Abstract
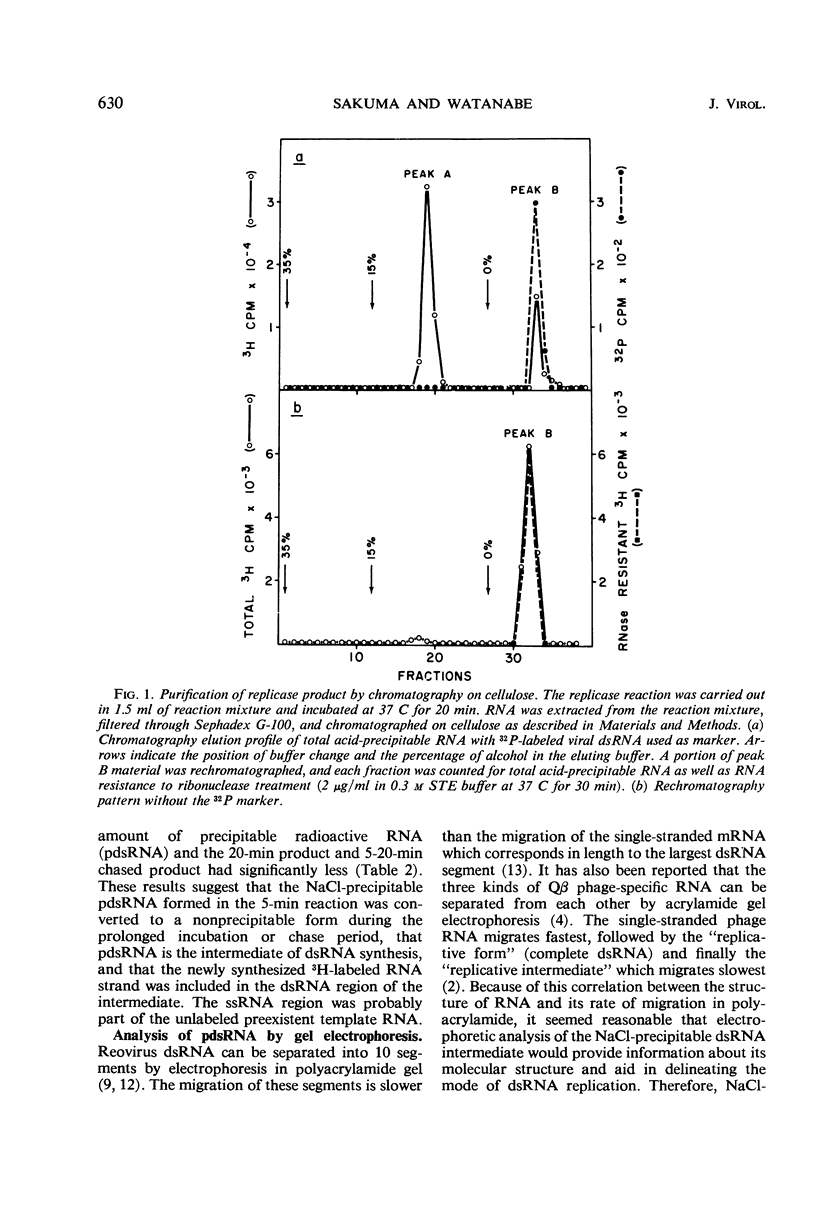
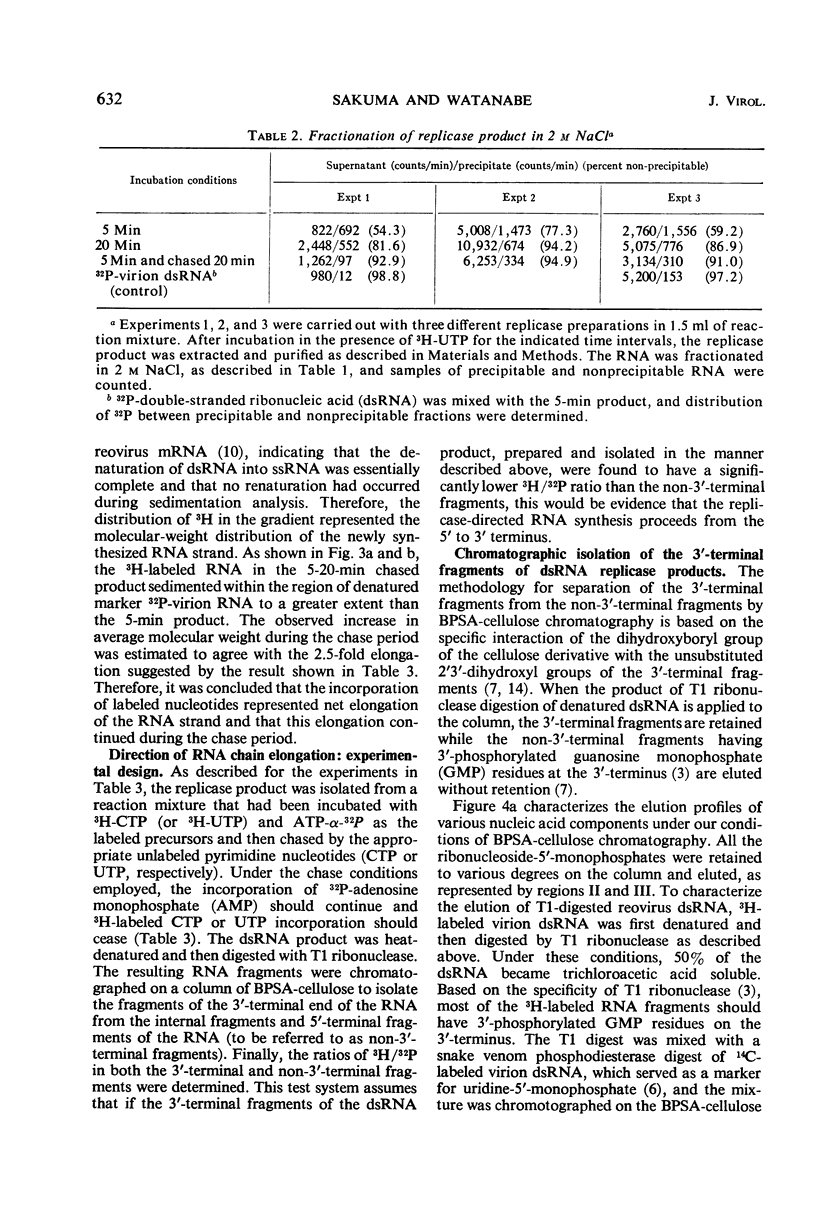
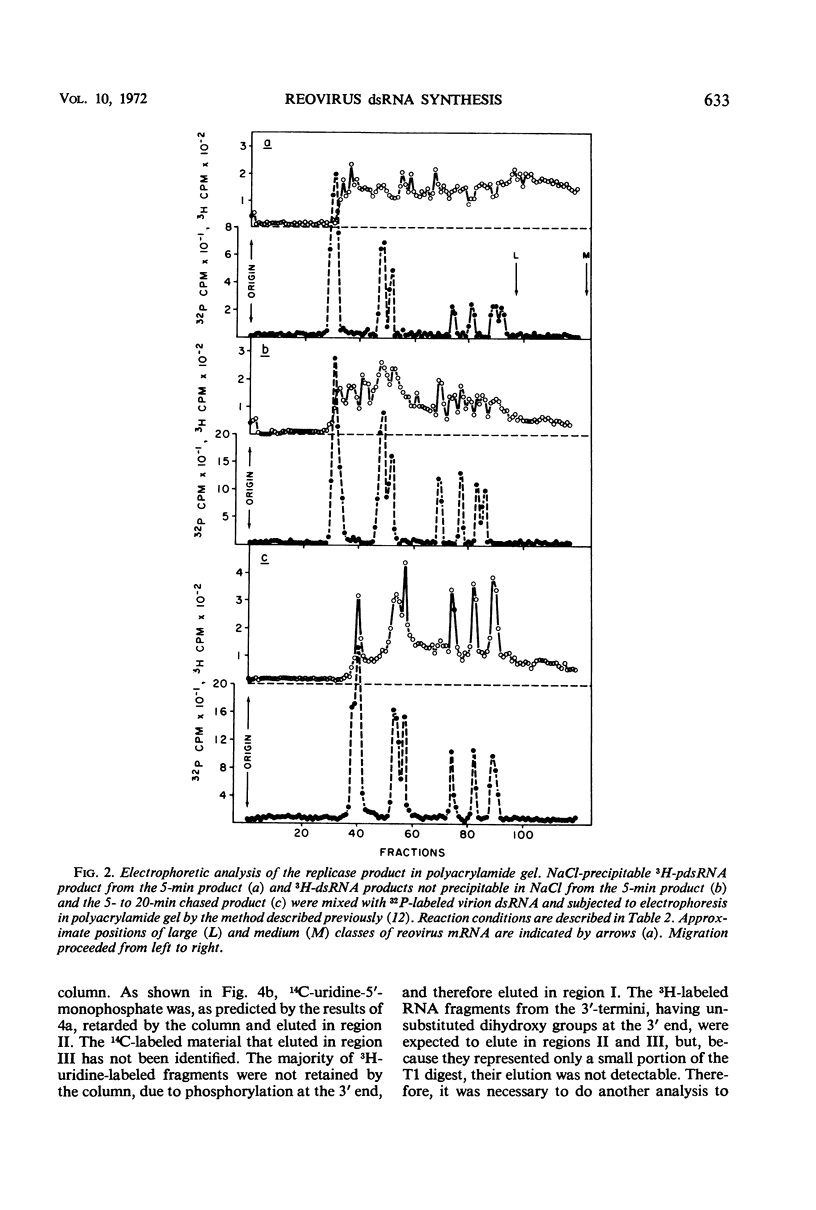
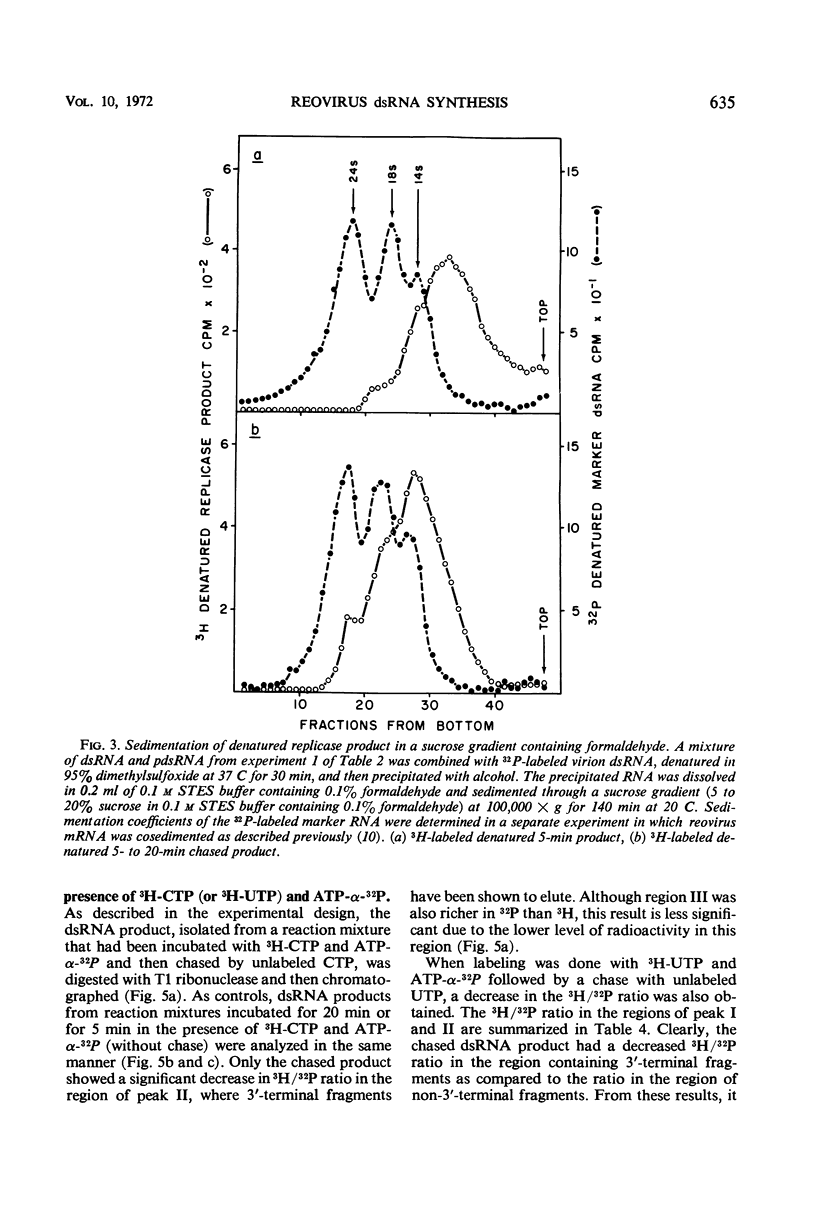
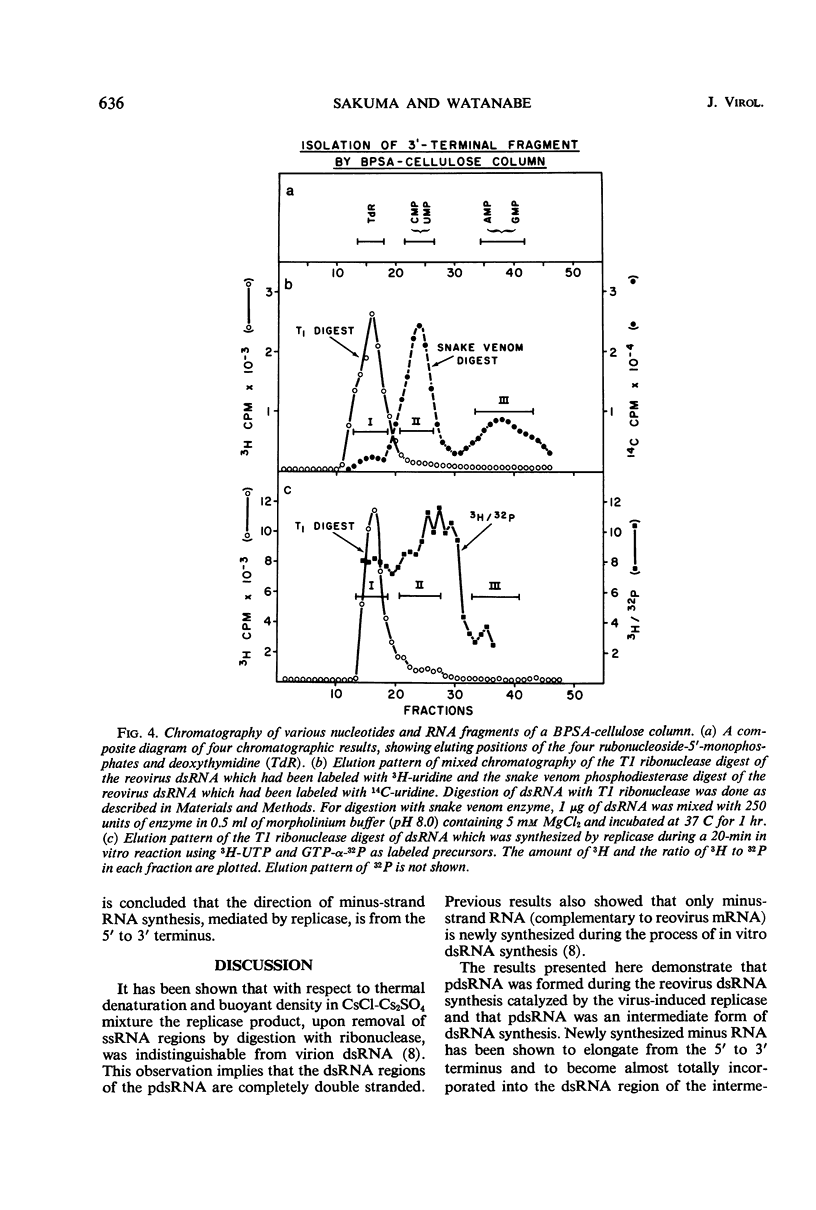
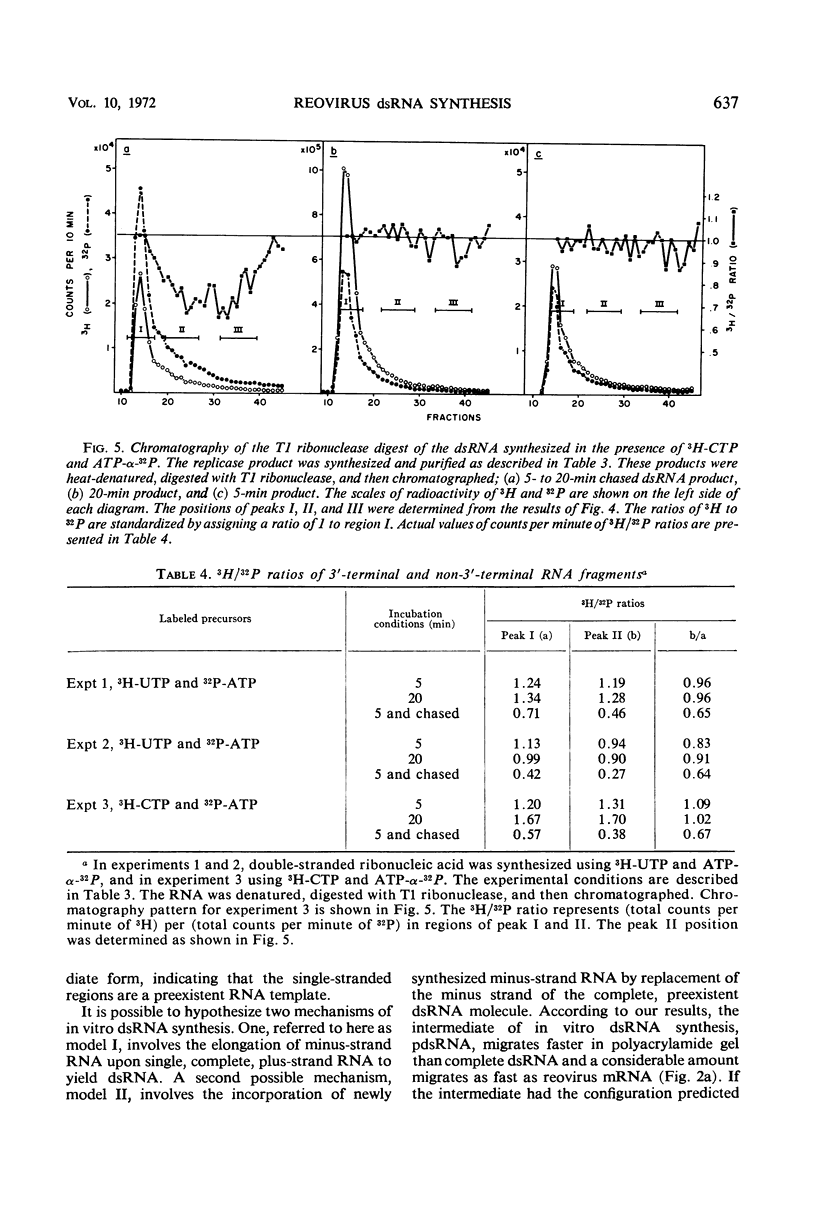
After the incubation of reovirus replicase reaction mixtures (containing labeled ribonucleoside triphosphates), partially double-stranded ribonucleic acid (pdsRNA) products were isolated by cellulose column chromatography followed by precipitation with 2 m NaCl. The pulse-labeled reaction product contained a significantly large amount of pdsRNA that became complete dsRNA as reaction time increased, indicating that pdsRNA was an intermediate of the replicase reaction. The newly synthesized RNA strand (3H-labeled) of the pdsRNA was resistant to ribonuclease digestion, suggesting that single-stranded RNA regions were part of a preexistent unlabeled RNA template. These observations, together with the electrophoretic behavior of the pdsRNA in polyacrylamide gel, are consistent with the hypothesis that dsRNA is synthesized by the elongation of a complementary RNA strand upon a preexistent template of single-stranded RNA (i.e., messenger RNA). The direction of the RNA strand elongation was determined by carrying out the replicase reaction in the presence of 3H-cytidine triphosphate (or 3H-uridine triphosphate) and adenine triphosphate-α-32P followed by a chase with excess unlabeled cytidine triphosphate (or uridine triphosphate). The dsRNA product was digested with T1 ribonuclease and the resulting 3′-terminal fragments were isolated by chromatography on a dihydroxyboryl derivative of cellulose. Examination of the ratio of 3H to 32P in these fragments indicated that RNA synthesis proceeded from the 5′ to 3′ terminus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Pace N. R., Spiegelman S. An analysis by gel electrophoresis of Q-beta-RNA complexes formed during the latent period of an in vitro synthesis. Proc Natl Acad Sci U S A. 1967 May;57(5):1474–1481. doi: 10.1073/pnas.57.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham P. T., Rosenberg M. The isolation of 3'-terminal polynucleotides from RNA molecules. Biochim Biophys Acta. 1971 Aug 26;246(2):337–340. doi: 10.1016/0005-2787(71)90143-2. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Watanabe Y., Graham A. F. Defective virions of reovirus. J Virol. 1970 Aug;6(2):226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Unilateral synthesis of reovirus double-stranded ribonucleic acid by a cell-free replicase system. J Virol. 1971 Aug;8(2):190–196. doi: 10.1128/jvi.8.2.190-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Gauntt C. J., Graham A. F. Reovirus-induced ribonucleic acid polymerase. J Virol. 1968 Sep;2(9):869–877. doi: 10.1128/jvi.2.9.869-877.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]


