Summary
The regulatory networks of the DNA damage response (DDR) encompass many proteins and posttranslational modifications. Here, we use mass spectrometry-based proteomics to analyze the systems-wide response to DNA damage by parallel quantification of the DDR-regulated phosphoproteome, acetylome and proteome. We show that phosphorylation-dependent signaling networks are regulated more strongly compared to acetylation. Among the phosphorylated proteins identified are many putative substrates of DNA-PK, ATM and ATR kinases, but a majority of phosphorylated proteins do not share the ATM/ATR/DNA-PK target consensus, suggesting an important role of downstream kinases in amplifying DDR signals. We show that the splicing-regulator phosphatase PPM1G is recruited to sites of DNA damage, while the splicing-associated protein THRAP3 is excluded from these regions. Moreover, THRAP3 depletion causes cellular hypersensitivity to DNA damaging agents, thus suggesting an important link between RNA metabolism and DNA repair. Our results broaden the knowledge of DNA damage signaling networks and identify novel components of the DDR.
Introduction
Cells are constantly exposed to external as well as internal insults that threaten the integrity of their genomes. In response to environmental and chemical genotoxic stress, they evoke an elaborate cellular response collectively known as the DNA damage response (DDR) to safeguard their genetic information (Ciccia and Elledge, 2010; Jackson and Bartek, 2009). Failure to repair damaged DNA can have dangerous, even fatal consequences to the cell or organism, and genomic instability can lead to neurodegenerative diseases, immune deficiency, cancer and premature aging (Ciccia and Elledge, 2010; Hoeijmakers, 2009; Jackson and Bartek, 2009). Chemical inhibitors of several enzymes involved in the DDR are currently under clinical investigation in the treatment of cancer (Jorgensen, 2009; Nitiss, 2009).
Phosphorylation of serines, threonines and tyrosines as well as acetylation of lysines are highly conserved posttranslational modifications (PTMs) that are involved in regulating many cellular processes, including the DDR. Advances in mass spectrometry (MS)-based quantitative proteomics now allow identification and quantification of many PTM sites, making systems-wide signaling analysis possible (Bodenmiller and Aebersold, 2011; Choudhary and Mann, 2010). However, nearly all large-scale PTM analyses performed to date have investigated only one type of PTM in response to a specific perturbation.
Here, we employ high-resolution MS to obtain a detailed picture of the systems response to DNA damage at the level of the phosphoproteome, acetylome and proteome, and identify hundreds of nuclear and non-nuclear DDR-regulated modification sites that were previously not implicated in DDR processes. We show that the deubiquitylase CYLD functions in the DDR to control NF-κB activation. Moreover, we demonstrate DDR-induced phosphorylation of RNA processing factors PPM1G and THRAP3, show that these proteins are recruited to and excluded from sites of DNA damage, respectively, and that THRAP3 promotes resistance to DNA damaging agents.
Results
Strategy for parallel quantification of DDR-regulated phosphoproteome, acetylome and proteome
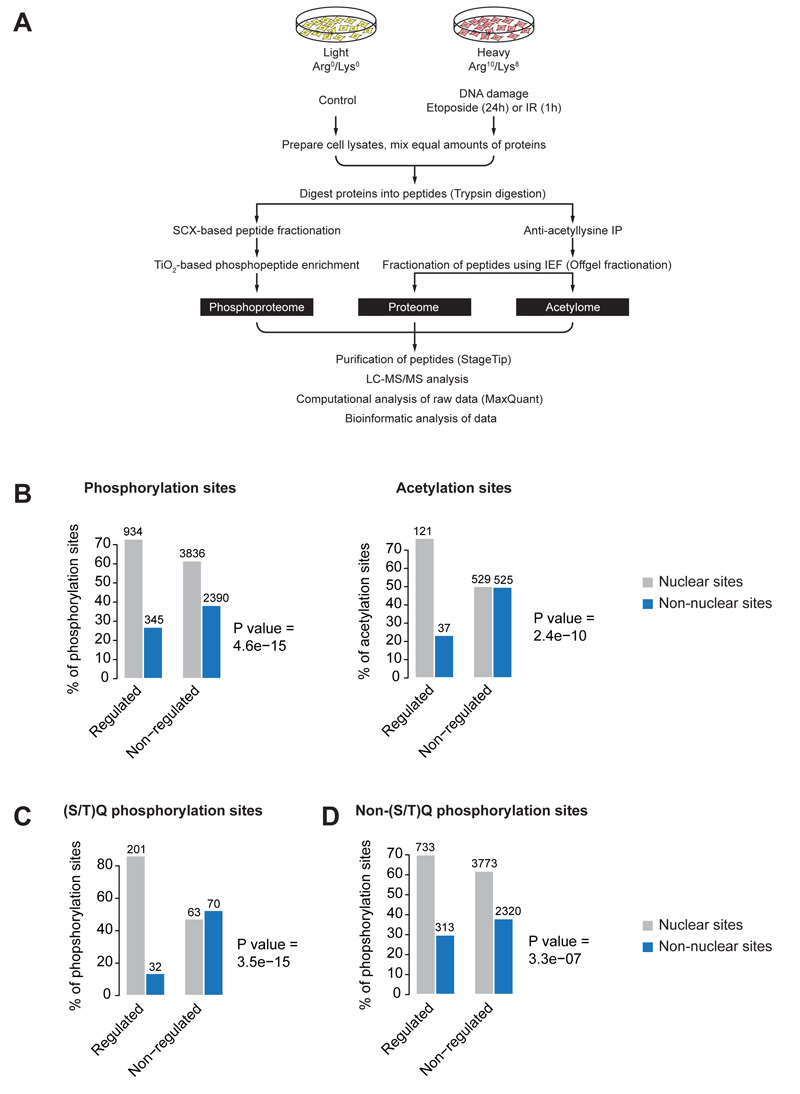
We combined high-resolution MS with stable isotope labeling with amino acids in cell culture (SILAC) (Ong et al., 2002) to quantify DNA damage-regulated changes of phosphoproteome, acetylome and proteome in human osteosarcoma (U2OS) cells. The “heavy” SILAC-labeled cell population was treated with etoposide for 24 hours (Fig. 1A), a topoisomerase II inhibitor that primarily causes double-strand DNA breaks (DSB) (Ross et al., 1984), while the “light” SILAC-labeled cell population was treated with DMSO and served as a control. Long-term treatment of cells with etoposide causes cell cycle arrest, which could also affect the PTM levels. Therefore, in parallel, we also analyzed changes in phosphorylation and acetylation in cells exposed to ionizing radiation (IR), at one hour after treatment (Fig. 1A). Equal amounts of proteins from the two SILAC-labeled cell populations were mixed and proteolysed using trypsin. Peptides were divided into two portions, one used for phosphoproteome measurement, the other used for acetylome and proteome analysis (Fig. 1A). Protein phosphorylation and acetylation were analyzed as described previously (Choudhary et al., 2009; Larsen et al., 2005; Olsen et al., 2006). Non-modified SILAC peptide pairs identified in the anti-acetyllysine immunoprecipitates were used to quantify relative protein abundance changes. All samples were analyzed on high resolution LTQ Orbitrap mass spectrometer, and data were processed with MaxQuant (Cox and Mann, 2008) at a fixed false-discovery rate (FDR) of 1%.
Figure 1.
Proteome-wide investigation of the DNA damage response. (A) Schematic presentation of the SILAC-based quantitative strategy. Phosphoproteome, acetylome and proteome were measured from SILAC-labeled U2OS cells treated with DNA damage inducing agents as outlined. (B) Cellular distribution of DDR-regulated phosphorylation and acetylation sites. The DDR-upregulated sites are significantly more localized to the nucleus compared to non-regulated sites. (C and D) Cellular distribution of DNA damage-upregulated phosphorylation sites that do or do not contain the S/TQ motif. The bar plots show nuclear or non-nuclear distribution of upregulated sites compared to non-regulated sites. p-values were calculated using Fisher exact test. (See also Figure S1)
Identification of systems-wide responses to DNA damage
We quantified 11,509 phosphorylation sites in cells treated with IR and 11,540 phosphorylation sites in etoposide-treated cells that were localized to a specific amino acid within the modified peptide sequence with high confidence (site-specific localization probability >75%; mean 0.95; Table S1). In addition, we quantified 1,848 acetylation sites, of which 1,796 were localized to a particular lysine with a site-specific localization probability >90% in cells treated with etoposide for 24 hours (Table S2). In parallel, we determined the relative protein abundances for 5,491 proteins (Table S3). We obtained good correlations between two independent biological experiments from cells treated with etoposide for phosphoproteome, acetylome and proteome (Fig. S1A).
In these experiments, only about 1% (53 out of 5,491) of quantified proteins were upregulated more than 2-fold, and about 3.5% (200 out of 5,491) were upregulated over 1.5-fold. This group of proteins contains well-known targets of the key tumor suppressor and DNA damage-activated transcription factor p53 (Lakin and Jackson, 1999), such as Sestrin-1, CDKN1A, CKAP2 and P53R2 (Table S3). Phosphorylation of about 8% of the modified sites was upregulated upon DNA damage (Table S1). These results show that the DDR is regulated more at the posttranslational level than by alterations in overall protein expression levels.
To determine phosphorylation sites that are robustly regulated by DNA damage, we applied a statistical cut-off by using SILAC ratios of non-phosphorylated peptides, which were identified as background in our phosphorylation experiments, and considered as non-regulated. We plotted the logarithmized SILAC ratios of non-phosphorylated peptides and determined the ratio corresponding to the 99% quantile (corresponding to a fold change of 1.85 for etoposide and 1.64 for IR; Fig. S1B). We only considered phosphorylation sites with over 2-fold increase as upregulated. Applying these statistical filters, we extracted 1,470 high confidence phosphorylation sites (combined from etoposide and IR experiments) that were upregulated after DNA damage (Table S1).
Changes in acetylation were overall less pronounced than those observed for phosphorylation. Therefore, a less strict cut-off (95% quantile of the ratio distribution of non-acetylated peptides, corresponding to a 1.42-fold change) was used for identifying DDR-regulated acetylations, and we considered sites with a 1.5-fold or more increase in acetylation as upregulated (Fig. S1B). Even with these relaxed criteria, only few sites showed increased acetylation within one hour after IR treatment, suggesting a slower kinetics of this modification compared to phosphorylation. Long-term treatment (24 hours) of cells with etoposide increased the acetylation of several proteins (Table S2), including MDC1, TIP49, p300, WRNIP1, RAD51AP1, TRIM28 and PCNA, which are known to be involved in the DDR. Several other proteins, including CCDC82 and SFRS2IP, whose role in the DDR is not yet defined were regulated both by acetylation and phosphorylation in response to DNA damage (Table S5).
We found that proteins exhibiting increased phosphorylation or acetylation upon DNA damage are significantly overrepresented in nuclear compartments as compared to proteins whose phosphorylation or acetylation is not increased after this perturbation (p=4.6e-15 for phosphorylation, and p=2.4e-10 for acetylation) (Fig. 1B). In particular, proteins containing DNA damage-induced phosphorylation sites that match the ATM/ATR/DNA-PK target consensus motif Ser/Thr-Gln (S/TQ) are predominantly nuclear, in agreement with the well-known role of these kinases in the nucleus after DNA damage (Fig. 1C; discussed further below). Nevertheless, a considerable fraction of proteins with DDR-dependent modifications, in particular phosphorylated proteins, are localized outside the nucleus (Fig. 1D), implying that phosphorylation-dependent DNA damage signaling also impacts on various non-nuclear cellular functions.
To further understand the global consequences of the DDR at a systems level, we performed Gene Ontology (GO) enrichment analysis (Fig. S2, Table S4). In the DNA damage-regulated phosphoproteome, GO terms “DNA metabolic process”, “DNA replication” and “double-strand break repair” were significantly overrepresented (Fig. S2A). By contrast, GO enrichment analysis of the proteome data indicated significant enrichment of terms related to cell cycle progression (Fig. S2B). Collectively, our proteomic screen demonstrates that all three modes of regulation – phosphorylation, acetylation and protein expression changes – act in a coordinated manner to establish the cellular DDR.
Analysis of DNA damage-induced phosphorylations
Activation of DNA damage signaling is initiated by the phosphatidylinositol 3-kinase related kinases (PIKKs) ATM, ATR and DNA-PK, which directly phosphorylate many substrates (Matsuoka et al., 2007). Activation of their downstream kinases triggers further phosphorylations of other proteins that are not direct PIKK substrates. Among the upregulated phosphorylation sites in our dataset, 265 sites occur at the specific ATM/ATR/DNA-PK consensus motif (S/TQ), and many of these are present on well-known DDR signaling components (Table S1). Notably, while some of these sites correspond to previously identified DNA damage-induced S/TQ target phosphorylations (Bennetzen et al., 2010; Bensimon et al., 2010; Matsuoka et al., 2007), about half of them have not previously been implicated in DNA damage signaling. Interestingly, a majority of DDR-regulated sites do not conform to S/TQ motifs, suggesting that they are phosphorylated by kinases acting downstream or independently of the PIKKs. In addition to detecting increased phosphorylation of well-known kinases involved in DDR signaling such as ATM, ATR, DNA-PK, CHK1 and CHK2, we identified DDR-regulated phosphorylations on other kinases such as p38MAPK, OXSR1 and BUB1. These latter three kinases are phosphorylated on SQ motifs, and are therefore likely direct ATM/ATR/DNA-PK targets.
Sequence properties of DDR-upregulated phosphorylation sites
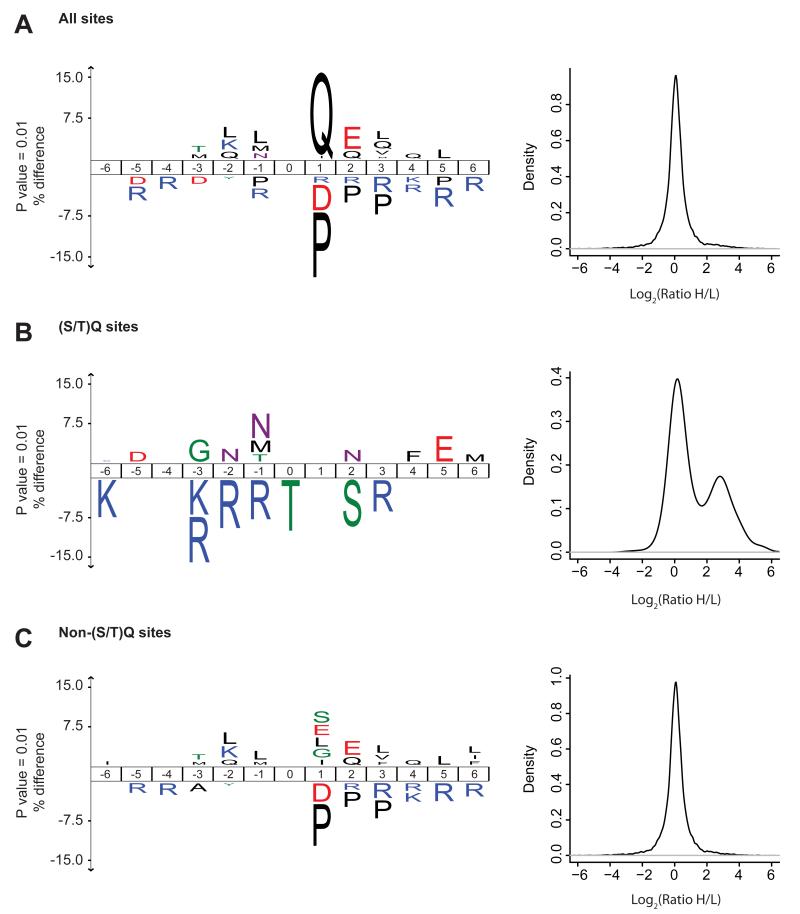
To gain insights into the sequence properties of the DDR-regulated phosphoproteome, we compared the amino acid sequences of phosphorylation sites that are upregulated upon DNA damage with non-regulated phosphorylation sites. In agreement with known roles of PIKKs in the DDR, these analyses showed significant overrepresentation of Gln (Q) at position +1 in sites showing increased phosphorylation (Fig. 2A). Indeed, we identified 607 phosphorylation sites with S/TQ motif (Fig. S3A, Table S1), and 44% of these sites showed a significant increase in phosphorylation after DNA damage (Table S1). To investigate whether the ATM/ATR/DNA-PK kinases might have additional preferences for amino acids flanking the target phosphorylation sites, we compared sequences surrounding the DDR-upregulated S/TQ phosphorylation sites alongside non-regulated sites with this motif. These data showed that positively charged Arg (R) in positions −1, −2, and −3 are underrepresented (Fig. 2B). We also analyzed the sequence properties of DDR-induced non-S/TQ sites, which are likely to be phosphorylated by kinases functioning downstream of ATM/ATR. Notably, these sites disfavored Pro (P) at position +1 (Fig. 2C). We also found many proteins that are dephosphorylated after DNA damage. Sequence motif analysis of these proteins revealed a significant enrichment of S/TP motif suggesting that phosphorylation of cyclin-dependent kinase and MAP kinase substrates is reduced after DNA damage (Bennetzen et al., 2010) (Fig. S3B).
Figure 2.
Sequence properties of DNA damage-upregulated phosphorylation sites. (A) The ice-Logo plot shows frequency of 6 amino acids flanking each side of phosphorylated residue. The amino acid sequences of DDR-regulated sites (both from etoposide and IR) were compared with the phosphorylation sites that do not change under these conditions. The numbers in the middle indicate the position of amino acid in peptide relative to the central, phosphorylated amino acid. Amino acids that are more frequently observed near the DDR-upregulated sites are indicated over the middle line, whereas the amino acids with lower frequency at these positions are indicated below the line. A significantly increased phosphorylation of sites conforming to the ATM/ATR/DNA-PK kinase motif (S/TQ) is observed upon DDR. The corresponding plot (right panel) shows distribution of SILAC ratios for phosphorylation sites conforming to the indicated phosphorylation motif. (B) DDR-regulated S/TQ sites disfavor arginine at positions preceding the phosphorylation sites. (C) Amino acid preference for non-S/TQ sites upregulated by DNA damage. (See also Figure S2 and S3)
DNA damage regulates multiple functional protein modules
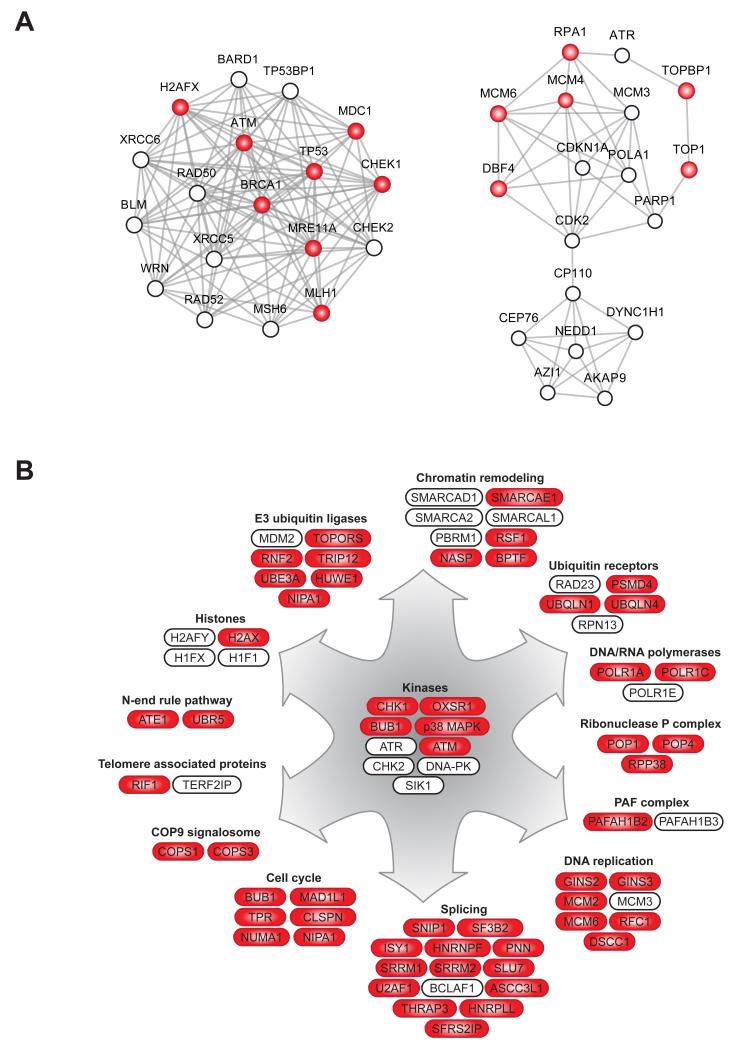
Network analysis of all proteins containing DDR-upregulated phosphorylation sites with the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (Szklarczyk et al., 2011) revealed that many of them are connected in tight interaction networks. A number of these proteins are well-known DDR components, providing independent evidence of the high quality of our quantitative mass spectrometric data (Fig. 3A). Nevertheless, a substantial proportion of DDR-regulated phosphoproteins do not show such connectivity, and their functional interactions in the DDR remain to be defined (Fig. S4).
Figure 3.
Functional modules of DDR-regulated phosphoroteins. (A) Network analysis of DDR-upregulated phosphoproteins using STRING (Szklarczyk et al.) showing connectivity among the proteins with increased phosphorylation upon DNA damage (STRING confidence score 0.7). Two of the highly interconnected subnetworks identified by MCODE are shown. (B) Schematic overview of cellular processes and involved proteins that show increased phosphorylation after DNA damage. Proteins with increased phosphorylation on S/TQ sites are colored in red whereas proteins without color show increased phosphorylation on non-S/TQ sites. (See also Figure S4)
In many cases, we found that multiple subunits of well-characterized protein complexes were phosphorylated upon DNA damage, many of them on S/TQ motifs (Fig. 3B). Such complexes include subunits of the Ribonuclease P complex (POP1, POP4 and RPP38) with a known role in RNA processing, but not previously involved in the DDR. Another example is several ubiquitin receptors such as UBQLN1, UBQLN4, RAD23 and PSMD4, and of these only a role for RAD23 in the DDR is known to date (Masutani et al., 1994). We also identified COPS1 and COPS3, two subunits of the COP9 signalosome that regulate de-neddylation of cullins and thus control activity of cullin-dependent ubiquitin ligases. Several nuclear E3 ubiquitin ligases also showed increased phosphorylation after DNA damage, including MDM2, HUWE1, TRIP12 and RNF2. In addition, phosphorylation of several components of the N-end rule pathway, which is important for degradation of protein substrates via N-terminal ubiquitylation, is increased in DNA damage-treated cells.
Crosstalk between the DDR and TNF-α/IL-1 signaling pathways
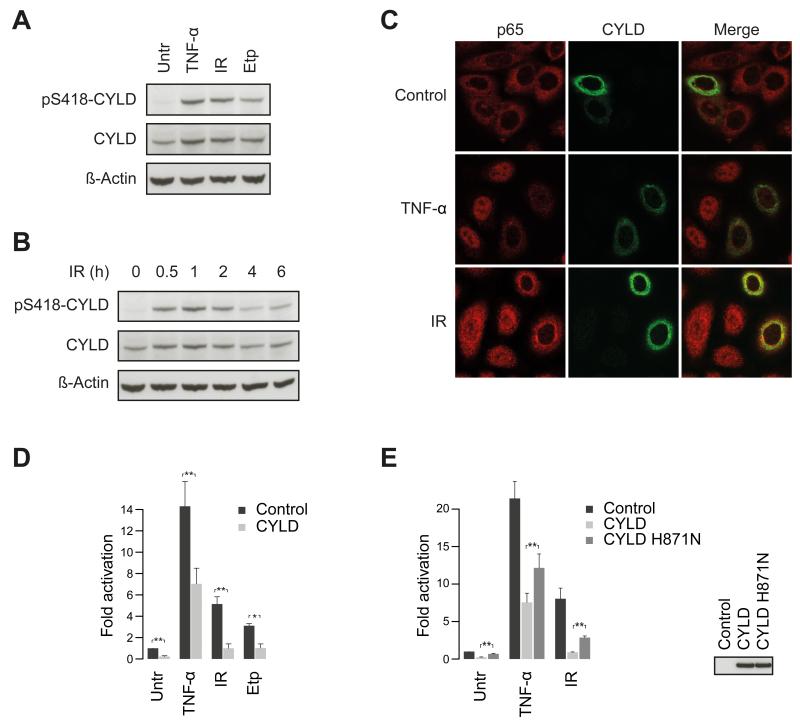
In our proteomic screen, we found DNA damage-induced phosphorylation of several well-known components of tumor necrosis factor alpha/interleukin 1 (TNF-α/IL-1) receptor signaling events that impinge on NF-κB activation, including TRAF2, p62, RIPK1, IKB-ε and CYLD (Table S1). CYLD, a prominent deubiquitylase, acts downstream of the TNF-α receptor to inhibit nuclear translocation of NF-κB and thus block activation of its transcriptional targets (Brummelkamp et al., 2003; Kovalenko et al., 2003; Trompouki et al., 2003). Activating the IKK complex upon TNF-α stimulation causes CYLD phosphorylation on Ser-418, which decreases its deubiquitylase activity and enhances NF-κB activation after TNF-α stimulation (Hutti et al., 2009; Reiley et al., 2005). We investigated CYLD phosphorylation by Western blotting with an antibody specific to CYLD phospho-Ser-418 (Fig. 4A). This revealed that IR-induced CYLD Ser-418 phosphorylation peaked ~1 hour after IR treatment and remained detectable at least 6 hours afterwards (Fig. 4B).
Figure 4.
CYLD regulates DNA damage-induced activation of NF-κB signaling. (A) Phosphorylation of CYLD Ser-418 by DNA damage inducing agents. Cells were treated with TNF-α, IR, or etoposide (Etp) and phosphorylation was analyzed using Ser-418 phospho-specific CYLD antibody. (B) Dynamics of DDR-induced CYLD phosphorylation. Cells were irradiated and allowed to recover for the indicated time points, and phosphorylation was detected as described above. (C) CYLD inhibits nuclear translocation of the p65 subunit of NF-κB. Cells were transfected with a plasmid encoding CYLD and nuclear translocation of p65 was analyzed by immunofluorescence microscopy after treatment with TNF-α or IR. (D and E) CYLD inhibits DNA damage-induced NF-κB transcriptional activity. Cells were transfected with the indicated CYLD constructs or empty vector and NF-κB activation was monitored with luciferase-based reporter assays. Error bars specify the standard deviation of the three independently performed experiments. p**<0.05. (See also Figure S5)
To test whether CYLD affects DDR-induced nuclear translocation of NF-κB, we monitored nuclear translocation of the NF-κB p65 subunit in human breast carcinoma cells (Cal-51). Comparing cells overexpressing CYLD with non-transfected cells showed that CYLD overexpression diminished nuclear translocation of NF-κB upon IR treatment (Fig. 4C). Stimulating cells with TNF-α or treating them with IR induced NF-κB transcriptional activity and, in each case this induction was impaired by CYLD overexpression (Fig. 4D). A catalytically inactive CYLD mutant (Kovalenko et al., 2003) had significantly reduced inhibitory activity towards NF-κB transcription than wild-type CYLD (Fig. 4E), demonstrating that CYLD enzymatic activity is required for these functions. Thus, CYLD plays a negative regulatory role in DNA damage-induced NF-κB activation. During the course of this study, a role of CYLD in DDR-induced NF-κB activity was reported (Niu et al., 2011; Wu et al., 2010). These data are in agreement with our results and support an important role of CYLD in this process. Our finding that multiple components of the TNF-α/IL-1 signaling pathway are phosphorylated in response to DNA damage implies substantial crosstalk between the DDR and TNF-α/IL-1 signaling pathway components (Fig. S5).
Involvement of PPM1G in the DDR
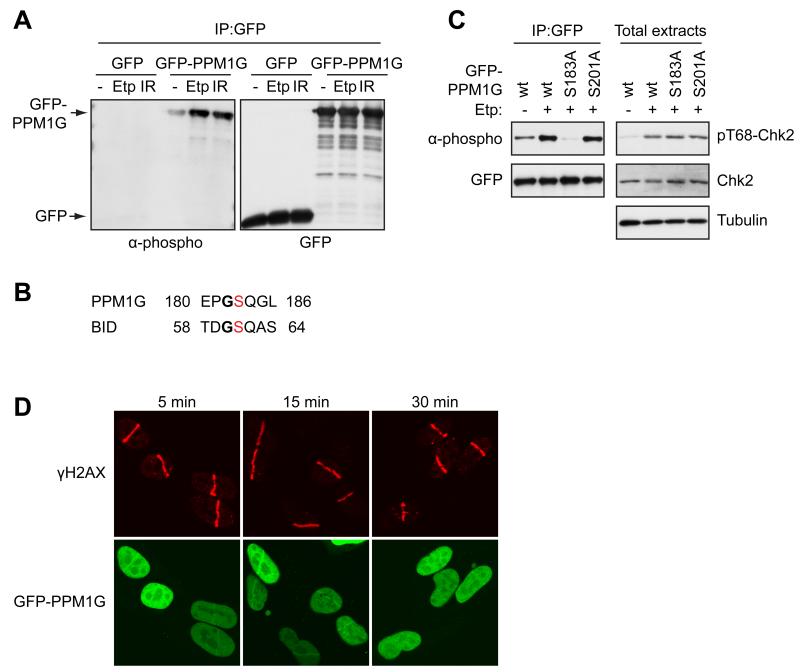
A recent genome-wide short-interfering RNA (siRNA) screen found that depletion of various RNA processing factors led to DNA damage induction (Lackner et al., 2011; Paulsen et al., 2009). We identified many DNA damage-induced phosphorylations on RNA processing factors, most of which have not hitherto been implicated in the DDR. One protein hit we selected for characterization was PPM1G (PP2Cγ), a nuclear member of the PP2C family of Ser/Thr phosphatases. In addition to positively regulating splicing (Allemand et al., 2007; Murray et al., 1999), PPM1G has been shown to bind histones and to act in vitro as a histone chaperone, and to promote cellular resistance to caffeine, suggesting potential roles in the DDR (Kimura et al., 2006). Notably, the PPM1G phosphorylation sites that we identified, Ser-183 and Ser-201, both conform to the SQ PIKK target consensus and reside in the acidic domain of PPM1G that is unique to it amongst its family members and is required for PPM1G function in alternative splicing (Allemand et al., 2007). By testing several phospho-specific antibodies, we found that one raised against Ser-61 of the Bid protein detected PPM1G expressed in cells (Fig. 5A). Moreover, detection of PPM1G with this antibody increased when cells were treated with etoposide or IR (Fig. 5A). In agreement with the context of Bid Ser-61 being most similar to that of PPM1G Ser-183 (Fig. 5B), antibody recognition of PPM1G was abrogated when Ser-183 but not Ser-201 was mutated to Ala (Fig. 5C). To see whether PPM1G might function at DNA damage sites, we subjected cells expressing GFP-PPM1G to laser micro-irradiation, which induces localized DNA damage in the irradiated areas (Lukas et al., 2003; Polo and Jackson, 2011). GFP-PPM1G was recruited to damage regions marked by the phosphorylated form of histone H2AX (γH2AX); its temporary recruitment being detectable within 5 minutes of irradiation and then persisting until 30 minutes (Fig. 5D). Consistent with previous reports (Allemand et al., 2007), siRNA-mediated depletion of PPM1G markedly reduced cell proliferation, which prevented us testing for potential effects of PPM1G in promoting cellular resistance to DNA damaging agents.
Figure 5.
PPM1G is phosphorylated in response to DNA damage and is recruited to DNA damage sites. (A) Detection of PPM1G phosphorylation by Ser-61 phospho-specific Bid antibody (α-phospho) in GFP immunoprecipitates from U2OS cells transfected with GFP or GFP-PPM1G plasmids and treated with 3 μM etoposide (Etp) for 2 hours or 10 Gy IR (1 hour recovery). Position of GFP-PPM1G and GFP is indicated by arrow. (B) Sequence alignment of the region encompassing Ser-183 and Ser-61 on PPM1G and Bid, respectively. (C) Western blotting analysis of GFP immunoprecipates and total cell extracts from U2OS cells transfected with wild-type GFP-PPM1G or indicated phospho-mutants and treated with etoposide (Etp) where indicated. (D) Recruitment of PPM1G to sites of DNA damage in U2OS cells transfected with GFP-PPM1G plasmid and fixed at indicated times following micro-irradiation.
DNA damage-regulated phosphorylation of THRAP3/TRAP150
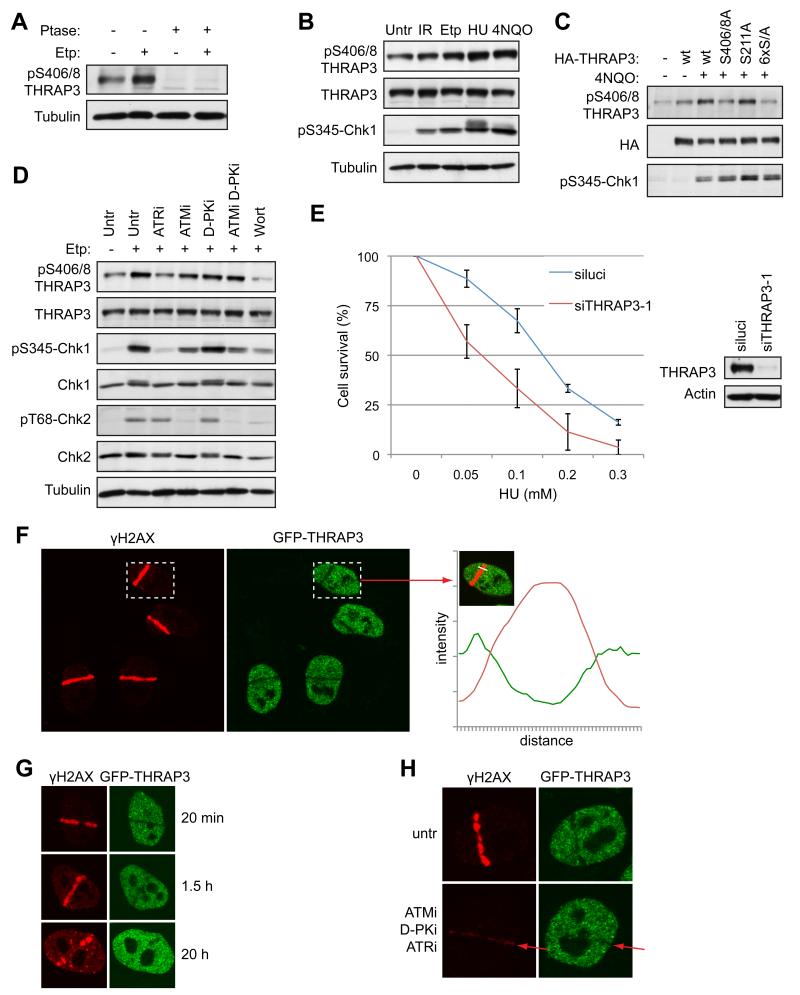
Another factor strongly phosphorylated in response to DNA damage in our screen was the transcription mediator subunit THRAP3 (TRAP150), which functions in RNA splicing and RNA degradation (Lee et al., 2010). THRAP3 is also found in a complex containing the proteins SNIP1, PNN, BCLAF1 and SKIIP, which regulates Cyclin D1 RNA stability (Bracken et al., 2008). Interestingly, SNIP1, BCLAF1 and PNN were also phosphorylated in our screen, suggesting that the whole complex may be regulated in response to DNA damage (Table S1). Notably, the THRAP3 phosphorylation sites Ser-210, 211, 399, 406, 408 we identified are evolutionary conserved from man to Xenopus (Fig. S6A), suggesting their functional importance. To detect THRAP3 phosphorylation, we raised a phospho-specific antibody against a di-phosphorylated peptide encompassing Ser-406 and Ser-408 and found that it detected THRAP3 in whole cell extracts in a manner that was abrogated by phosphatase treatment (Fig. 6A). Notably, although THRAP3 detection by the phospho-specific antibody was observed in extracts of untreated cells, it was increased when cells were treated with the DNA replication inhibitor hydroxyurea (HU) or with various DNA damaging agents, including etoposide, IR, and the UV-mimetic agent 4-nitroquinnoline oxide (4NQO) (Fig. 6A, B). Furthermore, as confirmation of antibody specificity, THRAP3 detection was significantly reduced when Ser-406 and Ser-408 but not Ser-211 were mutated to Ala (Fig. 6C).
Figure 6.
THRAP3 is phosphorylated in response to DNA damage and is excluded from DNA damage sites. (A) and (B) Phosphorylation of THRAP3 in cell extracts untreated or treated with etoposide (Etp; 3 μM, 2h), HU or 4NQO, which were treated with λ phosphatase (Ptase) where indicated. (C) Phosphorylation of wild-type HA-THRAP3 or indicated THRAP3 phospho-mutants in U2OS cells treated with 4NQO where indicated. (Note that antibody detection was not reduced further by mutating all 6 phosphorylation sites to Ala; 6×S/A). (D) Phosphorylation of THRAP3 in cells treated with inhibitors of ATM (ATMi), DNA-PK (D-PKi), ATR (ATRi) or wortmannin (Wort) for 1 hour, then treated with Etp (3 μM, 2h). (E) Clonogenic survival of cells transfected with siluci or siTHRAP3-1 and treated with HU at the indicated doses. Results are presented as an average from 3 experiments −/+ SEM. Depletion of THRAP3 is shown by Western blotting. (F) Exclusion of THRAP3 from sites of laser-induced DNA damage in HeLa-GFP-THRAP3 cells. Quantification of green (GFP-THRAP3) and red (γH2AX) was performed by Volocity software. The graph shows the intensity of fluorescence (y-axis) along the white line (x-axis) indicated by arrow. (G) Exclusion of THRAP3 from laser-damage in cells fixed at indicated times. (H) Exclusion of THRAP3 from DNA damage sites is inhibited in cells treated with inhibitors of ATM (ATMi), DNA-PK (D-PKi) and ATR (ATRi) for 1 hour prior to micro-irradiation. (untr = untreated; p = phospho). (See also Figure S6)
We noted that, like CHK1 Ser-345 phosphorylation, which is primarily targeted by ATR (Jazayeri et al., 2006), THRAP3 phosphorylation was most strongly increased upon HU and 4NQO treatment, suggesting it could also be mainly ATR dependent (Fig. 6B). To explore this potential relationship, we pretreated cells with selective ATM, ATR or DNA-PK inhibitors, with the pan-PIKK inhibitor wortmannin or with DMSO as control, then added etoposide to induce DNA damage (Fig. 6D). Analysis of THRAP3 Ser-406/408 phosphorylation revealed that its profile mirrored that of CHK1 Ser-345 phosphorylation, being inhibited when cells were pretreated with ATR inhibitor or wortmannin, weakly inhibited by the ATM inhibitor KU55933 (Hickson et al., 2004), but not by the DNA-PK inhibitor NU7441 (Veuger et al., 2003) (Fig. 6D). Similar results were obtained when THRAP3 phosphorylation was induced by HU (Fig. S6B). Thus, THRAP3 Ser-406/8 phosphorylation is largely mediated by ATR. Importantly, we found that THRAP3 depletion by two distinct siRNAs caused cells to be more sensitive to killing by HU treatment, which induces replication-fork stalling and DNA damage in S phase (Petermann and Helleday, 2010) (Fig. 6E, Fig. S6C).
THRAP3 is excluded from DNA damage sites
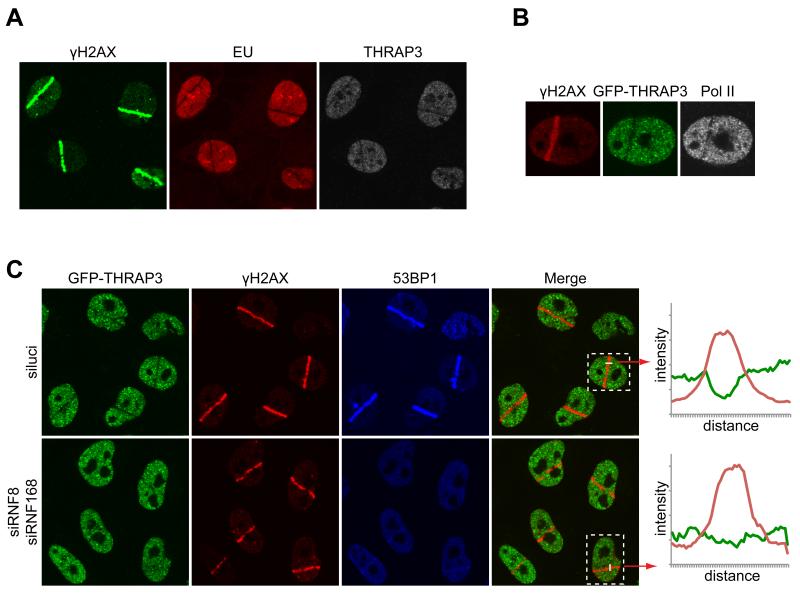
To further investigate the involvement of THRAP3 in the DDR, we laser micro-irradiated HeLa cells expressing THRAP3 tagged with GFP under the control of its endogenous chromosomal promoter (Poser et al., 2008). Strikingly, unlike most DDR factors previously analyzed, THRAP3 was excluded from micro-irradiated sites marked by γH2AX induction (Fig. 6F); this occurred rapidly, being detectable within 2-3 min of DNA damage induction (Fig. S6D), and then persisted for up to several hours (Fig. 6G); exclusion of endogenous THRAP3 was also observed in other cell types, including U2OS (Fig. 7A) and MRC5 cells (Fig. S6E). The exclusion kinetics, together with the fact that THRAP3 exclusion paralleled the presence of γH2AX (Fig. 6G), suggested that THRAP3 was excluded from sites of DNA DSBs. Notably, we also found that THRAP3 was excluded from 53BP1 OPT domains (Fig. S6F), that arise in G1 cells at sites of replication problems induced during the preceding S-phase (Harrigan et al., 2011; Lukas et al., 2011).
Figure 7.
Exclusion of THRAP3 is linked to transcriptional inhibition. (A) Incorporation of EU in U2OS cells is inhibited at sites of laser-induced DNA damage. (B) Exclusion of RNA PolII from sites of micro-irradiation (cells stained with phospho-Ser2-C-terminal domain specific antibody). (C) Exclusion of THRAP3 from laser-damage is reduced upon depletion of RNF8 and RNF168 (the efficiency of RNF8/RNF168 depletion is shown by abrogation of 53BP1 recruitment). Shown are representative fields of cells derived from three independent experiments. For the boxed cells, fluorescence intensity along the white line was measured by Volocity software and the resulting graphs are provided on the right. Quantifications for all cells in the fields are provided in Figure S7B
THRAP3 exclusion was not blocked by the proteasome inhibitor MG132, suggesting that it did not reflect localized THRAP3 degradation (Fig. S6G). By contrast, treating cells with a combination of ATM, ATR and DNA-PK inhibitors largely prevented THRAP3 exclusion (Fig. 6H; consistent with ATM, ATR and DNA-PK all being able to target H2AX, combined inhibition of all three kinases was necessary to virtually abolish γH2AX production). While this initially suggested that PIKK-mediated phosphorylation of THRAP3 might mediate its exclusion, mutating Ser-406/408 together with four other phosphorylation sites identified in our screen (Ser-210, 211 214 and 399) to Ala did not prevent THRAP3 exclusion (Fig. S6H). Notably, we found that deleting the amino- and carboxyl-terminal regions of THRAP3 – which are important for its role in RNA splicing and RNA degradation, respectively (Lee et al., 2010) – impaired THRAP3 exclusion from DNA damage regions (Fig. S6I). These findings, taken together with the fact that RNA polymerase II transcription is inhibited at flanking DSB sites (Shanbhag et al., 2010) led us to speculate that THRAP3 exclusion was a readout of reduced transcription and processing of nascent RNA transcripts. Consistent with this idea, transcription was inhibited at sites of laser-induced DNA damage as shown by lack of incorporation of 5-ethynyl uridine (EU) (Fig. 7A). Accordingly, the transcribing form of RNA polymerase (detected by an antibody against phosphorylated Ser-2 of the repeats in the carboxyl-terminal domain of its largest subunit) was excluded from sites of laser micro-irradiation (Fig. 7B). Moreover, THRAP3 exclusion was reduced by siRNA-mediated depletion of the ubiquitin E3 ligases RNF8 and RNF168 (Fig. 7C, Fig. S7B), which have previously been shown to be necessary for localized transcription inhibition at endonuclease-induced DSB sites (Shanbhag et al., 2010).
Discussion
Activation of cell signaling in response to cellular perturbations often involves complex signaling networks employing various proteins and PTMs. We used high-resolution MS to investigate cellular responses to DNA damage by parallel quantification of changes in protein phosphorylation, acetylation and protein abundance. Our results indicate that, at a systems level, cells employ various regulatory mechanisms encompassing different PTMs and proteome changes to respond to genotoxic stress. In-depth quantification of DDR-regulated phosphorylation and acetylation revealed that the magnitude of regulation differed for these two PTMs. DNA damage-induced phosphorylations typically displayed very rapid and robust increases, in particular for upregulated sites with S/TQ motifs, which are primarily phosphorylated by the DDR-regulated kinases ATM, ATR and DNA-PK. In contrast, the overall increase in site-specific acetylation levels in response to DNA damage was comparatively weak.
Recent mass spectrometry-based studies have investigated DNA damage-regulated changes in specific subsets of phosphorylation sites or cellular compartments (Bennetzen et al., 2010; Bensimon et al., 2010; Matsuoka et al., 2007). In addition to the large number of phosphorylations that are likely to be direct ATM/ATR/DNA-PK targets, our DDR-regulated phosphoproteome contains proteins that could be phosphorylated by other kinases and are present in nuclear as well as in non-nuclear compartments. Such kinases include CHK1 and CHK2, which play important DDR roles downstream of ATM/ATR (Reinhardt et al., 2007). Notably, the DNA damage-regulated phosphorylations on p38MAPK, BUB1 and OXSR1 conform to S/TQ motifs, suggesting that these kinases are directly phosphorylated and controlled by ATM/ATR/DNA-PK.
In our study, treating cells with DNA damaging agents increased phosphorylation of over 1,400 sites on several hundred proteins that participate in diverse cellular functions which highlighted the complexity of DDR-regulated signaling networks beyond the localized repair of the damaged DNA. Although we found that a great majority of targets with S/TQ motifs are predicted to localize to the nucleus, we also identified targets that are known to localize to non-nuclear compartments. Amongst this group is fumarate hydratase (FH), a mitochondrial protein and known tumour suppressor. Interestingly, this enzyme was recently reported to localize to the nucleus in response to DNA damage and to protect cells from deleterious effects of DNA DSBs (Yogev et al., 2010). Our data pinpoint a specific DDR-regulated site (Ser-46) on this protein that can be explored in this process. Furthermore, a large proportion of DNA damage-induced non-S/TQ sites that we identified are present on non-nuclear proteins, thus implicating downstream DDR kinases in amplifying and transmitting signals to proteins residing in extra-nuclear compartments. We hope that our provision of these and other high-confidence alterations in the phosphoproteome, acetylome and proteome in response to DNA damage will facilitate future investigations to determine how the DDR impinges on and regulates myriad cellular events.
Activation of NF-κB upon genotoxic stress is one of the best-known examples of signal transmission from the nucleus to the cytoplasm. In the past few years, several proteins, including NEMO (Wu et al., 2006), PIAS4/PIASY (Mabb et al., 2006), PARP1 (Stilmann et al., 2009), have been implicated in DNA damage-induced NF-κB activation. Our observation that several components of the TNF-α/IL-1 signaling pathway exhibited increased phosphorylation upon DNA damage suggests that DDR-induced NF-κB activation also involves some of these components.
While many cellular functions have now been connected to the DDR, it is only recently becoming apparent that there are important links between the DDR and RNA metabolism. For example, it has been established that transcription is inhibited in response to DNA damage, both generally and locally at DNA damage sites (Rockx et al., 2000; Shanbhag et al., 2010). Despite various RNA processing factors having been identified in our and other phosphoproteomic and functional DDR screens (Bennetzen et al., 2010; Bensimon et al., 2010; Matsuoka et al., 2007; Paulsen et al., 2009), only a few RNA-associated factors have so far been specifically examined for DDR links (Li and Manley, 2005; Moumen et al., 2005; Ramachandran et al., 2011). In this study, we have shown that the protein phosphatase PPM1G, which promotes pre-mRNA splicing, is phosphorylated in response to DNA damage and is rapidly and temporarily recruited to DNA damage sites. It will therefore be of interest to identify PPM1G target proteins, determine whether they are functionally involved in the DDR and whether their dephosphorylation by PPM1G affects DDR events. Another factor that we have characterized is THRAP3, which functions in RNA processing and in regulating RNA stability (Bracken et al., 2008; Lee et al., 2010). We have shown that THRAP3 is phosphorylated in response to DNA damage, with this primarily being mediated by ATR. Although our work focused on Ser-406/408, we note that our screen and others have identified additional DDR-induced THRAP3 phosphorylations.
While many DDR factors are recruited to DNA damage sites (Polo and Jackson, 2011), we have found that THRAP3 is excluded from such regions in a manner that parallels transcriptional inhibition. Previous work by Greenberg and colleagues has shown that transcriptional inhibition is induced when DNA DSBs are generated by an endonuclease at a chromosomal locus containing a transcriptional promoter linked to a reporter construct (Shanbhag et al., 2010), and is dependent on ATM activity and on the functions of RNF8 and RNF168. While our work demonstrates that THRAP3 exclusion at sites of laser micro-irradiation also requires RNF8/168, we could only abrogate THRAP3 exclusion from laser micro-irradiated regions when we simultaneously inhibited ATM, ATR and DNA-PK, probably because the concentrated and localized DNA damage triggered by micro-irradiation induces robust activation of the three DDR-PIKK enzymes. Notably, we have found that the THRAP3-binding proteins BCLAF1 (Fig. S7A) and PNN are also excluded from DNA damage sites (NL and SPJ, unpublished data), suggesting this whole protein complex is excluded along with RNA polymerase II. It will therefore be of interest to determine the functional interrelationships between these proteins and determine the role of these factors in the DDR. Finally, we note that the gene for THRAP3 is deleted in oral squamous cell carcinomas (Cha et al., 2011), and is one of the sites where hepatitis B virus DNA is integrated in some cases of human liver cancers (Paterlini-Brechot et al., 2003). Furthermore, recent reports show that mutations in spliceosome machinery are frequent in malignancies, such as myelodysplasia (Papaemmanuil et al., 2011; Yoshida et al., 2011), suggesting potential roles of other RNA splicing factors in maintaining genome stability and protecting against cancer.
Experimental procedures
Enrichment of phosphopeptides
For phosphoproteome analysis 10 mg of trypsin-digested peptides were fractionated using strong cation exchange chromatography (AKTA chromatography purifier system, Amersham Biosciences) as described previously (Olsen et al., 2006). A total of 12 peptide fractions and the flow through were collected and phosphopeptides were enriched using 2,5-dihydroxybenzoic acid (2,5-DHB) (Fluka) precoated Titanspheres (GL Sciences) (Larsen et al., 2005; Olsen et al., 2006). Phosphopeptides were eluted with 40% acetonitrile in 25% NH4OH-water (pH > 10.5) (Olsen et al., 2006). For acetylation enrichment, modified peptides were immuno-enriched using anti-acetyllysine antibodies, and acetylation analysis was performed as described previously (Choudhary et al., 2009).
Immunoprecipitation
Cells were lysed in NP-40 buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 450 mM NaCl, 1 mM EDTA) supplemented with protease inhibitors (Roche) and phosphatase inhibitors. Immunoprecipitations for GFP-tagged proteins and HA-tagged proteins were carried out with GFP-trap agarose beads (Chromotek) and EZview anti-HA affinity gel (Sigma) respectively for 2h at 4°C. Immunoprecipitated proteins were washed in NP-40 buffer and boiled in 2x Laemmli sample buffer. Samples were resolved by SDS-PAGE and analyzed by Western blotting with the indicated antibodies.
Western blotting
Total cell lysates were resolved on a 4-12% gradient or 8% SDS-PAGE gels and proteins were transferred onto nitrocellulose membranes. Membranes were blocked using 5% BSA solution in PBS supplemented with Tween-20 (0.1%). Secondary antibodies coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories) were used for immunodetection. The detection was performed with Novex ECL Chemiluminescent Substrate Reagent Kit (Invitrogen).
For further details about experimental procedures please refer to Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
We thank Emily Hsu, Dorte Bekker-Jensen and Heidi Cordula Grell for technical support, Niels Mailand and Simon Bekker-Jensen for providing CYLD plasmid. We thank Ina Poser and Tony Hyman for providing the stable HeLa BAC pool expressing GFP-tagged THRAP3. Their work is supported by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement 241548 (MitoSys Project). We are grateful to Prof. Neil Perkins for providing HA-THRAP3 plasmid and BCLAF1 and THRAP3 antibodies and to Prof. WY Tarn for providing THRAP3 deletion constructs. We thank members of the Mann Lab, the Jackson Lab and the department of proteomics at CPR for helpful discussions. We thank Kate Dry for critical reading of the manuscript. This work was supported by the Max Planck Society, by the Danish Research Council (FSS: 10-083519), and the Lundbeck Foundation (R48-A4699). SAW is supported by a postdoctoral grant from Danish Research Council (FSS: 10-085134). The Center for Protein Research is supported by a generous grant from the Novo Nordisk Foundation. NL was supported by funding from Cancer Research UK (CRUK, C6/A11224). Research in the SPJ Lab is funded by CRUK program grant C6/A11224, the European Community’s Seventh Framework Programme (GENICA and DDResponse) and core infrastructure funding was provided by CRUK (C11628/A6535) and the Wellcome Trust (075661).
References
- Allemand E, Hastings ML, Murray MV, Myers MP, Krainer AR. Alternative splicing regulation by interaction of phosphatase PP2Cgamma with nucleic acid-binding protein YB-1. Nat Struct Mol Biol. 2007;14:630–638. doi: 10.1038/nsmb1257. [DOI] [PubMed] [Google Scholar]
- Bennetzen MV, Larsen DH, Bunkenborg J, Bartek J, Lukas J, Andersen JS. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol Cell Proteomics. 2010;9:1314–1323. doi: 10.1074/mcp.M900616-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon A, Schmidt A, Ziv Y, Elkon R, Wang SY, Chen DJ, Aebersold R, Shiloh Y. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal. 2010;3:rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- Bodenmiller B, Aebersold R. Phosphoproteome resource for systems biology research. Methods Mol Biol. 2011;694:307–322. doi: 10.1007/978-1-60761-977-2_19. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Wall SJ, Barre B, Panov KI, Ajuh PM, Perkins ND. Regulation of cyclin D1 RNA stability by SNIP1. Cancer Res. 2008;68:7621–7628. doi: 10.1158/0008-5472.CAN-08-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Cha JD, Kim HJ, Cha IH. Genetic alterations in oral squamous cell carcinoma progression detected by combining array-based comparative genomic hybridization and multiplex ligation-dependent probe amplification. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:594–607. doi: 10.1016/j.tripleo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Mann M. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol. 2010;11:427–439. doi: 10.1038/nrm2900. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Belotserkovskaya R, Coates J, Dimitrova DS, Polo SE, Bradshaw CR, Fraser P, Jackson SP. Replication stress induces 53BP1-containing OPT domains in G1 cells. J Cell Biol. 2011;193:97–108. doi: 10.1083/jcb.201011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, Hahn WC, Cantley LC. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–472. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Jorgensen TJ. Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther. 2009;8:665–670. doi: 10.4161/cbt.8.8.8304. [DOI] [PubMed] [Google Scholar]
- Kimura H, Takizawa N, Allemand E, Hori T, Iborra FJ, Nozaki N, Muraki M, Hagiwara M, Krainer AR, Fukagawa T, et al. A novel histone exchange factor, protein phosphatase 2Cgamma, mediates the exchange and dephosphorylation of H2A-H2B. J Cell Biol. 2006;175:389–400. doi: 10.1083/jcb.200608001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Lackner DH, Durocher D, Karlseder J. A siRNA-based screen for genes involved in chromosome end protection. PLoS One. 2011;6:e21407. doi: 10.1371/journal.pone.0021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–7655. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- Lee KM, Hsu Ia, W., Tarn WY. TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation. Nucleic Acids Res. 2010;38:3340–3350. doi: 10.1093/nar/gkq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Manley JL. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005;122:365–378. doi: 10.1016/j.cell.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol. 2003;5:255–260. doi: 10.1038/ncb945. [DOI] [PubMed] [Google Scholar]
- Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS, Grofte M, Chan KL, Hickson ID, Bartek J, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–253. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol. 2006;8:986–993. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- Masutani C, Sugasawa K, Yanagisawa J, Sonoyama T, Ui M, Enomoto T, Takio K, Tanaka K, van der Spek PJ, Bootsma D, et al. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23. Embo J. 1994;13:1831–1843. doi: 10.1002/j.1460-2075.1994.tb06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123:1065–1078. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Murray MV, Kobayashi R, Krainer AR. The type 2C Ser/Thr phosphatase PP2Cgamma is a pre-mRNA splicing factor. Genes Dev. 1999;13:87–97. doi: 10.1101/gad.13.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Shi Y, Iwai K, Wu ZH. LUBAC regulates NF-kappaB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 2011;30:3741–3753. doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, Pellagatti A, Wainscoat JS, Hellstrom-Lindberg E, Gambacorti-Passerini C, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011;365:1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Saigo K, Murakami Y, Chami M, Gozuacik D, Mugnier C, Lagorce D, Brechot C. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene. 2003;22:3911–3916. doi: 10.1038/sj.onc.1206492. [DOI] [PubMed] [Google Scholar]
- Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009;35:228–239. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol. 2010;11:683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser I, Sarov M, Hutchins JR, Heriche JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods. 2008;5:409–415. doi: 10.1038/nmeth.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Tran DD, Klebba-Faerber S, Kardinal C, Whetton AD, Tamura T. An ataxia-telangiectasia-mutated (ATM) kinase mediated response to DNA damage down-regulates the mRNA-binding potential of THOC5. RNA. 2011;17:1957–1966. doi: 10.1261/rna.2820911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W, Zhang M, Wu X, Granger E, Sun SC. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–3895. doi: 10.1128/MCB.25.10.3886-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx DA, Mason R, van Hoffen A, Barton MC, Citterio E, Bregman DB, van Zeeland AA, Vrieling H, Mullenders LH. UV-induced inhibition of transcription involves repression of transcription initiation and phosphorylation of RNA polymerase II. Proc Natl Acad Sci U S A. 2000;97:10503–10508. doi: 10.1073/pnas.180169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W, Rowe T, Glisson B, Yalowich J, Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984;44:5857–5860. [PubMed] [Google Scholar]
- Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 2010;141:970–981. doi: 10.1016/j.cell.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilmann M, Hinz M, Arslan SC, Zimmer A, Schreiber V, Scheidereit C. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IkappaB kinase activation. Mol Cell. 2009;36:365–378. doi: 10.1016/j.molcel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39:D561–568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Wong ET, Shi Y, Niu J, Chen Z, Miyamoto S, Tergaonkar V. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell. 2010;40:75–86. doi: 10.1016/j.molcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD, Pines O. Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol. 2010;8:e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, Sato Y, Sato-Otsubo A, Kon A, Nagasaki M, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.