Abstract
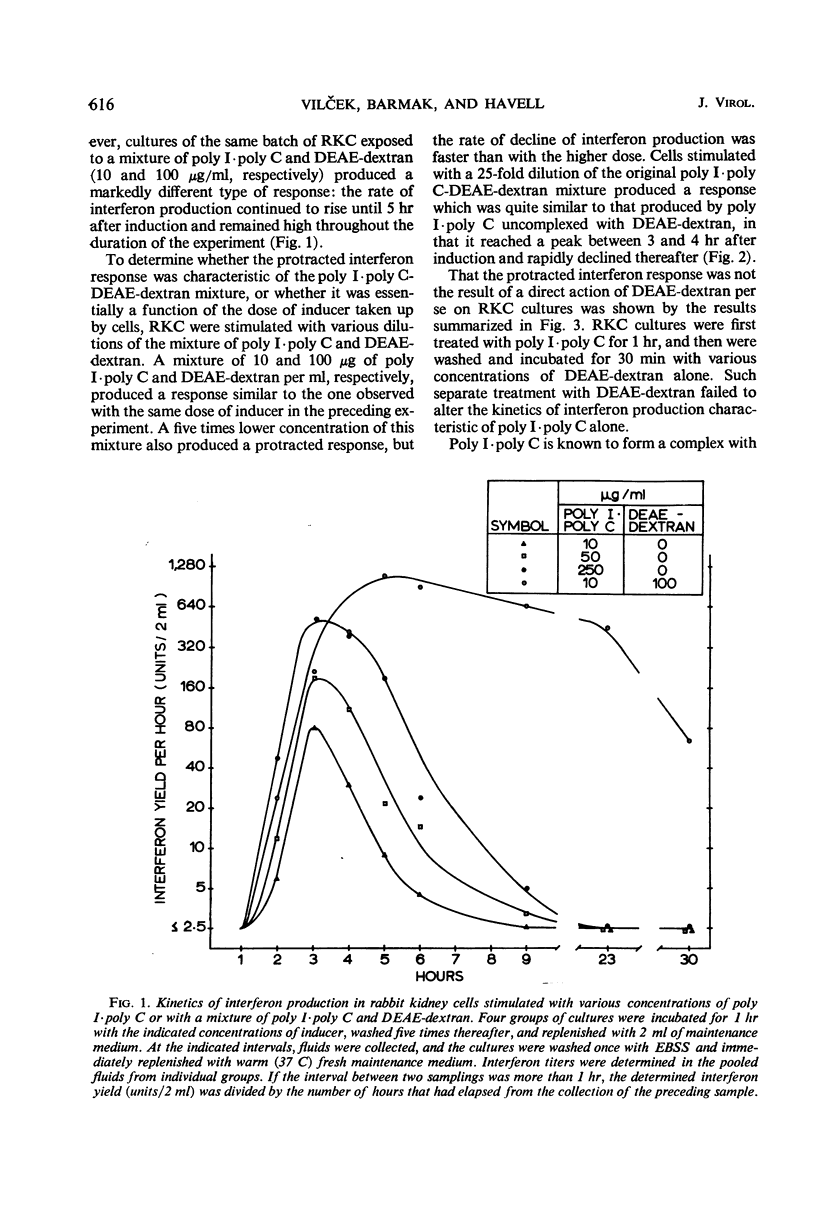
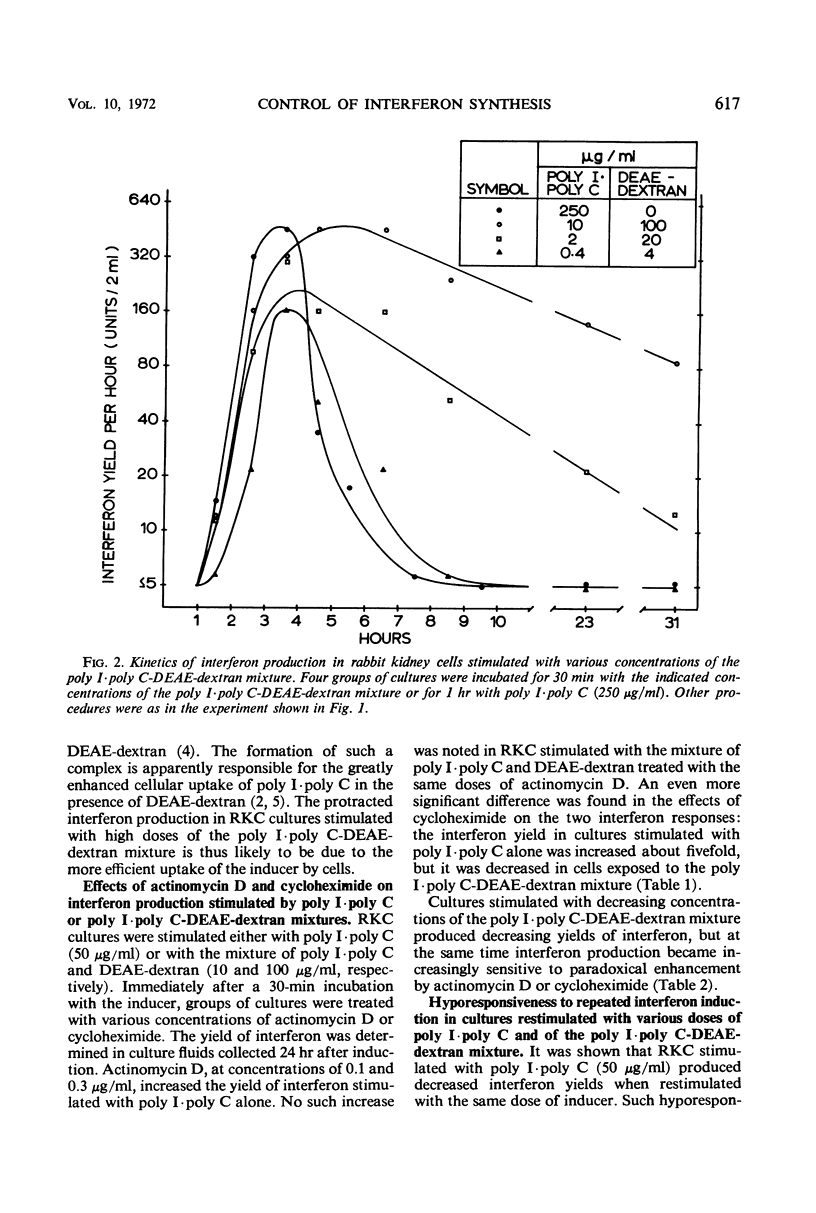
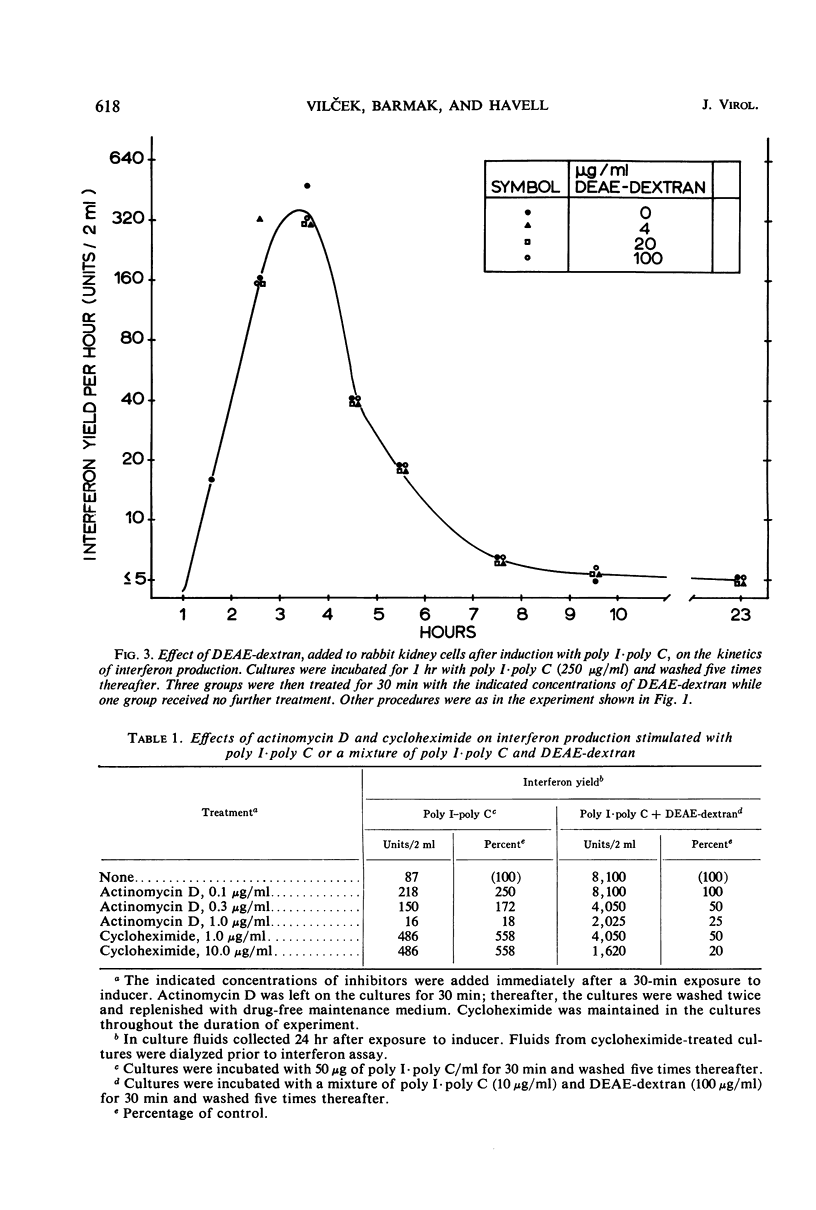
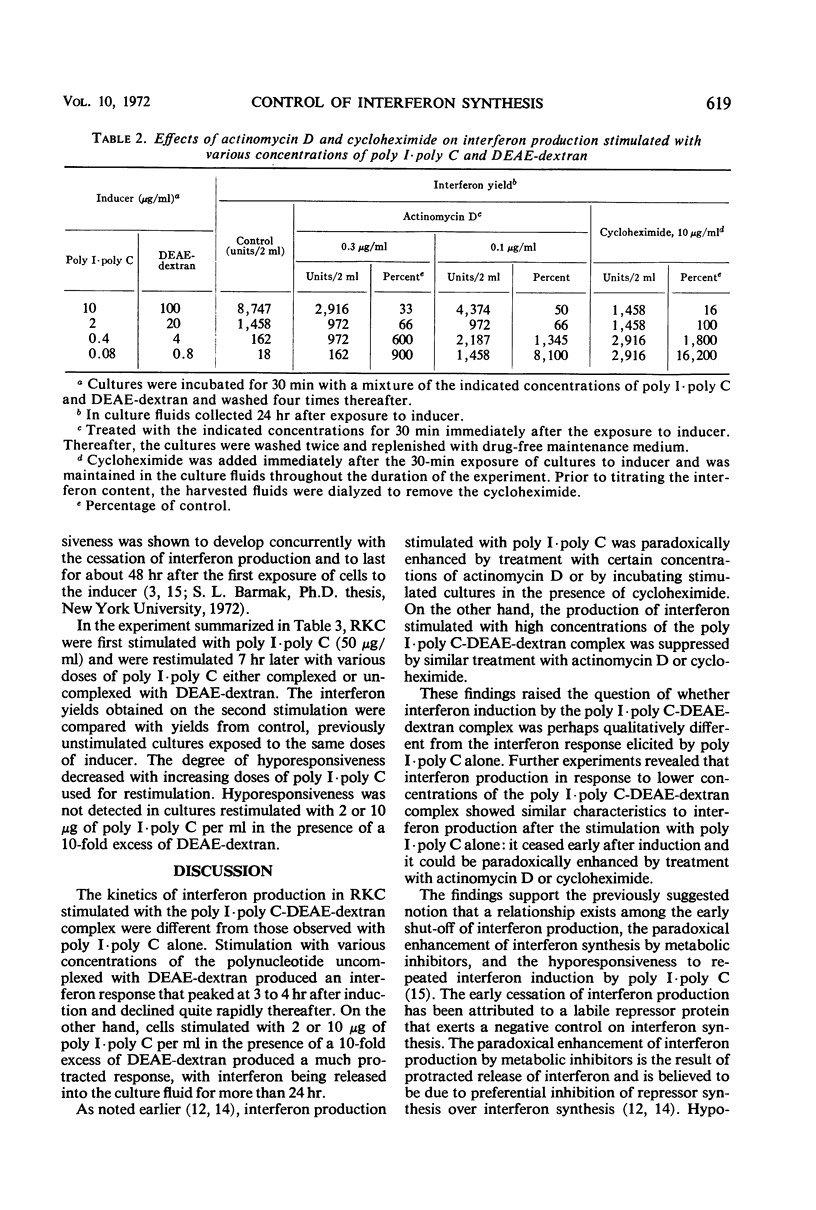
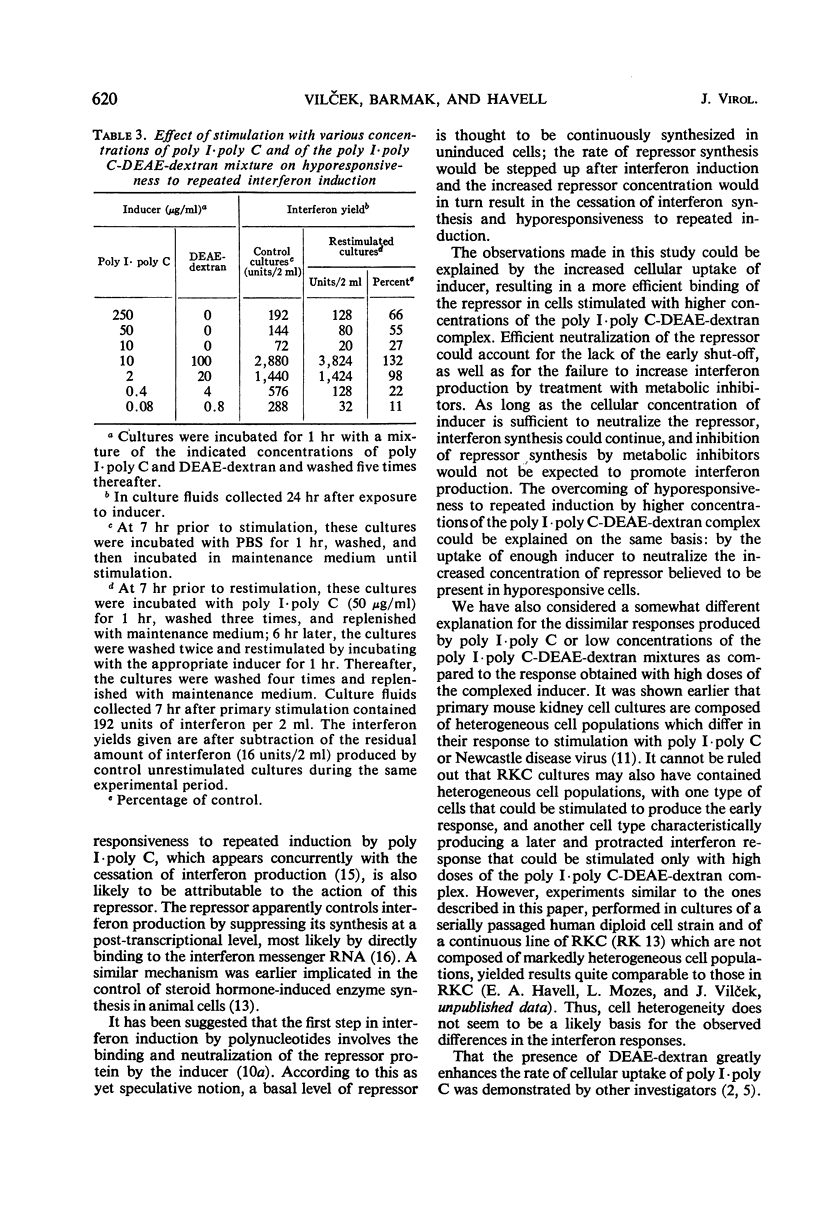
Interferon production in cultures of rabbit kidney cells (RKC) stimulated with 10 to 250 μg of polyinosinic-polycytidylic acid (poly I·poly C) per ml peaked at 3 to 4 hr after the exposure of cells to inducer and rapidly declined thereafter. On the other hand, RKC stimulated with poly I·poly C (10 or 2 μg/ml) in the presence of diethylaminoethyl (DEAE)-dextran (100 or 20 μg/ml, respectively) produced a protracted interferon response, with the release of interferon continuing for over 24 hr. The kinetics of interferon production in RKC stimulated with lower concentrations of the mixture of poly I·poly C and DEAE-dextran were similar to the response produced by poly I·poly C alone (10 to 250 μg/ml). Only the responses that terminated early were paradoxically enhanced by treatment with low doses of actinomycin D or with cycloheximide. Cells stimulated with 50 μg of poly I·poly C/ml showed hyporesponsiveness to a second interferon induction with poly I·poly C when restimulated 7 hr after primary induction. This hyporesponsiveness could be overcome by restimulating with higher concentrations of the poly I·poly C-DEAE-dextran complex. The results are compatible with the hypothesis that the early termination of interferon production and hyporesponsiveness to repeated induction with poly I·poly C are due to a cellular repressor exerting negative control on interferon synthesis, and that the increased cellular uptake of poly I·poly C in the presence of DEAE-dextran may effectively neutralize the repressor. These results also suggested that the often observed different kinetics and the varied effects of inhibitors of ribonucleic acid or protein synthesis on interferon responses in various cells and in cells stimulated with different inducers (such as with viruses as compared with polynucleotides) need not imply the existence of fundamentally different mechanisms of interferon production.
Full text
PDF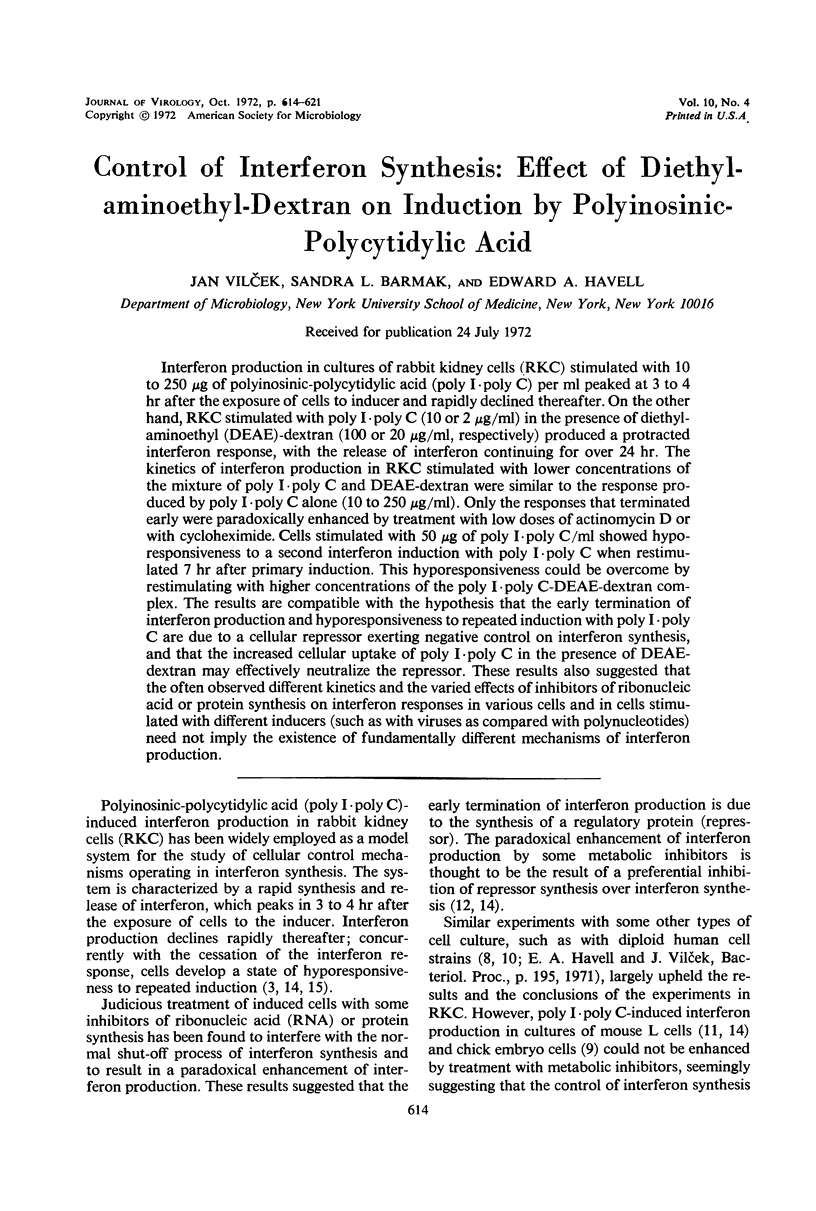


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausek G. H., Merigan T. C. Cell interaction with a synthetic polynucleotide and interferon production in vitro. Virology. 1969 Nov;39(3):491–498. doi: 10.1016/0042-6822(69)90097-x. [DOI] [PubMed] [Google Scholar]
- Billiau A. The refractory state after induction of interferon with double-stranded RNA. J Gen Virol. 1970 Jun;7(3):225–232. doi: 10.1099/0022-1317-7-3-225. [DOI] [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M. S., Bausek G. H., Merigan T. C. Interferon inducers in vitro: difference in sensitivity to inhbitiros of RNA and protein synthesis. Science. 1968 Aug 2;161(3840):465–468. doi: 10.1126/science.161.3840.465. [DOI] [PubMed] [Google Scholar]
- Ho M., Tan Y. H., Armstrong J. A. Accentuation of production of human interferon by metabolic inhibitors. Proc Soc Exp Biol Med. 1972 Jan;139(1):259–262. doi: 10.3181/00379727-139-36122. [DOI] [PubMed] [Google Scholar]
- Long W. F., Burke D. C. Interferon production by double-stranded RNA: a comparison of induction by reovirus to that by a synthetic double-stranded polynucleotide. J Gen Virol. 1971 Jul;12(1):1–11. doi: 10.1099/0022-1317-12-1-1. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Friedman R. M. Potentiation of human interferon production by superinduction. J Natl Cancer Inst. 1971 Oct;47(4):757–764. [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Distinguishing characteristics of the interferon responses of primary and continuous mouse cell cultures. J Gen Virol. 1971 Oct;13(1):35–50. doi: 10.1099/0022-1317-13-1-35. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ho M. Accentuation of interferon production by metabolic inhibitors and its dependence on protein synthesis. Virology. 1971 Jun;44(3):503–509. doi: 10.1016/0042-6822(71)90363-1. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H. Post-transcriptional control of interferon synthesis. J Virol. 1971 May;7(5):588–594. doi: 10.1128/jvi.7.5.588-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Interferon production in mice by double-stranded synthetic polynucleotides: induction or release? Virology. 1968 May;35(1):177–179. doi: 10.1016/0042-6822(68)90320-6. [DOI] [PubMed] [Google Scholar]


