival of endometriotic stromal cells, possibly leading to the development and persistence of pelvic endometriotic implants. Furthermore, PR is a robust regulator of STRA6 and CRABP2 gene expression (Fig. 4) (197). Under in vitro conditions, this PR effect was not ligand dependent (197). This finding fits well into the overall model of deficient PR action in endometriosis because endometriotic stromal cells are severely deficient of PR (157).
Progesterone action in endometriosis is less clear compared with that of normal endometrium or endometrial cancer. In addition, there are much more data that describe progesterone action in endometriotic stromal cells as opposed to endometriotic epithelial cells. Stromal PR deficiency is a major contributor to progesterone resistance and the blunted biochemical responses to progesterone that are observed. High levels of ERβ suppress ERα, thereby blocking estradiol-dependent induction of PR. Low expression of PR leads to defective RA production and action. As a result, endometriotic stromal cells survive longer than normal endometrial stromal cells. Defective RA production also leads to HSD17B2 deficiency and failure to metabolize estradiol, ultimately resulting in high tissue estradiol levels that promote the inflammatory process and pain of endometriosis (Fig. 4).
There are a number of questions that remain unanswered in this model of PR-RA action and deficiency. In ectopic endometriotic tissue and stromal cells, the RA-metabolizing enzyme CYP26B1 is strikingly higher, whereas another RA-metabolizing enzyme, CYP26A1, is deficient in eutopic endometrial tissue from endometriosis patients compared with disease-free women (157, 204). The roles of these enzymes in stromal and epithelial compartments in health and disease are not clear. The in vivo expression patterns of STRA6, CRAPBP2, or CYP26B1 in normal human endometrial stromal cells throughout the menstrual cycle are also currently unknown. It would be important to understand whether retinol, RA, or other retinoids are transported between endometrial stromal and epithelial cells in health and disease. What would be the molecular mechanisms responsible for retinoid trafficking in endometrium or its deficiency in endometriosis? What are the in vivo mechanisms for ligand-independent or dependent regulation of these processes by PR? Thus, exciting and interesting future directions emerge from the disease model depicted in Fig. 4.
VI. Uterine Fibroids and Progesterone
A. Clinical aspects of uterine leiomyoma
Uterine leiomyoma (fibroids) are benign smooth muscle tumors originating from the uterine myometrium (Fig. 5) and are the most common solid pelvic tumors as well as the most frequently reported indication for surgery in women. Uterine leiomyoma occur in up to 80% of all omen of reproductive age (205–207). African-American women develop uterine fibroids at a higher frequency and at earlier ages than Caucasian women (208). Uterine fibroids generally cause abnormal uterine bleeding, pressure-related symptoms, recurrent pregnancy loss, and in some cases infertility. Abnormal uterine bleeding (menorrhagia and metrorrhagia) is the main reason for women to seek treatment for leiomyoma and is the major cause for surgical intervention. In the United States, approximately 600,000 hysterectomies are performed annually with treatment of uterine fibroids accounting for approximately 40% of all hysterectomies (206, 209).
Figure 5.
Myometrium and leiomyoma. Myometrium exhibits organized smooth muscle cell bundles with dedicated and enriched vasculatures. Leiomyoma is composed of smooth muscle cells that are disorganized in storiform and whorl-appearing patterns with abundant extracellular collagen and significantly reduced vessel density, in comparison to matched myometrium.
The epidemiology of uterine fibroids parallels the changes observed during the woman's reproductive lifespan, tracking with changes in the levels of ovarian steroid hormones. Although there have been reports of fibroids in adolescents, no single case of fibroids has been reported in prepubertal girls. Symptomatic fibroids occur mostly between the ages of 30 and 40 yr, and prevalence increases with age. In most women, the symptoms are diminished by the time of menopause, when the ovarian production of steroid hormones wanes. However, symptoms may continue during HRT. HRT with estrogen and progestin has been shown to increase the size of fibroids in menopausal women (210–212). All of these observations indicate that leiomyoma are dependent on ovarian steroid hormones. Accordingly, the GnRH agonist leuprolide acetate induces hypogonadism and has been used to suppress the associated symptoms of fibroids.
B. Roles of estrogen and progesterone in leiomyoma growth
Although the initial steps in the pathogenesis of uterine fibroids are most likely due to chromosomal aberrations and/or specific gene mutations (213), their development is highly dependent on ovarian steroid hormones. Traditionally, estrogen has been considered the primary mitogenic factor in the uterus. It has been shown repeatedly that estrogens promote the growth of cultured fibroid cells (214–222). In contrast, progestin can be both growth promoting (214, 221–224) and growth inhibiting (225, 226) in vitro, depending on the culture conditions. In animal studies, exposure to exogenous estrogen can induce uterine fibroids in the mouse (227) and guinea pig (228), which can then be inhibited by progesterone. Moreover, the growth of spontaneously developed fibroids in the Eker rat, which carries a germ-line mutation of the tuberous sclerosis 2 gene (229–231), is stimulated by estrogen but not progesterone (232, 233).
Although the experimental evidence obtained from in vitro cell culture and animal model studies emphasizes the importance of estrogen over progestin in the pathogenesis of fibroids, a growing body of evidence from biochemical, histological, clinical, and pharmacological studies indicates that progesterone and PR play a key role in uterine fibroid growth and development (234). For example, the labeling index for proliferation markers such as Ki67 and proliferating cell nuclear antigen in fibroids peaks at the luteal/secretory phase, when progesterone is dominant (235–237). In alignment with proliferation activities, epidermal growth factor mRNA was found to be increased in leiomyoma only during the luteal/secretory phase of the cycle, suggesting its progesterone dependency (238). Pharmacological studies also indicate that progestin, and not estrogen, is the critical mitogen for this tumor. For instance, proliferation indices in the fibroids of postmenopausal women increased significantly with combined estrogen plus progestin replacement, but not with estrogen replacement alone (239). HRT was also shown to increase the growth of fibroids significantly when higher doses (5 mg/d) of MPA are used, compared with lower doses (2.5 mg/d) (240). When used as an add-back therapy in combination with GnRH agonists, progestins (MPA and norethindrone) attenuate or reverse the inhibitory effects of GnRH agonists on leiomyoma size (241, 242). The strongest current evidence for possible in vivo mitogenic effects of progesterone on fibroids comes from clinical trials in which four different antiprogestins consistently reduced tumor size (243–251).
C. Permissive role of estrogen to enhance progesterone action
In vivo, human fibroid cells are embedded in thick layers of extracellular matrix (ECM), which has been shown to influence critical cellular functions (252, 253). The fibroids in animal models (e.g., mouse, rat, and guinea pig) appear to lack the characteristic of ECM overproduction in human fibroids, suggesting a fundamental difference in their nature compared with the human tumor. The absence of the organotypic ECM may also underlie the dramatic changes in gene expression patterns in human fibroid cells grown on plastic dishes (254, 255) and for the discrepancy in progesterone actions in vivo and in vitro. An improved method that fills the gap between clinical and in vitro studies is the xenograft model, in which tissue fragments of human fibroids are placed under the subrenal capsule of immunodeficient mice (7). Surprisingly, the growth of fibroid xenografts depended on the combination of estradiol and progesterone, whereas estradiol or progesterone alone was unable to stimulate growth. Although estradiol is not a mitogen by itself, it is essential for the expression of PR, thereby supporting the progesterone action on fibroid cells (7).
When fibroid cells proliferate in response to estradiol plus progesterone, Ki67 is coexpressed with ERα and PR, suggesting that estradiol and progesterone can directly stimulate the proliferation of fibroid cells (Fig. 6) (7). In addition to cell proliferation, estradiol plus progesterone also stimulates the growth of fibroids via an accumulation of ECM. The production of ECM proteins such as collagen types I and III appears to be under the control of estrogen and progesterone (256–258). Additionally, cellular hypertrophy contributes to the hormone-induced growth of fibroids (259–261). In fibroid xenografts, the volume of fibroid cells was shown to increase 3-fold with estradiol plus progesterone treatment compared with estradiol alone, progesterone alone, or no hormone treatment (7). In summary, fibroids can increase their size via cell proliferation, ECM accumulation, and cellular hypertrophy, and all of these functions are under the control of progesterone with a permissive role of estradiol.
Figure 6.
Expression of ER and PR in fibroid xenografts and its correlation with proliferation. Fibroid xenografts were treated with estradiol (E2) plus progesterone (P4) for 2 wk. DAPI (blue), Ki67 (red), and ERα (upper panels) or PR (lower panels) immunofluorescent staining was done. Ki67-positive cells (arrows) are also positive for ERα and PR. E2 induces expression of PR, and P4 action via PR increases the size of fibroid through accumulation of ECM, proliferation, and hypertrophy of fibroid cells. [Modified from H. Ishikawa et al.: Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151:2433–2442, 2010 (7), with permission. © The Endocrine Society.]
D. Effect of pregnancy on leiomyoma
The effect of pregnancy on fibroids has been controversial. Despite the dramatic elevation in the circulating ovarian steroid levels, there is no conclusive evidence to explain the change in size of fibroids during pregnancy (262–264). However, the majority of longitudinal studies were performed in patients who were found to have fibroids on their obstetrical ultrasounds at the 16th to 18th weeks (262, 264, 265), whereas the greatest increase in the volume of uterine leiomyoma occurred before 10 wk gestation (263). Considering that cellular hypertrophy significantly contributes to tumor growth, the longitudinal studies that were conducted in the second and/or third trimesters likely missed this window of tumor volume increase in response to elevated progesterone levels. Hence, the absence of detectable growth in fibroids during pregnancy does not necessarily contradict the model of progesterone-dependent fibroid growth.
E. Therapeutic use of antiprogestins in leiomyoma
The original studies performed by Murphy and co-workers (243–245) in the 1990s suggested that RU486 might be used in the medical management of uterine leiomyoma. Pilot studies indicated that the size of leiomyoma decreased significantly after treatment with RU486. Early studies indicated that different doses of RU486 decreased leiomyoma size as well as the associated excessive uterine bleeding (246). A similar endometrial histology, characterized by hyperplastic glands and stroma, was observed in patients treated with the antiprogestins RU486 and asoprisnil (247). It was subsequently shown that asoprisnil also acts primarily as a progesterone antagonist in the endometrium (53).
To date, four antiprogestins—RU486, asoprisnil, proellex (CDB4124), and CDB2914—have proven to be useful in the medical management of uterine leiomyoma (248, 249). A number of investigators have attempted to avoid the side effect of endometrial hyperplasia by decreasing the dose of RU486 to 5 mg/d; this dose has been shown to successfully decrease leiomyoma size and uterine bleeding associated with these tumors (245, 250, 251). Importantly, treatment with RU486 given at a dose of 5 mg/d did not cause endometrial hyperplasia (251).
CDB4124 has been tested in two clinical trials in patients with symptomatic leiomyoma. The treatment group showed reduced blood loss and leiomyoma-related symptoms, as well as reduced leiomyoma volume (reviewed in Ref. 266). Clinical studies demonstrated that ulipristal acetate, CDB2914, controlled uterine bleeding by 13 wk in 91% of women with 5 mg/d and 92% with 10 mg/d, compared with 19% receiving placebo (6). Furthermore, total fibroid volumes decreased by 21% in the 5-mg group, 12% in the 10-mg group, and 3% in the placebo group. In another study, Donnez et al. (267) compared the efficacy and side-effect profile of ulipristal acetate to the GnRH agonist leuprolide acetate. Ulipristal acetate controlled bleeding in 90% of patients in the 5-mg group and in 98% of patients in the 10-mg group, compared with 89% with leuprolide acetate. In addition, amenorrhea occurred within 5–7 d with ulipristal acetate compared with 21 d with leuprolide acetate. Furthermore, hot flashes were reported in 11% of patients in the 5-mg group, 10% in the 10-mg group, and 40% in the leuprolide acetate group. Ulipristal acetate is now approved in Europe for the preoperative treatment of uterine fibroids. Clinical phase III studies are ongoing in the United States.
F. Paracrine interactions in leiomyoma
Uterine leiomyoma is thought to arise from a single transformed myometrial smooth muscle cell that forms a benign monoclonal tumor. Thus, unlike the endometrium, leiomyomas are comprised of primarily one cell type. The surrounding myometrium is also composed of smooth muscle cells. Thus, it is assumed that progesterone action in leiomyoma cells occurs in PR-positive cells. There are leiomyoma cells that do not express PR intermingled among the PR-positive cells; however, the effects of progesterone on these cells via paracrine communication are unknown. Recently, a distinct stem/reservoir cell-enriched population, designated as the leiomyoma-derived side population (LMSP), was identified to be responsible for cell proliferation and tumor growth in leiomyoma (268). LMSP cells represented 1% of the leiomyoma-derived cell population and expressed strikingly lower levels of ERα and PR compared with the majority (main population) of leiomyoma cells. Interestingly, incubating LMSP and unsorted myometrial smooth muscle cells in culture influenced the LMSP cells to have the capacity to grow into relatively large tumors when xenografted under the kidney capsule of immunocompromised mice. In contrast, the main population of leiomyoma cells grown in culture with unsorted myometrial cells, produced strikingly smaller tumors as xenografts (268). The exact changes that occur in the LMSPs while incubated with myometrial cells in vitro is unknown, and whether similar paracrine actions occur in vivo remains to be shown. To date, there is no evidence demonstrating the importance of paracrine actions in mediating progesterone action in leiomyoma growth. Current knowledge supports that the primary mode of action of progesterone is directly on PR-expressing leiomyoma cells.
VII. Breast Cancer and Progesterone
A. Clinical evidence for the role of progesterone in the breast
The human mammary gland consists of a series of branching ducts composed of luminal epithelial cells that line the central lumen and underlying myoepithelial cells that are adjacent to the basement membrane. Surrounding this epithelium is a collagenous stroma and adipose tissue. Estrogen and progesterone are essential for the development of the breast and actively promote cell proliferation during ductal development and pregnancy. As is the case for uterine fibroids, estrogen has been considered to be the primary mitogenic factor in the mammary gland. There is a wealth of evidence to support the critical role estrogen plays in the breast and its involvement in the pathogenesis of breast cancer (269). Comparatively, the role of progesterone in the human adult mammary gland and in cancer is less clear. However, preclinical and clinical evidence have demonstrated the pro-proliferative and antiproliferative roles of progesterone in the adult breast and breast cancer through autocrine and paracrine actions between PR-positive and PR-negative mammary cells. Recent studies have also demonstrated that progesterone expands a stem cell population that is sensitive to transformation (270–272).
Two major clinical studies, the Women's Health Initiative (WHI) and the Million Women study (MWS), demonstrated a protumorigenic role of progestins on breast cells (9–11, 273). Specifically, the WHI study, which was launched in 1991, included two hormone trials: one group of women with an intact uterus received estrogen and progestin, and another group of women without a uterus received estrogen alone. In the estrogen-alone trial, 5310 women took active conjugated equine estrogen pills (CEE or Premarin), and 5429 took placebo, and the risk for breast cancer development did not differ significantly between the two groups. In contrast, in women taking estrogen plus progestin (n = 8506) compared with placebo (n = 8102), the treated group had an increased incidence of breast cancer (11). An updated analysis shows that after an average of 5.6 yr, 245 women in the estrogen plus progestin group and 185 women in the placebo group developed breast cancer. The breast cancers in the estrogen plus progestin group had similar histological characteristics as those in the placebo group. However, the tumors in the estrogen plus progestin group tended to be larger and more advanced (e.g., had spread to the lymph nodes or elsewhere in the body).
The MWS involved more than one million United Kingdom women aged 50 yr or older. In a span of 5 yr, women received an invitation to attend a breast screening at one of 66 participating National Health Service Breast Screening Centers in the United Kingdom and to be enrolled in the study. The risk of breast cancer was higher in women taking HRT, and increased with duration of use. For example, 10-yr use of HRT is estimated to result in five additional breast cancers per 1000 users of estrogen-only preparations and 19 additional cancers per 1000 users of combination estrogen plus progestin (273). Thus, the results of these two large studies agreed that the risk for developing breast cancer is higher in postmenopausal women taking estrogen with progestin compared with estrogen alone.
In a unique study performed by Engman et al. (274), a group of premenopausal women taking the antiprogestin mifepristone (RU486), for the treatment of leiomyomas underwent fine-needle aspiration to acquire breast cell samples. Samples were taken at baseline and after 3 months of taking mifepristone or placebo. Proliferation of the mammary epithelium was significantly reduced after mifepristone treatment compared with baseline as measured by immunohistochemical staining of Ki67. These data further support a pro-proliferative role of progesterone in premenopausal women.
B. Mechanisms of progesterone action in normal mammary tissue
Transgenic mouse studies have unequivocally demonstrated that PR is essential for progesterone-dependent proliferation of mammary epithelial cells (MECs) (2). In the adult mammary gland, PR is not expressed in all cells; rather, 7–10% of the epithelial cells are PR-positive, and these cells are usually not proliferating (275–277). The proliferating cells reside nearby, suggesting that a paracrine mechanism supports progesterone-induced MEC proliferation (Fig. 7). This proposed paracrine mechanism was demonstrated more directly upon transplantation of a mixture of wild-type and PRKO MEC into a wild-type recipient. When PRKO MECs were put in close proximity to wild-type PR-positive MECs, proliferation and morphogenesis of the MECs were restored (278). It has been shown that TGFβ colocalizes with ER/PR and phosphorylated nuclear Smad2/3 and is involved in inhibiting proliferation of ER/PR-positive cells (279, 280). Immunohistochemical staining of human breast biopsy samples also showed that the proliferating cells are PR negative (281–283). One study used three-dimensional culture of primary normal human breast cells and found ER and PR to be heterogeneously coexpressed in luminal cells but not in myoepithelial cells (284). As observed in the mouse mammary gland, the majority of human breast cells that were proliferating were PR-negative.
Figure 7.
Progesterone action in the normal and malignant breast epithelium. In the normal mammary tissue and breast cancer, the primary target cell of progesterone action is in the glands or malignant epithelial cells, with no evidence of specifically organized stromal components that express PR. Only a small population of normal epithelial cells express PR. Proliferation in response to progesterone occurs in the cells that are negative for PR as a result of paracrine actions, whereas PR-expressing cells do not proliferate. In breast cancer cells, progesterone acts directly on PR-expressing cells and promotes proliferation. The role of surrounding stromal fibroblasts in mediating the progesterone-drive proliferation is unclear.
Several paracrine mediators that are responsible for proliferation of PR-negative cells in the breast have been identified using mouse models. Although initially defined as an essential factor for osteoclast differentiation and survival, receptor activator of nuclear factor κB (RANK) ligand (RANKL), also known as TNF (ligand) superfamily, member 11 (TNFSF11), has been shown to be required for cellular proliferation, survival, and alveologenesis of the mammary gland during pregnancy (285, 286). RANKL is a secreted cytokine that mediates its biological effects through its RANK receptor. In ovariectomized adult mice, treatment with estradiol plus progesterone or progesterone alone induced RANKL mRNA in the mammary epithelium; this effect was not seen in PRKO mice (4, 287). Furthermore, RANKL is expressed exclusively in PR-positive luminal epithelial cells of the breast (4). Beleut et al. (288) further demonstrated that progesterone-induced proliferation of the mammary epithelium in the adult mouse occurs in both an autocrine and paracrine manner. They found that an early wave of proliferation occurs in a small percentage of PR-positive cells within 24 h, followed by a subsequent larger, sustained phase of proliferation in PR-negative cells. The early autocrine phase was dependent on cyclin D1, and the second phase was dependent on RANKL. The role of paracrine communication was demonstrated by ectopically expressing RANKL in PRKO MECs and then transplanting the cells into cleared fat pads. This resulted in the rescue of ductal side branching and alveologenesis (288). In another study, bigenic mice expressed RANKL in the same spatial pattern as progesterone-induced endogenous RANKL in PRKO mice, demonstrating that even in the absence of PR, RANKL was able to rescue the PRKO phenotype in the mammary gland (289).
Studies have shown that progesterone can stimulate expansion of stem cells in the mouse mammary gland during the reproductive cycle and in pregnancy (270, 272). Mouse mammary stem cells are found in the basal compartment of the mammary epithelium, near ER/PR-positive cells (290, 291). It has been observed that when progesterone levels are elevated during diestrus or pregnancy, the pool of mammary stem cells expands (272). In transplantation experiments, exogenous progesterone increased the population of stem cells, whereas ovariectomy reduced stem cell number and reduced ductal outgrowths (270, 272). It has been suggested that estradiol primarily maintains PR expression, whereas progesterone promotes stem cell expansion. Finally, RANKL has been shown to mediate progesterone-induced expansion through paracrine signaling because administration of an anti-RANKL antibody decreased the clonigenicity of mammary stem cells (272). In normal human breast cells, the involvement of RANKL as a paracrine mediator of progesterone action is unclear. Graham et al. (284) demonstrated with a three-dimensional breast tissue culture system that RANKL was not induced by progesterone. Other paracrine mediators of progesterone action such as Wnt4 and amphiregulin were not induced directly by progesterone either. This discrepancy may be due to different in vivo microenvironments compared with in vitro culture conditions, or it may be due to species differences. The role of RANKL as a mediator of progesterone-driven proliferation of human MECs certainly requires further investigation.
C. Progesterone action in breast cancer cells
Progesterone action in normal mammary epithelium is distinct from that in breast cancer. Microarray analyses have shown that different pathways are regulated by progesterone in normal breast cells vs. breast cancer cells, with little overlap (284). It is thought that progesterone/progestins are able to both inhibit and stimulate proliferation of breast cancer cells. Unlike the paracrine action of progesterone in normal breast epithelial cells, there is an increase in the number of proliferating cells that are ER/PR-positive in breast cancer cells (282, 292). In addition, it has been observed in clinical samples that there is an increase in the percentage of proliferating ER/PR-positive cells in areas of normal tissue in breast cancer patients, in hyperplasias, and in ductal carcinoma in situ, which correlates with an increased risk of developing breast cancer (293, 294). Thus, a switch from paracrine- to autocrine-regulated progesterone action occurs upon neoplastic transformation of cells, leading to breast cancer (Fig. 7).
In mice, it has been demonstrated that progestins promote mammary tumor progression and growth. Carcinogen (7,12-dimethylbenz[α]anthracene)-induced mammary tumor development is highly dependent on progestins. In PRKO mice, there is a decrease in 7,12-dimethylbenz[α]anthracene-induced mammary tumors compared with wild-type mice (295, 296). ER/PR-positive mammary adenocarcinomas in female Balb/c mice require continuous progestin for growth (297, 298). BRCA1 deletion in the mouse mammary gland results in increased PR expression and a hyperproliferative response to progesterone. Upon treatment with RU486, development of these tumors is inhibited (8). Together, these studies strongly point toward a protumorigenic role of progesterone in mice.
Human breast cancer cell lines expressing PR have been used to elucidate mechanisms associated with the proliferative and tumorigenic roles of progesterone. Progestin action has even been studied in cell lines that do not express PR, but nonetheless respond to progestin with changes in cell behavior (299–301). In cancer cells, progesterone/progestins can both promote and inhibit proliferation. Specifically, a biphasic regulation of breast cancer cell growth by progesterone has been shown to occur. In one study, progesterone accelerated T47D-YB cells through the first mitotic cell cycle and subsequently arrested them in late G1 of the second cycle. The arrest was accompanied by decreased levels of cyclins D1, D3, and E; disappearance of cyclins A and B; and induction of the cyclin-dependent kinase inhibitors p21 and p27(Kip1). Furthermore, the retinoblastoma protein was hypophosphorylated and extensively down-regulated (302). In another study, it was shown that PR inhibited proliferation of human breast cancer cells by inducing MAPK phosphatase 1 (MKP-1/DUSP1), which catalyzes dephosphorylation and inactivation of MAPKs. Liganded PR increased promoter activity of MKP-1/DUSP1, bound to two PREs downstream of the MKP-1 transcription start site, and interacted with two Sp1 sites downstream of the transcription start to increase its expression, which subsequently decreased proliferation of breast cancer cells (269). Recently, it was reported that ERα interacts with PR at the cyclin D1/MYC promoters to stimulate proliferation and that the PR-ERα association was necessary for progestin-induced gene expression and proliferation (303). The antiestrogen ICI 182,780 blocked the formation of MPA-induced PR/ERα nuclear complexes, thereby inhibiting gene transcription and cell proliferation without affecting the activation and binding of PR at the gene promoter.
Nongenomic actions of PR, such as the rapid activation of signaling pathways, may also contribute to breast cancer cell proliferation. This idea has recently been reviewed by the same pioneering group that discovered that PR directly interacts with c-Src in breast cancer cells, subsequently activating the MAPK pathway (304). PR has been shown to rapidly activate the Src/p21Ras/Erk, PI3K/Akt, and JAK/STAT pathways, which contribute to the proliferative effects of progesterone in breast cancer cells (33, 297, 305, 306). PR activation of signaling pathways can also phosphorylate transcription factors such as Elk-1, which then transcriptionally activate cyclin D1 (305). Activation of signaling molecules by PR in turn leads to phosphorylation of PR, which promotes binding to its PREs or other elements on target DNA through tethering interactions with other transcription factors. It has been shown that phosphorylation of PR on Ser345 by EGFR/c-Src/MAPK promotes strong association with SP1 transcription factors and allows recruitment to SP1 sites on the p21 and EGFR promoters (307).
In advanced breast cancer, studies have suggested that progesterone may be inhibitory or suppressive in proliferation, invasion, metastasis, and inflammation processes (304). Furthermore, unliganded PR has been shown to suppress growth and inflammatory responses in breast cancer cells (308–310).
In all, these data demonstrate that progesterone can be pro-proliferative and protumorigenic; however, the actions of progesterone are dependent on the cellular context. Its action in normal mammary cells and in breast cancer of various stages can be quite different, and there are also species variations. As with other reproductive tissues, differences in the tissue microenvironment may explain the variety of observed responses to progesterone in normal mammary tissue and breast cancer.
D. Role of stroma in the breast
It has become increasingly evident that the microenvironment plays a key role in regulating the growth of normal or malignant epithelial cells. Fibroblasts of the stroma in any organ are important cells that synthesize, deposit, and remodel the ECM. Breast adipose fibroblasts also secrete numerous soluble paracrine growth factors that regulate cell proliferation and survival. The profound influence of the microenvironment is evidenced in autopsy studies where it was observed that the majority of middle-aged to older people who died from causes other than cancer frequently had precancerous lesions throughout their bodies (311–313). Furthermore, people with familial BRCA or APC mutations develop tumors in only specific organs although every cell in the body possesses the mutation. It has been proposed that the tissue-specific microenvironment around mutated cells promotes or limits the development of cancer.
The microenvironment of the breast epithelium consists of adipose tissue, fibroblasts, immune cells, endothelial cells, and ECM and is generally referred to as stroma (314). During mammary gland development in the mouse, complex interactions between epithelial cells and stromal cells occur (315, 316). Stromal cells secrete soluble growth factors that are stimulatory and/or inhibitory, and produce ECM proteins such as collagens, fibronectin, and laminin to regulate epithelial cells. Epithelial cells also secrete factors that influence proliferation and function of adjacent epithelial and stromal cells.
Up-regulated aromatase expression in breast adipose fibroblasts increases the tissue concentration of estradiol, which then activates a large number of carcinogenic genes via ERα in malignant epithelial cells (317). Clinically, aromatase inhibitors are the most effective hormonal treatment of ERα-positive breast tumors. We and others previously found that the majority of estrogen production in breast cancer tissue was accounted for by the aberrant activation of the proximal promoter I.3/II region in the breast adipose fibroblasts. PGE2 that is secreted in large amounts by malignant breast epithelial cells is the most potent known natural inducer of this promoter region (269), demonstrating a dynamic paracrine interaction between the breast epithelial cells and the stroma.
In the normal mammary gland or in breast cancer, there is relatively little known regarding the role of stroma in mediating progesterone action. It has been shown that progestins alone or together with estradiol do not influence proliferation of MECs grown in a three-dimensional culture system; however, addition of progestins within conditioned medium from mammary fibroblasts increases proliferation of the epithelial cells to a greater extent than with conditioned media alone. Hepatocyte growth factor was identified as the fibroblast-derived growth factor that, together with progestin, increased proliferation of MECs (318). Reciprocal transplantation of epithelium and stroma from wild-type and PRKO mice demonstrated that PR action in stroma was necessary for ductal growth. In addition, unusual distended endbuds were present in these outgrowths (319). In another study using a p53-null mammary transplant model, short-term (2 wk) exposure to estrogen and progesterone decreased mammary tumorigenesis by 70 to 88% (320) and decreased the proliferation of cells in the mammary glands by 85%. Interestingly, when p53-null MECs were transplanted into the cleared mammary fat pads of hormone-treated mice, the tumor-producing capabilities of the mammary cells were also decreased by 60% compared with the same cells transplanted into unexposed mice. The results of this study showed that the mammary stroma is affected by hormones and that this in turn could affect tumorigenesis of p53-null mammary cells. Curiously, although stromal fibroblasts express ERα, they do not express detectable levels of PR (321, 322), and thus the effects observed in these studies may be due to systemic effects of the progestins on the stroma rather than a direct effect.
Coculture studies demonstrated that fibroblasts derived from normal breast tissue (from reduction mammoplasty) inhibited the morphological conversion and growth of the preneoplastic cell lines, MCF10A and EIII8 (323). It has been shown that normal stroma controls epithelial cell polarity, and that when this control is lost, there is an increase in cell proliferation and tumorigenesis (324). In contrast, fibroblasts derived from breast tumors caused ductal-alveolar morphogenesis of both cell types (323). Caveolin-1 has been shown to mediate the effects of breast cancer-associated fibroblasts because mammary stromal fibroblasts deficient in calveolin-1 caused an up-regulation of RB/E2F-regulated genes, which confer a poor prognosis with enhanced epithelial-mesenchymal transition (325). It is apparent that breast stromal fibroblasts can change as well, depending on their microenvironment. It is not known whether progesterone plays a role in promoting these changes or whether the changes include regulation of PR or its downstream pathways. This is an area that remains unexplored.
VIII. Summary and Future Directions
The pleiotropic and complex actions of progesterone are evident in various reproductive tissues and in diseases of these tissues. Even within the same tissue, such as the uterus, progesterone can exert opposing physiological effects; for example, progesterone stimulates growth of leiomyomas but inhibits growth of the endometrium. Progesterone action is dependent on the cell type and context (normal vs. disease state). There are at least two isoforms of PR that are regulated at multiple levels, including mRNA expression, protein stability, nuclear translocation, and its ability to interact with DNA and coregulators. Posttranslational modifications of PR, including phosphorylation, sumoylation, and ubiquitination, and the accessibility of chromatin all play roles in regulating progesterone action.
The question remains as to why progesterone and its receptor are protective in endometrial tissue and tumorigenic in breast and leiomyoma tissues. The endometrium is a mucosa where circulating progesterone in blood vessels first comes in contact with multilayered and highly differentiated stromal cells rich in PR. Progesterone/PR action induces formation of stromal paracrine substances such as retinoids that act on epithelial cells to repress proliferation. This well-organized stromal-epithelial relationship, however, is not readily recognizable in breast or leiomyoma tissue (Fig. 8). It is highly likely that progesterone primarily acts through PR in the breast epithelial compartment or leiomyoma smooth muscle cells. The presence of a distinct stromal-epithelial interaction in the endometrium and the absence of such a well-defined and functional relationship in breast or leiomyoma could account for seemingly discrepant actions of progesterone and PR in these tissues (Fig. 8).
Figure 8.
Diverse actions of progesterone. Estradiol (E2) action via stromal ERα is critical for the development of endometrial cancer. By the same token, E2-ERα-dependent stimulation of stromal PR expression is the key protective mechanism against E2-induced carcinogenesis. Progesterone (P4) acts via stromal PR to oppose this carcinogenic effect of E2. Deficient P4 synthesis or defective stromal cell function may play key roles in the development of endometrial cancer. Interestingly, there is a progressive decline in stromal cell numbers from premalignant endometrial hyperplasia to high grade cancer, which is completely devoid of any stromal cells. In endometriosis, stromal cells are deficient in ERα but rich in ERβ. ERβ suppresses PR expression. In endometriosis, levels of PR are dramatically lower and response to P4 is blunted, resulting in promotion of survival and deficient epithelial differentiation. In breast cancer and uterine leiomyomas (fibroids), P4 acts directly via PR in malignant epithelial cells or leiomyoma smooth muscle cells with little evidence of specifically organized stromal components that mediate P4 action. In both tissues, P4 and PR are tumorigenic. In uterine leiomyoma smooth muscle cells, the primary role of E2 is to maintain PR expression.
In endometrial cancer and endometriosis, there is a general resistance to progesterone due to numerous factors, including a significant decrease in PR protein levels. In endometriosis, there is a decline in PR expression, and numerous PR responses are blunted. Differentiation of endometriotic stromal cells, a process named decidualization, is decreased in response to progesterone, the RA pathway is compromised, and there is an increase in local estrogen production that further aggravates the disease. PR action in the epithelial cells in endometriosis is not clear; however, the decreased responsiveness of stroma to progesterone has unfavorable effects on epithelial cells, such as failure to metabolize estradiol (Fig. 8).
In endometrial cancer, PR expression is maintained in malignant epithelial cells but decreases as aggressiveness increases. In addition, there is a diminishing population of stromal cells surrounding the malignant epithelial cells as histological grade increases (Fig. 3). Advanced grade endometrial cancer made of sheets of malignant cells with no stromal component has the lowest response rate to progestins. Because therapeutic response to progestins decreases with increasing aggressiveness, it is likely that stromal PR plays a key role in progestin responsiveness of endometrial cancer via regulating the production of paracrine compounds (e.g., RA), which act as tumor suppressors on epithelial cells.
In leiomyoma, progesterone promotes growth through increased proliferation of leiomyoma cells, increased deposition of ECM, and cell hypertrophy. These are strikingly different from progesterone action that maintains quiescence in the myometrium. Estrogen seems to play a permissive role by enhancing PR expression to augment progesterone action in this process of tumorigenesis. Intriguingly, recent data have demonstrated that cells with stem cell-like properties are found within leiomyoma, and that these cells enhance estrogen plus progesterone-dependent growth of leiomyoma tumors. In addition to smooth muscle cells, other cell types such as endothelial cells and fibroblasts are present in leiomyoma tissue. The biological significance of these cell types in terms of paracrine actions for estrogen or progesterone signaling to smooth muscle cells is unknown.
In normal mammary epithelium, PR-expressing cells do not proliferate; however, subsequent paracrine actions on PR-negative cells cause their proliferation. In breast cancer cells, progesterone can be pro-proliferative or antiproliferative depending on the length of exposure and numerous other factors. The most compelling evidence for the carcinogenic role of progesterone and its agonists came from the WHI study, indicating that treatment with the estrogen plus progestin combination increased postmenopausal breast cancer risk, whereas estrogen-only hormone replacement did not increase this risk (11). Thus, as in the case of leiomyoma growth, it is possible that estrogen and ERα seem to play a permissive role in breast carcinogenesis by inducing PR expression to augment progesterone action. To date, PR expression in breast stromal fibroblasts has not been studied in detail. The lack of a clear demonstration of functional PR in human breast stroma does not support a major role of a stromal-epithelial interaction that regulates progesterone signaling in breast cancer.
One of the major challenges in deciphering a clear role for progesterone in these tissues and diseases is that estrogen is always present and active. Estrogen plays a key role in the pathophysiology of each of these diseases and thus obscures the independent actions of progesterone. The discovery of progesterone as the major growth promoter in leiomyomas in a xenograft model shifts the current thinking that estrogen is the only steroid hormone that promotes growth. Perhaps there is a similar and more predominant role of progesterone in the breast and breast cancer, and estrogen, whereas it has mitogenic properties of its own, supports progesterone signaling to augment proliferation and cancer development. The WHI data support this concept.
Progesterone's mode of action within a specific disease is highly dependent on the microenvironment and external cues that act in a paracrine manner. This is evident in the diseases discussed in this review. A better understanding of the role of progesterone independent of that of estrogens is needed, as well as how the microenvironment influences PR function. Because current therapies for the diseases arising from progesterone-responsive tissues are inadequate, research in this area will provide a strong and convincing rationale to utilize new compounds that are emerging, such as PR modulators. Furthermore, new targets for therapy can be identified by studying the external influences that modify PR in a diseased cell. For example, inhibitors of major signaling pathways that have a direct impact on PR protein modification could be considered in combination treatment strategies. Endometrial cancer, endometriosis, leiomyoma, and breast cancer affect women of all ages and can have detrimental effects on reproductive potential, quality of life, and life span. Deciphering the mechanisms responsible for the beneficial actions of progesterone will provide the insight needed to provide innovative strategies to combat these diseases.
Acknowledgments
We acknowledge Dr. Jianjun Wei, Gynecological Pathologist at Northwestern University, for providing his expertise in histological analysis.
This work was supported by National Institutes of Health Grants R01HD044715 (to J.J.K.), R21CA127674 (to J.J.K.), R01CA154358 (to T.K.), R01HD064402 (to T.K.), R37HD36891 (to S.E.B.) and P01HD057877 (to J.J.K., T.K., and S.E.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APC
- Adenomatous polyposis coli
- atRA
- all trans-RA
- ChIP
- chromatin immunoprecipitation
- ECM
- extracellular matrix
- ER
- estrogen receptor
- Hand2
- heart and neural crest derivatives expressed 2
- HRT
- hormone replacement therapy
- HSD17B2
- 17β-hydroxysteroid dehydrogenase type 2
- LMSP
- leiomyoma-derived side population
- MEC
- mammary epithelial cell
- MPA
- medroxyprogesterone acetate
- PPAR
- peroxisome proliferator-activated receptor
- PR
- progesterone receptor
- PRE
- progesterone response element
- PRKO
- PR knockout
- RA
- retinoic acid
- RANK
- receptor activator of nuclear factor κB
- RANKL
- RANK ligand
- RAR
- RA receptor
- RBP
- retinol binding protein
- SF1
- steroidogenic factor-1.
References
- 1. Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. 2006. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol 102:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 3. Graham JD, Clarke CL. 1997. Physiological action of progesterone in target tissues. Endocr Rev 18:502–519 [DOI] [PubMed] [Google Scholar]
- 4. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. 2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- 6. Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, Terrill P, Osterloh I, Loumaye E. 2012. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366:409–420 [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. 2010. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151:2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. 2006. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314:1467–1470 [DOI] [PubMed] [Google Scholar]
- 9. Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, Aragaki AK, Ockene JK, Lane DS, Sarto GE, Rajkovic A, Schenken R, Hendrix SL, Ravdin PM, Rohan TE, Yasmeen S, Anderson G. 2009. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med 360:573–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML. 2008. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 299:1036–1045 [DOI] [PubMed] [Google Scholar]
- 11. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- 12. Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. 2010. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA 107:19272–19277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. 1998. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 139:4708–4713 [DOI] [PubMed] [Google Scholar]
- 14. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331:912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferenczy A. 1987. Anatomy and histology of the uterine corpus. 3rd ed New York: Springer-Verlag [Google Scholar]
- 16. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson-Rechavi M, Escriva Garcia H, Laudet V. 2003. The nuclear receptor superfamily. J Cell Sci 116:585–586 [DOI] [PubMed] [Google Scholar]
- 18. Beato M. 1989. Gene regulation by steroid hormones. Cell 56:335–344 [DOI] [PubMed] [Google Scholar]
- 19. Klein-Hitpass L, Tsai SY, Weigel NL, Allan GF, Riley D, Rodriguez R, Schrader WT, Tsai MJ, O'Malley BW. 1990. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell 60:247–257 [DOI] [PubMed] [Google Scholar]
- 20. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 21. Leonhardt SA, Edwards DP. 2002. Mechanism of action of progesterone antagonists. Exp Biol Med (Maywood) 227:969–980 [DOI] [PubMed] [Google Scholar]
- 22. McKenna NJ, Lanz RB, O'Malley BW. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- 23. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. 1993. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- 24. Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. 1993. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol [Erratum (1993) 7:1378] 7:1256–1265 [DOI] [PubMed] [Google Scholar]
- 25. McDonnell DP, Goldman ME. 1994. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem 269:11945–11949 [PubMed] [Google Scholar]
- 26. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. 2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- 27. Gronemeyer H. 1991. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet 25:89–123 [DOI] [PubMed] [Google Scholar]
- 28. Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. 1996. Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- 29. Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. 1996. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci USA 93:6169–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JJ, Buzzio OL, Li S, Lu Z. 2005. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod 73:833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 32. Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. 1998. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- 34. Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P. 1989. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433–442 [DOI] [PubMed] [Google Scholar]
- 35. Meyer ME, Quirin-Stricker C, Lerouge T, Bocquel MT, Gronemeyer H. 1992. A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem 267:10882–10887 [PubMed] [Google Scholar]
- 36. Lessey BA, Alexander PS, Horwitz KB. 1983. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology 112:1267–1274 [DOI] [PubMed] [Google Scholar]
- 37. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gronemeyer H, Meyer ME, Bocquel MT, Kastner P, Turcotte B, Chambon P. 1991. Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol 40:271–278 [DOI] [PubMed] [Google Scholar]
- 39. McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. 1994. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol 48:425–432 [DOI] [PubMed] [Google Scholar]
- 40. Edwards DP. 2000. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia 5:307–324 [DOI] [PubMed] [Google Scholar]
- 41. Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. 1999. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol 13:910–924 [DOI] [PubMed] [Google Scholar]
- 42. Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB. 2006. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol 20:2656–2670 [DOI] [PubMed] [Google Scholar]
- 43. Condon JC, Hardy DB, Kovaric K, Mendelson CR. 2006. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- 44. Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. 2003. Classification and pharmacology of progestins. Maturitas 46(Suppl 1):S7–S16 [DOI] [PubMed] [Google Scholar]
- 45. Gass EK, Leonhardt SA, Nordeen SK, Edwards DP. 1998. The antagonists RU486 and ZK98299 stimulate progesterone receptor binding to deoxyribonucleic acid in vitro and in vivo, but have distinct effects on receptor conformation. Endocrinology 139:1905–1919 [DOI] [PubMed] [Google Scholar]
- 46. Kobayashi S, Stice JP, Kazmin D, Wittmann BM, Kimbrel EA, Edwards DP, Chang CY, McDonnell DP. 2010. Mechanisms of progesterone receptor inhibition of inflammatory responses in cellular models of breast cancer. Mol Endocrinol 24:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cadepond F, Ulmann A, Baulieu EE. 1997. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 48:129–156 [DOI] [PubMed] [Google Scholar]
- 48. Spitz IM. 2003. Progesterone antagonists and progesterone receptor modulators: an overview. Steroids 68:981–993 [DOI] [PubMed] [Google Scholar]
- 49. Shibata H, Spencer TE, Oñate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. 1997. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res 52:141–165 [PubMed] [Google Scholar]
- 50. Jenster G, Spencer TE, Burcin MM, Tsai SY, Tsai MJ, O'Malley BW. 1997. Steroid receptor induction of gene transcription: a two-step model. Proc Natl Acad Sci USA 94:7879–7884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 2001. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc Natl Acad Sci USA 98:12426–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sartorius CA, Tung L, Takimoto GS, Horwitz KB. 1993. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem 268:9262–9266 [PubMed] [Google Scholar]
- 53. Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, Thompson SK, Stewart EL, Laping NJ, Williams SP, Bray JD. 2007. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol 21:1066–1081 [DOI] [PubMed] [Google Scholar]
- 54. Edwards DP, Leonhardt SA, Gass-Handel E. 2000. Novel mechanisms of progesterone antagonists and progesterone receptor. J Soc Gynecol Investig 7:S22–24 [DOI] [PubMed] [Google Scholar]
- 55. Shatnawi A, Tran T, Ratnam M. 2007. R5020 and RU486 act as progesterone receptor agonists to enhance Sp1/Sp4-dependent gene transcription by an indirect mechanism. Mol Endocrinol 21:635–650 [DOI] [PubMed] [Google Scholar]
- 56. Elger W, Bartley J, Schneider B, Kaufmann G, Schubert G, Chwalisz K. 2000. Endocrine pharmacological characterization of progesterone antagonists and progesterone receptor modulators with respect to PR-agonistic and antagonistic activity. Steroids 65:713–723 [DOI] [PubMed] [Google Scholar]
- 57. Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. 2005. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update 11:293–307 [DOI] [PubMed] [Google Scholar]
- 58. Yin P, Lin Z, Reierstad S, Wu J, Ishikawa H, Marsh EE, Innes J, Cheng Y, Pearson K, Coon JS, 5th, Kim JJ, Chakravarti D, Bulun SE. 2010. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res 70:1722–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yin P, Roqueiro D, Huang L, Owen JK, Xie A, Navarro A, Monsivais D, Coon JS, 5th, Kim JJ, Dai Y, Bulun SE. 2012. Genome-wide progesterone receptor binding: cell type-specific and shared mechanisms in T47D breast cancer cells and primary leiomyoma cells. PloS One 7:e29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, Demayo FJ. 2012. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 26:1428–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kratochwil K. 1971. In vitro analysis of the hormonal basis for the sexual dimorphism in the embryonic development of the mouse mammary gland. J Embryol Exp Morphol 25:141–153 [PubMed] [Google Scholar]
- 62. Kratochwil K, Schwartz P. 1976. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci USA 73:4041–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cunha GR, Lung B. 1978. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 205:181–193 [DOI] [PubMed] [Google Scholar]
- 64. Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. 1997. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94:6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chung D, Das SK. 2011. Mouse primary uterine cell coculture system revisited: ovarian hormones mimic the aspects of in vivo uterine cell proliferation. Endocrinology 152:3246–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kurita T, Wang YZ, Donjacour AA, Zhao C, Lydon JP, O'Malley BW, Isaacs JT, Dahiya R, Cunha GR. 2001. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ 8:192–200 [DOI] [PubMed] [Google Scholar]
- 67. Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. 2000. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod 62:831–838 [DOI] [PubMed] [Google Scholar]
- 68. Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. 2012. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J 26:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, Gold LI. 2005. The activation function-1 domain of estrogen receptor α in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 73:313–322 [DOI] [PubMed] [Google Scholar]
- 70. Pierro E, Minici F, Alesiani O, Miceli F, Proto C, Screpanti I, Mancuso S, Lanzone A. 2001. Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol Reprod 64:831–838 [DOI] [PubMed] [Google Scholar]
- 71. Bläuer M, Heinonen PK, Martikainen PM, Tomás E, Ylikomi T. 2005. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod 20:864–871 [DOI] [PubMed] [Google Scholar]
- 72. Mills AM, Longacre TA. 2010. Endometrial hyperplasia. Semin Diagn Pathol 27:199–214 [DOI] [PubMed] [Google Scholar]
- 73. Bokhman JV. 1983. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17 [DOI] [PubMed] [Google Scholar]
- 74. Clement PB, Young RH. 2002. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 9:145–184 [DOI] [PubMed] [Google Scholar]
- 75. Creasman WT, Eddy GL. 1990. Recent advances in endometrial cancer. Semin Surg Oncol 6:339–342 [DOI] [PubMed] [Google Scholar]
- 76. Siegel R, Ward E, Brawley O, Jemal A. 2011. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236 [DOI] [PubMed] [Google Scholar]
- 77. Creasman WT. 1997. Endometrial cancer: incidence, prognostic factors, diagnosis, and treatment. Semin Oncol 24 (1 Suppl 1):S1–140–S1–50 [PubMed] [Google Scholar]
- 78. Münstedt K, Grant P, Woenckhaus J, Roth G, Tinneberg HR. 2004. Cancer of the endometrium: current aspects of diagnostics and treatment. World J Surg Oncol 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sherman ME. 2000. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 13:295–308 [DOI] [PubMed] [Google Scholar]
- 80. Key TJ, Pike MC. 1988. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 57:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Siiteri PK. 1978. Steroid hormones and endometrial cancer. Cancer Res 38:4360–4366 [PubMed] [Google Scholar]
- 82. Roy D, Liehr JG. 1999. Estrogen, DNA damage and mutations. Mutat Res 424:107–115 [DOI] [PubMed] [Google Scholar]
- 83. Doherty JA, Weiss NS, Fish S, Fan W, Loomis MM, Sakoda LC, Rossing MA, Zhao LP, Chen C. 2011. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 20:1873–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hemminki K, Rajaniemi H, Lindahl B, Moberger B. 1996. Tamoxifen-induced DNA adducts in endometrial samples from breast cancer patients. Cancer Res 56:4374–4377 [PubMed] [Google Scholar]
- 85. Shibutani S, Ravindernath A, Suzuki N, Terashima I, Sugarman SM, Grollman AP, Pearl ML. 2000. Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis 21:1461–1467 [PubMed] [Google Scholar]
- 86. Kelley RM, Baker WH. 1961. Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med 264:216–222 [DOI] [PubMed] [Google Scholar]
- 87. Kistner RW, Griffiths CT, Craig JM. 1965. Use of progestational agents in the management of endometrial cancer. Cancer 18:1563–1579 [DOI] [PubMed] [Google Scholar]
- 88. Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. 1997. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer 79:320–327 [DOI] [PubMed] [Google Scholar]
- 89. Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. 2004. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol 95:133–138 [DOI] [PubMed] [Google Scholar]
- 90. Randall TC, Kurman RJ. 1997. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol 90:434–440 [DOI] [PubMed] [Google Scholar]
- 91. Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, Hatae M, Kodama S, Saito T, Terakawa N, Yaegashi N, Hiura M, Sakamoto A, Tsuda H, Fukunaga M, Kamura T. 2007. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol 25:2798–2803 [DOI] [PubMed] [Google Scholar]
- 92. Park H, Seok JM, Yoon BS, Seong SJ, Kim JY, Shim JY, Park CT. 2012. Effectiveness of high-dose progestin and long-term outcomes in young women with early-stage, well-differentiated endometrioid adenocarcinoma of uterine endometrium. Arch Gynecol Obstet 285:473–478 [DOI] [PubMed] [Google Scholar]
- 93. Perri T, Korach J, Gotlieb WH, Beiner M, Meirow D, Friedman E, Ferenczy A, Ben-Baruch G. 2011. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int J Gynecol Cancer 21:72–78 [DOI] [PubMed] [Google Scholar]
- 94. Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. 2011. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol 22:643–649 [DOI] [PubMed] [Google Scholar]
- 95. Decruze SB, Green JA. 2007. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 17:964–978 [DOI] [PubMed] [Google Scholar]
- 96. Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, Soper JT, Given FT. 1999. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol 17:1736–1744 [DOI] [PubMed] [Google Scholar]
- 97. Rodriguez GC, Rimel BJ, Watkin W, Turbov JM, Barry C, Du H, Maxwell GL, Cline JM. 2008. Progestin treatment induces apoptosis and modulates transforming growth factor-β in the uterine endometrium. Cancer Epidemiol Biomarkers Prev 17:578–584 [DOI] [PubMed] [Google Scholar]
- 98. Whitney CW, Brunetto VL, Zaino RJ, Lentz SS, Sorosky J, Armstrong DK, Lee RB. 2004. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 92:4–9 [DOI] [PubMed] [Google Scholar]
- 99. Kommoss F, Karck U, Prömpeler H, Pfisterer J, Kirkpatrick CJ. 1998. Steroid receptor expression in endometria from women treated with tamoxifen. Gynecol Oncol 70:188–191 [DOI] [PubMed] [Google Scholar]
- 100. Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. 2010. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev 12:CD007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Garavaglia E, Pella F, Montoli S, Voci C, Taccagni G, Mangili G. 2010. Treatment of recurrent or metastatic low-grade endometrial stromal sarcoma: three case reports. Int J Gynecol Cancer 20:1197–1200 [DOI] [PubMed] [Google Scholar]
- 102. Dupont NC, Disaia PJ. 2010. Recurrent endometrial stromal sarcoma: treatment with a progestin and gonadotropin releasing hormone agonist. Sarcoma 2010:353679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dahhan T, Fons G, Buist MR, Ten Kate FJ, van der Velden J. 2009. The efficacy of hormonal treatment for residual or recurrent low-grade endometrial stromal sarcoma. A retrospective study. Eur J Obstet Gynecol Reprod Biol 144:80–84 [DOI] [PubMed] [Google Scholar]
- 104. Landréat V, Paillocher N, Catala L, Foucher F, Descamps P, Levêque J. 2008. Low-grade endometrial stromal sarcoma of the uterus: review of 10 cases. Anticancer Res 28:2869–2874 [PubMed] [Google Scholar]
- 105. Beck TL, Singhal PK, Ehrenberg HM, Rose PG, Lele SB, Krivak TC, McBee WC., Jr 2012. Endometrial stromal sarcoma: analysis of recurrence following adjuvant treatment. Gynecol Oncol 125:141–144 [DOI] [PubMed] [Google Scholar]
- 106. Cheng X, Yang G, Schmeler KM, Coleman RL, Tu X, Liu J, Kavanagh JJ. 2011. Recurrence patterns and prognosis of endometrial stromal sarcoma and the potential of tyrosine kinase-inhibiting therapy. Gynecol Oncol 121:323–327 [DOI] [PubMed] [Google Scholar]
- 107. Demopoulos RI, Mesia AF, Mittal K, Vamvakas E. 1999. Immunohistochemical comparison of uterine papillary serous and papillary endometrioid carcinoma: clues to pathogenesis. Int J Gynecol Pathol 18:233–237 [DOI] [PubMed] [Google Scholar]
- 108. Darvishian F, Hummer AJ, Thaler HT, Bhargava R, Linkov I, Asher M, Soslow RA. 2004. Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic cases. Am J Surg Pathol 28:1568–1578 [DOI] [PubMed] [Google Scholar]
- 109. Kim JJ, Chapman-Davis E. 2010. Role of progesterone in endometrial cancer. Semin Reprod Med 28:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang S, Thiel KW, Leslie KK. 2011. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab 22:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kim JJ, Sefton EC, Bulun SE. 2009. Progesterone receptor action in leiomyoma and endometrial cancer. Prog Mol Biol Transl Sci 87:53–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wheeler DT, Bristow RE, Kurman RJ. 2007. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol 31:988–998 [DOI] [PubMed] [Google Scholar]
- 113. Kamoi S, Ohaki Y, Mori O, Kurose K, Fukunaga M, Takeshita T. 2008. Serial histologic observation of endometrial adenocarcinoma treated with high-dose progestin until complete disappearance of carcinomatous foci—review of more than 25 biopsies from five patients. Int J Gynecol Cancer 18:1305–1314 [DOI] [PubMed] [Google Scholar]
- 114. Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM. 1988. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol 158:796–807 [DOI] [PubMed] [Google Scholar]
- 115. Dai D, Wolf DM, Litman ES, White MJ, Leslie KK. 2002. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res 62:881–886 [PubMed] [Google Scholar]
- 116. Ueda M, Fujii H, Yoshizawa K, Abe F, Ueki M. 1996. Effects of sex steroids and growth factors on migration and invasion of endometrial adenocarcinoma SNG-M cells in vitro. Jpn J Cancer Res 87:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J, Kim JJ. 2011. Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1–59-mediated apoptosis. Horm Cancer 2:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. 2008. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology 149:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Di Nezza LA, Jobling T, Salamonsen LA. 2003. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol 89:325–333 [DOI] [PubMed] [Google Scholar]
- 120. Jaffe RC, Ferguson-Gottschall SD, Gao W, Beam C, Fazleabas AT. 2007. Histone deacetylase inhibition and progesterone act synergistically to stimulate baboon glycodelin gene expression. J Mol Endocrinol 38:401–407 [DOI] [PubMed] [Google Scholar]
- 121. Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, Sakamoto M, Inoue M. 2011. Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth. Clin Cancer Res 17:525–537 [DOI] [PubMed] [Google Scholar]
- 122. Shiozawa T, Nikaido T, Nakayama K, Lu X, Fujii S. 1998. Involvement of cyclin-dependent kinase inhibitor p27Kip1 in growth inhibition of endometrium in the secretory phase and of hyperplastic endometrium treated with progesterone. Mol Hum Reprod 4:899–905 [DOI] [PubMed] [Google Scholar]
- 123. Uchida H, Maruyama T, Nagashima T, Asada H, Yoshimura Y. 2005. Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 146:5365–5373 [DOI] [PubMed] [Google Scholar]
- 124. Uchida H, Maruyama T, Ono M, Ohta K, Kajitani T, Masuda H, Nagashima T, Arase T, Asada H, Yoshimura Y. 2007. Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 148:896–902 [DOI] [PubMed] [Google Scholar]
- 125. Watanabe J, Watanabe K, Jobo T, Kamata Y, Kawaguchi M, Imai M, Okayasu I, Kuramoto H. 2006. Significance of p27 as a predicting marker for medroxyprogesterone acetate therapy against endometrial endometrioid adenocarcinoma. Int J Gynecol Cancer 16(Suppl 1):452–457 [DOI] [PubMed] [Google Scholar]
- 126. Dai D, Kumar NS, Wolf DM, Leslie KK. 2001. Molecular tools to reestablish progestin control of endometrial cancer cell proliferation. Am J Obstet Gynecol 184:790–797 [DOI] [PubMed] [Google Scholar]
- 127. Paulssen RH, Moe B, Grønaas H, Orbo A. 2008. Gene expression in endometrial cancer cells (Ishikawa) after short time high dose exposure to progesterone. Steroids 73:116–128 [DOI] [PubMed] [Google Scholar]
- 128. Gielen SC, Hanekamp EE, Hanifi-Moghaddam P, Sijbers AM, van Gool AJ, Burger CW, Blok LJ, Huikeshoven FJ. 2006. Growth regulation and transcriptional activities of estrogen and progesterone in human endometrial cancer cells. Int J Gynecol Cancer 16:110–120 [DOI] [PubMed] [Google Scholar]
- 129. Smid-Koopman E, Blok LJ, Kühne LC, Burger CW, Helmerhorst TJ, Brinkmann AO, Huikeshoven FJ. 2003. Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J Soc Gynecol Investig 10:49–57 [PubMed] [Google Scholar]
- 130. Moe BG, Vereide AB, Orbo A, Sager G. 2009. High concentrations of progesterone and mifepristone mutually reinforce cell cycle retardation and induction of apoptosis. Anticancer Res 29:1053–1058 [PubMed] [Google Scholar]
- 131. Ørbo A, Moe BT, Grønaas H, Paulssen RH. 2009. Early effects of high concentrations of progesterone and mifepristone A gene expression study of endometrial cancer cells (Ishikawa). J Steroid Biochem Mol Biol 113:139–149 [DOI] [PubMed] [Google Scholar]
- 132. Press MF, Udove JA, Greene GL. 1988. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol 131:112–124 [PMC free article] [PubMed] [Google Scholar]
- 133. Snijders MP, de Goeij AF, Debets-Te Baerts MJ, Rousch MJ, Koudstaal J, Bosman FT. 1992. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil 94:363–371 [DOI] [PubMed] [Google Scholar]
- 134. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 135. Carmichael PL, Sardar S, Crooks N, Neven P, Van Hoof I, Ugwumadu A, Bourne T, Tomas E, Hellberg P, Hewer AJ, Phillips DH. 1999. Lack of evidence from HPLC 32P-post-labelling for tamoxifen-DNA adducts in the human endometrium. Carcinogenesis 20:339–342 [DOI] [PubMed] [Google Scholar]
- 136. Shibutani S, Suzuki N, Laxmi YR, Schild LJ, Divi RL, Grollman AP, Poirier MC. 2003. Identification of tamoxifen-DNA adducts in monkeys treated with tamoxifen. Cancer Res 63:4402–4406 [PubMed] [Google Scholar]
- 137. Yang S, Fang Z, Gurates B, Tamura M, Miller J, Ferrer K, Bulun SE. 2001. Stromal PRs mediate induction of 17β-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol 15:2093–2105 [DOI] [PubMed] [Google Scholar]
- 138. Arnold JT, Lessey BA, Seppälä M, Kaufman DG. 2002. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res 62:79–88 [PubMed] [Google Scholar]
- 139. Shi M, Zhang H, Li M, Xue J, Fu Y, Yan L, Zhao X. 2011. Normal endometrial stromal cells regulate survival and apoptosis signaling through PI3K/AKt/survivin pathway in endometrial adenocarcinoma cells in vitro. Gynecol Oncol 123:387–392 [DOI] [PubMed] [Google Scholar]
- 140. Sampey BP, Lewis TD, Barbier CS, Makowski L, Kaufman DG. 2011. Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures. Exp Mol Pathol 90:257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Tanwar PS, Zhang L, Roberts DJ, Teixeira JM. 2011. Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res 71:1584–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Giudice LC, Kao LC. 2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 143. Rock JA, Zacur HA, Dlugi AM, Jones HW, Jr, TeLinde RW. 1982. Pregnancy success following surgical correction of imperforate hymen and complete transverse vaginal septum. Obstet Gynecol 59:448–451 [PubMed] [Google Scholar]
- 144. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. 2004. In utero exposures and the incidence of endometriosis. Fertil Steril 82:1501–1508 [DOI] [PubMed] [Google Scholar]
- 145. Kennedy S. 1999. The genetics of endometriosis. Eur J Obstet Gynecol Reprod Biol 82:129–133 [DOI] [PubMed] [Google Scholar]
- 146. Garry R. 2004. The endometriosis syndromes: a clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum Reprod 19:760–768 [DOI] [PubMed] [Google Scholar]
- 147. Brosens I. 2004. Endometriosis rediscovered? Hum Reprod 19:1679–1680; author reply 1680–1681 [DOI] [PubMed] [Google Scholar]
- 148. Guzick DS, Silliman NP, Adamson GD, Buttram VC, Jr, Canis M, Malinak LR, Schenken RS. 1997. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine's revised classification of endometriosis. Fertil Steril 67:822–829 [DOI] [PubMed] [Google Scholar]
- 149. Stovall DW, Bowser LM, Archer DF, Guzick DS. 1997. Endometriosis-associated pelvic pain: evidence for an association between the stage of disease and a history of chronic pelvic pain. Fertil Steril 68:13–18 [DOI] [PubMed] [Google Scholar]
- 150. Olive DL, Schwartz LB. 1993. Endometriosis. N Engl J Med 328:1759–1769 [DOI] [PubMed] [Google Scholar]
- 151. Olive DL, Pritts EA. 2001. Treatment of endometriosis. N Engl J Med 345:266–275 [DOI] [PubMed] [Google Scholar]
- 152. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. 2004. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol 104:965–974 [DOI] [PubMed] [Google Scholar]
- 153. Attar E, Bulun SE. 2006. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril 85:1307–1318 [DOI] [PubMed] [Google Scholar]
- 154. Kettel LM, Murphy AA, Morales AJ, Ulmann A, Baulieu EE, Yen SS. 1996. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril 65:23–28 [DOI] [PubMed] [Google Scholar]
- 155. Murphy AA, Castellano PZ. 1994. RU486: pharmacology and potential use in the treatment of endometriosis and leiomyomata uteri. Curr Opin Obstet Gynecol 6:269–278 [PubMed] [Google Scholar]
- 156. Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN. 1999. Oestrogen receptor (ER)-α and ER-β isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod 5:651–655 [DOI] [PubMed] [Google Scholar]
- 157. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. 2000. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 85:2897–2902 [DOI] [PubMed] [Google Scholar]
- 158. Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. 2007. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 77:681–687 [DOI] [PubMed] [Google Scholar]
- 159. Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. 2005. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- 160. Sampson JA. 1927. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- 161. Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. 1996. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab 81:1118–1122 [DOI] [PubMed] [Google Scholar]
- 162. Noble LS, Simpson ER, Johns A, Bulun SE. 1996. Aromatase expression in endometriosis. J Clin Endocrinol Metab 81:174–179 [DOI] [PubMed] [Google Scholar]
- 163. Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. 1997. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
- 164. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 165. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. 2003. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- 166. Bulun SE. 2009. Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- 167. Taylor RN, Lebovic DI, Mueller MD. 2002. Angiogenic factors in endometriosis. Ann NY Acad Sci 955:89–100; discussion 396–406 [DOI] [PubMed] [Google Scholar]
- 168. Bartosik D, Viscarello RR, Damjanov I. 1985. Endometriosis as an autoimmune disease. Fertil Steril 43:351–3523979573 [Google Scholar]
- 169. Osteen KG, Sierra-Rivera E. 1997. Does disruption of immune and endocrine systems by environmental toxins contribute to development of endometriosis? Semin Reprod Endocrinol 15:301–308 [DOI] [PubMed] [Google Scholar]
- 170. Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. 2001. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod 16:1802–1808 [DOI] [PubMed] [Google Scholar]
- 171. Béliard A, Noël A, Foidart JM. 2004. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril 82:80–85 [DOI] [PubMed] [Google Scholar]
- 172. Hayes EC, Rock JA. 2002. COX-2 inhibitors and their role in gynecology. Obstet Gynecol Surv 57:768–780 [DOI] [PubMed] [Google Scholar]
- 173. Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. 1993. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril 60:75–79 [PubMed] [Google Scholar]
- 174. Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. 2007. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- 175. Vercellini P, Crosignani P, Somigliana E, Viganò P, Frattaruolo MP, Fedele L. 2011. ‘Waiting for Godot’: a commonsense approach to the medical treatment of endometriosis. Hum Reprod 26:3–13 [DOI] [PubMed] [Google Scholar]
- 176. Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. 1996. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril 65:299–304 [PubMed] [Google Scholar]
- 177. Vercellini P, Cortesi I, Crosignani PG. 1997. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril 68:393–401 [DOI] [PubMed] [Google Scholar]
- 178. Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. 2005. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev 26:423–438 [DOI] [PubMed] [Google Scholar]
- 179. Sun HS, Hsiao KY, Hsu CC, Wu MH, Tsai SJ. 2003. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology 144:3934–3942 [DOI] [PubMed] [Google Scholar]
- 180. Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- 181. Bulun SE, Adashi E. 2011. The physiology and pathology of the female reproductive axis. Williams textbook of endocrinology. Editors, Melmed S, Polonsky KS, Larsen PR, Kronenberg HM; 12th ed Philadelphia: Saunders; Chap 17, pp 604–608 [Google Scholar]
- 182. Brosens JJ, Hayashi N, White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 183. Fazleabas A, Brudney A, Chai D, Langoi D, Bulun S. 2003. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril 80(Suppl 2):820–827 [DOI] [PubMed] [Google Scholar]
- 184. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. 2006. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 [DOI] [PubMed] [Google Scholar]
- 185. Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, Xue Q, Reierstad S, Innes J, Thung S, Kim JJ, Xu E, Bulun SE. 2007. Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab 92:4459–4466 [DOI] [PubMed] [Google Scholar]
- 186. Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. 2012. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 97:E35–E43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Osteen KG, Bruner-Tran KL, Eisenberg E. 2005. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril 83:529–537 [DOI] [PubMed] [Google Scholar]
- 188. Casey ML, MacDonald PC, Andersson S. 1994. 17β-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest 94:2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Mustonen MV, Isomaa VV, Vaskivuo T, Tapanainen J, Poutanen MH, Stenbäck F, Vihko RK, Vihko PT. 1998. Human 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J Clin Endocrinol Metab 83:1319–1324 [DOI] [PubMed] [Google Scholar]
- 190. Cheng YH, Yin P, Xue Q, Yilmaz B, Dawson MI, Bulun SE. 2008. Retinoic acid (RA) regulates 17β-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab 93:1915–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tseng L, Gurpide E. 1974. Estradiol and 20α-dihydroprogesterone dehydrogenase activities in human endometrium during the menstrual cycle. Endocrinology 94:419–423 [DOI] [PubMed] [Google Scholar]
- 192. Tseng L, Gurpide E. 1975. Induction of human endometrial estradiol dehydrogenase by progestins. Endocrinology 97:825–833 [DOI] [PubMed] [Google Scholar]
- 193. Satyaswaroop PG, Wartell DJ, Mortel R. 1982. Distribution of progesterone receptor, estradiol dehydrogenase, and 20α-dihydroprogesterone dehydrogenase activities in human endometrial glands and stroma: progestin induction of steroid dehydrogenase activities in vitro is restricted to the glandular epithelium. Endocrinology 111:743–749 [DOI] [PubMed] [Google Scholar]
- 194. Kauppila A, Vierikko P, Isotalo H, Rönnberg L, Vihko R. 1984. Cytosol estrogen and progestin receptor concentrations and 17 β-hydroxysteroid dehydrogenase activities in the endometrium and endometriotic tissue. Effects of hormonal treatment. Acta Obstet Gynecol Scand Suppl 123:45–49 [DOI] [PubMed] [Google Scholar]
- 195. Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. 2007. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol 196:391.e1–e7; discussion 391.e7–e8 [DOI] [PubMed] [Google Scholar]
- 196. Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE. 1998. Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J Clin Endocrinol Metab 83:4474–4480 [DOI] [PubMed] [Google Scholar]
- 197. Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. 2010. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab 95:E300–E309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Brunton L, Lazo J, Parker K. 2006. Goodman and Gilman's: the pharmacological basis of therapeutics. 11th ed New York: McGraw-Hill, Medical Publishing Division [Google Scholar]
- 199. Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315:820–825 [DOI] [PubMed] [Google Scholar]
- 200. Mark M, Ghyselinck NB, Chambon P. 2006. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 46:451–480 [DOI] [PubMed] [Google Scholar]
- 201. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. 2007. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Napoli JL. 1999. Retinoic acid: its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol 63:139–188 [DOI] [PubMed] [Google Scholar]
- 203. Noy N. 2000. Retinoid-binding proteins: mediators of retinoid action. Biochem J 348:481–495 [PMC free article] [PubMed] [Google Scholar]
- 204. Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, Bulun SE. 2011. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod 26:2157–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. 1997. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 90:967–973 [DOI] [PubMed] [Google Scholar]
- 206. Myers ER, Barber MD, Gustilo-Ashby T, Couchman G, Matchar DB, McCrory DC. 2002. Management of uterine leiomyomata: what do we really know? Obstet Gynecol 100:8–17 [DOI] [PubMed] [Google Scholar]
- 207. Parker WH. 2007. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 87:725–736 [DOI] [PubMed] [Google Scholar]
- 208. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. 2003. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188:100–107 [DOI] [PubMed] [Google Scholar]
- 209. Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. 1994. Hysterectomy in the United States, 1988–1990. Obstet Gynecol 83:549–555 [DOI] [PubMed] [Google Scholar]
- 210. Sener AB, Seçkin NC, Ozmen S, Gökmen O, Doğu N, Ekici E. 1996. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril 65:354–357 [DOI] [PubMed] [Google Scholar]
- 211. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. 2002. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women—a 3-year study. Maturitas 43:35–39 [DOI] [PubMed] [Google Scholar]
- 212. Palomba S, Sena T, Noia R, Di Carlo C, Zullo F, Mastrantonio P. 2001. Transdermal hormone replacement therapy in postmenopausal women with uterine leiomyomas. Obstet Gynecol 98:1053–1058 [DOI] [PubMed] [Google Scholar]
- 213. Williams AJ, Powell WL, Collins T, Morton CC. 1997. HMGI(Y) expression in human uterine leiomyomata. Involvement of another high-mobility group architectural factor in a benign neoplasm. Am J Pathol 150:911–918 [PMC free article] [PubMed] [Google Scholar]
- 214. Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 2001. 17-β Estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol 186:414–424 [DOI] [PubMed] [Google Scholar]
- 215. Shimomura Y, Matsuo H, Samoto T, Maruo T. 1998. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab 83:2192–2198 [DOI] [PubMed] [Google Scholar]
- 216. Park SH, Ramachandran S, Kwon SH, Cha SD, Seo EW, Bae I, Cho C, Song DK. 2008. Upregulation of ATP-sensitive potassium channels for estrogen-mediated cell proliferation in human uterine leiomyoma cells. Gynecol Endocrinol 24:250–256 [DOI] [PubMed] [Google Scholar]
- 217. Butler PD, Sims PF, Wild DG. 1979. Abnormal ribosome assembly in a mutant of Escherichia coli. Biochem J 182:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M. 2000. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 141:3852–3861 [DOI] [PubMed] [Google Scholar]
- 219. Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. 1995. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig 2:542–551 [DOI] [PubMed] [Google Scholar]
- 220. Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T, Dixon D. 2008. A low concentration of genistein induces estrogen receptor-α and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod 23:1873–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E, Yin P, Clardy J, Chakravarti D, Bulun S, Kim JJ. 2009. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab 94:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kim JJ, Sefton EC. 2012. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol 358:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Matsuo H, Maruo T, Samoto T. 1997. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab 82:293–299 [DOI] [PubMed] [Google Scholar]
- 224. Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, Maruo T. 2006. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab 91:1296–1304 [DOI] [PubMed] [Google Scholar]
- 225. Maruo T, Ohara N, Wang J, Matsuo H. 2004. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update 10:207–220 [DOI] [PubMed] [Google Scholar]
- 226. Yamada T, Nakago S, Kurachi O, Wang J, Takekida S, Matsuo H, Maruo T. 2004. Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod 19:815–821 [DOI] [PubMed] [Google Scholar]
- 227. Newbold RR, Moore AB, Dixon D. 2002. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES). Toxicol Pathol 30:611–616 [DOI] [PubMed] [Google Scholar]
- 228. Lipschotz A, Vargas L., Jr 1941. Structure and origin of uterine and extragenital fibroids induced experimentally in the guinea pig by prolonged administration of estrogens. Cancer Res 1:236–249 [Google Scholar]
- 229. Hino O, Kobayashi T, Tsuchiya H, Kikuchi Y, Kobayashi E, Mitani H, Hirayama Y. 1994. The predisposing gene of the Eker rat inherited cancer syndrome is tightly linked to the tuberous sclerosis (TSC2) gene. Biochem Biophys Res Commun 203:1302–1308 [DOI] [PubMed] [Google Scholar]
- 230. Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. 1994. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 91:11413–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. 1995. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Genet 9:70–74 [DOI] [PubMed] [Google Scholar]
- 232. Walker CL, Hunter D, Everitt JI. 2003. Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes Chromosomes Cancer 38:349–356 [DOI] [PubMed] [Google Scholar]
- 233. Burroughs KD, Fuchs-Young R, Davis B, Walker CL. 2000. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod 63:1322–1330 [DOI] [PubMed] [Google Scholar]
- 234. Cermik D, Arici A, Taylor HS. 2002. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril 78:979–984 [DOI] [PubMed] [Google Scholar]
- 235. Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM. 1993. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol 169:78–85 [DOI] [PubMed] [Google Scholar]
- 236. Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. 1989. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 160:637–641 [DOI] [PubMed] [Google Scholar]
- 237. Tiltman AJ. 1985. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol 4:89–96 [DOI] [PubMed] [Google Scholar]
- 238. Harrison-Woolrych ML, Charnock-Jones DS, Smith SK. 1994. Quantification of messenger ribonucleic acid for epidermal growth factor in human myometrium and leiomyomata using reverse transcriptase polymerase chain reaction. J Clin Endocrinol Metab 78:1179–1184 [DOI] [PubMed] [Google Scholar]
- 239. Lamminen S, Rantala I, Helin H, Rorarius M, Tuimala R. 1992. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest 34:111–114 [DOI] [PubMed] [Google Scholar]
- 240. Palomba S, Sena T, Morelli M, Noia R, Zullo F, Mastrantonio P. 2002. Effect of different doses of progestin on uterine leiomyomas in postmenopausal women. Eur J Obstet Gynecol Reprod Biol 102:199–201 [DOI] [PubMed] [Google Scholar]
- 241. Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, Roark M, Steinkampf MP. 1993. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 76:1217–1223 [DOI] [PubMed] [Google Scholar]
- 242. Friedman AJ, Daly M, Juneau-Norcross M, Rein MS, Fine C, Gleason R, Leboff M. 1993. A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add-back” regimens for women with leiomyomata uteri. J Clin Endocrinol Metab 76:1439–1445 [DOI] [PubMed] [Google Scholar]
- 243. Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. 1993. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab 76:513–517 [DOI] [PubMed] [Google Scholar]
- 244. Murphy AA, Morales AJ, Kettel LM, Yen SS. 1995. Regression of uterine leiomyomata to the antiprogesterone RU486: dose-response effect. Fertil Steril 64:187–190 [PubMed] [Google Scholar]
- 245. Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. 2003. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol 101:243–250 [DOI] [PubMed] [Google Scholar]
- 246. Murphy LL, Mahesh VB. 1985. Selective release of follicle-stimulating hormone and luteinizing hormone by 5α-dihydroprogesterone and 3α,5α- tetrahydroprogesterone in pregnant mare's serum gonadotropin-primed immature rats exposed to constant light. Biol Reprod 32:795–803 [DOI] [PubMed] [Google Scholar]
- 247. Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K. 2007. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod 22:1696–1704 [DOI] [PubMed] [Google Scholar]
- 248. Steinauer J, Pritts EA, Jackson R, Jacoby AF. 2004. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol 103:1331–1336 [DOI] [PubMed] [Google Scholar]
- 249. Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. 2007. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 87:1399–1412 [DOI] [PubMed] [Google Scholar]
- 250. Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. 2005. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol 12:227–233 [DOI] [PubMed] [Google Scholar]
- 251. Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. 2006. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 108:1381–1387 [DOI] [PubMed] [Google Scholar]
- 252. Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, Korecki C, Iatridis J, Catherino WH, Tuan RS, Dhillon N, Leppert P, Segars JH. 2008. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol 198:474.e1–474.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Nurmenniemi S, Sinikumpu T, Alahuhta I, Salo S, Sutinen M, Santala M, Risteli J, Nyberg P, Salo T. 2009. A novel organotypic model mimics the tumor microenvironment. Am J Pathol 175:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Zaitseva M, Vollenhoven BJ, Rogers PA. 2006. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod 12:187–207 [DOI] [PubMed] [Google Scholar]
- 255. Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ. 1996. Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod 2:823–828 [DOI] [PubMed] [Google Scholar]
- 256. Stewart EA, Friedman AJ, Peck K, Nowak RA. 1994. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 79:900–906 [DOI] [PubMed] [Google Scholar]
- 257. Ohara N. 2009. Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin Exp Obstet Gynecol 36:10–11 [PubMed] [Google Scholar]
- 258. Morikawa A, Ohara N, Xu Q, Nakabayashi K, DeManno DA, Chwalisz K, Yoshida S, Maruo T. 2008. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod 23:944–951 [DOI] [PubMed] [Google Scholar]
- 259. Ito F, Kawamura N, Ichimura T, Tsujimura A, Ishiko O, Ogita S. 2001. Ultrastructural comparison of uterine leiomyoma cells from the same myoma nodule before and after gonadotropin-releasing hormone agonist treatment. Fertil Steril 75:125–130 [DOI] [PubMed] [Google Scholar]
- 260. Kawamura N, Ito F, Ichimura T, Shibata S, Umesaki N, Ogita S. 1997. Correlation between shrinkage of uterine leiomyoma treated with buserelin acetate and histopathologic findings of biopsy specimen before treatment. Fertil Steril 68:632–636 [DOI] [PubMed] [Google Scholar]
- 261. Crow J, Gardner RL, McSweeney G, Shaw RW. 1995. Morphological changes in uterine leiomyomas treated by GnRH agonist goserelin. Int J Gynecol Pathol 14:235–242 [DOI] [PubMed] [Google Scholar]
- 262. Neiger R, Sonek JD, Croom CS, Ventolini G. 2006. Pregnancy-related changes in the size of uterine leiomyomas. J Reprod Med 51:671–674 [PubMed] [Google Scholar]
- 263. Rosati P, Exacoustòs C, Mancuso S. 1992. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med 11:511–515 [DOI] [PubMed] [Google Scholar]
- 264. Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S, Diamond MP. 2006. Volume change of uterine myomas during pregnancy: do myomas really grow? J Minim Invasive Gynecol 13:386–390 [DOI] [PubMed] [Google Scholar]
- 265. Aharoni A, Reiter A, Golan D, Paltiely Y, Sharf M. 1988. Patterns of growth of uterine leiomyomas during pregnancy. A prospective longitudinal study. Br J Obstet Gynaecol 95:510–513 [DOI] [PubMed] [Google Scholar]
- 266. Spitz IM. 2009. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol 21:318–324 [DOI] [PubMed] [Google Scholar]
- 267. Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, Nouri K, Selvaggi L, Sodowski K, Bestel E, Terrill P, Osterloh I, Loumaye E. 2012. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 366:421–432 [DOI] [PubMed] [Google Scholar]
- 268. Ono M, Qiang W, Serna VA, Yin P, Coon JS, 5th, Navarro A, Monsivais D, Kakinuma T, Dyson M, Druschitz S, Unno K, Kurita T, Bulun SE. 2012. Role of stem cells in human uterine leiomyoma growth. PloS One 7:e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. 2011. Cracking the estrogen receptor's posttranslational code in breast tumors. Endocr Rev 32:597–622 [DOI] [PubMed] [Google Scholar]
- 270. Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465:798–802 [DOI] [PubMed] [Google Scholar]
- 271. Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. 2009. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150:3318–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. 2010. Progesterone induces adult mammary stem cell expansion. Nature 465:803–807 [DOI] [PubMed] [Google Scholar]
- 273. Beral V. 2003. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419–427 [DOI] [PubMed] [Google Scholar]
- 274. Engman M, Skoog L, Söderqvist G, Gemzell-Danielsson K. 2008. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum Reprod 23:2072–2079 [DOI] [PubMed] [Google Scholar]
- 275. Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. 2002. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489 [DOI] [PubMed] [Google Scholar]
- 276. Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. 2002. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol 80:137–148 [DOI] [PubMed] [Google Scholar]
- 277. Silberstein GB, Van Horn K, Shyamala G, Daniel CW. 1996. Progesterone receptors in the mouse mammary duct: distribution and developmental regulation. Cell Growth Diff 7:945–952 [PubMed] [Google Scholar]
- 278. Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. 1998. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA 95:5076–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Barcellos-Hoff MH, Ewan KB. 2000. Transforming growth factor-β and breast cancer: mammary gland development. Breast Cancer Res 2:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Grimm SL, Rosen JM. 2006. Stop! In the name of transforming growth factor-β: keeping estrogen receptor-α-positive mammary epithelial cells from proliferating. Breast Cancer Res 8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Clarke RB, Howell A, Potten CS, Anderson E. 1997. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991 [PubMed] [Google Scholar]
- 282. Rosen JM. 2003. Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis 18:3–9 [DOI] [PubMed] [Google Scholar]
- 283. Russo J, Ao X, Grill C, Russo IH. 1999. Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat 53:217–227 [DOI] [PubMed] [Google Scholar]
- 284. Graham JD, Mote PA, Salagame U, Balleine RL, Huschtscha LI, Clarke CL. 2009. Hormone-responsive model of primary human breast epithelium. J Mammary Gland Biol Neoplasia 14:367–379 [DOI] [PubMed] [Google Scholar]
- 285. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. 1999. RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. 2000. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41–50 [DOI] [PubMed] [Google Scholar]
- 287. Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, Lydon JP. 2008. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 149:6236–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. 2010. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107:2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Mukherjee A, Soyal SM, Li J, Ying Y, He B, DeMayo FJ, Lydon JP. 2010. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J 24:4408–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. 2006. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98:1011–1014 [DOI] [PubMed] [Google Scholar]
- 291. Vaillant F, Asselin-Labat ML, Shackleton M, Lindeman GJ, Visvader JE. 2007. The emerging picture of the mouse mammary stem cell. Stem Cell Rev 3:114–123 [DOI] [PubMed] [Google Scholar]
- 292. Anderson E. 2002. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Khan SA, Rogers MA, Obando JA, Tamsen A. 1994. Estrogen receptor expression of benign breast epithelium and its association with breast cancer. Cancer Res 54:993–997 [PubMed] [Google Scholar]
- 294. Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP. 1999. Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 155:1811–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Chatterton RT, Jr, Lydon JP, Mehta RG, Mateo ET, Pletz A, Jordan VC. 2002. Role of the progesterone receptor (PR) in susceptibility of mouse mammary gland to 7,12-dimethylbenz[a]anthracene-induced hormone-independent preneoplastic lesions in vitro. Cancer Lett 188:47–52 [DOI] [PubMed] [Google Scholar]
- 296. Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW. 1999. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59:4276–4284 [PubMed] [Google Scholar]
- 297. Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffé E, Schillaci R, Elizalde PV. 2007. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol 21:1335–1358 [DOI] [PubMed] [Google Scholar]
- 298. Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, Charreau EH, Elizalde PV. 2003. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol 23:1095–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Krämer EA, Seeger H, Krämer B, Wallwiener D, Mueck AO. 2005. The effects of progesterone, medroxyprogesterone acetate, and norethisterone on growth factor- and estradiol-treated human cancerous and noncancerous breast cells. Menopause 12:468–474 [DOI] [PubMed] [Google Scholar]
- 300. Krämer EA, Seeger H, Krämer B, Wallwiener D, Mueck AO. 2006. The effect of progesterone, testosterone and synthetic progestogens on growth factor- and estradiol-treated human cancerous and benign breast cells. Eur J Obstet Gynecol Reprod Biol 129:77–83 [DOI] [PubMed] [Google Scholar]
- 301. Cooley A, Matthews L, Zelivianski S, Hardy A, Jeruss JS. 2012. Effect of infertility treatment and pregnancy-related hormones on breast cell proliferation in vitro. Hum Reprod 27:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. 1997. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol 11:1593–1607 [DOI] [PubMed] [Google Scholar]
- 303. Giulianelli S, Vaqué JP, Soldati R, Wargon V, Vanzulli SI, Martins R, Zeitlin E, Molinolo AA, Helguero LA, Lamb CA, Gutkind JS, Lanari C. 2012. Estrogen receptor α mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res 72:2416–2427 [DOI] [PubMed] [Google Scholar]
- 304. Obr AE, Edwards DP. 2012. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 357:4–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. 2007. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol 21:359–375 [DOI] [PubMed] [Google Scholar]
- 306. Ballaré C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. 2003. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol 23:1994–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Faivre EJ, Daniel AR, Hillard CJ, Lange CA. 2008. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol 22:823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. 2005. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol 19:574–587 [DOI] [PubMed] [Google Scholar]
- 309. Sartorius CA, Shen T, Horwitz KB. 2003. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat 79:287–299 [DOI] [PubMed] [Google Scholar]
- 310. Hardy DB, Janowski BA, Chen CC, Mendelson CR. 2008. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol 22:1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA. 1987. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer 56:814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Folkman J, Kalluri R. 2004. Cancer without disease. Nature 427:787. [DOI] [PubMed] [Google Scholar]
- 313. Bissell MJ, Hines WC. 2011. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Tlsty TD, Coussens LM. 2006. Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150 [DOI] [PubMed] [Google Scholar]
- 315. Woodward TL, Xie JW, Haslam SZ. 1998. The role of mammary stroma in modulating the proliferative response to ovarian hormones in the normal mammary gland. J Mammary Gland Biol Neoplasia 3:117–131 [DOI] [PubMed] [Google Scholar]
- 316. Fendrick JL, Raafat AM, Haslam SZ. 1998. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia 3:7–22 [DOI] [PubMed] [Google Scholar]
- 317. Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. 2012. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab 23:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Sunil N, Bennett JM, Haslam SZ. 2002. Hepatocyte growth factor is required for progestin-induced epithelial cell proliferation and alveolar-like morphogenesis in serum-free culture of normal mammary epithelial cells. Endocrinology 143:2953–2960 [DOI] [PubMed] [Google Scholar]
- 319. Humphreys RC, Lydon J, O'Malley BW, Rosen JM. 1997. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endocrinol 11:801–811 [DOI] [PubMed] [Google Scholar]
- 320. Rajkumar L, Kittrell FS, Guzman RC, Brown PH, Nandi S, Medina D. 2007. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res 9:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Shyamala G, Barcellos-Hoff MH, Toft D, Yang X. 1997. In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol 63:251–259 [DOI] [PubMed] [Google Scholar]
- 322. Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. 2005. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577–3588 [DOI] [PubMed] [Google Scholar]
- 323. Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. 2001. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res 61:1320–1326 [PubMed] [Google Scholar]
- 324. Polyak K, Kalluri R. 2010. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol 2:a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, Daumer KM, Zhou J, Wang C, Katiyar S, Xu H, Bosco E, Quong AA, Aronow B, Witkiewicz AK, Minetti C, Frank PG, Jimenez SA, Knudsen ES, Pestell RG, Lisanti MP. 2009. Caveolin-1−/− null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol 174:746–761 [DOI] [PMC free article] [PubMed] [Google Scholar]