Abstract
Disruptions in the social environment, such as social isolation, are distressing and can induce various behavioral and neural changes in the distressed animal. We conducted a series of experiments to test the hypothesis that long-term social isolation affects brain plasticity and alters behavior in the highly social prairie vole (Microtus ochrogaster). In Experiment 1, adult female prairie voles were injected with a cell division marker, 5-bromo-2′-deoxyuridine (BrdU), and then same-sex pair-housed (control) or single-housed (isolation) for 6 weeks. Social isolation reduced cell proliferation, survival, and neuronal differentiation and altered cell death in the dentate gyrus of the hippocampus and the amygdala. In addition, social isolation reduced cell proliferation in the medial preoptic area and cell survival in the ventromedial hypothalamus. These data suggest that long-term social isolation affects distinct stages of adult neurogenesis in specific limbic brain regions. In Experiment 2, isolated females displayed higher levels of anxiety-like behaviors in both the open field and elevated plus maze tests and higher levels of depression-like behavior in the forced swim test than controls. Further, isolated females showed a higher level of affiliative behavior than controls, but the two groups did not differ in social recognition memory. Together, our data suggest that social isolation not only impairs cell proliferation, survival, and neuronal differentiation in limbic brain areas, but also alters anxiety-like, depression-like, and affiliative behaviors in adult female prairie voles. These data warrant further investigation of a possible link between altered neurogenesis within the limbic system and behavioral changes.
Keywords: BrdU, Ki67, NeuN, TUNEL, Amygdala, Hippocampus, Social affiliation
Introduction
Over the past decades, a substantial amount of evidence has emerged supporting the notion that adult neurogenesis—cell division leading to the addition of new neurons in adulthood (Gross, 2000)— occurs in a variety of mammalian species (Dayer et al., 2005; Fowler et al., 2005; Gould et al., 1997; Huang et al., 1998; Smith et al., 2001), including humans (Eriksson et al., 1998). The two main brain regions that continuously give rise to adult-generated neurons are the subventricular zone (SVZ) and the subgranular zone of the dentate gyrus (DG) of the hippocampus. Cells born in the SVZ migrate along the rostral migratory stream to the olfactory bulb (OB), where the vast majority then differentiate into neurons and integrate into the existing circuitry (Lledo and Saghatelyan, 2005; Ming and Song, 2005). Similarly, most cells born in the subgranular zone migrate at a short distance within the DG and then undergo neuronal differentiation and integration (Christie and Cameron, 2006; Ming and Song, 2005). Distinct stages of adult neurogenesis may be modulated by a variety of endogenous (e.g., trophic factors, neurotransmitters, and hormones) and non-social exogenous (e.g., physical activity, environmental complexity, and acute stress) factors (Grote and Hannan, 2007; Ming and Song, 2005; Veenema et al., 2007). More recently, social environmental factors have also been documented to influence some aspects of adult neurogenesis in the DG. In particular, aversive social experiences (e.g., exposure to an aggressive conspecific or social isolation housing) reduce (Czeh et al., 2007; Gould et al., 1997; Thomas et al., 2007; Westenbroek et al., 2004), whereas positive social stimuli (e.g., exposure to male pheromones or to a conspecific pup) increase, the number of adult-generated cells in the hippocampus and SVZ (Furuta and Bridges, 2009; Mak et al., 2007; Ruscio et al., 2008).
Although most studies of adult neurogenesis have focused on the SVZ/OB and DG, adult-generated cells have also been documented in other non-traditional neurogenic brain regions (Fowler et al., 2008; Gould, 2007; Migaud et al., 2010). While the occurrence of adult neurogenesis in these brain regions remains controversial, several studies have reported the existence of new neurons in the neocortex (Dayer et al., 2005), piriform cortex (Bernier et al., 2002) and striatum (Bedard et al., 2006), as well as various limbic structures, including the amygdala (AMY) (Akbari et al., 2007; Bernier et al., 2002; Fowler et al., 2002; Okuda et al., 2009), medial preoptic area (MPOA) (Akbari et al., 2007), and hypothalamus (Fowler et al., 2002; Huang et al., 1998; Kokoeva et al., 2005). The occurrence of adult neurogenesis within the limbic system is especially intriguing as these brain regions play a role in mediating social behaviors, including parental, agonistic, affiliative, and mating behaviors (Cushing et al., 2003; Kollack-Walker and Newman, 1995; Lonstein et al., 1998; Wang et al., 1997). Few studies, however, have examined the expression and functional significance of new neurons in these brain regions.
The prairie vole (Microtus ochrogaster), a species frequently used to study the neurobiology of social behaviors (Young et al., 2011), has recently been utilized to study adult neurogenesis. Such studies have documented adult-generated cells in traditional and non-traditional neurogenic brain regions sensitive to changes in the social environment (Fowler et al., 2002; Ruscio et al., 2008; Smith et al., 2001). For example, pup exposure increased hippocampal cell proliferation in male and female prairie voles (Ruscio et al., 2008). Furthermore, alterations in the social environment influenced cell birth and death in a brain region-specific manner in adult female prairie voles (Fowler et al., 2002; Smith et al., 2001). Although these studies demonstrate site- and stimulus-specific effects of the social environment on adult-generated cells, little is known about whether long-term social isolation affects different stages of adult neurogenesis and whether adult-generated neurons exhibit long-term survival in prairie voles. In the present study, we examined the consequences of chronic social isolation in adulthood on cell proliferation, cell survival, neuronal differentiation, and cell death, in both traditional (e.g., DG) and non-traditional (e.g., AMY, MPOA, and VMH) neurogenic brain regions in female voles. In addition, we investigated the effects of chronic social isolation on behaviors relevant to exploration, anxiety, depression, affiliation, and social recognition. Finally we also examined the effects of such isolation on basal levels of corticosterone (CORT). Establishing the effects of social isolation on neurogenesis and behavior will guide further research to investigate a possible link between the two in adult social animals.
Materials and methods
Animals
Female prairie voles (M. ochrogaster), offspring of our laboratory breeding colony, were weaned at 21 days of age and housed in same-sex pairs in plastic cages (13×29×16 (H) cm) in a temperature- (21±1 °C) and light-controlled environment (14:10 light-dark cycle with lights on at 0700) with ad libitum access to food and water. All experimental procedures were approved by the Animal Care and Use Committee of Florida State University and conformed to the guidelines set forth by the NIH.
Experiment 1: Effects of chronic social isolation on cell proliferation and survival, neuronal differentiation, and cell death
Adult females (90–120 days of age) were injected intraperitoneally (ip) with a cell division marker, 5-bromo-2′-deoxyuridine (BrdU, 100 mg/kg; Sigma: St. Louis, MO), once daily (between 0800 and 1000 h) for 14 consecutive days. This injection paradigm has previously been used to label a large cohort of newly proliferated cells without causing neurotoxicity (Cameron and McKay, 2001; Lie et al., 2002). Following the last BrdU injection, subjects were randomly assigned into one of two treatment groups: same-sex pair-housed (control, subject remains with the familiar cage mate) or single-housed (isolation). Control and isolation animals were placed into new cages at the beginning of treatment. Six weeks later, subjects were deeply anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde solution. The brains were harvested, post-fixed for 2 h, and then stored in 30% sucrose in 0.1 M phosphate buffer until coronally sectioned at 40 μm using a sliding microtome. To assess cell survival, one set of brain sections (section interval of 240 μm) was incubated in rat anti-BrdU antibody (Table S1 in Supplement 1) and the staining was visualized with diaminobenzidine (DAB) using a previously established method (Fowler et al., 2002). To assess cell proliferation, a second set of brain sections was incubated in rabbit anti-Ki67 antibody (Table S1 in Supplement 1) and the staining was visualized using DAB (Wojtowicz and Kee, 2006). The nuclear protein Ki67, an endogenous proliferation marker, is expressed in dividing cells throughout the entire mitotic process (Scholzen and Gerdes, 2000). A third set of brain sections was used to assess neuronal differentiation. Tissue was incubated in rat anti-BrdU and mouse anti-NeuN antibodies to produce a double fluorescent-label (Table S1 in Supplement 1). NeuN is expressed in the nuclei of mature neurons (Mullen et al., 1992). Lastly, a fourth set of brain sections was processed to assess cell death, using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)—a technique commonly used to detect DNA fragmentation that occurs during apoptosis. Tissue was labeled using the TACS 2 TdT-Fluor In Situ Apoptosis Detection Kit, following the procedures recommended by the manufacturer (Trevigen: Gaithersburg, MD). Each stain resulted in clearly labeled nuclei and visible structural landmarks (such as fiber bundles), as observed in our previous studies (Fowler et al., 2002, 2003), allowing for identification of select brain regions.
BrdU-immunoreactive (BrdU-ir) cells were quantified in the granular cell layer, hilus, and molecular cell layer of the DG; basolateral, central, cortical, and medial nuclei of the AMY; MPOA; and VMH using a Zeiss Axioskop II microscope with the optical fractionator workflow (for stereological sampling parameters see Table S2 in Supplement 1) of the Stereo Investigator software (MBF Bioscience, Chicago, IL). BrdU-ir cells were also observed in the SVZ, particularly at the innermost ependymal cell layer lining the lateral ventricle. As these cells are better quantified in sagittal brain sections (Fowler et al., 2002; Smith and Luskin, 1998), we focused on the DG and the limbic brain regions mentioned above. Due to a low overall cell count, Ki67- and TUNEL-labeled cells were quantified using a modified version of the optical fractionator method (West et al., 1991). Briefly, Ki67-ir cells were counted in every sixth section (which occurred at 240 μm intervals) throughout each of the aforementioned regions, except the SVZ. The sum of the counted cells across all sections for each animal was multiplied by six to obtain an estimate of the total number of Ki67-ir cells within the corresponding region (for detailed methods, see Leuner et al., 2009). Similarly, TUNEL-labeled cells were counted in every twelfth section (480 μm intervals) throughout the AMY and DG and these total cell counts were multiplied by 12 to obtain an estimate of the total number of TUNEL-labeled cells. Because the numbers of BrdU-labeled cells in the MPOA and VHM were low, BrdU/NeuN fluorescent-labeled cells were only quantified in the granular cell layer of the DG and in the AMY. Two anatomically-matched brain sections per animal were scanned using a Leica TCS SP2 AOBS laser confocal microscope, as previously described (Fowler et al., 2005). An average of 40 hippocampal and 80 amygdaloid cells per animal were evaluated for BrdU/NeuN double-labeling. All microscope slides were coded to conceal treatment group identity until every section had been analyzed.
Experiment 2: Effects of chronic social isolation on behavior and baseline levels of corticosterone
As data from Experiment 1 indicated that 6 weeks of social isolation significantly altered adult neurogenesis in selected brain regions, we investigated the effects of social isolation on several types of behavior. Control and isolated females (as treated in Experiment 1) were tested in the open field (OF), social affiliation (AFF), elevated plus maze (EPM), and forced swim (FS) tests. The OF and AFF tests were conducted on day 1 of behavioral testing with a minimum of 2 h between tests. The EPM and FS tests were conducted on days 2 and 3, respectively. Animals were allowed to acclimate to the behavioral testing room for at least 30 min prior to testing. Behavioral testing started around 0800 h.
The 10-minute OF test was conducted, as previously described (Pan et al., 2009), to evaluate exploratory and anxiety-like behaviors. The plastic apparatus used measured 56×56×20 (H) cm and had a visual line grid that divided the apparatus into 16 squares, each measuring 14×14 cm. Each subject was placed into the center of the OF, and anxiety-like (i.e., frequency of center entries and duration spent in the center or corners) and locomotor (i.e., frequency of line crosses) behaviors were quantified.
The AFF test was established previously in male prairie voles (Pan et al., 2009). The testing apparatus consisted of two plexiglass chambers (13×28×16 (H) cm) connected by a clear hollow tube (16 L×7.5 radius cm). While one chamber remained empty, an unrelated stimulus female (approximately 70 days of age) was loosely tethered in the other chamber. The subject was placed into the empty chamber and allowed to move freely throughout the apparatus. During the 60-minute test, photobeam light sensors coupled with a customized computer program automatically recorded the subject’s frequency of chamber entries (indicator of locomotion) and the duration spent in each chamber. In addition, the test was video recorded and the duration of each subject’s direct body contact with the stimulus female (i.e., affiliative behavior) was later quantified.
The 5-minute EPM test was conducted to assess anxiety-like behaviors (Grippo et al., 2007b, 2008). The testing apparatus was elevated 45 cm off the ground and consisted of two open (35×6.5 cm) and two closed (35×6.5×15 (H) cm) arms that crossed in the middle. Each subject was placed onto the center of the EPM facing a closed arm. Anxiety-like behaviors (i.e., latency to enter the open arm and duration in the open and closed arms) and an index of locomotion (i.e., total arm entries) were recorded and subsequently quantified.
The FS test was conducted to assess depression-like behaviors (Grippo et al., 2008, 2009). A clear tank (25×45×20 (H) cm) was filled with tap water (32±1 °C) to a depth of 13 cm. Each subject was placed into the tank for the 5-minute test. Depression-like behaviors (i.e., latency to immobility, frequency of immobility bouts, and the total immobility duration) and an index of locomotion (i.e., swim duration) were recorded and quantified.
An additional cohort of subjects was created and tested in the social recognition (SOC) test to assess an animal’s recognition memory (Winslow, 2003; Zhao et al., 2009) as well as to measure baseline levels of plasma corticosterone (CORT). Each subject was placed into the experimental cage (25×45×20 (H) cm) and allowed to habituate for 10 min. Thereafter, an unrelated juvenile female (approximately 30–40 days of age) was introduced into the experimental cage for 5 min (trial 1, T1) and then removed. Thirty minutes later, the same stimulus juvenile was reintroduced for 5 min (trial 2, T2). This process was repeated once more to total three times (T1, T2, T3). During the fourth trial (New), a new unrelated juvenile was introduced. All 5-minute interaction periods were video recorded; the frequency and duration of the subject’s behaviors including olfactory investigation (i.e., sniffing of the juvenile’s anogenital or head region), close pursuit (i.e., closely following the juvenile), and escape behavior (i.e., moving away from the juvenile) were quantified.
Two days after the SOC test, subjects were deeply anesthetized (between 0900 and 1200 h) and their cardiac blood was collected, via right atrium incision, in a tube containing 20 μl of EDTA. Blood samples were centrifuged for 20 min at 4 °C at 6000 rpm, and plasma was diluted 1:8 in assay buffer and processed for CORT measurement using a radioimmunoassay kit (Coat-A-Count, Siemens Healthcare Diagnostics Inc., Los Angeles, CA), as described in a previous study (Stowe et al., 2005). According to the manufacturer, the assay detection limit is 5.7 ng/ml. The antiserum is highly specific for rat corticosterone (100% crossreactivity) and has extremely low crossreactivity to other compounds, such as progesterone (0.42%), aldosterone (0.15%), and testosterone (0.04%).
Data analysis
Differences in the number of BrdU- and Ki67-ir cells across treatment groups and brain regions were analyzed using a two-way ANOVA followed by a Student–Neuman–Keul’s (SNK) post-hoc test. Group differences in the number of BrdU- and Ki67-ir cells within the AMY subnuclei and DG subregions, the percentage of BrdU/NeuN double-labeled cells in the AMY and granular cell layer of the DG, and the number of TUNEL-labeled cells were analyzed by independent-samples t-tests. As the number of apoptotic and proliferating cells are significantly correlated (Amrein et al., 2004), and the ratio of cell death/proliferation may indicate the differential influence of social isolation on cell death, the ratio of TUNEL-ir/Ki67-ir cells in the AMY and DG was analyzed by a t-test.
For all behavioral tests, a trained observer blind to treatment used the J-Watcher V1.0 software (Macquarie University and UCLA; http://www.jwatcher.ucla.edu/) to score behaviors. Group differences across the behavioral measurements in the OF, EPM, FS, and AFF tests were analyzed by nonparametric Mann–Whitney U tests. Two animals were excluded from the behavioral analyses because they frequently showed abnormal behaviors, including spinning in circles and falling over in the home cage. The behavioral measures obtained in the SOC test were analyzed using a repeated measures ANOVA, followed by a SNK post-hoc test. Group differences in CORT levels were analyzed using an independent samples t-test. All statistical analyses were performed using PASW Statistic 18 software. Data were expressed as mean±SEM, and p<0.05 was considered statistically significant. To determine effect size, partial-eta squared, ηp2, for ANOVAs and Cohen’s d, d, for independent t-tests were calculated.
Results
Effects of social isolation on cell survival and proliferation, neuronal differentiation, and cell death
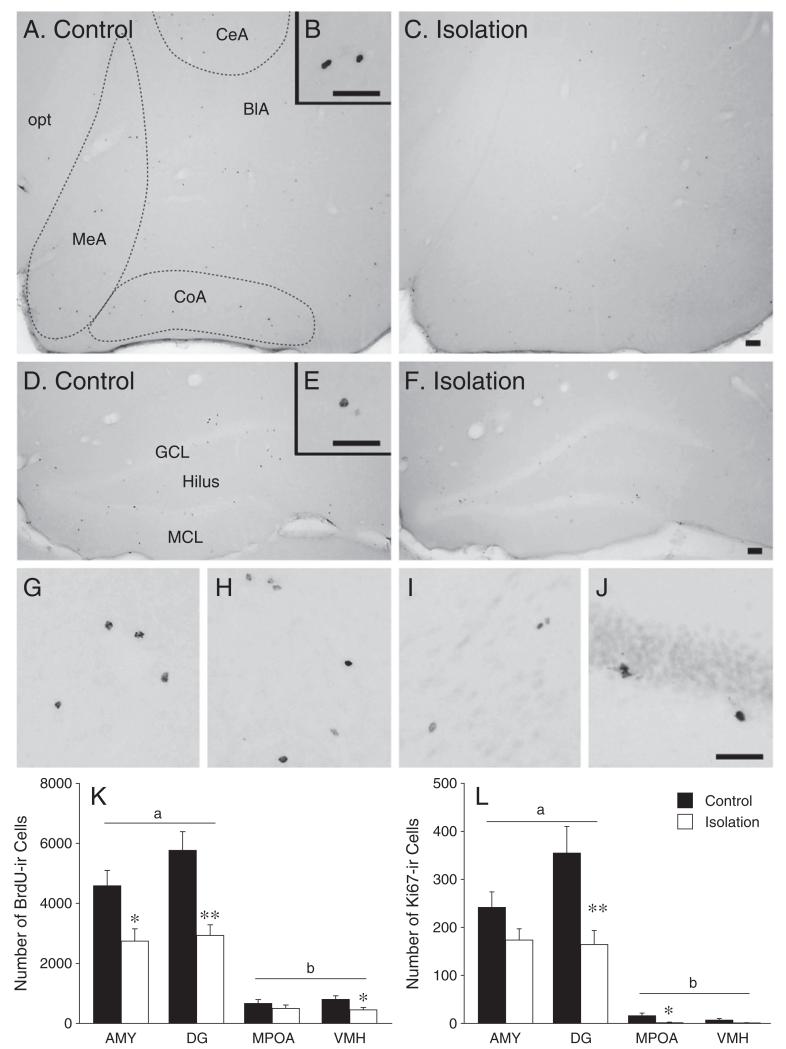
Social isolation reduced cell survival, as indicated by BrdU-immunoreactivity (F (1,64)=26.84, p<0.001, ηp2=0.30), in a brain region-specific manner (F (3,64)=6.46, p<0.01, ηp2=0.23; Fig. 1). Post-hoc analysis showed that isolated females had fewer BrdU-ir cells in the AMY (Figs. 1A-C, K), DG (Figs. 1D-F, K), and VMH (Figs. 1G and K), but not the MPOA (Figs. 1H and K), than control females. BrdU-immunoreactivity also varied across brain regions (F (3,64)=62.91, p<0.001, ηp2=0.75); post-hoc analysis showed that the number of BrdU-labeled cells was similar between the AMY and DG but was significantly greater in these regions than in the MPOA and VMH (Fig. 1K). Analysis of cell survival within the AMY revealed that isolated females had significantly fewer BrdU-ir cells in the basolateral, central, and medial, but not the cortical, nuclei than control females (Table 1). Isolation also reduced BrdU-immunoreactivity across all DG subregions (Table 1).
Fig. 1.
Chronic social isolation in adulthood affected long-term cell survival (assessed by BrdU-labeling) and proliferation (assessed by Ki67-labeling) in the brains of female prairie voles. Compared to pair-housed females (control, n=9), single-housed females (isolation, n=9) showed fewer BrdU-labeled cells in the amygdala (AMY) (A–C), dentate gyrus (DG) of the hippocampus (D–F), and ventromedial hypothalamus (VMH) (G), but not in the medial preoptic area (H, K). In addition, the number of BrdU-labeled cells in the AMY and DG was significantly higher than that in the medial preoptic area (MPOA) and VMH (K). Socially isolated females (n=8) also showed fewer Ki67-labeled cells in the DG (J) and MPOA, compared to pair-housed females (n=8; L). Finally, the number of Ki67-labeled cells in the AMY (I) was similar to the number in the DG, but higher than that in the MPOA and VMH (L). opt: optic tract; BlA: basolateral, CeA: central, CoA: cortical, and MeA: medial nuclei of the AMY. *p<0.05 and **p<0.01. Alphabetic characters represent the results of the post-hoc test. Error bars represent SEM. Scale bar=500 μm.
Table 1.
Number of cells labeled for proliferation markers in the subregions of the amygdala and hippocampus.
| Brain area | BrdU-labeling |
Ki67-labeling |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Isolation | p | d a | Control | Isolation | p | d | |
| Amygdala | ||||||||
| Basolateral | 915.9±112.3b | 553.2 ±96.0 | <0.05 | 1.16 | 48.8 ±13.4 | 31.5 ±6.2 | ns (0.26) | 0.58 |
| Central | 914.2±110.6 | 521.0 ±129.7 | <0.05 | 1.09 | 30.6 ±4.6 | 20.3 ± 5.5 | ns (0.16) | 0.73 |
| Cortical | 1589.0 ±245.0 | 1047.6 ±136.8 | ns (0.07) | 0.91 | 128.3 ±22.4 | 92.3 ± 14.5 | ns (0.20) | 0.68 |
| Medial | 1153.5±134.7 | 625.8 ±101.3 | <0.01 | 1.48 | 33.8 ±10.4 | 29.3 ± 5.7 | ns (0.71) | 0.19 |
| Hippocampus | ||||||||
| Granular cell layer | 3403.9 ±393.0 | 1657.0 ±226.2 | <0.01 | 1.82 | 48.0 ±11.6 | 18.0 ±6.4 | <0.05 | 1.13 |
| Hilus | 931.4± 166.4 | 461.7 ±88.7 | <0.05 | 1.17 | 228.8 ±40.2 | 99.8 ±21.5 | <0.05 | 1.41 |
| Molecular cell layer | 1431.1 ±233.8 | 811.5 ±105.8 | <0.05 | 1.14 | 78.0 ±15.0 | 46.5 ±11.9 | ns (0.12) | 0.82 |
Cohen’s d.
Mean ± SEM.
Social isolation reduced cell proliferation, as indicated by Ki67-immunoreactivity (F (1,56)=14.03, p<0.001, ηp2=0.20), in a brain region-specific manner (F (3,56)=5.22, p<0.001, ηp2=0.22). Post-hoc analysis showed that isolated females had fewer Ki67-ir cells in the DG (Fig. 1J) and MPOA, but not the AMY (Fig. 1I) and VHM, than control females (Fig. 1L). Cell proliferation also varied depending on brain region (F (3,56)=50.95, p<0.001, ηp2=0.73); the number of Ki67-ir cells was similar between the AMY and DG but was significantly greater in these regions than in the MPOA and VMH (Fig. 1L). Furthermore, isolated females had fewer Ki67-ir cells in the central nucleus of the AMY, as well as in the hilus of the DG, compared to control females (Table 1).
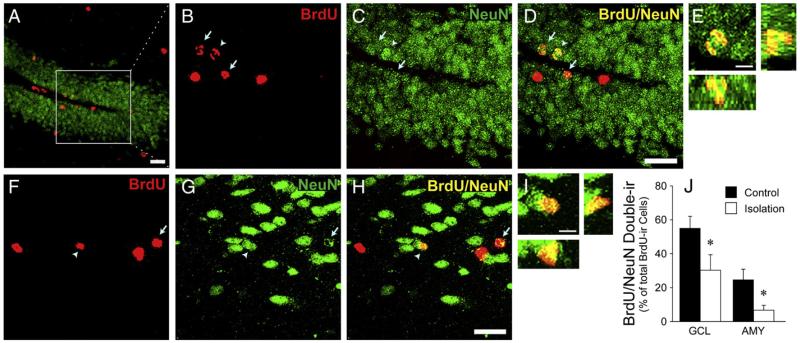
BrdU/NeuN double-labeled cells were quantified in the granular cell layer of the DG (Figs. 2A-E) and the AMY (Figs. 2F-I) to evaluate neuronal differentiation. Isolated females had a lower percentage of BrdU-ir cells that co-labeled for NeuN in the granular cell layer of the DG (t (16)=2.17, p<0.05, d=1.03) and AMY (t (16)=2.46, p<0.05, d=1.17) than control females (Fig. 2J).
Fig. 2.
Stacked confocal microscopy images illustrating labeled cells in the granular cell layer of the hippocampus (A–E) and amygdala (AMY) (F–I). Cells were labeled for BrdU (red), the mature neuronal marker NeuN (green), or both (yellow). The white box in Panel A indicates the area of the DG with high magnification (B–D; stack thickness=10 μm). Arrows indicate cells double-labeled for BrdU/NeuN. Arrowheads indicate the double-labeled cell shown in the magnified image in the DG (E) and AMY (I; stack thickness =8 μm). 3D co-localization of BrdU and NeuN is demonstrated using views along the y–z axis (right) and x–z axis (below). Scale bar=20 μm (A–D and F–H) or 5 μm (E and I). (J) Isolated females (n=8) had significantly lower percentages of BrdU-ir cells that co-labeled with NeuN in the hippocampal granular cell layer (GCL) and AMY, compared to control females (n=10). *p<0.05. Error bars represent SEM.
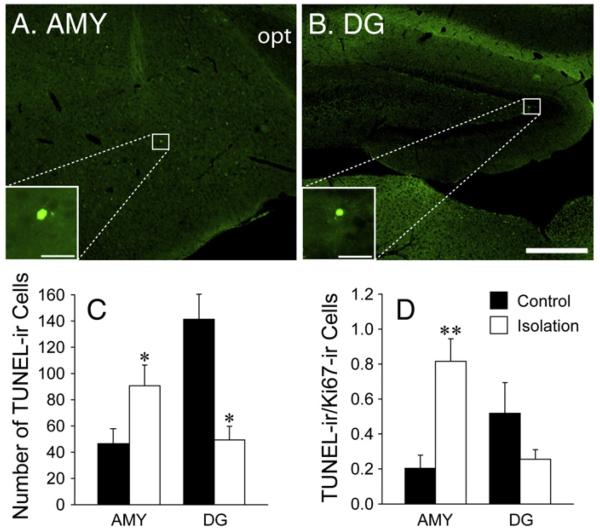
Isolated females also had a higher number of apoptotic (TUNEL-ir) cells in the AMY (t (15)=2.51, p<0.05, d=1.22), but a lower number in the DG (t (16)=2.71, p<0.05, d=1.28), than control females (Figs. 3A-C). A greater ratio of TUNEL/Ki67 was found in the AMY (t (15)=3.24, p<0.01, d=1.57), but not DG, in isolated females compared to controls (Fig. 3D).
Fig. 3.
Photo images illustrating TUNEL-labeled cells in the AMY (A) and the hippocampus (B). White boxes in Panels A and B indicate cells visualized under high magnification. opt, optic tract. Scale bar=250 μm (A and B) or 25 μm (insets in A and B). (C) Number of TUNEL-labeled cells in the AMY and DG between pair-housed (control, n=8) and single-housed (isolation, n=9) females. (D) Ratio of TUNEL-ir/Ki67-ir cells in the AMY and DG between control and isolated females. *p<0.05 and **p<0.01. Error bars represent SEM.
Effects of social isolation on anxiety- and depression-like behaviors and basal CORT levels
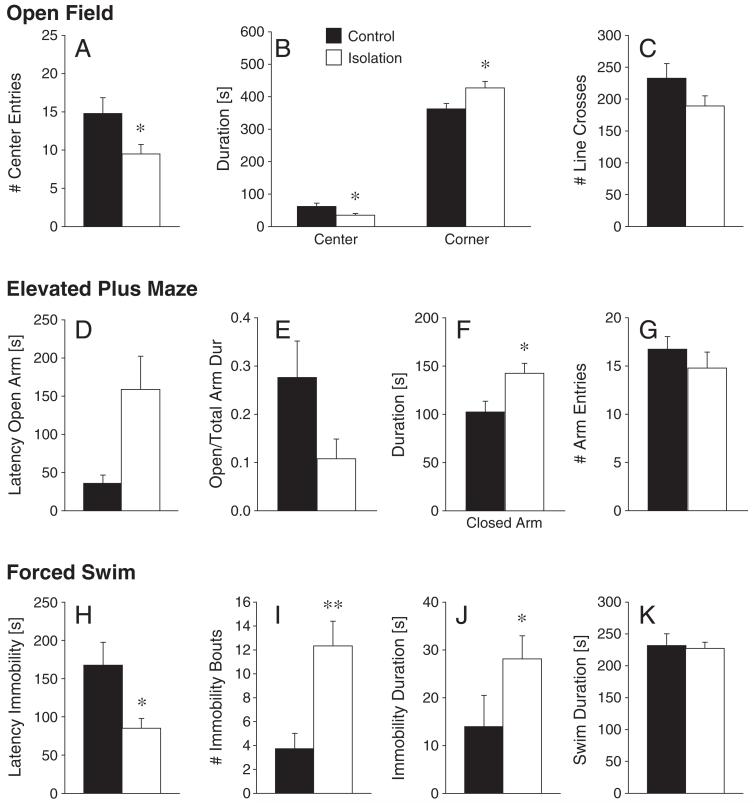
In the OF test, isolated females exhibited fewer center entries (Z=1.93, p<0.05; Fig. 4A) and spent less time in the center (Z=2.01, p<0.05) and more time in the corners (Z=2.12, p<0.05;Fig. 4B) compared to control females. The two groups did not differ in locomotion, as indicated by the frequency of line crosses (Z=1.58, p=0.12; Fig. 4C). In the EPM test, isolated females did not differ from control females in the latency to enter the open arm (Z=1.64, p=0.10; Fig. 4D). While the ratio of open to total arm duration (Z=171, p=0.087; Fig. 4E) only showed a trend to be higher in control females compared to isolated females, isolated females spent more time in the closed arms (Z=2.17, p<0.05; Fig. 4F) than control females. No group differences were observed in the open arm duration (Z=1.56, p=0.12) or in the frequency of arm entries (Z=0.69, p=0.49; Fig. 4G). In the FS test, isolated females showed a reduced latency to immobility behavior compared to control females (Z=2.16, p<0.05; Fig. 4H). Isolation also increased the number of immobility bouts (Z=3.1, p<0.01; Fig. 4I) and the immobility duration (Z=2.00, p<0.05; Fig. 4J). Swim duration (Z=0.80, p=0.42; Fig. 4K) did not differ between treatment groups. Lastly, there was no difference in the basal levels of circulating CORT between control (1217.8±187.3 ng/ml) and isolated females (1045.7±141.2 ng/ml).
Fig. 4.
Chronic social isolation in adulthood affected anxiety- and depression-like behaviors of female prairie voles. In the open field test, single-housed females (isolation, n=13) made fewer center entries (A) and spent less time in the center and more time in the corners of the apparatus (B), compared to the pair-housed females (control, n=13). The two groups did not differ in locomotor activity (C). In the elevated plus maze test, single-housed females (n=11) did not differ from control females in the latency to enter the open arm (n=13, D). While the ratio of open to total arm duration tended to be higher in control females (E), isolation females spent more time in the closed arms than control females (F). The two groups did not differ in the total number of arm entries (G). In the forced swim test, single-housed females (n=15) had a shorter latency to immobility (H) and a higher number of immobility bouts (I) than control females (n=11). Single-housed females also spent more time immobile than control females (J), while locomotion (K) did not differ between the two groups. *p<0.05 and **p<0.01. Error bars represent SEM.
Effects of social isolation on affiliative behaviors and social recognition
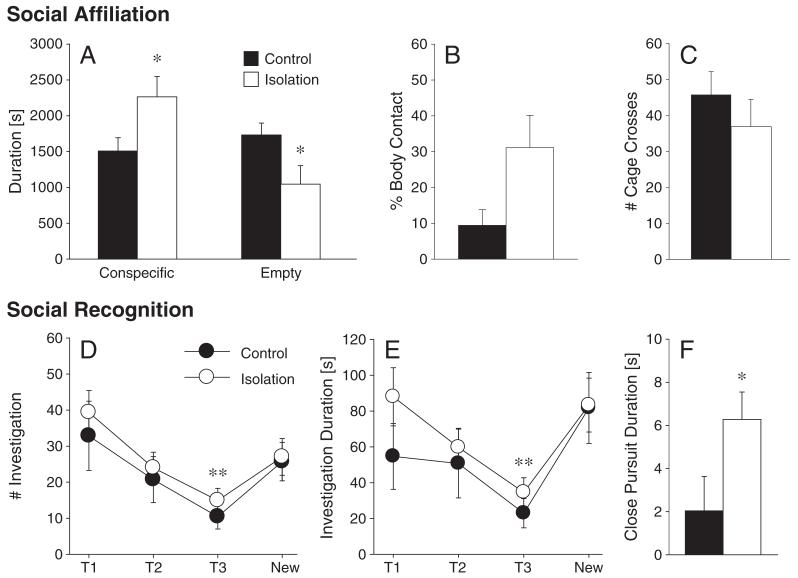
During the social affiliation test, isolated females spent more time in the cage containing a conspecific (Z=2.39, p<0.05) and less time in the empty cage (Z=2.40, p<0.05) than control females (Fig. 5A). In addition, isolation tended to increase the duration of body contact with the conspecific (Z=1.74, p=0.08; Fig. 5B). No group difference was found in locomotor activity (Z=1.28, p=0.20; Fig. 5C). In the social recognition test, there was a main effect of exposure (T1, T2, T3, New) on the frequency (F (3,48)=8.25, p<0.01, ηp2=0.34) and duration (F (3,48)=6.11, p<0.01, ηp2=0.28) of olfactory investigation (Figs. 5D and E). Post-hoc analysis revealed that the frequency and duration of olfactory investigation at T3 were significantly lower than those observed during the other trials (T1, T2, and New) (Figs. 5D and E). There was no treatment effect or treatment by exposure interaction on the frequency or duration of olfactory investigation. However, isolated females showed a greater duration of close pursuit behavior than control females (F (1,16)=16.61, p<0.05, ηp2=0.21; Fig. 5F). No group differences were observed in the frequency and duration of escape behavior.
Fig. 5.
In the two-chambered social affiliation test, single-housed females (isolation, n=11) spent more time in the cage containing a conspecific young adult female and less time in the empty cage than control females (n=11; A). Single-housed females also tended to spent more time in direct body contact with the conspecific compared to the control females (B). No group differences were found in locomotor activity (C). The social recognition test consisted of three 5-minute exposures to the same juvenile (T1, T2, and T3), followed by a 5-minute exposure to a new juvenile (New). The inter-exposure interval was 30 min. Single-housed (n=11) and control females (n=7) did not differ in the frequency and duration of olfactory investigation of the juvenile female (D, E). For both treatment groups, trial 3 (T3) differed significantly from the other trials for frequency and duration of olfactory investigation. Furthermore, close pursuit duration was higher in single-housed as compared to control females (F). *p<0.05 and **p<0.01. Error bars represent SEM.
Discussion
Social isolation, which is especially stressful for social animals (Stowe et al., 2005), can induce various neural and behavioral changes in the distressed animal and may even contribute to the occurrence of psychopathologies (e.g., depression) in humans (Fone and Porkess, 2008; Hall, 1998). Previous studies have shown that social isolation affects neurogenesis in the hippocampus of various rodent species. For example, post-weaning isolation-rearing reduces hippocampal cell proliferation, survival, and neuronal differentiation in juvenile male mice and rats (Ibi et al., 2008; Lu et al., 2003), and social isolation-housing for 3 weeks reduces hippocampal cell survival in adult female rats (Westenbroek et al., 2004). Extending these findings, our data showed that long-term social isolation housing in adults significantly reduced cell proliferation and survival in the DG of female prairie voles. Furthermore, this is, to our knowledge, the first study to report that long-term social isolation also decreased cell survival in the VMH and basolateral, central, and medial nuclei of the AMY, as well as cell proliferation in the MPOA, indicating that the social isolation-induced impairment in adult-generated cells is not limited to the hippocampus. Furthermore, early studies examining the effects of social environmental factors (e.g., social defeat and offspring interactions) on fate specification focused mainly on the DG and/or SVZ/OB (Czeh et al., 2007; Furuta and Bridges, 2009; Mak et al., 2007; Ruscio et al., 2008; Thomas et al., 2007). Our data expand these findings, showing that adult-generated neurons exhibit long-term survival (6–8 weeks) in prairie voles and that social isolation reduced the number of BrdU/NeuN double-labeled cells in the DG and in the AMY. These data indicate that social factors may also affect neuronal differentiation in non-traditional neurogenic brain regions. Finally, social isolation influenced the rate of cell death in a region-specific manner; it increased cell death in the AMY, but decreased it in the DG. More interestingly, the TUNEL/Ki67 ratio revealed that social isolation increased cell death independent of cell proliferation in the AMY. In the DG, however, the high rate of cell death in the control group may have been driven by a high rate of cell proliferation (Amrein et al., 2004). It is important to note that female prairie voles are induced ovulators and only experience an estrogen surge after 24–48 h of exposure to a conspecific male or male-associated cue (Cohen-Parsons and Carter, 1987). Therefore, observed group differences in cell proliferation and survival, neuronal differentiation, and cell death in the present study are likely not due to group differences in circulating estrogen levels. It should also be noted that although social isolation prevented physical interaction with a conspecific, single-housed females were housed in the same vivarium space as other females and were exposed to olfactory, auditory, and visual stimuli of conspecifics. Therefore, observed effects of social isolation on adult neurogenesis and behaviors in the present study should be interpreted as effects due to the lack of physical interactions with a same-sex conspecific.
Interestingly, a previous study in female prairie voles demonstrated that social isolation had no effect on hippocampal cell proliferation or cell survival in either the DG or AMY (Fowler et al., 2002). However, in the present study social isolation decreased cell proliferation in the DG and cell survival in both the DG and AMY. These discrepancies may be explained by several notable differences in the methodologies and procedures of the two studies. For example, in addition to receiving different BrdU injection paradigms, control subjects were housed with a familiar female cage mate in the present study, but with an unfamiliar female conspecific in the previous study (Fowler et al., 2002). It is possible that housing with a familiar versus unfamiliar female affected adult-generated cells differently. Further, the current study assessed the effect of long-term social isolation (6 weeks) on cell proliferation, while the previous study assessed the effect of acute social isolation (24 h) on cell proliferation. The length of chronic social isolation also differed notably between the two studies, lasting for 6 weeks in the current versus 3 weeks in the previous study. Therefore, it is also possible that the length of isolation housing plays a significant role in modulating changes in adult neurogenesis.
While the majority of studies in adult neurogenesis have focused on the DG and SVZ/OB, recent studies have shown that adult-generated cells in non-traditional neurogenic brain regions can also be influenced by endogenous and environmental factors (Fowler et al., 2002; Huang et al., 1998; Okuda et al., 2009) and that these new neurons may be important in cognitive and behavioral functions (Gheusi et al., 2009). One interesting finding from our study is that among the brain regions examined, the AMY had a much higher level of cell proliferation and survival than the MPOA and VMH. Previous studies have reported newly proliferated cells in the adult AMY (Akbari et al., 2007; Bernier et al., 2002; Fowler et al., 2002; Okuda et al., 2009) and suggested that stem cells may be present in non-neurogenic regions of the adult brain (Palmer et al., 1999). However, it is still unknown whether stem cells are present within the adult AMY. Nevertheless, an early study in prairie voles reported BrdU-labeled cells in the adult AMY 30 min following an acute BrdU injection and showed that these cells displayed morphological characteristics of local cell division (i.e., BrdU-ir cells were present in doublets or clusters), suggesting that these cells likely proliferated locally within the AMY (Fowler et al., 2003). Interestingly, in the current study, the AMY and DG showed a similar level of cell proliferation and long-term survival following the social environmental manipulation. These data provide evidence to support the notion that adult-generated cells exist in brain areas other than the DG and SVZ/OB (Fowler et al., 2002; Gould, 2007; Migaud et al., 2010; Shapiro et al., 2009) and indicate a substantial level of cell proliferation and survival in the adult AMY. As the AMY has been implicated in a variety of behavioral and cognitive functions (Cushing et al., 2003; Kollack-Walker and Newman, 1995; Lonstein et al., 1998; Wang et al., 1997), adult-generated neurons in this region may play an important role in regulating such functions—a notion similar to the suggested involvement of adult-generated SVZ/OB or DG neurons in olfactory discrimination and spatial learning and memory, respectively (Bruel-Jungerman et al., 2005; Clelland et al., 2009; Enwere et al., 2004; Gheusi et al., 2000; Kempermann and Gage, 2002; Shors et al., 2001).
Disruptions in the social environment such as social or physical stress, which usually lead to an increase in circulating CORT, impair cell proliferation and/or survival in the DG (Czeh et al., 2007; Gould et al., 1997; Paizanis et al., 2007; Thomas et al., 2007). Additionally, CORT has been shown to influence adult neurogenesis as treatment with glucocorticoids (Cameron et al., 1998) decreases, whereas adrenalectomy (Cameron and Gould, 1994; Gould et al., 1992) or treatment with a glucocorticoid receptor antagonist (Oomen et al., 2007) increases BrdU-labeling in the DG of rats. In a previous study, long-term social isolation did not alter basal levels of circulating CORT in prairie voles (Grippo et al., 2007a). This finding was replicated in the present study. Together, these data seem to suggest that the observed isolation-induced neural plasticity is likely not mediated via a mechanism involving CORT. However, one limitation in the present study is that CORT was only measured at a single time point. Since short-term/acute social isolation was reported to increase circulating CORT in female prairie voles (Kim and Kirkpatrick, 1996), we cannot exclude the possibility that a transient increase in CORT levels immediately following isolation may play a role in influencing neurogenesis in the adult vole brain. Further, it is also possible that social isolation, a mild stressor, induces fluctuations in circulating CORT, which could have been missed by our single time-point measurement and involved in mediating cell birth and/or death. Therefore, our CORT data are not conclusive. Future studies should carefully examine CORT levels at multiple time points following social isolation.
In addition to its effects on neural plasticity, social isolation altered anxiety- and depression-like behaviors in female prairie voles. Consistent with previous studies in voles (Grippo et al., 2007b, 2008; Pan et al., 2009) and in other rodent species (Wright et al., 1991), our data indicate that social isolation induced an anxiogenic response—indicative of an increased level of anxiety (Bridges and Starkey, 2004; Ferdman et al., 2007). Further, social isolation facilitated depression-like behaviors in female prairie voles, as has been reported in previous studies (Grippo et al., 2008, 2009). A novel, interesting finding in the present study is that social isolation increased affiliative behavior without altering social recognition in female prairie voles. These data suggest that it is unlikely that enhanced social affiliation was due to impaired social recognition. The presence of a conspecific may have anxiolytic consequences by eliminating the stress-related up-regulation of CORT and decreasing anxiety levels in socially isolated animals (DeVries et al., 2007; Kikusui et al., 2006). Given that no group differences were found in basal CORT levels, the observed changes in affiliation may be driven by an increase in social-seeking, rather than by anxiety-like, behaviors. In support of this notion, socially isolated male Mongolian gerbils (Meriones unguiculatus) display an increase in social investigation and passive social interaction with conspecifics (Shimozuru et al., 2008), socially isolated marmosets (Callithrix geoffroyi) display increased levels of affiliative behaviors (Smith et al., 2011), and isolated rats show a conditioned place preference for a cage associated with a con-specific, suggesting that the restriction of social experience may make social interactions more rewarding (Douglas et al., 2004).
Adult-generated neurons in the brain may play an important role in cognitive and behavioral functions (Imayoshi et al., 2009). For example, new OB neurons facilitate olfactory discrimination and individual recognition (Enwere et al., 2004; Mak and Weiss, 2010) while those in the hippocampus are important in the acquisition or long-term retention of a spatial learning and memory task, object recognition, and associative memory formation (Clelland et al., 2009; Jessberger et al., 2009; Shors et al., 2001). New neurons in the hypothalamus are involved in energy balance (Kokoeva et al., 2005) and those in the SVZ seem to mediate maternal behavior (Furuta and Bridges, 2009). In the AMY, the addition of new neurons is important in establishing and/or maintaining structural sexual dimorphisms in rats (Ahmed et al., 2008). Therefore, we have good reason to speculate that impaired adult neurogenesis might contribute to the behavioral changes noted following social isolation. It is intriguing to see that social isolation altered adult neurogenesis in several nuclei of the AMY that have been implicated in distinct functions. For example the basolateral nucleus plays a role in fear conditioning (Vazdarjanova and McGaugh, 1999), the central nucleus is involved in the stress response (Roozendaal et al., 1990), and the cortical and medial nuclei are important for olfactory discrimination/memory (Ferguson et al., 2001) and social behaviors (Kirkpatrick et al., 1994; Maras and Petrulis, 2008). Therefore, social isolation-induced alterations in neurogenesis in each of the nuclei in the AMY may affect different behaviors, respectively. The possible neurogenesis-dependent mechanism as well as the causal relationship between the observed changes in region-specific adult neurogenesis and altered behavior should be further examined. Finally, although the current study focused on females, given the similar effects of social isolation rearing on anxiety-like behaviors in male prairie voles (Pan et al., 2009) and the enhanced hippocampal cell proliferation by pup exposure in both males and females (Ruscio et al., 2008), one may assume that chronic social isolation has similar effects on adult neurogenesis and behaviors in male prairie voles. This speculation needs to be examined in further studies.
Supplementary Material
Acknowledgments
We are grateful to Dr. Kimberly A. Young, Kelly Lei, and Adam S. Smith for their critical reading of the manuscript and to Louis V. Frosch, Kyle L. Gobrogge, and Sarah Huth for their technical assistance. In addition, we thank Adam S. Smith for his assistance with the plasma corticosterone radioimmunoassay. This research was supported by NIH grants MHR01-89852 and MHR01-58616 to ZXW.
Abbreviations
- AFF test
affiliation test
- AMY
amygdala
- BrdU
5-bromo-2′-deoxyrudine
- DG
dentate gyrus
- EPM test
elevated plus maze test
- FS test
forced swim test
- MPOA
medial preoptic area
- OB
olfactory bulb
- OF test
open field test
- SOC test
social recognition test
- SVZ
subventricular zone
- VMH
ventromedial hypothalamus
Footnotes
Author contributions
CL and ZW designed the research; CL, XJ and YL performed the research; CL analyzed the data; CL and ZW wrote the manuscript. All authors contributed to and have approved the final version of the manuscript.
Conflict of interest
The authors have no financial disclosures and have no potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.yhbeh.2012.03.005.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari EM, Chatterjee D, Levy F, Fleming AS. Experience-dependent cell survival in the maternal rat brain. Behav. Neurosci. 2007;121:1001–1011. doi: 10.1037/0735-7044.121.5.1001. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Lipp HP. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur. J. Neurosci. 2004;20:3342–3350. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp. Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges NJ, Starkey NJ. Sex differences in Mongolian gerbils in four tests of anxiety. Physiol. Behav. 2004;83:119–127. doi: 10.1016/j.physbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur. J. Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiol. Behav. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic inter-neurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner: social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav. Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J. Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J. Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J. Comp. Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res. Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res. Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. A niche for adult neurogenesis in social behavior. Behav. Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007a;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry. 2007b;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress. Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin. Exp. Pharmacol. Physiol. 2007;34:533–545. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit. Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J. Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev. Growth Differ. 2009;51:379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr., Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur. J. Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol. Psychiatry. 1996;40:918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav. Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J. Comp. Neurol. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp. Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus) Neuroscience. 2008;156:425–435. doi: 10.1016/j.neuroscience.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur. J. Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Paizanis E, Kelai S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochem. Res. 2007;32:1762–1771. doi: 10.1007/s11064-007-9330-0. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Differential effect of lesioning of the central amygdala on the bradycardiac and behavioral response of the rat in relation to conditioned social and solitary stress. Behav. Brain Res. 1990;41:39–48. doi: 10.1016/0166-4328(90)90052-g. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav. Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ng K, Zhou QY, Ribak CE. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav. 2009;14(Suppl. 1):74–80. doi: 10.1016/j.yebeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Effects of isolation-rearing on the development of social behaviors in male Mongolian gerbils (Meriones unguiculatus) Physiol. Behav. 2008;94:491–500. doi: 10.1016/j.physbeh.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev. Dyn. 1998;213:220–227. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm. Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol. Behav. 2011;104:955–961. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol. Behav. 2005;86:369–378. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J. Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J. Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, Buwalda B, Neumann ID, Koolhaas JM, Lucassen PJ. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J. Neuroendocrinol. 2007;19:489–498. doi: 10.1111/j.1365-2826.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, TerHorst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res. Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Curr. Protoc. Neurosci. 2003;16 doi: 10.1002/0471142301.ns0816s22. Chapter 8, Unit 8. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.