Abstract
There are currently no proven effective treatments that can improve recovery of function in spinal cord injury (SCI) patients. Many therapeutic compounds have shown promise in pre-clinical studies, but clinical trials have been largely unsuccessful. P-glycoprotein (Pgp, Abcb1b) is a drug efflux transporter of the blood–spinal cord barrier that limits spinal cord penetration of blood-borne xenobiotics. Pathological Pgp upregulation in diseases such as cancer causes heightened resistance to a broad variety of therapeutic drugs. Importantly, several drugs that have been evaluated for the treatment of SCI, such as riluzole, are known substrates of Pgp. We therefore examined whether Pgp-mediated pharmacoresistance diminishes delivery of riluzole to the injured spinal cord. Following moderate contusion injury at T10 in male Sprague–Dawley rats, we observed a progressive, spatial spread of increased Pgp expression from 3 days to 10 months post-SCI. Spinal cord uptake of i.p.-delivered riluzole was significantly reduced following SCI in wild type but not Abcb1a-knockout rats, highlighting a critical role for Pgp in mediating drug resistance following SCI. Because inflammation can drive Pgp upregulation, we evaluated the ability of the new generation dual anti-inflammatory drug licofelone to promote spinal cord delivery of riluzole following SCI. We found that licofelone both reduced Pgp expression and enhanced riluzole bioavailability within the lesion site at 72 h post-SCI. This work highlights Pgp-mediated drug resistance as an important obstacle to therapeutic drug delivery for SCI, and suggests licofelone as a novel combinatorial treatment strategy to enhance therapeutic drug delivery to the injured spinal cord.
Key words: drug resistance, licofelone, P-glycoprotein, riluzole, SCI
Introduction
Over the past several decades, research in the field of spinal cord injury (SCI) has identified a number of neuroprotective compounds that can improve recovery of locomotor function in animal models of SCI.1 However, despite promising results in pre-clinical studies, translation of these therapies to the clinic has been largely unsuccessful. To date, none of the therapeutic interventions that have been evaluated in clinical trials have led to sufficiently robust functional improvement in human SCI patients. Despite an immense need, there are still no proven effective therapeutic interventions available to improve functional recovery following SCI.
P-glycoprotein (Pgp, Abcb1), a key component of the blood–spinal cord barrier (BSCB), is a drug transporter with a well-known role in the formation of drug resistance in a variety of pathological conditions, most famously in cancer.2,3 Pgp is a member of the adenosine triphosphate (ATP)-binding cassette (ABC) transporter family of proteins that actively transport substrates between different biological compartments, and which are particularly important for normal function of blood–tissue barriers.4 Pgp is expressed in multiple tissues of the body, including on the luminal membrane of capillary endothelial cells lining the blood–brain and blood–spinal cord barriers.5 Here, this highly promiscuous transporter limits the blood-to-central nervous system (CNS) penetration of diverse compounds, including endogenous substrates (e.g., opioid peptides, bile acids, beta-amyloid), as well as a large number of therapeutic drugs.5 It has been known for decades that cancer cells can overexpress Pgp and thereby become simultaneously cross-resistant to multiple types of chemotherapeutic drugs.2,6–8 More recently, several pre-clinical and clinical studies have shown that in neurological diseases characterized by high levels of excitotoxicity, oxidative stress, and inflammation in the brain, Pgp can become pathologically overexpressed at the blood–brain barrier, dramatically diminishing delivery of therapeutic drugs to the CNS.9–14 This Pgp-mediated pharmacoresistance is now recognized as a major barrier to the effective treatment of not only cancer, but of multiple neurological diseases as well.15
Significantly, several drugs that have been evaluated as potential SCI interventions, or that are used clinically for the treatment of pain in SCI patients, are known substrates of Pgp (Table 1)16–53. Among these drugs is riluzole, an anti-glutamatergic compound that is United States Food and Drug Administration (FDA) approved for the treatment of amyotrophic lateral sclerosis (ALS). Riluzole is currently being evaluated in a phase I clinical trial as an acute SCI intervention.44 Milane and colleagues have shown that pathological overexpression of Pgp in the brain diminishes blood-to-brain delivery of riluzole in a mouse model of ALS expressing mutant human superoxide dismutase 1 (SOD1).9 More recently, elevated Pgp expression has also been reported in spinal cords of human ALS patients.54 It is therefore plausible that if spinal cord drug resistance develops following traumatic SCI, the delivery and therapeutic efficacy of riluzole might also be decreased in SCI patients. This drug resistance would have dramatic clinical implications with the capacity to impact not only the current riluzole clinical trial, but the efficacy of other neuroprotective drugs for SCI, as well as the treatment of patients with chronic SCI pain and spasticity (Table 1).
Table 1.
Several Drugs that Have Been Evaluated for the Treatment of Spinal Cord Injury are Substrates of P-Glycoprotein
| Drug | Pre-clinical evidence of therapeutic benefits for SCI | Clinical trials and/or clinical use | Evidence that P-glycoprotein activity affects therapeutic efficacy and/or CNS Disposition |
|---|---|---|---|
| Methylprednisolone sodium succinate (MPSS) | MPSS exhibits neuroprotective effects in pre-clinical models of acute SCI16–20 | NASCIS I: No benefit of MPSS over naloxone or placebo21 NASCIS II: Post-hoc analysis detected modest neurological improvement in a subset of patients receiving MPSS 3–8 h post-injury;22,23 subsequently criticized because of questionable statistical analysis and lack of clinically significant functional outcome measures24,25 NASCIS III: No significant difference in motor recovery or FIM with 24 h or 48 h MPSS treatment26 The clinical use of MPSS for acute SCI has greatly declined, as the evidence suggesting harmful medical side effects of high-dose MPSS is more consistent than evidence suggesting its therapeutic benefit.27 |
Methylprednisolone is a Pgp substrate28 Intravascular delivery of MPSS results in lower spinal cord bioavailability than intrathecal delivery in pigs; spinal cord penetration of i.p. MPSS is increased in Pgp knockout mice.29 Pgp inhibition with cyclosporin-A increased CSF levels of i.v.-delivered MPSS in the pig.30 |
| Minocycline | Neuroprotective effects for acute SCI31–34 Attenuated neuropathic pain in SCI rats35 |
Phase I/II trial, Minocycline and Perfusion Pressure Augmentation in Acute Spinal Cord Injury (NCT00559494)36 Current status: Currently recruiting |
Minocycline is a substrate and inhibitor of Pgp37 |
| Riluzole | Neuroprotective effects for pre-clinical models of acute SCI38–41 Reversed neuropathic pain behavior in SCI rats42 Attenuated spastic muscle activity in rats43 |
Phase I trial, Safety of Riluzole in Patients with Acute Spinal Cord Injury (NCT00876889)44 Current status: Ongoing, but not recruiting participants |
Riluzole is a Pgp substrate37 Pgp overexpression in the mSOD1 mouse brain decreases brain concentrations of i.p.-delivered riluzole9 |
| Nimodipine | Improved spinal cord axonal function and blood flow in pre-clinical SCI models45 | Phase III RCT (France): Compared with MPSS and placebo, no difference detected between groups (study likely underpowered)46 Blind RCT (France): Compared to MPSS, no neurological differences detected between groups47 |
Nimodipine is a Pgp substrate48 |
| Lamotrigine | - | Lamotrigine is used clinically as a pain treatment in SCI patients49,50 | Lamotrigine is a Pgp substrate51,52 |
| Amitriptyline | - | Amytriptyline is used clinically as a pain treatment in SCI patients49 | Amitriptyline is a Pgp substrate53 |
SCI, spinal cord injury; CNS, central nervous system; FIM, functional independence measure; NASCIS, North American Spinal Cord Injury Study; CSF, cerebrospinal fluid; RCT, randomized controlled trial.
Pharmacological inhibition of Pgp activity is not a viable therapeutic solution to overcome drug resistance, because of harmful side effects associated with the clinical use of Pgp inhibitors.15,55 Therefore, a more attractive strategy is to antagonize the molecular mechanisms driving Pgp overexpression.15 Recent studies have revealed that cyclooxygenase (COX) signaling upregulates Pgp expression in rodent seizure models as well as in experimental models of cancer.56–61 As heightened COX activity is a major component of the secondary injury cascade following SCI, we sought to investigate the possibility that this signaling pathway also underlies Pgp overexpression within the injured spinal cord.62,63 COX inhibitors possess deleterious side effects including high incidence of gastrointestinal and cardiovascular side effects, as well as the shunting of arachidonic acid (AA) into the parallel, pro-inflammatory 5-lipoxygenase (5-LOX) pathway.64–72 Therefore, as a more clinically viable strategy, we instead sought to evaluate the efficacy of the dual COX/5-LOX inhibitor licofelone, a new generation anti-inflammatory drug that has successfully passed phase III clinical trials for osteoarthritis in Europe, with improved gastrointestinal (GI) tolerability compared with classical COX inhibitors.73,74
Using a clinically relevant model of contusion/compression injury in rats, we have investigated spatial and temporal Pgp expression in the injured spinal cord, and examined the role of Pgp in mediating blood-to-spinal cord delivery of riluzole. Furthermore, we have evaluated the ability of the dual anti-inflammatory drug licofelone to diminish Pgp-mediated drug resistance within the injured spinal cord. Here, we describe the existence of a novel and important obstacle to therapeutic drug delivery following SCI. We also present a novel combinatorial treatment strategy to overcome Pgp-mediated drug resistance and promote drug delivery to the injured spinal cord.
Methods
Animals and surgeries
All applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol used in this study was approved by The University of Texas Health Science Center Institute for Animal Care and Use Committee.
Animals
A total of 81 adult female Sprague–Dawley rats weighing 250–300 g were used for studies of Pgp expression without drug treatment. A total of 101 adult male Sprague–Dawley rats weighing 250–300 g were used for all studies utilizing treatment with licofelone and/or riluzole. All wild type (WT) Sprague–Dawley rats were purchased from Harlan Laboratories (Houston, Texas).
Transgenic mice
Adult female Abcb1a/b constitutive double knockout (KO) mice (FVB.129P2-abcb1atm1Borabcb1btm1BorN12, n=5) and WT mice (FVB.129P2, n=5) were purchased from Taconic (Germantown, NY).
Transgenic rats
Four-week-old male Abcb1a KO rats (SD-abcb1atm1sage, n=15) were purchased from SAGE Labs (St. Louis, MO). KO rats were generated using zinc finger nuclease (ZFN) gene-targeting technology, as previously described.75 Briefly, ZFN targeting the genetic sequence of interest is microinjected into the nucleus of a single-cell rat embryo. This induces cleavage of the nucleic acid target site and yields embryos lacking expression of the targeted gene.
All transgenic animals exhibited normal phenotypes, and were indistinguishable from WT animals. WT mice and rats were purchased separately from transgenic mice and rats, and were not derived from littermates of transgenic animals. All studies were blinded and randomized.
SCI surgery
All surgical procedures were performed on animals that were deeply anesthetized with a ketamine cocktail (ketamine [80 mg/kg], xylazine [10 mg/kg], acepromazine [0.75 mg/kg]) at a dose of 0.01 ml/kg body weight.76 SCI surgeries were performed as previously described.77,78 Incisions were made on the animals' dorsal skin and overlying muscles and the vertebral column was exposed. A laminectomy was performed at thoracic level 10 (T10) and the vertebral column was stabilized using forceps that grasp the ventral surface of the lateral spinous processes at vertebral levels T9 and T11. Using an Infinite Horizon Spinal Impactor Device (Precision Systems and Instrumentation, LLC, Fairfax Station, VA), moderate contusion/compression injuries were delivered to the T10 spinal cord using 150 kdynes of force with a 1 sec dwell. The dura mater remained closed for the entire duration of SCI surgeries. Immediately following SCI, the overlying muscles were sutured and the skin was securely closed using stainless steel wound clips.
Postoperative animal care
Beginning on the day of surgery, animals received Baytril injections (2.5 mg/kg s.c., b.i.d.) for 10 days to prevent postoperative infections. Beginning the day after SCI surgery, each rat's urinary bladder was manually expressed two to three times daily until the animal recovered the ability to void its bladder. As a rule, bladder care was discontinued for an individual animal when it exhibited an already-voided bladder on two consecutive bladder care sessions. To minimize pain, animals received buprenorphine injections (0.02 mg/kg s.c., b.i.d.) for 1 week following surgery. To prevent dehydration, animals received 0.9% saline (3-5 mL s.c.) daily for 5 days following surgery.
Exclusion criteria
To maximize reproducibility of results, spinal cord-injured animals were excluded from this study if the force of impact to the spinal cord fell outside a range between 150 and 175 kdynes.78 Additionally, injured animals exhibiting a Basso, Beattie, and Bresnahan open field locomotor score>2 on postoperative day 1 were also excluded.79 Based on the above-described criteria, 14 animals were excluded from this study.
Drug treatment
Licofelone (Santa Cruz Biotechnology, cat. #sc-207826, lot #J1411) was suspended in 0.5% carboxymethylcellulose in 0.9% saline (vehicle) to a final concentration of 50 mg/mL, and administered to animals via oral gavage (100 mg/kg). Riluzole (Sigma-Aldrich, cat. #R116, lot #057K3900V) was dissolved in ethanol to a concentration of 50 mg/mL, and this stock solution was stored at −20°C for up to 3 months. Riluzole stock solution was diluted with vehicle to a final working concentration of 2.5 mg/mL, and administered to animals (8 mg/kg i.p.). All working drug solutions were prepared fresh daily. Experimenters were blinded to drug sample source.
Tissue processing
All animals were deeply anesthetized with beuthanasia (390 mg/mL pentobarbital sodium, 50 mg/mL phenytoin sodium) (75 mg/kg i.p.). For all of the following experiments (except microarray and quantitative reverse transcription polymerase chain reaction [qRT-PCR] studies, for which perfusion was not performed), animals were transcardially perfused with ice-cold saline. Tissue was then harvested, immediately snap-frozen on either dry ice or liquid nitrogen, and stored at −80°C.
Microarray analysis
Spinal cord tissue from T10 was harvested immediately following euthanasia and snap-frozen in liquid nitrogen. Total RNA from spinal cord tissue was isolated using the mirVana miRNA Isolation Kit (Ambion). RNA was applied to RatRef 12 whole genome arrays (Illumina). Arrays were hybridized, washed, and scanned by The University of Texas Medical School Microarray Core Laboratory. Data was analyzed with Illumina BeadStudio software. Group data were compared by Student's t test.
Quantitative real-time PCR
DNase-treated total RNA, isolated as described, was reverse transcribed using the High Capacity Reverse transcriptase kit (Ambion). The cDNA was amplified in the presence of gene specific primers and fluorescent probes with TaqMan Fast Universal reagents (Applied Biosystems, Inc.). Abcb1b (Rn01529260_m1) expression was examined and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (Gapdh, Rn01775763_g1) was used as an internal control. Expression levels of Abcb1b were normalized to Gapdh expression levels. Data collection and Ct analysis was performed with a StepOne Plus real-time thermocycler and associated software (Applied Biosystems, Inc.). Data were analyzed by one way ANOVA, followed by the Bonferroni post test.
Immunohistochemistry
For all immunohistochemical procedures, frozen spinal cord tissue was cryosectioned in the coronal plane to a thickness of 10 μm. Sections were directly mounted to gelatinized slides, air dried, and post-fixed in 4% paraformaldehyde for 15 min at 4°C, then in 1:2 acetic acid: methanol solution for 10 min at −20°C.
Pgp staining
Following post-fixation, sections were washed three times for 10 min in tris-buffered saline (TBS, 10 mM Tris, pH 8.0; 150 mM NaCl), then incubated for 30 min in H2O2 buffer (0.6% H2O2 in TBS) at room temperature. Sections were washed twice for 10 min in TBS, then blocked with blocking-permeabilization buffer (BPB) (5% normal horse serum in TBS, 0.1% Triton-X-100) for 1 h. Sections were then incubated in BPB containing primary antibody (1:250 mouse monoclonal anti-P-glycoprotein C219, Calbiochem) for 2 h at room temperature, then washed three times for 10 min in TBS. Samples were incubated in BPB containing 1:200 biotinylated anti-mouse IgG antibody (Vector Labs) for 3 h at room temperature, then washed three times for 10 min in TBS. Samples were processed with the Vectastain ABC Kit (Vector Labs), and immunoreactivity was then visualized by incubation in diaminobenzidine (DAB) solution. Samples were counter-stained with hematoxylin to visualize nuclei. Sections were dehydrated, mounted, and cover-slipped with Fluoromount-G (Fisher).
Immunofluorescence
Following post-fixation, sections were washed three times for 10 min in TBS, then nonspecific IgG was blocked with 5% normal serum for 1 h. Sections were then incubated in TBS containing primary antibodies against P-glycoprotein (1:250 mouse monoclonal anti-P-glycoprotein C219, Calbiochem), glial fibrillary acidic protein (GFAP) (1:1000 rabbit polyclonal, Dako), von Willebrand factor (1:1500 rabbit polyclonal, Sigma-Aldrich), ED2/CD163 (1:750 mouse monoclonal, Serotec), or 5-lipoxygenase (1:1000 goat polyclonal, Novus Biologicals) overnight at 4°C on a rotating shaker. Sections were washed three times for 10 min in TBS, and incubated with Alexa Fluor conjugated secondary antibodies (1:500, Invitrogen) for 3 h. Sections were washed, dried, and cover-slipped with Fluoromount-G. Every sixth section in the series was chosen for analysis.
For all immunohistochemical experiments, labeled sections were imaged using an Olympus BX61 wide-field upright microscope with fluorescence optics with a SPOT Flex microscope digital camera. For Figures 3B and 3C, images were captured using an Olympus FluoView 1000 confocal microscope (sample box size of 800×800 pixels, under a 40×objective). For Figure 3B, both image panels were captured using identical exposure settings for qualitative comparison of Pgp immunoreactivity.
FIG. 3.
P-glycoprotein (Pgp) expression is localized to blood vessels 7 days following spinal cord injury (SCI). Pgp immunoreactivity (green) co-localizes with blood vessel endothelial cell markers within the spinal cord lesion site 1 week post-SCI (n=6). (A) Pgp co-localizes with spinal cord vasculature and not with macrophages. von Willebrand factor (vWF), blue; ED2, rat mature macrophage antigen, red. Scale bar=100 μm. (B) Vascular Pgp immunoreactivity is increased at 1 week post-SCI compared with uninjured controls (n=6). Scale bar=100 μm. (C) Pgp does not co-localize with astrocytes. Glial fibrillary acidic protein (GFAP), red. Scale bar=50 μm.
Immunoblotting
Tissue samples were homogenized in Tissue Protein Extraction Reagent (T-PER, Thermo Scientific) containing protease inhibitor cocktail tablets (Complete Mini, Roche) using an electric tissue homogenizer. Lysate was centrifuged at 14,000g for 30 min at 4°C, and the supernatant was removed and stored at −80°C. Protein concentration of samples was determined using a Pierce BCA Protein Assay (Thermo Scientific), using bovine serum albumin as the standard. Approximately 50–100 μg of tissue lysate was resolved onto polyacrylamide Tris-HEPES-SDS gels (Thermo Scientific) under reducing conditions. HeLa cell lysate (Santa Cruz Biotechnology) was used as a positive control for immunodetection of Pgp. Equality of loading was confirmed by Coomassie Brilliant Blue staining of gels. Proteins were transferred to Immobilon-FL PVDF membranes (Millipore) using a semi-dry transfer apparatus. For Pgp detection, transfer buffer was prepared without methanol to optimize large protein transfer efficiency. Following transfer, nonspecific antigens were blocked by incubation in Odyssey Blocking Buffer (LI-COR Biosciences) for 1 h at 4°C. Blots were then incubated in blocking buffer containing primary antibodies at the following concentrations:
P-glycoprotein (C219): 1:250 mouse monoclonal, Calbiochem (#513710)
Mdr-1 (D-11): 1:100 goat polyclonal, Santa Cruz Biotechnology (#sc-55510)
Beta-actin: 1:20,000 mouse monoclonal, Abcam (#ab6276)1:20,000 rabbit polyclonal, Abcam (#ab8227)
Cyclooxygenase-1: 1:200 mouse monoclonal, Cayman Chemical (#160110)
Cyclooxygenase-2: 1:200 rabbit polyclonal, Cayman Chemical (#160126)
5-Lipoxygenase: 1:250 mouse monoclonal, BD Biosciences (#610694)
Membranes were incubated in primary antibodies at room temperature for 1 h, or at 4°C overnight. Blots were then washed in TBS containing 0.1% Tween-20 (TBST), five times for 5 min, then incubated in blocking buffer containing species-specific IRDye-conjugated infrared secondary antibodies (1:500, LI-COR Biosciences). Blots were imaged using the Odyssey Infrared Imaging System (LI-COR Biosciences). This detection method has the advantages of high sensitivity of detection and increased signal-to-noise ratio over chemiluminescence, as well as capability to detect two different antigens on the same blot simultaneously. Band intensity was quantified using Odyssey software, and data were analyzed using Student's t test (when two groups were compared), or by one way ANOVA followed by the Bonferroni post test (when more than two groups were compared).
High-performance liquid chromatography (HPLC)
All reagents used for HPLC experiments were HPLC-grade (ChromaSolv, Sigma-Aldrich). Riluzole stock solutions (1 mM) were prepared by dissolving riluzole in 1:1 H2O:CH3OH. Stock solutions were stored for up to 3 months at −20°C. Serial dilutions of riluzole stock solutions were prepared and known amounts of riluzole were injected onto the HPLC system in the following quantities: 0.05, 0.1, 0.5, 1, and 2 nmoles. A calibration curve was generated using Empower 3 Chromatography Data Software program (Waters). The R2 value obtained for the riluzole calibration curve was 0.998.
Tissue extraction of riluzole
Animals were injected with riluzole (i.p.), and euthanized 2 h later. Animals were transcardially perfused with ice-cold saline, and spinal cords were immediately harvested, rinsed, and snap-frozen on dry ice.
Spinal cord riluzole extraction
A 1.5 cm segment of spinal cord tissue centered around the lesion site was homogenized, and riluzole was extracted with ethyl acetate as previously described.80 Samples were re-suspended in 100 μL mobile phase buffer.
Plasma riluzole extraction
Whole blood was obtained by cardiac puncture immediately following euthanasia, and plasma was recovered by centrifugation at 1,300g for 10 min at 4°C. Plasma samples were diluted 1:2 in 0.01 M phosphate buffer, pH 7.4 and riluzole was recovered via solid-phase extraction as previously described.81 Samples were re-suspended in 75 μL mobile phase buffer and filtered through Millex-GV4 0.22 μM membranes (Millipore) before injection onto the HPLC system.
HPLC separation and analysis
The HPLC system consisted of a Waters 515 HPLC pump, a Waters 996 Photodiode Array Detector, and a CI-10B integrator (LDC Analytical). Separation was achieved through a ZORBAX Extend-C18 column (4.6×150 mm) (Agilent Technologies) with a 20-mm ODS pre-column (Custom LC, Inc.). The mobile phase consisted of 68% CH3OH and 32% of (1% triethylamine [TEA] in H2O, adjusted to pH 3.2 with H3PO4). The flow was isocratic with a constant flow rate of 0.5 mL/min. The sample injection volume was 100 μL (spinal cord samples) or 75 μL (plasma samples). Absorbance was continuously monitored at a wavelength of 254 nm. Riluzole peaks (retention time=∼9 min) were identified and integrated at 264 nm, and quantified using previously run calibration curves. Naïve spinal cord and plasma samples were spiked with 0.5 nmoles riluzole and extracted, then analyzed by HPLC to determine percent recovery of riluzole (63.2% in spinal cord; 76.4% in plasma). Riluzole concentrations in spinal cord samples were normalized to plasma concentrations to obtain a spinal cord/plasma ratio for each animal. Riluzole concentrations were analyzed by one way ANOVA followed by the Student–Newman–Keuls post test.
Statistical analysis
All statistical analysis was performed using SigmaPlot 11 (Systat Software, Inc., Chicago, IL) and GraphPad Prism 5. Student's t test was used for comparisons between two groups. One way ANOVA followed by the Bonferroni post test was used for comparisons among more than two groups. p values<0.05 were considered statistically significant.
Results
Expression of ABC transporter genes after SCI
Previous work has shown that the BSCB undergoes multiple structural and functional alterations after traumatic SCI, leading to a loss of functional integrity and passive diffusion of blood-borne molecules into the spinal cord tissue.82–85 However, expression of active transporters in the BSCB following SCI has remained largely uninvestigated. We performed microarray analysis to detect changes in expression of ABC transporter genes within the spinal cord lesion site at 1 week after SCI compared with uninjured controls (Table 2). Microarray data revealed increased expression of several gene family members at 1 week post-SCI. Of these, Abcb1b, encoding the Abcb1b isoform of Pgp, exhibited the most dramatic upregulation with 10-fold higher expression levels versus controls (+10.05-fold, p<0.001). (At the time of this study, the Illumina microarray chip did not contain a probe against the Abcb1a isoform.) Additionally, expression of several ABC transporter genes, such as Abcc2 (−13.92-fold, p<0.001), were found to be decreased in the acutely injured spinal cord.
Table 2.
Differential Expression of ABC Transporter Genes 7 Days after Spinal Cord Injury
| Gene | Synonym | Fold change vs. uninjured |
|---|---|---|
| Abca3 (predicted) | 1.97 | |
| Abca5 | −2.59 | |
| Abcb1 | Mdr1; Pgy1; Abcb1b | 10.05 |
| Abcb4 | Mdr2; Pgy3 | 6.07 |
| Abcb6 | −1.71 | |
| Abcb7 | 2.06 | |
| Abcb9 | Tap1 | −3.91 |
| Abcc2 | −13.92 | |
| Abcc3 | Mlp2; Mrp3 | 2.14 |
| Abcc6 | Mrp6 | −3.44 |
| Abcc8 | Sur | −3.00 |
| Abcd2 | Aldr | −5.54 |
| Abcd3 | Pmp70; Pxmp1 | −1.55 |
| Abcd4 (predicted) | 2.27 | |
| Abcf2 (predicted) | −1.59 | |
| Abcf3 (predicted) | −1.38 | |
| Abcg1 | Abc8 | 3.36 |
| Abcg2 | −1.62 | |
| Abcg4 (predicted) | −1.51 |
Microarray gene expression data for ABC transporter family members exhibiting significantly altered expression 7 days following SCI in the rat T10 spinal cord. Gene expression for SCI group (n=6) is shown as fold change versus levels of uninjured control group (n=6). Abcb1b (bold) encodes rat P-glycoprotein. p<0.01 versus uninjured controls for all gene expression data included in this table.
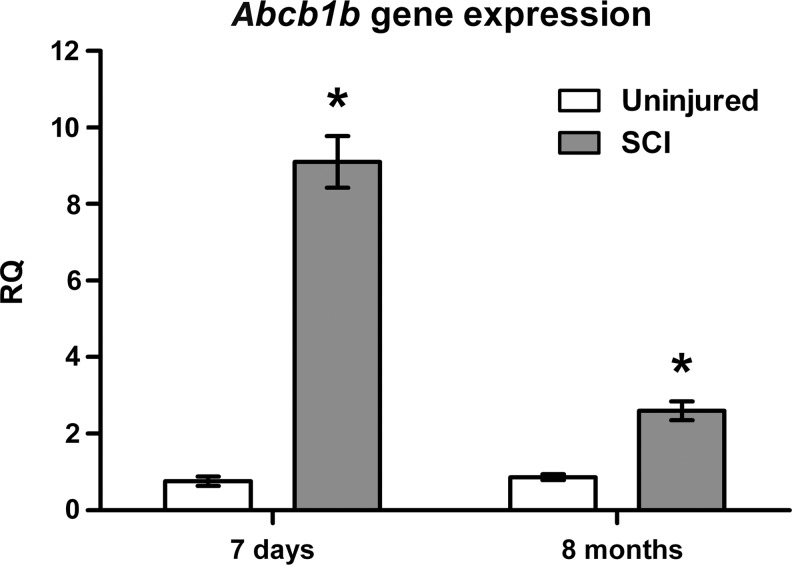
To further examine Pgp expression levels within the spinal cord at both acute and chronic time points after injury, we performed quantitative real time PCR (qRT-PCR) analysis of Abcb1b expression (Fig. 1). Our results revealed a 12-fold increase in the lesion site of rats 7 days post-SCI (relative quantification [RQ]=9.10±0.67) compared with expression in uninjured control animals (0.76±0.12). Moreover, Abcb1b gene expression was also increased three-fold in the spinal cord lesion site of rats with chronic injuries, 8 months post-SCI (RQ=2.60±0.24) compared with age-matched, uninjured controls (0.87±0.077), suggesting that upregulation of Pgp expression is sustained long into the chronic phase of injury.
FIG. 1.
Expression of Abcb1b is increased during acute and chronic spinal cord injury (SCI). qRT-PCR gene expression data for T10 spinal cord tissue from rats 7 days (n=3) and 8 months post-SCI (n=5), as well as age-matched, uninjured controls (n=4–5). RQ, relative quantity of Abcb1b expression (fold change) normalized to Gapdh expression. All data are mean±S.E.M. *p<0.001 versus uninjured, age-matched controls.
Expression of P-glycoprotein within the injured spinal cord
Because our gene expression data indicated that spinal cord Pgp expression is upregulated at acute and chronic time points following injury, we next examined immunohistochemical localization of Pgp in order to characterize whether injury alters its sites of expression within the spinal cord. In the brain, Pgp expression is restricted to the luminal membrane of capillary endothelial cells.5 Similarly, we observed Pgp immunoreactivity only on blood vessel endothelial cells in the uninjured spinal cord (Fig. 2A and B). Immunohistochemical staining revealed strong Pgp localization on the luminal surface of capillary endothelial cells in the spinal cord (Fig. 2C), consistent with previous findings.87 We found that at 1 week post-SCI, Pgp immunoreactivity was increased but remained restricted to capillary endothelial cells, and did not co-localize with other cell types such as macrophages or astrocytes (Fig. 3). Because Pgp expression remained localized to spinal cord blood vessels after injury, for subsequent studies we took spinal cord Pgp levels (as determined by immunoblotting) to indicate Pgp expression levels on blood capillary endothelial cells, consistent with previous studies.87
FIG. 2.
P-glycoprotein (Pgp) expression is localized to blood vessels in the intact spinal cord. (A) Coronal spinal cord section with Pgp immunoreactivity in green. Cartoon indicates field of view (red-bounded box). Scale bar=250 μm. (B) Pgp immunoreactivity (green) co-localizes with the endothelial marker von Willebrand factor (vWF, red), but not with the astrocytic marker glial fibrillary acidic protein (GFAP, blue) in uninjured spinal cord tissue (n=6). Scale bar=100 μm. (C) Pgp immunoreactivity (brown) is localized to the luminal surface of capillary endothelial cells (arrowhead) in uninjured spinal cord tissue (n=6). Nuclei are stained blue. Scale bar=10 μm.
Although humans express just one Pgp isoform (ABCB1), mice and rats express two distinct Pgp gene products: Abcb1a and −1b. Both of these isoforms are expressed in the rodent CNS; however, Abcb1a is the predominantly expressed Pgp isoform in the brain and spinal cord.88 We next sought to verify the efficacy of the primary antibody for immunodetection of Pgp. Total protein samples from uninjured spinal cord tissue of Abcb1A/b−/− mice (Fig. 4A) and Abcb1a−/− rats (Fig. 4B) were analyzed (double Abcb1a/b KO rats were not available at the time of this study). Pgp immunoreactivity was not detectable in samples from KO animals, confirming the utility of the anti-Pgp antibody for subsequent protein expression studies.
FIG. 4.
P-glycoprotein (Pgp) is undetectable in spinal cords of Abcb1a/b−/− mice and Abcb1a−/− rats. Representative immunoblots of total protein extract from spinal cord tissue of uninjured wild-type and Pgp knockout mice (A) and rats (B). Immunoreactivity against Pgp is conspicuously absent in samples from knockout mice and rats. Upper band in A (indicated by arrowhead) is due to nonspecific binding. Anti-Pgp antibody recognizes both Abcb1a and −1b isoforms. n=5 for all groups.
We next investigated the temporal expression profile of Pgp within the spinal cord lesion site at several acute and chronic time points after injury. Pgp expression levels were quantified via immunoblot analysis at 72 h, 7 days, 4 months, and 10 months post-SCI, because of previous observations in our laboratory revealing enhanced inflammatory signaling activity at these time points after injury (R. Grill, unpublished observations). We observed that Pgp expression was significantly higher within the lesion site of injured animals than in thoracic cord tissue of uninjured controls, at 3 days (uninjured=1.00±0.09, SCI=2.92±0.63, p=0.0073), 7 days (uninjured=1.00±0.15, SCI=1.61±0.29, p<0.001), 4 months (uninjured=1.00±0.07, SCI=1.93±0.27, p=0.011), and 10 months (uninjured=1.00±0.05, SCI=4.36±0.67, p<0.001) following SCI (Fig. 5A). These findings support our gene expression data (Fig. 1), providing further evidence that Pgp expression is increased in the spinal cord lesion site in both the acute and chronic phases of injury.
FIG. 5.
Spinal cord P-glycoprotein (Pgp) expression is increased during acute and chronic spinal cord injury (SCI). Pgp levels in spinal cord tissue were quantified by immunoblot analysis and normalized to beta-actin. Representative immunoblots are shown below each graph (U=Uninjured; S=SCI). Cartoons in (B) and (C) indicate locations of spinal cord tissue analyzed. (A) Pgp expression is significantly higher in the spinal lesion site of rats at 3 days (n=7), 7 days (n=8), 4 months (n=6), and 10 months (n=5) post-SCI compared with age-matched controls (n=6-8). (B) In the cervical spinal cord, Pgp expression is significantly increased at 7 days, 4 months, and 10 months following SCI. (C) In the lumbar spinal cord, Pgp expression is significantly increased at 4 months and 10 months following SCI. All data are mean±S.E.M. and normalized to uninjured, age-matched controls. *p<0.05 versus uninjured, age-matched controls.
We also evaluated Pgp levels in spinal cord regions distant from the initial site of injury. We found that Pgp expression was significantly increased within the cervical enlargement (C6-C7) of the spinal cord at 7 days (uninjured=1.00±0.16, SCI=1.89±0.15, p=0.002), 4 months (uninjured=1.00±0.11, SCI=1.97±0.33, p=0.021), and 10 months (uninjured=1.00±0.11, SCI=1.61±0.16, p=0.009) post-SCI (Fig. 5B). Furthermore, Pgp expression was increased in the lumbar enlargement (L3-L4) of the spinal cord at 4 months (uninjured=1.00±0.07, SCI=2.60±0.42, p=0.004) and 10 months (uninjured=1.00±0.07, SCI=1.93±0.27, p=0.011) after injury (Fig. 5C). Therefore, the SCI-associated increase in Pgp expression is not merely restricted to the lesion site; rather, our data suggest that increased Pgp expression is a progressive and spreading phenomenon, which may reflect a larger, ongoing BSCB pathology secondary to SCI.
Role of P-glycoprotein in spinal cord riluzole bioavailability after SCI
Riluzole is a drug that has neuroprotective and antiglutamatergic properties and is FDA-approved for the treatment of ALS.89 Milane and colleagues demonstrated that Pgp overexpression in the brain of a transgenic mouse model of ALS (G86R mSOD1 mice) decreases the brain disposition of systemically delivered riluzole.9 Because riluzole is currently being investigated as an acute SCI intervention in a phase I clinical trial, we next examined whether spinal cord riluzole bioavailability is altered following SCI.44 In order to avoid the confounding effects of “passive” BSCB leakiness, we first chose to examine drug disposition at 3 weeks, a time point by which passive barrier properties are thought to be largely re-established following SCI.74,90–92
Three weeks post-SCI, rats were given a single i.p. bolus of riluzole, and riluzole concentrations in whole spinal cord tissue and plasma were quantified. Spinal cord riluzole levels were normalized to plasma drug levels, as previously described, to accurately reflect spinal cord riluzole disposition.9 We found that at 3 weeks after SCI, spinal cord riluzole disposition was significantly lower than that of uninjured controls (uninjured=100±9.21%, SCI=74.1±5.20%, p=0.031) (Fig. 6A). Riluzole concentrations in spinal cord tissue and plasma were both lower in injured animals than in uninjured controls (plasma: uninjured=100±4.95%, SCI=69.7±6.66%, p=0.003; spinal cord: uninjured=100±5.19%, SCI=52.3±6.00%, p<0.001) (Fig. 6B); however, normalized spinal cord/plasma ratios remained significantly lower in SCI animals (Fig. 6A), supporting our hypothesis of the development of drug resistance in the injured spinal cord.
FIG. 6.
Spinal cord riluzole bioavailability is decreased 21 days after spinal cord injury (SCI). (A) Spinal cord/plasma riluzole ratios were significantly lower in animals 3 weeks post-SCI (n=7) than those of uninjured controls (n=7). (B) Raw riluzole concentrations in plasma and spinal cord tissue were both significantly decreased in injured rats compared with uninjured controls. All data are mean±S.E.M., expressed as percent of control values. *p<0.05 versus controls.
To assess whether the observed reduction in riluzole disposition might be mediated by Pgp, we next assessed riluzole bioavailability in Abcb1a KO rats following SCI. Because Abcb1a−/− rats do not express Pgp at the blood–brain barrier93 or the BSCB (Fig. 4), they are an ideal tool with which to study spinal cord Pgp function in a rat SCI model. For this study, we chose to examine riluzole uptake at an earlier time point after injury, falling within the 2 week treatment period of riluzole that is currently being evaluated in human SCI patients.44 Because we were unsure how well Abcb1a KO rats would tolerate riluzole treatment in the earlier, acute phase of injury, we elected to examine riluzole disposition at the subacute time point of 10 days post-SCI. Ten days after injury, WT and Abcb1a−/− (KO) rats received a single i.p. bolus of riluzole; spinal cord and plasma riluzole concentrations were then quantified (Fig. 7). We found that the spinal cord/plasma riluzole ratio was significantly lower in WT rats 10 days post-SCI compared with uninjured WT controls (WT uninjured=100±10.2%, WT SCI=66.2±2.84%, p=0.005) (Fig. 7A). This effect was caused by a significant injury-induced decrease in spinal cord riluzole concentrations (Fig. 7B), despite no change in plasma drug levels (Fig. 7C). In contrast, there was no detectable difference in spinal cord/plasma riluzole levels between KO injured and uninjured groups (KO uninjured=91.6±5.13%, KO SCI=97.2±6.35%, p=0.005) (Fig. 7A). Notably, riluzole spinal cord disposition was significantly higher in KO rats than in WT rats at 10 days post-SCI (p=0.019), confirming that Pgp plays a critical role in diminishing spinal cord riluzole disposition following SCI.
FIG. 7.
Spinal cord riluzole bioavailability is decreased 10 days after spinal cord injury (SCI) in wild type (WT) but not Abcb1a-/- rats. (A) Spinal cord/plasma riluzole ratios were significantly lower in WT rats with SCI (n=10) than those of WT uninjured controls (n=9). There was no significant difference between spinal cord riluzole disposition in uninjured (n=6) and injured (n=7) knockout (KO) rats. However, normalized riluzole levels were significantly higher in injured KO rats compared with injured WT rats. (B) Spinal cord riluzole levels were significantly reduced in WT rats with SCI (68.4±1.10%) versus WT uninjured controls (100±11.1%, p=0.006). There was no significant effect of injury on spinal cord riluzole levels in KO rats (KO uninjured=84.5±4.83%; KO SCI=75.5±1.77%). (C) Plasma riluzole levels were significantly lower in KO rats with SCI (79.3±5.39%) than in WT rats with SCI (105±4.74%, p=0.009). WT uninjured=100±5.83%; KO uninjured=92.3±3.75%). All data are mean±S.E.M., expressed as a percentage of WT, uninjured controls. *p<0.05. WT, wild-type; KO, Abcb1a knockout; U, uninjured; SCI, 10 days post-SCI.
Licofelone attenuates P-glycoprotein overexpression and enhances riluzole bioavailability after SCI
Pekcec and colleagues demonstrated an important role for prostaglandin E2 (PGE2) in both the driving of Pgp overexpression, and the development of resistance to the anti-epileptic drug phenobarbital, in the brain following seizure.94 We have previously found that an acute SCI-induced spike in spinal cord PGE2 levels is abolished by oral treatment with the dual COX/5-LOX inhibitor licofelone (J. Dulin et al., in press). We therefore evaluated whether treatment with licofelone could reduce the effects of injury on spinal cord Pgp expression and riluzole bioavailability during the earliest time point at which we have observed Pgp upregulation. Animals with SCI were treated with either licofelone (100 mg/kg) or vehicle via oral gavage beginning at 3 h post-SCI, then once daily for 3 days. At 72 h post-SCI, Pgp expression within the T10 lesion site was significantly increased in the injured, vehicle-treated group compared with uninjured controls (uninjured=1.00±0.090, SCI+vehicle=3.06±0.344, p<0.001) (Fig. 8). Importantly, Pgp expression was significantly lower in injured, licofelone-treated animals than in injured, vehicle-treated animals (SCI+licofelone=1.98±0.264, p=0.008).
FIG. 8.
Licofelone treatment attenuates P-glycoprotein (Pgp) overexpression 72 h following spinal cord injury (SCI). Immunoblots of T10 spinal cord samples from rats 72 h post-SCI. Pgp expression levels are normalized to beta-actin and expressed as a percentage of uninjured controls (n=8). Pgp levels in the spinal lesion site of injured, vehicle-treated rats are significantly higher than in uninjured controls (n=10). Pgp levels of injured, licofelone-treated rats are significantly lower than in vehicle-treated rats (n=15), but are still significantly higher than uninjured levels (p=0.022). Representative immunoblots are pictured below graph. All data are mean±S.E.M. *p<0.05.
We next examined the effects of licofelone treatment on spinal cord riluzole disposition. Animals were treated with licofelone or vehicle beginning at 3 h after injury, then once daily for 3 days. All animals also received riluzole (i.p.) on the same dosing schedule. At 72 h post-injury, spinal cord/plasma ratios of riluzole were assessed (Fig. 9). Our results showed that spinal cord/plasma riluzole ratios were significantly lower at 72 h post-SCI relative to uninjured controls (uninjured=100±3.41%, SCI+vehicle=81.1±4.53%, p=0.060). We have previously demonstrated that normalized spinal cord riluzole levels are also significantly decreased in T10 spinal tissue at 10 days post-SCI (Fig. 7), as well as in whole spinal cords at 21 days post-SCI (Fig. 6). Notably, we observed that normalized spinal cord riluzole levels of licofelone-treated animals at 72 h post-SCI were significantly higher than those of vehicle-treated animals (SCI+licofelone=129±14.8%, p=0.020), providing evidence that the attenuation of Pgp expression by licofelone may enhance spinal cord drug delivery following injury.
FIG. 9.
Licofelone treatment enhances spinal cord riluzole bioavailability 72 h after spinal cord injury (SCI). (A) Spinal cord/plasma riluzole ratios were significantly decreased 72 h after SCI in vehicle-treated animals (n=6) versus uninjured controls (n=7). Normalized spinal cord riluzole levels were significantly higher in injured animals treated with licofelone+riluzole (n=8) than in injured, vehicle-treated animals. (B) Compared with raw spinal cord riluzole levels of uninjured control rats (100±3.00%), those of both SCI groups were significantly lower (SCI+vehicle=74.4±8.70%, p=0.022; SCI+licofelone=71.4±8.31%, p=0.016). (C) In contrast, there was no reduction in plasma riluzole concentrations in the injured, vehicle-treated group (93.4±13.2%) compared with uninjured controls (100±3.92%). However, the licofelone-treated group exhibited significantly lower plasma riluzole levels (58.4±8.46%) than both uninjured controls (p=0.036) and injured, vehicle-treated animals (p=0.049). All data are mean±S.E.M., expressed as percentage of uninjured controls. *p<0.05.
Discussion
Potential roles for other ABC transporters in the injured spinal cord
In the current study, we have focused on SCI-induced alterations in expression of the drug transporter Pgp, because of its well-documented role in cancer and various neurological diseases.9–13,55,95–98 However, our results indicate that several other ABC transporter genes are also differentially expressed following SCI (Table 2). It is, therefore, possible that more than one member of this large and diverse family may affect spinal cord drug disposition following injury. At 1 week post-SCI, we observed a 14-fold decrease in expression of Abcc2, which encodes the multidrug resistance-associated protein 2 (Mrp2). Like Pgp, Mrp2 is expressed on the luminal membrane of brain capillary endothelial cells and possesses broad substrate specificity, contributing to efflux of drugs and other compounds.99 Pgp and Mrp2 have some overlap in function; for example, upregulated Pgp functions in a compensatory role for antiepileptic drug transport in rats deficient in Mrp2.100 However, whether reduced activity of this transporter might influence riluzole disposition, for example, in the current study, remains to be determined. Active drug transport at the BSCB is not entirely understood, as it is an extraordinarily complex system composed of multiple, tightly regulated family members that often exhibit functional redundancy.101 Therefore, the pathological consequences of SCI may very well include altered expression and/or activity of multiple drug transporters. Future studies are needed to examine the roles of other drug transporter proteins in the injured spinal cord and the extent to which they might alter the CNS disposition of different bioactive drugs.
Given the broad functional diversity of the ABC transporter family, it is conceivable that some of these proteins, which perform biological functions other than drug transport, might play different roles in SCI pathology. For example, the Abcd2 gene product, the adrenoleukodystrophy-related protein (Aldrp), is expressed in peroxisomal membranes, where it is vital for the transport of very long-chain fatty acids (VLCFA) during normal fatty acid oxidation.102 Dysfunction of Abcd1 and Abcd2 results in accumulation of VLCFA, causing lipotoxicity-induced, demyelinating inflammation of the brain.103 Interestingly, it has recently been shown that this lipotoxic effect of VLCFA accumulation is mediated through 5-LOX activity and enhanced production of the pro-inflammatory cysteinyl leukotrienes (CysLTs) and leukotriene B4 (LTB4).104 It is therefore plausible that the substantial downregulation of Abcd2 during acute SCI may contribute to a similar VLCFA-mediated lipotoxic effect, thus exacerbating inflammation and secondary damage in the days following injury. Developing an understanding of the multifaceted contributions of ABC transporter family members to SCI pathophysiology remains an ongoing topic of investigation in our laboratory.
Increased P-glycoprotein expression and activity in the injured spinal cord
Here, we report that Pgp expression is increased within the spinal lesion site from as early as 72 h post-SCI, to as far as 10 months post-SCI (Fig. 5A). Because of practical limitations, we have only assessed protein expression levels at four acute and chronic time points: 72 h, 7 days, 4 months, and 10 months post-SCI. We have not yet assessed whether Pgp upregulation may occur even earlier than 72 h following SCI. Similarly, we have not examined Pgp expression at time points between 1 week and 4 months. During this large time window, the BSCB is generally thought to regain much of the functional integrity that was lost upon injury to the cord; however, some reports have demonstrated prolonged BSCB dysfunction for months following injury.105,106 In the context of the sparse and incomplete information that has been gained about BSCB function during the subacute and chronic phases of SCI, it will be intriguing to see whether future investigations reveal a relationship between the temporal dynamics of BSCB leakiness and the expression patterns of selective transport systems such as Pgp. Characterizing the relationship between “passive” barrier dysfunction (e.g., loss of tight junctional integrity) and differential regulation of “active” barrier function (i.e., Pgp upregulation) will increase our understanding of how changes in BSCB properties contribute to outcome following traumatic injury to the spinal cord.
We have detected a progressive, spatial spread of increased Pgp expression in regions of the spinal cord centimeters away from the initial site of injury (Fig. 5B and C). Our results indicate that Pgp expression is significantly increased in cervical (C6-C7) spinal cord tissue as early as 1 week after injury at T10, an observation that appears to be sustained for as long as 10 months (Fig. 5B). Similarly, lumbar spinal cord, Pgp expression is significantly higher than controls at both 4 and 10 months post-SCI (Fig. 5C). Together, these findings indicate that the injury-induced increase in Pgp expression is not merely restricted to the T10 lesion site. Rather, this appears to be a progressive and spatially expanding phenomenon, which may reflect a greater, ongoing BSCB pathology secondary to SCI. Patel and colleagues have previously demonstrated BSCB dysfunction in the chronic phase of SCI, as evidenced by enhanced vascular permeability within the lesion site, and in spinal cord tissue away from the epicenter of injury, for up to 8 weeks post-injury.107 Together with these findings, our observations of progressive alterations in Pgp expression suggest the presence of a progressive, spreading BSCB pathology that encompasses both “active” as well as “passive” components of barrier function. As BSCB integrity may be an important determinant of functional outcome following SCI,105 further investigation is needed in order to gain a better understanding of the progression of vascular changes following SCI.
Notably, we have demonstrated that Pgp plays a key role in diminishing the blood-to-spinal cord penetration of the neuroprotective drug riluzole following traumatic SCI (Fig. 7). This is a clinically important observation, as riluzole is currently being evaluated in a phase I clinical trial as an intervention for SCI.44 Our data provide strong support for the existence of Pgp-mediated drug resistance within the injured spinal cord, a phenomenon that acts to diminish the spinal cord bioavailability of systemically administered riluzole following SCI. Moreover, because many drugs that have been evaluated as neuroprotective treatments for acute SCI are Pgp substrates (Table 1), it is possible that increased spinal cord Pgp expression could also impact the efficacy of systemic delivery for a variety of other potentially effective drugs for SCI as well.
Curiously, we observed that plasma riluzole levels were also significantly reduced at 3 weeks after SCI in WT animals (Fig. 6B). We speculate that this observation might be caused by pathological alterations in drug pharmacokinetics (e.g., metabolism or excretion) during this subacute period of injury. Milane and colleagues showed that Abcb1a−/− mice exhibited higher plasma riluzole levels following i.p. injection, presumably because of a reduced biliary excretion rate;108 in contrast, our observations of reduced plasma levels might be explained by increased excretion of riluzole from the plasma compartment. This may be because of SCI-induced upregulation of Pgp in the liver or kidneys, or to otherwise increased drug metabolism and/or excretion secondary to injury. This is a plausible explanation, in light of the clinical evidence showing that drug pharmacokinetics are often altered in SCI patients.109 Further studies will be needed to examine the effects of injury on systemic drug absorption, disposition, and metabolism, as this will provide valuable information about the optimization of treatment regimens for SCI patients.
Our conclusion that Pgp mediates the SCI-associated reduction in spinal cord riluzole uptake is based on the finding that Abcb1a KO rats exhibit no change in uptake following injury, unlike their WT counterparts (Fig. 7). However, we cannot rule out the possibility that phenotypic differences between the strains, because of the use of non-littermate WT controls, might obfuscate interpretation of these results. Although it is unlikely that the effects of within-strain variability would underlie the robust effects reported here, further studies should be performed utilizing littermate controls in order to confirm that these effects are attributed to loss of Pgp.
In these studies, female rats were utilized for the characterization of Pgp gene- and protein-expression profiles, whereas male rats were utilized for all experiments assessing riluzole disposition and licofelone treatment. The primary reason for the use of male rats in the latter studies is our emerging interest on the impact of SCI on the blood–testis barrier,77 causing our laboratory to switch to the use of male rats during the course of this study. Although previous studies have indicated that there are gender-associated differences in Pgp transport activity in body tissues of experimental animals and humans,110 we have not yet investigated whether there is a differential impact of SCI on Pgp expression and/or function in male and female rats. We have compared baseline Pgp expression in uninjured male and female rats, as well as at 72 h after SCI, and we have detected no gender-associated differences (data not shown). However, we cannot rule out the possibility that other gender-specific differences (e.g., differential expression of other drug transporters, or differential Pgp expression levels at later time points after injury) may exist. Regardless, all experiments reported here that assessed riluzole disposition, as well as the effects of licofelone, utilized only male rats, and, therefore, were not confounded by possible sex-dependent effects. Further studies will be needed in order to explore the question of whether males and females exhibit differences in the response of drug transporter systems following SCI.
Potschka and colleagues have demonstrated that following seizure, extracellular glutamate induces an intracellular signaling cascade via COX/PGE2 that drives the upregulation of Pgp expression in the brain.56 More recently, other groups have also reported COX-dependent regulation of Pgp expression in cancer cells21–23 and rodent models of seizure.87,94 Here, we have shown that acute treatment with the COX/5-LOX inhibitor licofelone attenuates the upregulation of Pgp expression by SCI (Fig. 8), suggesting similar regulatory mechanisms within the injured spinal cord. Based on previous reports, Pgp upregulation in the CNS appears to be a common response under neurological conditions characterized by inflammation, excitotoxicity, and oxidative stress.111 It is, therefore, interesting to consider whether enhanced Pgp expression might serve as an endogenous protective response in the injured or diseased CNS. Perhaps this reinforcement of active transport across the barrier is spurred in part by passive deficits in barrier integrity resulting from trauma.105,106 Increased extravasation of circulating substances through the BSCB would lead to accumulation of toxic blood-borne compounds and endogenous metabolites; therefore, an increase in efflux transport activity at the blood–spinal cord interface would function to protect the CNS tissue by contributing to clearance of toxic metabolites. This interplay between “active” and “passive” barrier properties is an area that certainly warrants further investigation, as a better understanding of the response of the BSCB to SCI will be crucial for the development of more effective targeted therapies.
Importantly, we have demonstrated that licofelone treatment can also enhance the spinal cord uptake of systemically delivered riluzole at 72 h post-SCI (Fig. 9). We speculate that this finding may provide a novel therapeutic avenue to circumvent spinal cord drug resistance and improve therapeutic efficacy of systemic drug treatment following SCI. In the absence of a systematic study comparing Pgp expression following COX inhibition with combined COX/5-LOX inhibition in SCI rats, it is difficult to speculate whether licofelone treatment would be more efficacious than treatment with a classical COX inhibitor such as ibuprofen. However, licofelone possesses desirable qualities compared with currently used NSAIDs, including reduced incidence of GI bleeding and ulcers,112 as well as superior anti-inflammatory properties compared with COX inhibitors alone.74 Furthermore, both COX and 5-LOX signaling contribute to the acute secondary pathology of SCI;64,113,114 therefore, inhibiting both arms of the AA signaling cascade may confer greater benefits than COX inhibition. Licofelone has also passed phase III clinical trials for osteoarthritis in Europe,74 making it an attractive candidate for further translational SCI studies.
Based on the results of this study, we propose a working model (Fig. 10) in which Pgp expression at the BSCB is upregulated following traumatic SCI, resulting in a reduction in spinal cord bioavailability of systemically delivered drugs that are Pgp substrates. We propose that this is a novel pathophysiological mechanism arising early after SCI that imparts the spinal cord with drug resistance, thus establishing a major, previously unknown barrier to effective therapeutic intervention for SCI. Our observations hold important implications not only for the acute delivery of neuroprotective drugs immediately after SCI, but also for the long-term clinical management of pain and muscle spasticity in chronic SCI patients. Overcoming Pgp-mediated drug resistance, thereby enhancing spinal cord delivery of systemically administered neuroprotective drugs after SCI, will provide a significant therapeutic advantage to patients who otherwise have little hope for functional recovery.
FIG. 10.
Working model of P-glycoprotein (Pgp)-mediated drug resistance in the injured spinal cord. (A) Under normal conditions, Pgp is expressed on the luminal surface of blood capillary endothelial cells of the blood–spinal cord barrier (BSCB). Blood-borne drug molecules, such as riluzole, must diffuse through the endothelial cell in order to enter spinal cord tissue. However, the drug efflux transport activity of Pgp pumps drugs back into the bloodstream at a discrete rate, thereby limiting disposition of drug molecules into the spinal cord parenchyma. (B) Following SCI, pro-inflammatory cyclooxygenase (COX) signaling within the damaged spinal cord tissue drives Pgp overexpression at the BSCB. This increased Pgp expression and drug transport activity results in diminished drug delivery to the spinal cord. Treating rats with the dual COX/5-lipoxygenase (5-LOX) inhibitor licofelone after SCI attenuates spinal cord Pgp overexpression, thus enhancing spinal cord drug delivery and efficacy of treatment.
Furthermore, we also speculate that this work has defined a new combinatorial mechanism with applications to human disease beyond SCI research. Pgp overexpression is a recognized obstacle to effective treatment of neurological diseases such as ALS9,54 and epilepsy,14 as well as multiple types of cancer.55 The ability to target the molecular signaling pathways driving Pgp upregulation with licofelone may therefore be a highly useful strategy with potential to impact any pathological condition in which Pgp activity reduces efficacy of drug treatment. Importantly, we have shown that licofelone treatment can enhance blood-to-spinal cord delivery of riluzole during acute SCI. We anticipate that this finding has potential to develop into a new combinatorial treatment strategy to attenuate the expression of post-SCI drug resistance and improve therapeutic efficacy for riluzole, as well as a variety of other neuroprotective drugs.
Acknowledgments
This work is supported by grants from the National Institutes of Health (R01 NS049409), Paralyzed Veterans of America (#2511), The Gillson-Longenbaugh Foundation, Mission Connect, a project of The Institute for Rehabilitation and Research (TIRR) Foundation, and the Center for Clinical and Translational Sciences, funded by National Institutes of Health Clinical and Translational Award (TL1 RR024147) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Center for Research Resources or the National Institutes of Health. This research was performed in partial fulfillment of the requirements for the Ph.D. degree from The University of Texas Graduate School of Biomedical Sciences at Houston; The University of Texas Health Science Center at Houston, Texas. The authors gratefully acknowledge Dr. Michael Blackburn for his generosity in the use of his HPLC system.
Author Disclosure Statement
No competing financial interests exist.
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center (UTH NIH Assurance Number: A3413-01).
References
- 1.Kwon B.K. Okon E. Hillyer J. Mann C. Baptiste D. Weaver L.C. Fehlings M.G. Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma. 2011;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman M.M. Pastan I. Ambudkar S.V. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 1996;6:610–617. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]
- 3.Merino V. Jiménez–Torres N.V. Merino–Sanjuán M. Relevance of multidrug resistance proteins on the clinical efficacy of cancer therapy. Curr. Drug Deliv. 2004;1:203–212. doi: 10.2174/1567201043334650. [DOI] [PubMed] [Google Scholar]
- 4.Fromm M.F. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci. 2004;25:423–429. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Begley D.J. P-glycoprotein: the prototypical BBB efflux transporter. In: Taylor E.M., editor. Efflux Transporters and the Blood–Brain Barrier. Nova Science Publishers, Inc.; New York: 2005. pp. 107–135. [Google Scholar]
- 6.Baldini N. Scotlandi K. Barbanti–Bròdano G. Manara M.C. Maurici D. Bacci G. Bertoni F. Picci P. Sottili S. Campanacci M. Serra M. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N. Engl. J. Med. 1995;333:1380–1385. doi: 10.1056/NEJM199511233332103. [DOI] [PubMed] [Google Scholar]
- 7.Marie J.P. Zittoun R. Sikic B.I. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood. 1991;78:586–592. [PubMed] [Google Scholar]
- 8.Tan B. Piwnica–Worms D. Ratner L. Multidrug resistance transporters and modulation. Curr. Opin. Oncol. 2000;12:450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Milane A. Fernandez C. Dupuis L. Buyse M. Loeffler J.P. Farinotti R. Meininger V. Bensimon G. P-glycoprotein expression and function are increased in an animal model of amyotrophic lateral sclerosis. Neurosci. Lett. 2010;472:166–170. doi: 10.1016/j.neulet.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Spudich A. Kilic E. Xing H. Kilic U. Rentsch K.M. Wunderli–Allenspach H. Bassetti C.L. Hermann D.M. Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat. Neurosci. 2006;9:487–488. doi: 10.1038/nn1676. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S. Hartz A.M. Elmquist W.F. Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr. Pharm. Des. 2011;17:2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Klerk O.L. Willemsen A.T. Bosker F.J. Bartels A.L. Hendrikse N.H. den Boer J.A. Dierckx R.A. Regional increase in P-glycoprotein function in the blood–brain barrier of patients with chronic schizophrenia: a PET study with [(11)C]verapamil as a probe for P-glycoprotein function. Psychiatry Res. 2010;183:151–156. doi: 10.1016/j.pscychresns.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Breedveld P. Beijnen J.H. Schellens J.H. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol. Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Bauer B. Hartz A.M. Pekcec A. Toellner K. Miller D.S. Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood–brain barrier through glutamate and cyclooxygenase-2 signaling. Mol. Pharmacol. 2008;73:1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 15.Potschka H. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol. Ther. 2010;125:118–127. doi: 10.1016/j.pharmthera.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Amar A.P. Levy M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 17.Tator C.H. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J. Spinal Cord Med. 1996;19:206–214. doi: 10.1080/10790268.1996.11719436. [DOI] [PubMed] [Google Scholar]
- 18.Tator C.H. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery. 1998;42:696–707. doi: 10.1097/00006123-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Young W. Bracken M.B. The Second National Acute Spinal Cord Injury Study. J. Neurotrauma. 1992;9(Suppl 1):S397–405. [PubMed] [Google Scholar]
- 20.Zeidman S.M. Ling G.S. Ducker T.B. Ellenbogen R.G. Clinical applications of pharmacologic therapies for spinal cord injury. J. Spinal Disord. 1996;9:367–380. [PubMed] [Google Scholar]
- 21.Bracken M.B. Shepard M.J. Hellenbrand K.G. Collins W.F. Leo L.S. Freeman D.F. Wagner F.C. Flamm E.S. Eisenberg H.M. Goodman J.H. Perot P.L., Jr. Green B.A. Grossman R.G. Meagher J.N. Young W. Fischer B. Clifton G.L. Hunt W.E. Rifkinson N. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J. Neurosurg. 1985;63:704–713. doi: 10.3171/jns.1985.63.5.0704. [DOI] [PubMed] [Google Scholar]
- 22.Bracken M.B. Shepard M.J. Collins W.F., Jr. Holford T.R. Baskin D.S. Eisenberg H.M. Flamm E. Leo–Summers L. Maroon J.C. Marshall L.F. Perot P.L., Jr. Piepmeier J. Sonntag V. Wagner F.C., Jr. Wilberger J.L. Winn H.R. Young W. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 23.Bracken M.B. Shepard M.J. Collins W.F. Holford T.R. Young W. Baskin D.S. Eisenberg H.M. Flamm E. Leo–Summers L. Maroon J. Marshall L.F. Perot P.L., Jr. Piepmeier J. Sonntag V. Wagner F.C. Wilberger J.E. Winn H.R. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 24.Hurlbert R.J. Hamilton M.G. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can. J. Neurol. Sci. 2008;35:41–45. doi: 10.1017/s031716710000754x. [DOI] [PubMed] [Google Scholar]
- 25.Tator C.H. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- 26.Bracken M.B. Shepard M.J. Holford T.R. Leo–Summers L. Aldrich E.F. Fazl M. Fehlings M.G. Herr D.L. Hitchon P.W. Marshall L.F. Nockels R.P. Pascale V. Perot P.L., Jr. Piepmeier J. Sonntag V.K. Wagner F. Wilberger J.E. Winn H.R. Young W. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J. Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 27.Hawryluk G.W. Rowland J. Kwon B.K. Fehlings M.G. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg. Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- 28.Saitoh H. Hatakeyama M. Eguchi O. Oda M. Takada M. Involvement of intestinal P-glycoprotein in the restricted absorption of methylprednisolone from rat small intestine. J. Pharm. Sci. 1998;87:73–75. doi: 10.1021/js970163u. [DOI] [PubMed] [Google Scholar]
- 29.Koszdin K.L. Shen D.D. Bernards C.M. Spinal cord bioavailability of methylprednisolone after intravenous and intrathecal administration: the role of P-glycoprotein. Anesthesiology. 2000;92:156–163. doi: 10.1097/00000542-200001000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Bernards C.M. Cyclosporine–A-mediated inhibition of p-glycoprotein increases methylprednisolone entry into the central nervous system. Spinal Cord. 2006;44:414–420. doi: 10.1038/sj.sc.3101863. [DOI] [PubMed] [Google Scholar]
- 31.Festoff B.W. Ameenuddin S. Arnold P.M. Wong A. Santacruz K.S. Citron B.A. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J. Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.M. Yune T.Y. Kim S.J. Park D.W. Lee Y.K. Kim Y.C. Oh Y.J. Markelonis G.J. Oh T.H. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 33.Stirling D.P. Khodarahmi K. Liu J. McPhail L.T. McBride C.B. Steeves J.D. Ramer M.S. Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yune T.Y. Lee J.Y. Jung G.Y. Kim S.J. Jiang M.H. Kim Y.C. Oh Y.J. Markelonis G.J. Oh T.H. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J. Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchand F. Tsantoulas C. Singh D. Grist J. Clark A.K. Bradbury E.J. McMahon S.B. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur. J. Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 36.University of Calgary. Minocycline and Perfusion Pressure Augmentation in Acute Spinal Cord Injury. 2007. http://clinicaltrials.gov/show/NCT00559494. [Aug 26;2012 ]. http://clinicaltrials.gov/show/NCT00559494 NLM Identifier: NCT00559494.
- 37.Milane A. Fernandez C. Vautier S. Bensimon G. Meininger V. Farinotti R. Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood–brain barrier. J. Neurochem. 2007;103:164–173. doi: 10.1111/j.1471-4159.2007.04772.x. [DOI] [PubMed] [Google Scholar]
- 38.Fehlings M.G. Agrawal S. Role of sodium in the pathophysiology of secondary spinal cord injury. Spine (Phila Pa 1976) 1995;20:2187–2191. doi: 10.1097/00007632-199510001-00002. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz G. Fehlings M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. 2001;94:245–256. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz G. Fehlings M.G. Secondary injury mechanisms of spinal cord trauma: a novel therapeutic approach for the management of secondary pathophysiology with the sodium channel blocker riluzole. Prog Brain Res. 2002;137:177–190. doi: 10.1016/s0079-6123(02)37016-x. [DOI] [PubMed] [Google Scholar]
- 41.Wang S.J. Wang K.Y. Wang W.C. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neuroscience. 2004;125:191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Hama A. Sagen J. Antinociceptive effect of riluzole in rats with neuropathic spinal cord injury pain. J. Neurotrauma. 2011;28:127–134. doi: 10.1089/neu.2010.1539. [DOI] [PubMed] [Google Scholar]
- 43.Kitzman P.H. Effectiveness of riluzole in suppressing spasticity in the spinal cord injured rat. Neurosci. Lett. 2009;455:150–153. doi: 10.1016/j.neulet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 44.The Methodist Hospital System. Safety of Riluzole in Patients With Acute Spinal Cord Injury. 2009. http://clinicaltrials.gov/show/NCT00876889. [Aug 26;2012 ]. http://clinicaltrials.gov/show/NCT00876889 NLM Identifier: NCT00876889.
- 45.Fehlings M.G. Tator C.H. Linden R.D. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J. Neurosurg. 1989;71:403–416. doi: 10.3171/jns.1989.71.3.0403. [DOI] [PubMed] [Google Scholar]
- 46.Petitjean M.E. Pointillart V. Dixmerias F. Wiart L. Sztark F. Lassié P. Thicoïpé M. Dabadie P. Medical treatment of spinal cord injury in the acute stage [in French] Ann. Fr. Anesth. Reanim. 1998;17:114–122. doi: 10.1016/s0750-7658(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 47.Pointillart V. Petitjean M.E. Wiart L. Vital J.M. Lassié P. Thicoïpé M. Dabadie P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71–76. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L. Liu X.D. Xie L. Wang G.J. P-glycoprotein restricted transport of nimodipine across blood–brain barrier. Acta Pharmacol. Sin. 2003;24:903–906. [PubMed] [Google Scholar]
- 49.Baastrup C. Finnerup N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 50.Wiffen P.J. Derry S. Moore R.A. Lamotrigine for acute and chronic pain. Cochrane Database Syst. Rev. 2011;2:CD006044. doi: 10.1002/14651858.CD006044.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luna–Tortós C. Fedrowitz M. Löscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;55:1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 52.Potschka H. Fedrowitz M. Löscher W. P-Glycoprotein-mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood–brain barrier: evidence from microdialysis experiments in rats. Neurosci. Lett. 2002;327:173–176. doi: 10.1016/s0304-3940(02)00423-8. [DOI] [PubMed] [Google Scholar]
- 53.Uhr M. Steckler T. Yassouridis A. Holsboer F. Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood–brain barrier deficiency due to mdr1a P-glycoprotein gene disruption. Neuropsychopharmacology. 2000;22:380–387. doi: 10.1016/S0893-133X(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 54.Jablonski M.R. Jacob D.A. Campos C. Miller D.S. Maragakis N.J. Pasinelli P. Trotti D. Selective increase of two ABC drug efflux transporters at the blood–spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol. Dis. 2012;47:194–200. doi: 10.1016/j.nbd.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coley H.M. Overcoming multidrug resistance in cancer: clinical studies of p-glycoprotein inhibitors. Methods Mol. Biol. 2010;596:341–358. doi: 10.1007/978-1-60761-416-6_15. [DOI] [PubMed] [Google Scholar]
- 56.Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010;51:1333–1347. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu B. Qu L. Tao H. Cyclo-oxygenase 2 up-regulates the effect of multidrug resistance. Cell Biol. Int. 2009;34:21–25. doi: 10.1042/CBI20090129. [DOI] [PubMed] [Google Scholar]
- 58.Szczuraszek K. Materna V. Halon A. Mazur G. Wróbel T. Kuliczkowski K. Maciejczyk A. Zabel M. Drag M. Dietel M. Lage H. Surowiak P. Positive correlation between cyclooxygenase-2 and ABC-transporter expression in non-Hodgkin's lymphomas. Oncol. Rep. 2009;22:1315–1323. doi: 10.3892/or_00000570. [DOI] [PubMed] [Google Scholar]
- 59.Xia W. Zhao T. Lv J. Xu S. Shi J. Wang S. Han X. Sun Y. Celecoxib enhanced the sensitivity of cancer cells to anticancer drugs by inhibition of the expression of P-glycoprotein through a COX-2-independent manner. J. Cell. Biochem. 2009;108:181–194. doi: 10.1002/jcb.22239. [DOI] [PubMed] [Google Scholar]
- 60.Ye C.G. Wu W.K. Yeung J.H. Li H.T. Li Z.J. Wong C.C. Ren S.X. Zhang L. Fung K.P. Cho C.H. Indomethacin and SC236 enhance the cytotoxicity of doxorubicin in human hepatocellular carcinoma cells via inhibiting P-glycoprotein and MRP1 expression. Cancer Lett. 2011;304:90–96. doi: 10.1016/j.canlet.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Zrieki A. Farinotti R. Buyse M. Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm. Res. 2008;25:1991–2001. doi: 10.1007/s11095-008-9596-1. [DOI] [PubMed] [Google Scholar]
- 62.Rafati D.S. Geissler K. Johnson K. Unabia G. Hulsebosch C. Nesic–Taylor O. Perez–Polo J.R. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J. Neurosci. Res. 2008;86:566–580. doi: 10.1002/jnr.21508. [DOI] [PubMed] [Google Scholar]
- 63.Resnick D.K. Graham S.H. Dixon C.E. Marion D.W. Role of cyclooxygenase 2 in acute spinal cord injury. J. Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 64.Rainsford K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 65.Smith W.L. DeWitt D.L. Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 66.Fiorucci S. Meli R. Bucci M. Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem. Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 67.Hirsch F.R. Lippman S.M. Advances in the biology of lung cancer chemoprevention. J. Clin. Oncol. 2005;23:3186–3197. doi: 10.1200/JCO.2005.14.209. [DOI] [PubMed] [Google Scholar]
- 68.Mao J.T. Tsu I.H. Dubinett S.M. Adams B. Sarafian T. Baratelli F. Roth M.D. Serio K.J. Modulation of pulmonary leukotriene B4 production by cyclooxygenase-2 inhibitors and lipopolysaccharide. Clin. Cancer Res. 2004;10:6872–6878. doi: 10.1158/1078-0432.CCR-04-0945. [DOI] [PubMed] [Google Scholar]
- 69.Mao J.T. Roth M.D. Serio K.J. Baratelli F. Zhu L. Holmes E.C. Strieter R.M. Dubinett S.M. Celecoxib modulates the capacity for prostaglandin E2 and interleukin-10 production in alveolar macrophages from active smokers. Clin. Cancer Res. 2003;9:5835–5841. [PubMed] [Google Scholar]
- 70.Okunishi K. Peters–Golden M. Leukotrienes and airway inflammation. Biochim. Biophys. Acta. 2011;1810:1096–1102. doi: 10.1016/j.bbagen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szczeklik A. Mechanism of aspirin-induced asthma. Allergy. 1997;52:613–619. doi: 10.1111/j.1398-9995.1997.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 72.Ye Y.N. Wu W.K. Shin V.Y. Bruce I.C. Wong B.C. Cho C.H. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 73.Alvaro–Gracia J.M. Licofelone—clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis. Rheumatology (Oxford) 2004;43(Suppl. 1):i21–25. doi: 10.1093/rheumatology/keh105. [DOI] [PubMed] [Google Scholar]
- 74.Cicero A.F. Laghi L. Activity and potential role of licofelone in the management of osteoarthritis. Clin. Interv. Aging. 2007;2:73–79. doi: 10.2147/ciia.2007.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geurts A.M. Cost G.J. Rémy S. Cui X. Tesson L. Usal C. Ménoret S. Jacob H.J. Anegon I. Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol. Biol. 2010;597:211–225. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 76.Herrera J.J. Haywood–Watson R.J., 2nd Grill R.J. Acute and chronic deficits in the urinary bladder after spinal contusion injury in the adult rat. J. Neurotrauma. 2010;27:423–431. doi: 10.1089/neu.2009.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dulin J.N. Moore M.L. Gates K.W. Queen J.H. Grill R.J. Spinal cord injury causes sustained disruption of the blood–testis barrier in the rat. PLoS One. 2011;6:e16456. doi: 10.1371/journal.pone.0016456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheff S.W. Rabchevsky A.G. Fugaccia I. Main J.A. Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 79.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 80.Maltese A. Maugeri F. Drago F. Bucolo C. Simple determination of riluzole in rat brain by high-performance liquid chromatography and spectrophotometric detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;817:331–334. doi: 10.1016/j.jchromb.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 81.Colovic M. Zennaro E. Caccia S. Liquid chromatographic assay for riluzole in mouse plasma and central nervous system tissues. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;803:305–309. doi: 10.1016/j.jchromb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Beggs J.L. Waggener J.D. Transendothelial vesicular transport of protein following compression injury to the spinal cord. Lab. Invest. 1976;34:428–439. [PubMed] [Google Scholar]
- 83.Noble L.J. Mautes A.E. Hall J.J. Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. J. Comp. Neurol. 1996;376:542–556. doi: 10.1002/(SICI)1096-9861(19961223)376:4<542::AID-CNE4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 84.Noble L.J. Wrathall J.R. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- 85.Sinescu C. Popa F. Grigorean V.T. Onose G. Sandu A.M. Popescu M. Burnei G. Strambu V. Popa C. Molecular basis of vascular events following spinal cord injury. J. Med. Life. 2010;3:254–261. [PMC free article] [PubMed] [Google Scholar]
- 86.Cordon–Cardo C. O'Brien J.P. Casals D. Rittman–Grauer L. Biedler J.L. Melamed M.R. Bertino J.R. Multidrug–resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. U. S. A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Vliet E.A. Zibell G. Pekcec A. Schlichtiger J. Edelbroek P.M. Holtman L. Aronica E. Gorter J.A. Potschka H. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58:404–412. doi: 10.1016/j.neuropharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Hartz A.M. Bauer B. ABC transporters in the CNS – an inventory. Curr. Pharm. Biotechnol. 2011;12:656–673. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- 89.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 90.Pan W. Kastin A.J. Gera L. Stewart J.M. Bradykinin antagonist decreases early disruption of the blood–spinal cord barrier after spinal cord injury in mice. Neurosci. Lett. 2001;307:25–28. doi: 10.1016/s0304-3940(01)01904-8. [DOI] [PubMed] [Google Scholar]
- 91.Pan W. Kastin A.J. Increase in TNFalpha transport after SCI is specific for time, region, and type of lesion. Exp. Neurol. 2001;170:357–363. doi: 10.1006/exnr.2001.7702. [DOI] [PubMed] [Google Scholar]
- 92.Pan W. Csernus B. Kastin A.J. Upregulation of p55 and p75 receptors mediating TNF-alpha transport across the injured blood–spinal cord barrier. J. Mol. Neurosci. 2003;21:173–184. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- 93.Chu X. Zhang Z. Yabut J. Horwitz S. Levorse J. Li X.Q. Zhu L. Lederman H. Ortiga R. Strauss J. Li X. Owens K.A. Dragovic J. Vogt T. Evers R. Shin M.K. Characterization of multidrug resistance 1a/P-glycoprotein knockout rats generated by zinc finger nucleases. Mol. Pharmacol. 2012;81:220–227. doi: 10.1124/mol.111.074179. [DOI] [PubMed] [Google Scholar]
- 94.Pekcec A. Unkrüer B. Schlichtiger J. Soerensen J. Hartz A.M. Bauer B. van Vliet E.A. Gorter J.A. Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J. Pharmacol. Exp. Ther. 2009;330:939–947. doi: 10.1124/jpet.109.152520. [DOI] [PubMed] [Google Scholar]
- 95.Furuno T. Landi M.T. Ceroni M. Caporaso N. Bernucci I. Nappi G. Martignoni E. Schaeffeler E. Eichelbaum M. Schwab M. Zanger U.M. Expression polymorphism of the blood–brain barrier component P-glycoprotein (MDR1) in relation to Parkinson's disease. Pharmacogenetics. 2002;12:529–534. doi: 10.1097/00008571-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Vogelgesang S. Cascorbi I. Schroeder E. Pahnke J. Kroemer H.K. Siegmund W. Kunert–Keil C. Walker L.C. Warzok R.W. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Langford D. Grigorian A. Hurford R. Adame A. Ellis R.J. Hansen L. Masliah E. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J. Neuropathol. Exp. Neurol. 2004;63:1038–1047. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- 98.Bartels A.L. Willemsen A.T. Kortekaas R. de Jong B.M. de Vries R. de Klerk O. van Oostrom J.C. Portman A. Leenders K.L. Decreased blood–brain barrier P-glycoprotein function in the progression of Parkinson's disease, PSP and MSA. J. Neural. Transm. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wijnholds J. Multidrug resistance-associated proteins and efflux of organic anions at the blood–brain and blood cerebrospinal fluid barriers. In: Taylor E.M., editor. Efflux Transporters and the Blood–Brain Barrier. Nova Biomedical Books; New York: 2005. pp. 137–156. [Google Scholar]
- 100.Hoffmann K. Löscher W. Upregulation of brain expression of P-glycoprotein in MRP2-deficient TR(-) rats resembles seizure-induced up-regulation of this drug efflux transporter in normal rats. Epilepsia. 2007;48:631–645. doi: 10.1111/j.1528-1167.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 101.Begley D.J. ABC transporters and the blood–brain barrier. Curr. Pharm. Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 102.Kim W.S. Weickert C.S. Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 103.Pujol A. Ferrer I. Camps C. Metzger E. Hindelang C. Callizot N. Ruiz M. Pàmpols T. Giròs M. Mandel J.L. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004;13:2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- 104.Khan M. Singh J. Gilg A.G. Uto T. Singh I. Very long-chain fatty acid accumulation causes lipotoxic response via 5-lipoxygenase in cerebral adrenoleukodystrophy. J. Lipid Res. 2010;51:1685–1695. doi: 10.1194/jlr.M002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen D.M. Patel C.B. Ahobila–Vajjula P. Sundberg L.M. Chacko T. Liu S.J. Narayana P.A. Blood–spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Whetstone W.D. Hsu J.Y. Eisenberg M. Werb Z. Noble–Haeusslein L.J. Blood–spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel C.B. Cohen D.M. Ahobila–Vajjula P. Sundberg L.M. Chacko T. Narayana P.A. Effect of VEGF treatment on the blood–spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J. Neurotrauma. 2009;26:1005–1016. doi: 10.1089/neu.2008.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milane A. Tortolano L. Fernandez C. Bensimon G. Meininger V. Farinotti R. Brain and plasma riluzole pharmacokinetics: effect of minocycline combination. J. Pharm. Pharm. Sci. 2009;12:209–217. doi: 10.18433/j36c78. [DOI] [PubMed] [Google Scholar]
- 109.Segal J.L. Brunnemann S.R. Clinical pharmacokinetics in patients with spinal cord injuries. Clin. Pharmacokinet. 1989;17:109–129. doi: 10.2165/00003088-198917020-00004. [DOI] [PubMed] [Google Scholar]
- 110.Bebawy M. Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr. Drug Metab. 2009;10:322–328. doi: 10.2174/138920009788498996. [DOI] [PubMed] [Google Scholar]
- 111.Hong H. Lu Y. Ji Z.N. Liu G.Q. Up-regulation of P-glycoprotein expression by glutathione depletion-induced oxidative stress in rat brain microvessel endothelial cells. J. Neurochem. 2006;98:1465–1473. doi: 10.1111/j.1471-4159.2006.03993.x. [DOI] [PubMed] [Google Scholar]
- 112.Bias P. Buchner A. Klesser B. Laufer S. The gastrointestinal tolerability of the LOX/COX inhibitor, licofelone, is similar to placebo and superior to naproxen therapy in healthy volunteers: results from a randomized, controlled trial. Am. J. Gastroenterol. 2004;99:611–618. doi: 10.1111/j.1572-0241.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 113.Genovese T. Mazzon E. Rossi A. Di Paola R. Cannavò G. Muià C. Crisafulli C. Bramanti P. Sautebin L. Cuzzocrea S. Involvement of 5-lipoxygenase in spinal cord injury. J. Neuroimmunol. 2005;166:55–64. doi: 10.1016/j.jneuroim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 114.Genovese T. Rossi A. Mazzon E. Di Paola R. Muià C. Caminiti R. Bramanti P. Sautebin L. Cuzzocrea S. Effects of zileuton and montelukast in mouse experimental spinal cord injury. Br. J. Pharmacol. 2008;153:568–582. doi: 10.1038/sj.bjp.0707577. [DOI] [PMC free article] [PubMed] [Google Scholar]