Introduction
The myotubularin family of phosphatases (EC 3.1.3.48) comprises 16 members, 9 of which contain the canonical dual specificity protein tyrosine phosphatase active site CX5R motif (Robinson et al., 2006; Hnia et al., 2011). These phosphatases all theoretically catalyze the dephosphorylation of phosphatidylinositol 3-phosphate (PtdIns(3)P) and phosphatidylinositol-3,5-bisphosphate (PtdIns(3,5)P2) thereby forming phosphatidylinositol and phosphatidylinositol-5-phosphate (PtdIns(5)P) respectively. The other 7 members lack the CX5R motif and are not catalytically active. We initially isolated the PtdIns(3)P phosphatase (EC 3.1.3.64) from rat brain homogenates and found that it was present in two forms: a 65-kDa monomeric catalytic subunit and a dimer comprised of the catalytic subunit and an 85 kDa adaptor subunit initially designated as 3-PAP for 3-phosphatase adaptor protein (Caldwell et al., 1991; Nandurkar et al., 2001; Nandurkar et al., 2003). The gene encoding this phosphatase was isolated by positional cloning of the gene mutated in X-linked myotubular myopathy (Laporte et al., 1996). The gene was designated as MTM1. Subsequently a large family of related proteins has been found, and they are designated as myotubularin related proteins (MTMR1 to MTMR15) as shown in figure 1. The previously described 3-PAP is now designated as MTMR12. In addition to forming homodimers, the catalytically active proteins form heterodimers with the inactive myotubularins. In general the formation of heterodimers alters function by increasing activity or altering cellular localization (Kim et al., 2003; Robinson et al., 2005; Berger et al., 2006; Mruk et al., 2011). The formation of heterodimers is important as illustrated by the findings described concerning active MTMR2 and its inactive partner MTMR13. In this case mutations in either subunit cause a form of Charcot-Marie-Tooth disease (type 4B) (Azzedine et al., 2003). While all members catalyze the same reactions as shown in figure 1, they are not redundant (Laporte et al., 2003). Presumably, each controls a distinct pool of PtdIns(3)P or PtdIns(3,5)P2 within cells.
Figure 1.
Schematic representation of the myotubularin family of phosphatases.
In the current report we describe a closely related subfamily of active myotubularins MTMR6, MTMR7, and MTMR8 all of which partner with the same inactive subunit MTMR9. This situation is unique in that it appears that the three active subunits compete with each other for binding to MTMR9. Another unusual feature is that MTMR8 is expressed only in primates and thus importantly is absent in mice. We previously characterized the interaction of mouse MTMR7 and MTMR9 (Mochizuki et al., 2003; Dang et al., 2004) and showed that they associate both in vitro and in cells. We plan to characterize the interactions of the human proteins in future studies. Previous studies have shown an association between MTMR6 and MTMR9 in mouse and Caenorhabditis elegans (Mochizuki et al., 2003; Dang et al., 2004). Two studies implicate MTMR6 as a regulator of apoptosis. By RNA microarray analysis increased MTMR6 expression was observed in chronic lymphocytic leukemia cells with increased resistance to irradiation-induced apoptosis (Vallat et al., 2003) whereas RNAi of MTMR6 in HeLa cells promoted apoptosis (Mackeigan et al., 2005).
Results and Discussion
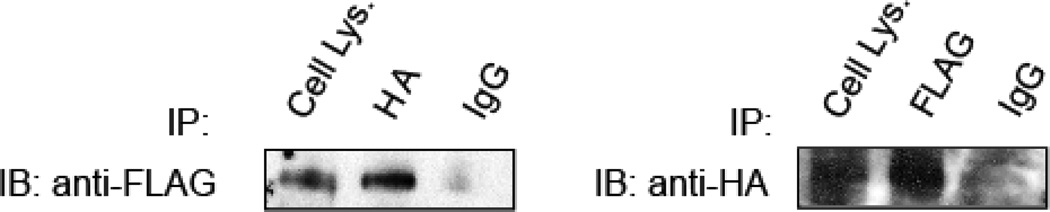
We cloned cDNAs encoding human MTMR6 and MTMR9 (Zou et al., 2009) and prepared FLAG and GST tagged constructs for protein expression. Purified GST-MTMR6 immobilized on glutathione agarose beads bound FLAG-MTMR9. In the converse experiment we showed that MTMR9-FLAG captured MTMR6. We expressed HA-MTMR6 and FLAG MTMR9 in HeLa cells to confirm the interaction in vivo. We immunoprecipitated the proteins from HeLa lysates using anti-FLAG and anti-HA antibodies and showed that the proteins were complexed in cells (Fig. 2). We performed immunofluorescence experiments, and demonstrated that the proteins co-localize and that complex formation does not alter cellular localization (Zou et al., 2009).
Figure 2.
Western blots of HeLa cell lysates from cells transfected with HA-MTMR6 and FLAG-MTMR9 blotted with anti-FLAG and anti-HA antibodies, respectively. Adapted from Zou et al., 2009.
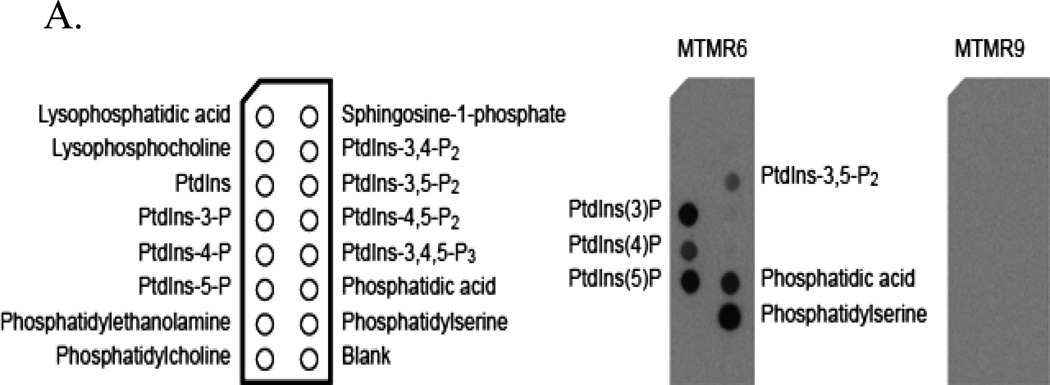
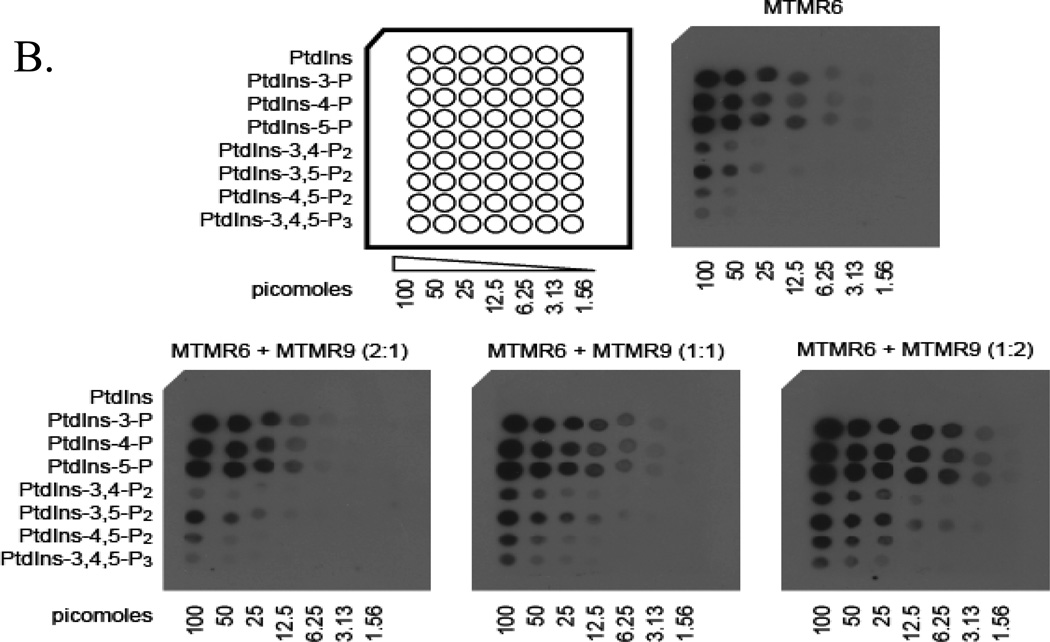
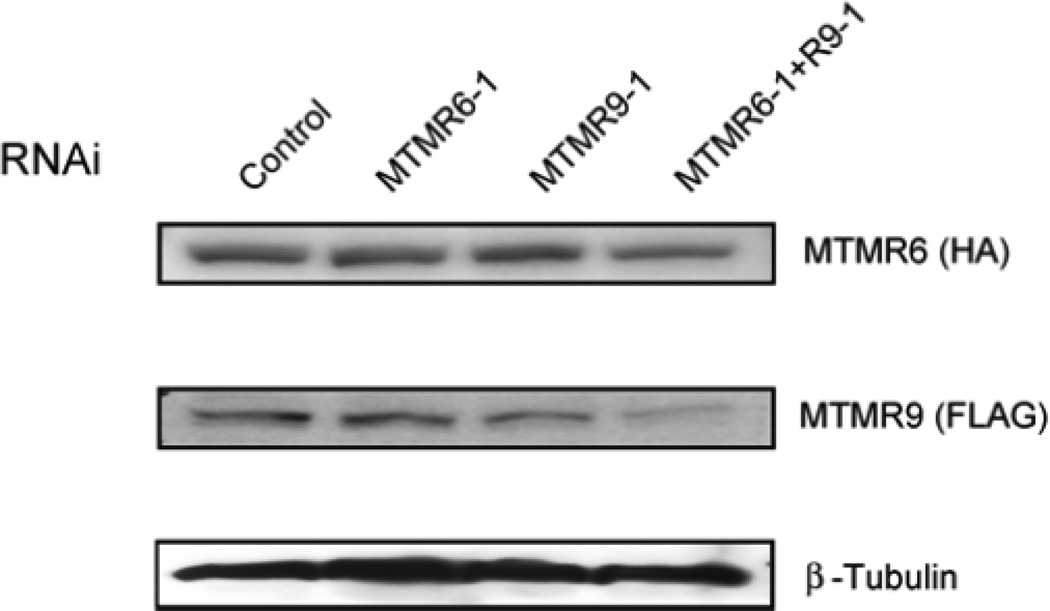
We next determined the effect of complex formation on catalytic activity using vesicles comprised of 50% phosphatidylcholine/ 50% phosphatidylserine with radiolabeled PtdIns [32P-3]P. Complex formation increased catalytic activity by 7-fold. We also found that two nonsubstrate lipids phosphatidylinositol 4-phosphate and phosphatidylinositol 5-phosphate further increased the activity of the complex (Zou et al., 2009). We next determined the effect of complex formation on lipid binding using protein-lipid overlay assays with PIP strips (Echelon Bioscience). In this experiment myotubularins were incubated with PIP strips spotted with 100 pmol of the indicated lipid and binding was detected by Western blotting the strips using specific antibodies. MTMR6 bound all monophosphorylated phosphatidylinositols as shown in figure 3A. It also bound bisphosphate phosphatidylinositols to a lesser extent. MTMR9 did not bind these lipids but complex formation between MTMR6 and MTMR9 greatly increased lipid binding to MTMR6 (Fig. 3B). This is most apparent in the case where MTMR9 was added in a 2:1 molar ratio to MTMR6. The most striking finding was that complex formation increased the stability of both binding partners. In these experiments cycloheximide was added at time zero to stop protein synthesis. The subsequent survival of proteins was measured by immunoblotting cell extracts using the antibodies indicated. Both proteins displayed prolonged survival when in a complex. RNAi experiments gave similar results: RNAi of either MTMR6 or MTMR9 when the proteins were not in a complex caused modest decreases in stability (Fig. 4) but when in a complex with RNAi of both proteins survival was decreased markedly.
Figure 3.
(A) Phospholipid binding of MTMR6 and MTMR9. Proteins were detected by Western blotting PIP strips. (B) The phospholipid binding of MTMR6 is increased by MTMR9. Reproduced from Zou et al., 2009.
Figure 4.
The stability of MTMR6 and MTMR9 is decreased upon RNAi of either MTMR6 or MTMR9. Cell extracts were Western blotted for MTMR6, MTMR9, or β-tubulin as a loading control.
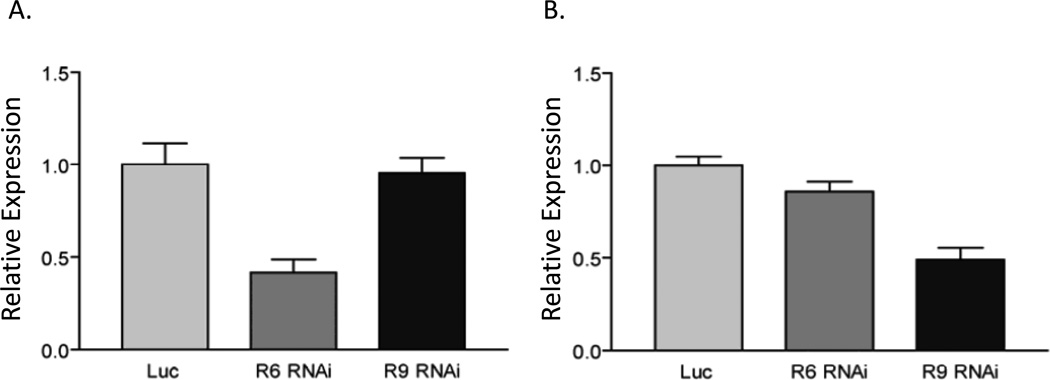
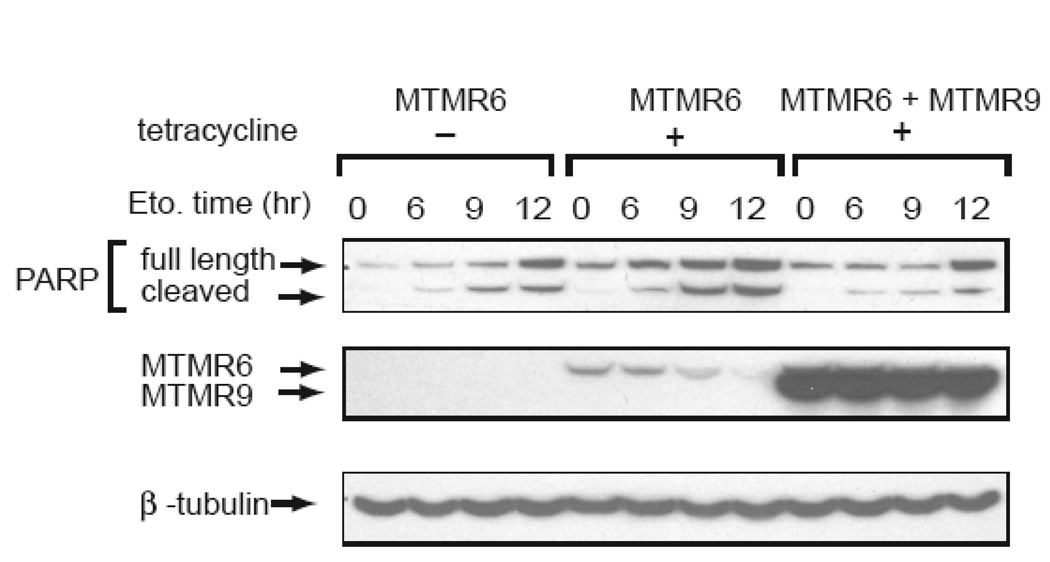
A study of inositol phosphatases that decrease apoptosis was carried out by performing RNAi of phosphatases in HeLa cells. Among those that inhibited apoptosis were MTMR6, MTMR7, and MTMR8 (Mackeigan et al., 2005). We measured apoptosis after etoposide treatment by FACS analysis of MTMR6 and the MTMR6/MTMR9 complex. Increased apoptosis is not seen in MTMR6 expressing cells but the degree of apoptosis is markedly increased in cells expressing the complex (Zou et al., 2009). As shown in figure 5, the RNAi is very specific for each protein. SiRNA of MTMR6 reduced the expression level of MTMR6 but not that of MTMR9, and siRNAi of MTMR9 did not affect the level of expression of MTMR6. We also tested whether or not overexpression of MTMR6 or MTMR6 plus MTMR9 could protect cells from apoptosis. Cells were treated with etoposide to induce apoptosis for 0, 6, 9, and 12 hours. As expected, we observed decreased poly(ADP-ribose) polymerase (PARP) cleavage when MTMR6 is overexpressed in the presence of excess MTMR9, indicating a protective role of the MTMR6/MTMR9 complex in apoptosis (Fig. 6).
Figure 5.
Expression of MTMR6 (A) or MTMR9 (B) in HeLa cells. HeLa cells were treated with SiRNA against luciferase (control), MTMR6, or MTMR9 for 24 hours. RNA from 1 × 106 cells was purified with TRIzol® plus (Invitrogen) and subjected to qRT-PCR. Data were normalized to GAPDH expression levels. Relative expression levels were compared with expression levels of MTMR6 or MTMR9 in the control cells.
Figure 6.
Etoposide treatment of HEK-293 cells – HEK-293 TRex cells stably transfected with MTMR6 or MTMR9 were grown to 90–95% confluence. For expression of both MTMR6 and MTMR9, the plasmid with MTMR6 construct was transiently transfected into MTMR9 stably transfected cells, and the MTMR9 construct was transiently transfected into MTMR6 stably transfected cells. Cells were grown for two days with or without 0.5 µg/ml of tetracycline. For induction of apoptosis, 100 µM etoposide was added to the media for the indicated times. Cells were harvested and sonicated briefly to disrupt all cell membranes. Particulate debris was removed and Samples (20 µg) were loaded onto SDS-PAGE gels and transferred for Western blotting.
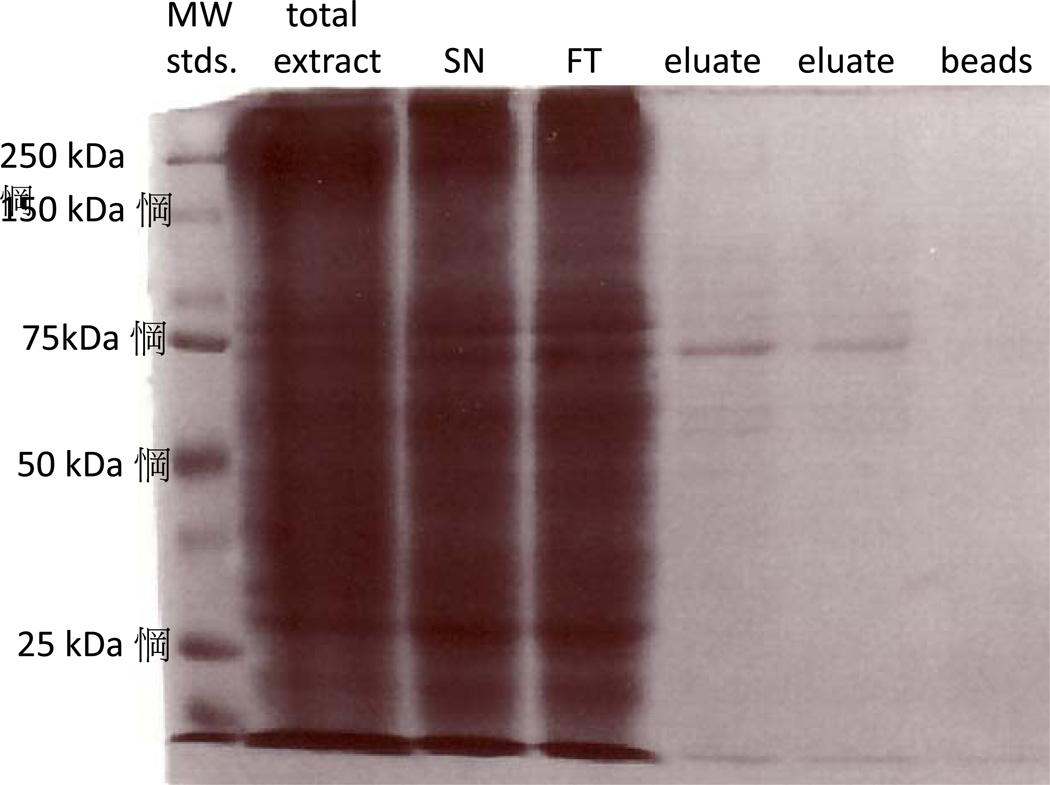
We next characterized the interactions of MTMR8 and MTMR9. Both proteins were purified for enzymatic studies. Purification of FLAG-tagged MTMR8 from Sf9 cells is shown in figure 7. Coomasse blue staining shows a single band in the FLAG peptide eluate. We prepared cDNAs encoding HA-tagged MTMR8 and FLAG-tagged MTMR9 for expression in HeLa cells. That these proteins are in a complex is illustrated by the fact that they co-immunoprecipitate (data not shown). We also determined the effect of complex formation on protein stability after treatment with cycloheximide to block further protein synthesis. As was the case for the MTMR6/MTMR9 complex, the formation of the MTMR8/MTMR9 complex also stabilized both proteins (data not shown).
Figure 7.
Purification of MTMR8 from Sf9 cells. FLAG-tagged MTMR8 was expressed in Sf9 cells in serum-free Sf900 medium (Invitrogen). Aliquots of the total cellular extract (total extract), the soluble fraction of the extract (SN), the flow through (FT), FLAG-peptide-eluted protein (eluate), and residual protein bound to the FLAG-agarose beads were subjected to SDS-PAGE and stained with coomassie.
We next studied the relative ability of these proteins to hydrolyze PtdIns(3)P vs PtdIns(3,5)P2. PtdIns(3)P was modestly cleaved by MTMR6 and complex formation with MTMR9 only increased activity by 1.7-fold. In contrast MTMR8 was much more active towards PtdIns(3)P and complex formation with MTMR9 further increased activity by 4.3-fold. When PtdIns(3,5)P2 was used as substrate, MTMR6 showed modest activity that was increased by 38-fold in the MTMR6/MTMR9 complex. In contrast MTMR8 and its complex with MTMR9 was much less active towards PtdIns(3,5)P2. When we measured cellular levels of PI5-P (Zou et al., 2007) we found that cells expressing MTMR6/MTMR9 display a large increase in PI(5)P while those expressing the MTMR8/MTMR9 complex do not, thus confirming the enzyme assay results in cells. In cells expressing the MTMR8/MTMR9 complex the level of PI(3)P was reduced as measured by HPLC, also confirming the enzyme assay data.
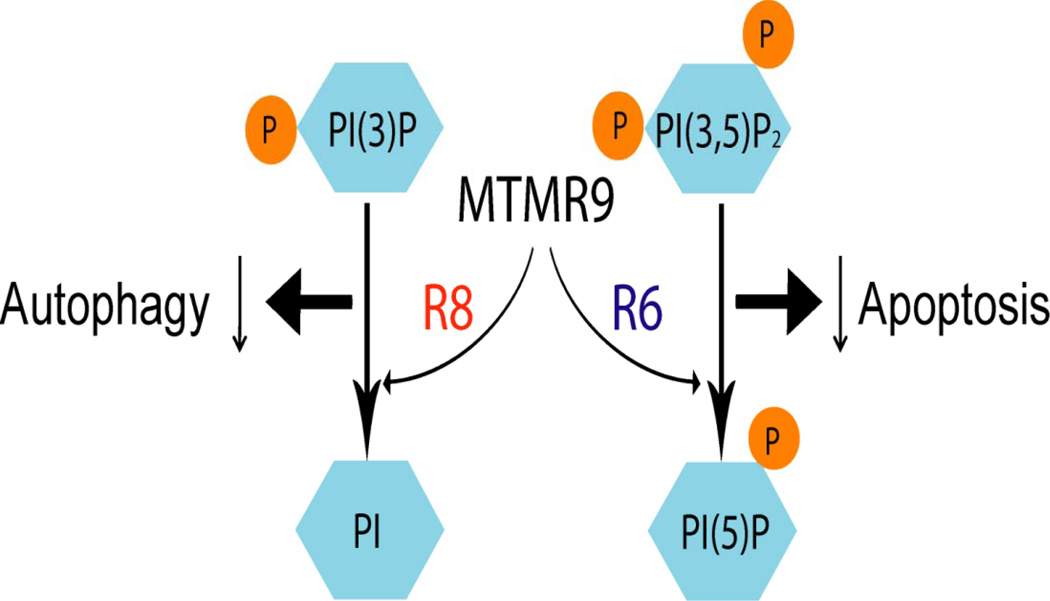
Since PtdIns(3)P is a critical factor in autophagosome formation we postulated that the MTMR8/MTMR9 complex would inhibit autophagy. This prediction was shown using degradation of p62 (data not shown). A protein designated as p62 is degraded during autophagy and thus in cells with reduced autophagy p62 levels are increased while when autophagy occurs levels of p62 decrease. Levels of p62 increased 2-fold when MTMR8/MTMR9 was expressed in cells whereas RNAi of these proteins caused a 50% decline in p62 levels (data not shown). Serum starvation induces autophagy. When HeLa cells expressing HA-MTMR8 and MTMR9 were placed in serum-free medium, the complex between these proteins was completely dissociated by 2 hours as determined by immunoprecipitation of MTMR9 followed by Western blotting of the immune precipitate with anti-HA. Therefore the different substrate preferences of these two MTM complexes leads to differing functions as illustrated in figure 8. The MTMR8/MTMR9 complex functions to reduce autophagy while the MTMR6/MTMR9 complex inhibits apoptosis.
Figure 8.
Proposed functions of MTMR8 plus MTMR9 (inhibits autophagy ) while MRMR6 plus MTMR9 (inhibits apoptosis).
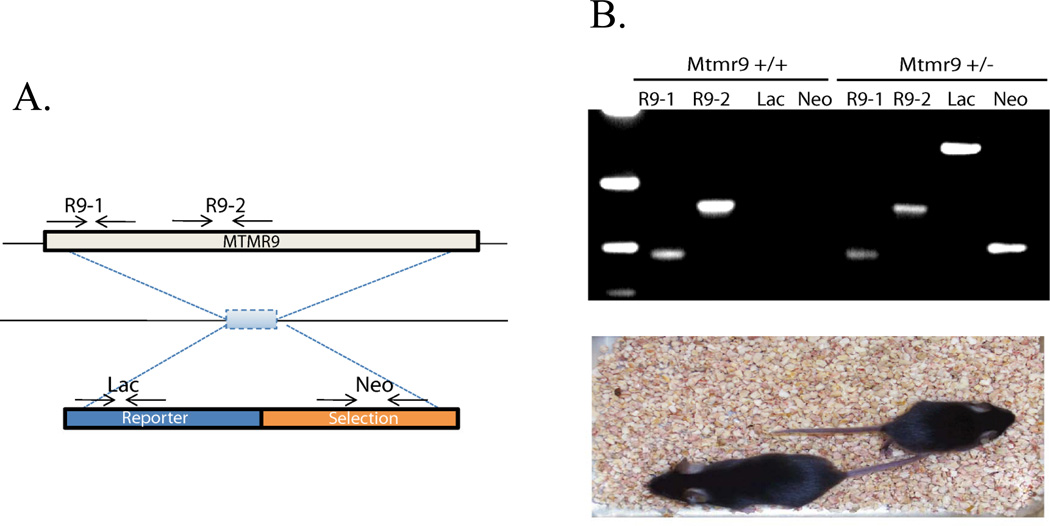
The MTMR6, MTMR7, and MTMR8 subfamily is unique in that all three active members compete for complex formation with the same inactive member MTMR9. Thus we created a mouse knockout of MTMR9 which would be predicted to interfere with the functions of both active members. We acquired a targeting construct from the KOMP mouse knockout project. This construct deletes the entire coding sequence of MTMR9 replacing it with a lacZ gene and neomycin resistance (neo) cassettes. We used Velocigene technology from Regeneron (Dechiara et al., 2010) to create MTMR9 chimeric mice. PCR genotyping of wild-type and heterozygous mice is shown in figure 9. PCR products reflecting the presence of the MTMR9 gene were detected in Mtmr9+/+ mice but no products were formed using primers for lacZ or neo in these mice. Mtmr9 heterozygotes gave the expected products using the Lac and Neo primers in addition to products using primers specific for Mtmr9. The heterozygous mice were much smaller than their wild-type littermates as is also seen in figure 9. Furthermore, these heterozygous animals displayed a highly penetrant phenotype with seizures, severe ataxia, and shivering behavior independent of locomotion. A high incidence of death occurs in the Mtmr9+/− mice within the first two weeks of life. Of the 311 mice born from mating the chimeric males, 167 died prior to being genotyped. Of those that survived long enough to be genotyped, 79 did not carry the gene trap and 65 did. Eight of these 65 animals confirmed as carrying the gene trap allele survived to adulthood. We postulate that the strong phenotype in heterozygotes results from the fact that all of these MTMR proteins are very unstable when not in a heterodimeric complex. Thus heterozygous animals suffer from near homozygous deficiency of this family of MTM proteins. An additional curious phenotype in these animals is a continuous growth of the teeth whereby the teeth must be pruned every few days in young animals and later weekly in order to prevent an inability to feed because of the overgrown teeth.
Figure 9.
Generation of MTMR9 knockout mice. (A) Diagram of the targeting construct which deletes the entire MTMR9 coding sequence. It contains a LacZ reporter gene and neomycin resistance cassette. The PCR primers used to genotype the animals are indicated by arrows. The R9-1 and R9-2 primers are used to detect the wild type MTMR9 allele, and the Lac and Neo primers are used to detect the presence of the knockout gene cassette. (B) PCR of wild type and an Mtmr9 heterozygote. The lower panel shows the small size of the heterozygous mouse compared to a wild type littermate.
The small size of the Mtmr9+/− animals suggests a possible eating defect that is intriguing since a form of familial obesity in Japan has been strongly linked to polymorphisms in the MTMR9 gene (Yanagiya et al., 2007). These authors also showed that mRNA levels for MTMR9 in rats that were starved were increased while the mRNA level in rats fed a high fat diet was decreased. In other preliminary studies we have shown that MTMR6 expression in brain appears to be mainly in glial cells rather than in neurons. On histological analysis, sciatic nerves of Mtmr9+/− mice appeared normal, so demyelination does not seem to account for the phenotype observed. In future studies we hope to breed the heterozygous mice in an effort to generate an Mtmr9 null mouse. We currently have 5 adult heterozygous mice (4 males and one female), which are being bred. It is possible that this goal may not be achieved as homozygosity may be incompatible with life.
Summary
We have investigated a previously little-studied subfamily of related active MTM proteins namely MTMR6, MTMR7, and MTMR8. All three of these proteins partner with the same inactive MTMR9 thus potentially creating competition. We find that complex formation in each case greatly stabilizes all of the proteins. In the absence of complex formation all of the proteins are markedly unstable. We examined the substrate specificity of the proteins and found that the MTMR8/MTMR9 complex prefers PtdIns(3)P as substrate while the MTMR6/MTMR9 complex prefers PtdIns(3,5)P2. In view of this difference the two complexes serve different functions in cells. The MTMR8/MTMR9 complex serves to inhibit autophagy while the MTMR6/MTMR9 complex inhibits apoptosis. We have also created a mouse heterozygous for Mtmr9 that displays a very strong phenotype with small size, ataxia, seizures, unrestrained growth of the teeth, and in most animals premature death. The heterozygous mice express markedly reduced levels of all of these MTM proteins presumably reflecting the instability of the monomeric proteins. This instability results in levels of the proteins that are much less than that expected in heterozygous animals.
Acknowledgments
The authors would like to thank Deborah Laflamme for technical assistance. This work was supported by grants from the National Institutes of Health (HL-16634-43 to P.W.M.; DK075618, to D.B.W.; and DK52574, to the Histology Core Facility) and the Children’s Discovery Institute (MD-II-2009-174, to M.P.W.).
References
- Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–1153. doi: 10.1086/375034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, Berger I, Schaffitzel C, Tersar K, Volkmer B, Suter U. Multi-level regulation of myotubularin-related protein-2 phosphatase activity by myotubularin-related protein-13/set-binding factor-2. Hum Mol Genet. 2006;15:569–579. doi: 10.1093/hmg/ddi473. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Lips DL, Bansal VS, Majerus PW. Isolation and characterization of two 3-phosphatases that hydrolyze both phosphatidylinositol 3-phosphate and inositol 1,3-bisphosphate. J Biol Chem. 1991;266:18378–18386. [PubMed] [Google Scholar]
- Dang H, Li Z, Skolnik EY, Fares H. Disease-related myotubularins function in endocytic traffic in Caenorhabditis elegans. Mol Biol Cell. 2004;15:189–196. doi: 10.1091/mbc.E03-08-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Poueymirou WT, Auerbach W, Frendewey D, Yancopoulos GD, Valenzuela DM. Producing fully ES cell-derived mice from eight-cell stage embryo injections. Methods Enzymol. 2010;476:285–294. doi: 10.1016/S0076-6879(10)76016-X. [DOI] [PubMed] [Google Scholar]
- Hnia K, Kretz C, Amoasii L, Bohm J, Liu X, Messaddeq N, Qu CK, Laporte J. Primary T-tubule and autophagy defects in the phosphoinositide phosphatase Jumpy/MTMR14 knockout mice muscle. Adv Enzyme Regul. 2011 doi: 10.1016/j.advenzreg.2011.09.007. (in press). [DOI] [PubMed] [Google Scholar]
- Kim SA, Vacratsis PO, Firestein R, Cleary ML, Dixon JE. Regulation of myotubularinrelated (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc Natl Acad Sci U S A. 2003;100:4492–4497. doi: 10.1073/pnas.0431052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte J, Bedez F, Bolino A, Mandel JL. Myotubularins, a large disease-associated family of cooperating catalytically active and inactive phosphoinositides phosphatases. Hum Mol Genet. 2003;12(Spec No 2):R285–R292. doi: 10.1093/hmg/ddg273. [DOI] [PubMed] [Google Scholar]
- Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13:175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7:591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Majerus PW. Characterization of myotubularin-related protein 7 and its binding partner, myotubularin-related protein 9. Proc Natl Acad Sci U S A. 2003;100:9768–9773. doi: 10.1073/pnas.1333958100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. The myotubularin family of lipid phosphatases in disease and in spermatogenesis. Biochem J. 2011;433:253–262. doi: 10.1042/BJ20101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandurkar HH, Caldwell KK, Whisstock JC, Layton MJ, Gaudet EA, Norris FA, Majerus PW, Mitchell CA. Characterization of an adapter subunit to a phosphatidylinositol (3)P 3-phosphatase: identification of a myotubularin-related protein lacking catalytic activity. Proc Natl Acad Sci U S A. 2001;98:9499–9504. doi: 10.1073/pnas.171306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandurkar HH, Layton M, Laporte J, Selan C, Corcoran L, Caldwell KK, Mochizuki Y, Majerus PW, Mitchell CA. Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein; 3-PAP. Proc Natl Acad Sci U S A. 2003;100:8660–8665. doi: 10.1073/pnas.1033097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 2006;16:403–412. doi: 10.1016/j.tcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Robinson FL, Dixon JE. The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem. 2005;280:31699–31707. doi: 10.1074/jbc.M505159200. [DOI] [PubMed] [Google Scholar]
- Vallat L, Magdelenat H, Merle-Beral H, Masdehors P, Potocki de Montalk G, Davi F, Kruhoffer M, Sabatier L, Orntoft TF, Delic J. The resistance of B-CLL cells to DNA damage-induced apoptosis defined by DNA microarrays. Blood. 2003;101:4598–4606. doi: 10.1182/blood-2002-06-1743. [DOI] [PubMed] [Google Scholar]
- Yanagiya T, Tanabe A, Iida A, Saito S, Sekine A, Takahashi A, Tsunoda T, Kamohara S, Nakata Y, Kotani K, Komatsu R, Itoh N, Mineo I, Wada J, Masuzaki H, Yoneda M, Nakajima A, Miyazaki S, Tokunaga K, Kawamoto M, Funahashi T, Hamaguchi K, Tanaka K, Yamada K, Hanafusa T, Oikawa S, Yoshimatsu H, Nakao K, Sakata T, Matsuzawa Y, Kamatani N, Nakamura Y, Hotta K. Association of single-nucleotide polymorphisms in MTMR9 gene with obesity. Hum Mol Genet. 2007;16:3017–3026. doi: 10.1093/hmg/ddm260. [DOI] [PubMed] [Google Scholar]
- Zou J, Chang SC, Marjanovic J, Majerus PW. MTMR9 increases MTMR6 enzyme activity, stability, and role in apoptosis. J Biol Chem. 2009;284:2064–2071. doi: 10.1074/jbc.M804292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Marjanovic J, Kisseleva MV, Wilson M, Majerus PW. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc Natl Acad Sci U S A. 2007;104:16834–16839. doi: 10.1073/pnas.0708189104. [DOI] [PMC free article] [PubMed] [Google Scholar]